Abstract
Polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) are persistent organic pollutants (POPs) that are present as complex mixtures in all environmental compartments, including aquatic ecosystems. However, little is known about the effects of such complex mixtures on teleost behaviour. In this study, zebrafish (Danio rerio) were chronically exposed to an environmentally relevant mixture (MIX) containing 22 PCB and 7 PBDE congeners through diet from 5 days post fertilization onwards. MIX-exposed F0 fish produced offspring (F1 and F2 generations) that were fed using plain food and grown until adulthood. In each generation, five behavioural traits (i.e. boldness, activity, sociality, exploration and anxiety) were evaluated by the mean of different experimental set-ups. Two distinct behavioural syndromes were identified: boldness, positively correlated to activity and exploration; and anxiety, associated with low sociality. F0 fish did not display any behavioural disruption resulting from POP exposure whereas F1 MIX fish were bolder than fish from other generations but did not differ significantly from F1 controls. F2 MIX fish displayed a higher anxiety syndrome than F2 controls. This is of particular importance since such behavioural changes in offspring generations may have persistent ecological consequences, may affect fitness and hence cause detrimental effects on wild fish populations exposed to POP mixtures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Following the increase in industrial production, several thousands of chemicals have emerged since the twentieth century. Among all of them, persistent organic pollutants (POPs) gather molecules that are hydrophobic, lipophilic, little or no metabolized by organisms and have a long half-life in the environment (McFarland and Clarke 1989; Jones and de Voogt 1999; Gray 2002; Luo et al. 2007). Polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) are two POPs’ families of 209 organic congeners each (McFarland and Clarke 1989; Rahman et al. 2001). PCBs were used since the 1930s in several industrial applications (e.g. dielectric fluids in electrical capacitors, transformers and hydraulic systems) (Environment PUN 1999) while PBDEs were being used since the 1970s as flame retardants (e.g. in plastics, furniture, house equipment, electrical and electronic devices, textiles and other household products) (United Nations Environment Programme 2012). To these ends, PCBs and PBDEs were produced as complex mixtures of numerous congeners, e.g. Aroclor, Kanechlor or Clophen in the case of PCBs (Kannan 2000) and e.g. penta-, octa- and decaBDE for PBDEs (Darnerud et al. 2001; Rahman et al. 2001; De Wit 2002). As concerns about their toxicity were increasing, regulatory rules were adopted to progressively ban their use since the 1970s for PCBs and in the early 2000s for PBDEs (Breivik et al. 2007; Kemmlein et al. 2009).
During their use, pollution of aquatic and terrestrial environment by PCBs and PBDEs was reported through wastewater discharges, leaching fields or atmospheric fallout (Jensen et al. 1969; Darnerud et al. 2001; Breivik et al. 2007; Luo et al. 2007; Robinson et al. 2017). This resulted in contamination of aquatic and terrestrial ecosystems and organisms (Rusiecki et al. 2008; Couderc et al. 2015; Holma-Suutari et al. 2016). Since their ban, environmental concentrations have been reduced; however, due to their persistence, they are still detected in all environmental compartments around the world, for example by the remobilization of sediments during trawling, dredging or flood episodes (Puzyn and Mostrag 2012). The effects of PCBs and PBDEs on organisms are diverse, depending on the chemical properties of the congeners to which they are exposed. In particular, they have been shown to act as endocrine disrupters, mainly affecting thyroid hormone (TH) pathway (Safe et al. 2001; Khan et al. 2002; Legler and Brouwer 2003; Yang et al. 2005; Otake et al. 2007; Calò et al. 2010), and capable of interfering with stress and sexual steroid metabolism as well (Hany et al. 1999; Jørgensen et al. 2002; Aluru et al. 2004; Yang et al. 2005; Calò et al. 2010; Kumar et al. 2020). Disruption of endocrine pathways can have profound consequences on individual fitness. Exposure to POPs was shown for instance to negatively impact growth and reproduction (Daouk et al. 2011; Horri 2018; Horri et al. 2018), and to interfere with brain development and function resulting in behavioural alterations such as increase in anxiety and cognitive decline in human and animal models (Kodavanti 2005; Dingemans et al. 2011; Macaulay et al. 2015; Gonzalez et al. 2016; Klocke and Lein 2020).
In the current literature, biological effects mentioned above were mainly studied following acute exposure to rather high concentrations of single compounds when compared to that measured in the environment up to µg/g (e.g. Billsson et al. 1998; Orn et al. 1998; Mccarthy et al. 2003; Wang et al. 2012; Gonzalez et al. 2016; Lovato et al. 2016). These high concentration exposures are useful to identify biological pathways affected by these pollutants but provide less relevant insights about consequences for organisms in environmental conditions. Indeed, pollutants generally occur in the wild as mixture of a large number of compounds present at low concentrations and they induce lifelong chronic exposure to complex mixtures (Barakat et al. 2002; Law et al. 2006; Puzyn and Mostrag 2012; Robinson et al. 2017).
Besides, it was shown that PCBs and PBDEs can be maternally transferred to F1 offspring and this transfer may elicit behavioural defects observed in F1 offspring after parental exposure (Daouk et al. 2011; Yu et al. 2011; Chen et al. 2012, 2017; Péan et al. 2013; Alfonso et al. 2019a; Blanc et al. 2021). Furthermore, data showed that effects of chemical exposure can be observed beyond the F1 generation as a possible consequence of epigenetic modifications, and result in multi or transgenerational behavioural effects (Xin et al. 2015; Carvan et al. 2017). In the same research project as the present paper, we previously reported that exposure to an environmental mixture of PCBs and PBDEs interferes with anxiety-like behaviour of unexposed F2 offspring (Alfonso et al. 2019a) and further disrupt mitochondrial function, lipid metabolism and neurotransmission in the brain (Blanc et al. 2021).
In zebrafish (Danio rerio) juveniles and adults, one of the most frequently used test in (eco)toxicological studies, the novel-tank diving (or bottom-dwelling test), allows to measure the anxiety level of fish, i.e. the more time the fish spend at the bottom of the tank, the more anxious they are (Egan et al. 2009). Modification of anxiety level can interfere with many essential behaviours and therefore modify individual fitness of ecological importance. However, animal behavioural repertoires rely on a wider set of traits (e.g. foraging, social interaction, environment exploration, aggression, boldness) which can all be affected by external stressors and are all important for individual fitness and recruitment and so for population sustainability (Réale et al. 2007). Some of these traits could covary forming a behavioural syndrome (Sih et al. 2004). For instance, bold individuals are generally more aggressive and explore faster their environment. Despite their high ecological relevance, effects of environmental chemicals on multiple behavioural traits and behavioural syndromes are, however, understudied (e.g. Klaminder et al. 2019; Martin et al. 2019; Hamilton et al. 2021).
In the present study, we intended to evaluate disruption of a set of behavioural traits following dietary exposure to an environmentally relevant mixture of POPs (PCBs and PBDEs). The main goals were to investigate (1) whether ecologically relevant behavioural traits of interest (i.e. anxiety, boldness, activity, exploration and sociality) of exposed individuals are affected and (2) how unexposed offspring (F1 and F2) can be further impacted. Fish from all generations (F0, F1 and F2) were individually screened for different behavioural traits in a multi-testing approach. A multi-parametric analysis was then performed to study the relationship between the different behavioural traits and to assess the potential impact of this exposure on behavioural syndromes in zebrafish.
Material and methods
Fish reproduction and rearing
The study was performed on the zebrafish wild type strain TU (ZFIN ID: ZDB-GENO-990623–3) kept at the Ifremer Fish Ecophysiology Platform (L’Houmeau, 17 France) and originated from the European Zebrafish Resources Centre (EZRC, Karlsruhe, Germany).
Fish were reared at 27 ± 1 °C and at pH = 7.5 ± 0.5 under a 14 h/10 h light/dark cycle, with light onset at 9 a.m. Physical water conditions were maintained constant during the experiment and were within recommended ranges (Lawrence 2007). Eggs were obtained at around 180 days post fertilization (dpf) by random pairwise mating using adults (6 pairs, mixed spawns of 10 eggs from each spawn to reach 60 eggs per Petri dish × 5 replicates) (Alfonso et al. 2019a). At least 5 replicates of 30 adult individuals each were reared per treatment per generation (i.e. 5*30 = 150 individuals/treatment per generation). During the rearing from larval to adult stage, the stocking density was 0.12 kg/m3 during the larval stage while it was 9 kg/m3 for adults. Larvae were initially reared in Petri dishes at a rate of 60 larvae per Petri dish. At 5 dpf, the groups of 60 larvae were transferred from their Petri dishes to separate 1-L tanks (AquaSchwarz, Göttingen, Germany). At 15 dpf, the groups of larvae were transferred to tubes inserted inside separate 10-L rearing tanks (Aquatic Habitat, Apopka, FL; USA) disposed on flow-through racks and were then freed into the tanks at 27 dpf (Vignet et al. 2014).
MIX diet preparation, F0 exposure and F1-F2 regime
The MIX diet was spiked with 22 PCB congeners (CB-8, -18, -28, -31, -44, -49, -52, -77, -101, -105, -110, -118, -128, -132, -138, -149, -153, -156,—170, -180, -187 and -194) and 7 PBDE congeners (BDE-28, -47, -99, -100, -153, -183 and -209) all with purity above 98% (AccuStandard Inc., New Haven, USA). PCBs and PBDE congeners were selected as being the most representative in marine biota, and in concentrations reflecting profiles observed in mussels from the Seine Estuary in France (Abarnou et al. 2000). They cover a large range of chlorine (2–8) or bromine (3–10) substitutions and hydrophobicity, with log Kow (octanol/water partition coefficient) from 5.07 to 7.80 for PCBs and 6.7 to 12.1 for PBDEs. (Abarnou et al. 2000; OSPAR 2009, 2013). Following the same authors, relative proportions of congeners were chosen to simulate environmentally relevant mixtures (Abarnou et al. 2000; OSPAR 2009, 2013). Full description of MIX diet preparation is given in Alfonso et al. (2019a). Briefly, MIX diet was prepared after dilution of to each age (SDS100 to SDS400 (Special Diet Service, France) and Inicio + 500 μm (Biomar, France)). Control diet was prepared in the same manner, with addition of isooctane only. In the MIX diet (n = 12), total concentrations summed across 22 PCB and 7 PBDE congeners were 1932.3 ± 90.4 ng/g (mean ± SE) wet weight (ww) for ∑PCBs and 479.8 ± 50.8 ng/g ww for ∑PBDEs. In the control diet (n = 16), total concentrations were 7.9 ± 3.5 ng/g ww for ∑PCBs and 0.92 ± 0.36 ng/g ww for ∑PBDEs. Such values underline that raw material used to produce plain food likely contained low contaminated fish products, further they also show no cross-contamination between treatments (Alfonso et al. 2019a). POP concentrations were measured using high performance liquid chromatography (see Horri et al. (2018) for details). Substitution numbers, targeted and actual diet concentrations for each congener are given in Alfonso et al. (2019a). F0 fish were exposed using food of appropriate size following the same schedule as described in Horri et al. (2018). They were fed from their first meal (5 dpf) with either a control or a MIX diet until the end of the experiment. F1 and F2 generations were fed from first feeding (5 dpf) until behavioural measurements using the same commercial plain food as indicated above. The fish were fed their respective treatment diet around 09:30 and 16:30. Freshly hatched artemia were also provided to all fish regardless of their treatment around 13:30 each day.
PCB and PBDE concentrations were measured in F0 and F1 fish. The results were presented previously in Alfonso et al. (2019a) and confirmed actual contamination of F0 fish and the absence of significant cross-contamination between MIX and control diets during exposure. Maternal transfer of the MIX from F0 to F1 was observed in early-life stages; however, concentrations had fallen down to control levels at adulthood, when the behavioural experiments were performed (Horri et al. 2018; Alfonso et al. 2019a).
Behavioural experiments
Videos were recorded with an analogue camera ICD-48E (Ikegami) and a 2.1–13.5 lens (Fujinon) linked to a computer with an acquisition card and EthoVision XT 10.1 software (Noldus, The Netherlands). Data extraction and analyses were performed automatically using the EthoVision XT 10.1 software.
Behavioural experiments were performed in a dedicated challenge room maintained at 27 ± 1 °C, with a photoperiod synchronized with the rearing room. The four following behavioural experiments were conducted in reproducing adults at 210 dpf in F0 and at 110 dpf in F1 and F2 generations. Control and treated fish were tested simultaneously with each generation and the different generations were investigated separately for evident practical reasons. For each generation, 40 fish per treatment were challenged in groups of 10 in a risk-taking test which was performed on the first day of experimentation (day 1) and allowed to determine individual boldness. Thereafter, the novel tank diving test (day 2), the shoal preference test (day 2) and the Z-maze exploration test (day 3) were conducted individually on a subset of 20 fish per treatment (i.e. 10 bold and 10 shy). Sampling size of the study has been defined according to similar behavioural experiments in zebrafish from us and others (Egan et al. 2009; Péan et al. 2013; Rey et al. 2013; Vignet et al. 2014). All tests used were previously described and validated (see details in the next section). The fish were tested from 09:00 to 18:00 each day at maximum depending on the duration of the test of the day, alternating the fish of the two treatments. Care was taken to have an even distribution of treatments and “Latency to exit shelter” (see below) over the day to avoid any bias related to the time of the day. Fish order was kept constant over the 3 days of test. During the whole experimental period, fish were kept in isolation in a 1 L tank in visual contact with each other and were fed once a day with freshly hatched artemia in late afternoon at the end of the testing day. On day 4, at the same time of the day for each generation (between 10 and 12 pm), fish were euthanized by placing them during 1 min into a 1 L container filled with 200 mL of system water and 700 μL of a benzocaine stock solution (50 g.L−1 in 100% ethanol; Benzocaine Sigma, St Quentin Fallavier, France). Individual standard length (from tip of head to beginning of caudal fin rays, mm) and body mass (mg) were measured and sex was determined. The sex ratio of tested fish (30:70 female: male ratio) varied between generations but not between treatments within generations.
Day 1: boldness screening using group risk taking test (RT)
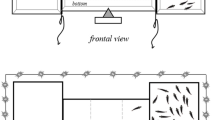
To assess boldness, we used a risk-taking test in group adapted from Huntingford et al. (2010), which was successfully implemented for various fish species, including zebrafish (Rey et al. (2013)). In the morning before the test, 4 groups of 10 fish were randomly selected from 4 different tanks of each treatment and placed into 3 L tanks and transferred from the rearing room to the challenge room for 4 h of acclimation. The order of testing for risk-taking test has been defined by starting from the replicate groups 1 to 4 by alternating groups from the two treatments (i.e. control 1, MIX 1, control 2, MIX2, […], MIX4). In the beginning of the afternoon, each group of 10 fish was placed for a 10-min habituation period in a dark shelter with dark walls and a sliding door (Fig. 1A, 20 cm in height, 20 cm in width and 14 cm in length). After this habituation period, the sliding door was gently removed allowing fish to exit the shelter via a 3 cm diameter hole located in the centre of an opaque partitioning plate, and to explore the appended open area during a screening period of 15 min (20 cm in height, 20 cm in width and 26 cm in length; Fig. 1A). When a fish exited the shelter, it was gently caught with a net and placed into a numbered 1 L tank, keeping track of the rank and timing of exit (Latency to exit shelter, s). These fish were considered bold. Fish that remained inside the shelter after this screening period were considered shy and assigned maximal latency (i.e. latency to exit shelter = 900 s).
Description of the four set-up used for measuring behavioural traits in top view. A Risk taking test apparatus composed of a shelter area (1) and an open area (2); B novel tank diving test and shoal preference test multi-compartment apparatus: a closed compartment (1) accommodates six fish isolated by a transparent window (blue line), and a sliding opaque door (black line) to the other part of the tank which contains the individual focal fish (2). The latter compartment (2) serves first as a novel tank to evaluate fish anxiety-like behaviour during the habituation period before the opaque sliding door is opened to run the shoal preference test. The area comprised between the transparent window and the dotted red line represents the social area (3); C Z-maze apparatus used to evaluate fish exploration, which contains a starting area that also served as a shelter (1). Quadrant (2) represents the first quadrant and quadrant (3) the last one used to calculate the exploration index. See text for apparatus dimensions
The proportion of bold fish was calculated for each treatment, within each generation to compare the boldness level of MIX vs. control groups. Latency to exit the shelter (s) was used to characterize the individual boldness as a continuous variable. Among the total of 40 fish tested per treatment for each generation, a subset of 10 fish (11 for F1) per boldness level and per treatment were selected (i.e. 10 bold and 10 shy for each treatment of each generation). Shy fish selected were the ones that did not exit the shelter while selection of the bold fish was made in order to cover the variability of behavioural response displayed during the test (i.e. selection of both fish exiting the shelter quickly and fish exiting more slowly). It was ensured that selected fish presented the same distribution of latency to exit the shelter as their whole population of origin (i.e. within each treatment and each generation). These fish were further challenged in the novel tank diving test, shoal preference and exploration tests, detailed below.
Day 2: anxiety-like behaviour (novel tank diving test; NT) and sociality (shoal preference test; SP) tests
Novel tank diving test (NT) and shoal preference (SP) tests were run in the same multi-compartment apparatus (15 cm in height (filled up to 10 cm), 10 cm in width and 40 cm in length; Fig. 1B) and fish position was recorded over 12 min. NT, measuring anxiety-like behaviour, and SP, measuring social behaviour, were respectively adapted from Egan et al. (2009) and Bertram et al. (2018). First, each individual fish was placed in the novel tank compartment (15 cm in height, 10 cm in width and 30 cm in length). After 7 min, a sliding opaque door was gently opened to allow visual contact with the shoal compartment (15 cm in height, 10 cm in width and 10 cm in length) containing 3 males and 3 females of unfamiliar individuals matched in age and size from stock population of the laboratory (wild type strain TU zebrafish). Preliminary tests showed us that the behaviour of these shoals remained stable over at least 5 runs without any visible sign of suffering and making possible to synchronize the changes of shoals and of the treatment studied. Consequently, shoals were changed every 5 runs.
In this manner, the period before the shoal preference test served as novel tank diving test and vertical positioning of the fish was tracked the first 6 min of recording, followed by one extra minute that served as transition between tests. For analysis, the tank was virtually separated into two areas according to Alfonso et al. (2019a): the top area including one half of the volume and bottom area including the other half. The time spent in top area (s) and distance travelled (cm) were the variables of interest for evaluating anxiety-like behaviour and measuring activity level respectively.
For SP test, position of focal fish was tracked during minutes 7 to 12, resulting in 5 min of monitoring. The tank was virtually separated into two areas to highlight a social area that includes one third of the volume near the shoal (Fig. 1B) (Bertram et al. 2018). Time spent in social area (s) and latency to go in the social area (s) was recorded.
Day3: exploration (Z-maze; ZM) test
To evaluate the ability of fish to explore their environment, a Z-maze adapted from Chapman et al. (2010) was used. The Z-maze (70 cm in length, 45 cm in width and 15 cm in height) consisted of a starting area which served as a shelter during the test (3/4 of an arm), and of four other arms (Fig. 1C). Each arm was divided virtually into four quarters of equal size, referred to as quadrants. The maze thus consisted of 17 quadrants (one adjacent to refuge zone and 16 for the 4 other arms (see Fig. 1C). A fish was introduced into the starting area (equivalent to three quadrants, covered with a black cover and ending with a sliding door) and left for 2 min in the starting area; then the door was gently opened and fish movements were recorded for a 5 min period. The variables measured were the time taken to leave the starting area and thus to enter in the Z-maze (latency to enter in Z-maze, s). We also determined the number of quadrants into which the fish ventured within the maze (17 indicating that the fish reached the end of the maze) and an exploration index was calculated as follow:
Individuals with high exploration index explored faster their environment than individuals with low value. Time spent in the shelter (after first exit, s) was also recorded.
Statistical analysis
All statistical analyses were carried out at 95% level of significance using the R software 3.1.0 (R Development Core Team 2021). Values are represented as mean ± SEM. Prior to statistical analysis, around 4 individuals per generation were removed (11/124 individuals tested, resulting in 9%) with no overall difference between treatments (5 fish for control and 6 fish for MIX). These exclusions were due to death of two fish over trial, or acquisition default of video recording or extreme freezing behaviour among one of the three tests due to excessive stress caused by handling.
Fish weight and length were compared between generations and treatments with a factorial ANOVA followed by post hoc Tukey HSD. The behavioural responses were analysed using a generalized linear mixed model (GLMM), as described below (Bates et al. 2014). The choice of the data family to be applied for each variable (i.e. gaussian, gamma or binomial) was done according to the distribution of the data. To encompass the variability in the data, the experimenter was added as a random factor into the analysis.
For the group risk taking test, the proportion of bold fish and the latency to exit shelter (for all fish tested) was analysed using the treatment (control vs. MIX) and generation (F0, F1 and F2) as fixed factors, using GLMM with binomial and gamma family for the two variables, respectively. When significant, post hoc Tukey HSD tests were performed for comparing between generations and treatments.
For the other behavioural responses, we first compared one by one all the behavioural variables depending on treatment within each generation using univariate parametric (T-test) or non-parametric (Wilcoxon test) tests depending on the normality of data distribution. Second, principal component analysis (PCA) was performed with individual data from all generations measured during the three tests: time spent in top area (s) and distance travelled (cm) (novel tank test); time spent in social area (s) and latency to go in social area (s) (shoal preference test); latency to enter in Z-maze (s), exploration index and time spent in shelter (s) (Z-maze test). PCA analysis was run using the ade4 package (Dray and Dufour 2007) and individual PCA scores were downloaded for the first two axes: PCA scores axis 1 and PCA scores axis 2. In behavioural studies, this method allows to merge the information from different variables into one, the PC score (e.g. Castanheira et al. 2013; Millot et al. 2014; Alfonso et al. 2019b).
A GLMM was then performed on the PC scores, using Gaussian family for both axes. GLMM was run using the factors treatment, generation, latency to exit shelter in risk taking test, and fish sex and length as fixed factors, as well as the interactions between all factors. The best model for each PC axis was selected based on the Akaike information criterion (AIC) and is presented below. Sex and length were discarded from both models since no significant difference was observed (data not shown).
-
Model 1: PCA score (axis 1) ~ generation + latency to exit shelter (RT)
-
Model 2: PCA score (axis 2) ~ generation + treatment + generation:treatment
Model 1 reached best significance when latency to exit shelter was included only with bold fish (i.e. fish that exited the shelter during risk taking test). Hence, model 1 was only applied to bold fish (i.e. continuous variable instead of semi continuous variable). Model 2 was performed with all fish (bold and shy) since latency to exit shelter was not significant in the model. For the second model, post hoc Tukey HSD test was performed to compare between generations and treatments. Finally, a visual inspection of the residuals was carried out for each model and revealed no violation of the statistical assumptions.
Results
Fish weight and length were different between generations (factorial ANOVA, F = 172.6, df = 2, p < 0.001 for weight and F = 104.5, df = 2, p < 0.001 for length) but not between treatments within generation (F = 1.5, df = 1, p = 0.22 for weight and F = 0.21, df = 1, p = 0.65 for length). F0 fish were heavier (F0: 446 ± 14 mg; F1: 233 ± 10 mg; F2: 239 ± 20 mg) and longer (F0: 28.0 ± 0.3 mm; F1: 22.7 ± 0.2 mm; F2: 22.4 ± 0.4 mm) than the two other generations, which is logical since experiments were performed at 210 and 120 dpf in FO and F1/F2 generation respectively.
Behavioural responses
Boldness screening
During the risk-taking test, an average of 37% (from 22.5 to 52.5% across generations and treatments) of tested fish exited the shelter. There was no effect of the generation, treatment or interaction between the two factors for the proportion of fish that exited the shelter (GLMM, p > 0.05 for all factors; Fig. 2A).
Behavioural responses during the risk-taking test. A Mean proportion of fish that exited the shelter and were qualified as bold and B latency to exit shelter for each treatment (control: black and MIX: blue) within each generation (mean ± SEM). F0: n = 19 control/n = 18 MIX; F1: n = 21 control/n = 22 MIX; F2: n = 17 control/n = 16 MIX; different letters indicate significant difference between generations and treatments (GLMM, Tukey HSD: p < 0.05)
Among fish that exited the shelter, the latency to exit the shelter did not differ between treatments (GLMM, t = 0.42, p = 0.67) but there was a generation effect. F1 fish were bolder than F0 while F2 and F0 did not differ (F0–F1: t = − 2.076 and p = 0.038; F0–F2: t = 0.207 and p = 0.84). Within each generation, there was no difference between treatments (p > 0.05 for all generations). Nonetheless, F1 MIX, but not F1 controls, was bolder than both F0 and F2 fish whatever the treatment in these generations (Fig. 2B).
Behavioural responses: multivariate analysis
None of the variables measured was significantly different between controls and MIX whatever the generation using univariate analyses, with the exception of the increase in time spent in top area during the novel tank diving test for F2 MIX compared to controls (Fig. S1).
In some cases, such univariate approach proved to be not sensitive enough to reveal subtle changes. Therefore, we performed a multivariate analysis using PCA and GLMM. The PCA axis 1, which explained 24% of the variability observed, was significantly driven by distance travelled (NT) — a proxy of activity, by latency to enter the Z-maze — a proxy of boldness, and by the exploration index (ZM) which reflected individual exploration (Fig. 3). A behavioural syndrome was observed with the significant correlation of PCA axis 1 scores and boldness. High scores on the PCA axis 1 characterized proactive individuals (high activity, high boldness and fast exploration) compared to low values which are typical of reactive individuals (low activity, shyness and slow exploration) according to Réale et al. (2010). The PCA scores were significantly affected by the generation and by the latency to exit the shelter in the risk-taking test (GLMM, Table 1) but no treatment effect was observed. Among fish that exited the shelter, bolder individuals that left the shelter quickly during the risk-taking test were more active and explored their environment faster than shyer individuals that left the shelter later during the risk-taking test, whatever the treatment (Fig. 4).
Relationship between the PCA score (axis 1) and the latency to exit shelter during the risk-taking test for each generation (F0: red, F1 green and F2: blue; LMM, see Table 2 for statistics)
By contrast, PCA axis 2, which explained 21% of the variability observed, was significantly driven by time spent in top area (NT), time spent in social area (SP), the time spent in shelter (ZM), and the latency to enter in Z-maze (ZM). PCA axis 2 could thus be considered reflecting the anxiety state of the individual fish. Individuals with high scores spent less time in top area, less time in social area (SP) and more time in the shelter during the Z-maze and could be considered anxious fish compared to individuals characterized by low scores (Fig. 3). For axis 2, the PCA scores were affected by the generation, the treatment, and by the interaction between generation and treatment (Table 2). Post hoc tests indicated that in F0 and F1 generations, MIX and control fish displayed the same anxiety level (p = 0.99 and p = 0.57 for F0 and F1 respectively) whereas MIX F2 were more anxious than control F2 (p < 0.001; Fig. 5).
PCA scores (axis 2) for each treatment (control: black and MIX: blue) within each generation (mean ± SEM). F0: n = 19 control/n = 18 MIX; F1: n = 21 control/n = 22 MIX; F2: n = 17 control/n = 16 MIX; different letters indicate significant difference between generations and treatments (GLMM, post hoc Tukey HSD: p < 0.05)
Discussion
In the present study, the analysis highlighted two distinct behavioural syndromes in zebrafish: boldness, positively correlated to activity and exploration; and anxiety, associated with low sociality. Chronic exposure to an environmental relevant mixture of PCBs and PBDEs did not clearly affect the boldness syndrome while it increased the level of the anxiety-like syndrome in the F2 MIX generation. Behavioural changes observed in this study were generation-dependant and are later discussed in an ecological context.
As shown in Horri et al. (2018), the measurements of PCB and PBDE concentrations in Control fish showed no external or cross-contamination. Environmental relevance of exposure was confirmed by the fact that, at the end of the experiment, PCB and PBDE levels in F0 MIX fish were in the range of those reported in fish from European industrialized estuaries (Law et al. 2006; Robinson et al. 2017). Maternal transfer of PCBs and PBDEs occurred in MIX larvae, while the concentrations were very low (at background level) and similar between treatments in F1 adults (Alfonso et al. 2019a). Even if there are no more PCBs and PBDEs in F1 MIX adults, the MIX treatment may have exerted an effect on F2 generation because of the exposure in F1 at the embryonic stage of germ cell that later produce the gametes. Indeed, primordial germ cells are detected as early as 8 h post fertilization (hpf) and migrate to reach presumptive gonad site at 24 hpf (Yoon et al. 1997; Weidinger et al. 1999). Therefore, the present study covers three generations that were exposed to the POP mixture in three distinct ways: fish were directly exposed through diet for F0 fish and after maternal transfer for F1 with a body burden decreasing overtime (Alfonso et al. 2019a). In F1, germ cells which will later on produce gametes for F2 which are already present in larvae were exposed to MIX at early stages.
In animals, behavioural traits such as boldness, exploration or sociality are important because they are related to different biological processes and coping abilities that can affect fitness and life history (Smith and Blumstein 2008; Wilson et al. 2019). In the present study, the proportion of bold fish was overall consistent with previous studies published in zebrafish (Rey et al. 2013; Alfonso et al. 2020), and there was no difference between treatments whatever the generation considered. The latency to exit the shelter during RT was overall similar for the three generations in control fish, but it was significantly lower in F1 MIX compared to the other generations, suggesting a bolder behaviour. However, this was not directly confirmed by comparisons to the within generation control. This may be explained by very small differences in boldness between treatments in F1 that would have need higher sample size to be clearly highlighted, or simply that the mixture did not significantly affect fish boldness.
Boldness is one of the most studied behavioural traits and is generally positively correlated with other traits such as activity, exploration and aggressiveness, and negatively correlated with sociality (Réale et al. 2010), forming a behavioural syndrome (Sih et al. 2004). Interestingly, in the present study, using PCA analysis, we observed a first behavioural syndrome that links to boldness (RT), activity (NT) and exploration (ZM). This is consistent with the previous literature (Biro and Stamps 2010; Wilson et al. 2010; Geffroy et al. 2015). Furthermore, this behavioural syndrome was observed in all generations without difference between treatments, suggesting that the exposure to the POP mixture did not alter the relationship between the different traits, unlike what was observed for other pollutants such as insecticide or fluoxetine (Montiglio and Royauté 2014). This is consistent with the results of univariate analyses indicating that variables contributing to PCA axis 1 (the one corresponding to boldness syndrome) were not affected by treatment whatever the generation. The relationships between the different behavioural traits were, however, different across generations. Indeed, for the same level of boldness in group risk taking test, F0 fish were more active and more explorative in the other challenges than F2 fish, which themselves were more active and more explorative than F1. An explanation for such a difference could be the age at which fish were screened. F0 were screened at 210 dpf whereas F1 and F2 were screened at 110 dpf; however, several elements advocate against this hypothesis. The first reason is that F0 and F2 are more alike than they are alike F1 while F1 and F2 have been tested at the same age. Second, individual consistency of behavioural traits over a long period of time, up to 10 months, was reported in zebrafish adults (Rey et al. 2013). Similarly, in a previous work, we showed that behavioural effects of polycyclic aromatic hydrocarbons were similar at 60 and 180 dpf (Vignet et al. 2014). Altogether, this led us to set aside this explanation. Another explanation for such differences could be due to the experimental design of the study. In fact, for evident reasons, F0, F1 and F2 fish were challenged at different time periods — several months apart, over the course of the study. Although the same experimental protocol was applied, we cannot ensure that there was no external factor (e.g. atmospheric pressure) that could have played a role in the differences we observe between generations. Causes underlying the observed differences between generations still remain to be clarified.
As explained before, PCB and PBDE exposure did not disrupt the behavioural syndrome between boldness, activity and exploration, but triggered an increase in boldness in F1 MIX compared to the other generations. This trend in the data needs to be further investigated since no difference in boldness level was observed between MIX and control F1, suggesting no massive alteration of boldness due to PCB and PBDE exposure. Nevertheless, studies are reporting boldness disruption due to pollutant exposure, as well as disruption of behavioural syndromes (Montiglio and Royauté 2014). PCBs and PBDEs are known to affect biological processes that are linked to boldness behaviour, such as neurotransmitter signalling (Wang et al. 2015, 2016, 2018; Tanaka et al. 2018; Blanc et al. 2021). This is of particular interest since changes in boldness, and the associated behaviours such as locomotor activity and exploration tendencies, may alter fitness and have ecological consequences (Smith and Blumstein 2008; Conrad et al. 2011; Saaristo et al. 2018). For instance, being more active and explorative, bold fish tend to capture more preys but this risky behaviour tends to increase the odds of being predated. It is here important to emphasize that even if no behavioural disruption was observed in F0 MIX, previous studies, using the same exposure protocol and environmental mixture, showed that MIX F0 displayed alterations in energy homeostasis, a higher growth rate and lower spawning probability than F0 controls (Horri et al. 2018; Blanc et al. 2021), which may point to more general physiological disruptions.
Furthermore in this study, we investigated the anxiety-like behaviour of zebrafish using the novel tank diving test, an assay validated and extensively used in behavioural neuroscience, neuropsychopharmacology, neurotoxicology and (eco)toxicology (Levin et al. 2007; Egan et al. 2009; Maximino et al. 2010; Bailey et al. 2013; Kalueff et al. 2014). First, the anxiety-like behaviour of fish was consistent with what has been observed in previous studies for zebrafish using a similar test system (Vignet et al. 2014; Alfonso et al. 2019a), supporting that fish handling, change in feeding regime, isolation from congeners and testing in risk-taking test occurring the day before did not significantly affect fish behavioural response. In Alfonso et al. (2019a), we have previously shown that anxiety-like behaviour was higher in F2 MIX compared to their respective controls, and that this may be a consequence of upregulation of glutamatergic signalling within F2 MIX brains (Blanc et al. 2021). This behavioural disruption has been already described after exposure to POP congeners such as 6-OH-BDE-47 (Macaulay et al. 2015) or after direct embryonic exposure to CB-126 (Glazer et al. 2016). It is, however, generally described with a single measure of anxiety (usually by monitoring bottom dwelling behaviour) and therefore not related to other behavioural traits, such as exploration, activity or sociality. In the present study, we have shown that a high anxiety-like behaviour level (i.e. low time spent in the top area of the tank), as classically measured using the novel tank diving test, was also related to a low sociality behaviour during the shoal preference test and to a propensity to spend more time in the shelter during the Z-maze experiment. These data form another behavioural syndrome in relation to anxiety. Interestingly, bottom-dwelling behaviour measured in the novel diving test could also sometimes be related to boldness and exploration behaviours, i.e. an increase in the time spent in the top area reflecting a bolder behaviour (Thörnqvist et al. 2018; Mustafa et al. 2019). However, in the present study, a multi-testing and multi-variate analysis showed no clear correlation between bottom-dwelling behaviour and variables linked to activity, exploration or boldness. This suggests that this behaviour, specifically measured in the novel diving test, is indeed related to anxiety-like behaviour. Therefore, the syndrome related to the PCA axis 2, which includes the variable time spent in top area of the novel tank diving test, can be assimilated to an anxiety-like disorder; fish displaying a chronic anxiety-like state regardless of the context (i.e. novel tank, Z-maze and shoal preference test).
In the present study, F2 MIX displayed a higher anxiety syndrome than F2 controls, whereas F0 MIX and F1 MIX did not display any particular increased anxiety-like behaviour in comparison with their respective F0 and F1 controls. Sociality, as part of the anxiety-like syndrome, was reduced in F2 MIX compared to the respective controls. A decrease in social behaviour was previously observed in eastern mosquitofish after exposure to 17β-trenbolone (Bertram et al. 2018) while shoaling behaviour was increased in Betta splendens males following exposure to an antiandrogen (Dzieweczynski et al. 2018). Sociality is an important trait in gregarious species, it favours communication and reduces the predation risk (Godin et al. 1988; Krause and Ruxton 2002). Therefore, lower sociality resulting from exposure to pollutants can cause deleterious effects for gregarious fish populations. Besides, anxiety per se may also affect important activities such as foraging or migration. For instance, following exposure to oxazepam, salmon smolt becomes less anxious, making them easy prey during downstream migration (Klaminder et al. 2019). Interestingly, a recent study highlighted that fish exposed to high temperature displayed lower sociality and greater anxiety, as we here observed for F2 (Angiulli et al. 2020), suggesting that similar biological pathways may be involved.
Overall, the effects of a parental exposure to an environmentally relevant mixture of POPs were highly dependent on the generation considered, as previously reported (Alfonso et al. 2019a; Blanc et al. 2021). Indeed, chronic exposure of F0 fish did not alter their own behavioural responses and there was no clear impact on boldness and associated behaviour in F1 or F2 generations (exploration and activity). However, F2 MIX displayed higher anxiety-like behaviour than F2 controls. As recently reviewed by Bell and Hellmann (2019), the changes from one offspring generation to the following one may be similar, amplified or opposed. The generation-dependent diversity of behavioural changes is likely related to the different exposure scenario and the possibly associated epigenetic modifications that can convey biological information across generations (Alfonso et al. 2019a; Blanc et al. 2021). In this regard, contrasting effects between generations may reflect maladaptation following a conflict between parental information conveyed by the germ cells to adapt to the presence of the stressor, and information acquired by the offspring from an environment that became stressor-free (Herman et al. 2014; Bell and Hellmann 2019; Blanc et al. 2021). Therefore, there is a need for multi and transgenerational studies to investigate how organisms cope with the continuous or discontinuous presence of environmental stressors, including chemical pollutants, and to which extent this can affect the fate of fish populations. In the present study, by measuring many behavioural traits together, we were able to be more precise concerning the characterization of behavioural disruptions resulting from POP exposure, hereby increasing the understanding of potential ecological consequences. However, in addition to behavioural measurements, physiological (e.g. cortisol, neurotransmitters levels) and integrative measurements (e.g. growth, reproduction and survival) are of utmost importance to have a complete overview of pollutant effects, as well as to estimate and model the dynamic and the fate of natural populations to better adapt management plans (Geffroy et al. 2020).
Data availability
The datasets used during the current study are available from the corresponding author on reasonable request.
References
Abarnou A, Burgeot T, Chevreuil M et al (2000) Les contaminants organiques. Quels risques pour le monde vivant ?. Editions IFREMER
Alfonso S, Blanc M, Joassard L et al (2019a) Examining multi- and transgenerational behavioral and molecular alterations resulting from parental exposure to an environmental PCB and PBDE mixture. Aquat Toxicol. https://doi.org/10.1016/j.aquatox.2018.12.021
Alfonso S, Sadoul B, Gesto M et al (2019b) Coping styles in European sea bass: the link between boldness, stress response and neurogenesis. Physiol Behav 207:76–85. https://doi.org/10.1016/j.physbeh.2019.04.020
Alfonso S, Peyrafort M, Cousin X, Bégout ML (2020) Zebrafish Danio rerio shows behavioural cross-context consistency at larval and juvenile stages but no consistency between stages. J Fish Biol 1–11. https://doi.org/10.1111/jfb.14310
Aluru N, Jorgensen EH, Maule AG, Vijayan MM (2004) PCB disruption of the hypothalamus-pituitary-interrenal axis involves brain glucocorticoid receptor downregulation in anadromous Arctic charr. Am J Physiol - Regul Integr Comp Physiol 287:787–793. https://doi.org/10.1152/ajpregu.00091.2004
Angiulli E, Pagliara V, Cioni C et al (2020) Increase in environmental temperature affects exploratory behaviour, anxiety and social preference in Danio rerio. Sci Rep 10:1–12. https://doi.org/10.1038/s41598-020-62331-1
Bailey J, Oliveri A, Levin ED (2013) Zebrafish model systems for developmental neurobehavioral toxicology. Birth Defects Res Part C - Embryo Today Rev 99:14–23
Barakat AO, Kim M, Qian Y, Wade TL (2002) Organochlorine pesticides and PCB residues in sediments of Alexandria Harbour, Egypt. Mar Pollut Bull 44:1426–1434. https://doi.org/10.1016/S0025-326X(02)00313-2
Bates D, Mächler M, Bolker B, Walker S (2014) Fitting linear mixed-effects models using lme4. 67. https://doi.org/10.18637/jss.v067.i01
Bell AM, Hellmann JK (2019) An integrative framework for understanding the mechanisms and multigenerational consequences of transgenerational plasticity. Annu Rev Ecol Evol Syst 50:97–118. https://doi.org/10.1146/annurev-ecolsys-110218-024613
Bertram MG, Saaristo M, Martin JM et al (2018) Field-realistic exposure to the androgenic endocrine disruptor 17Β-trenbolone alters ecologically important behaviours in female fish across multiple contexts. Environ Pollut 243:900–911. https://doi.org/10.1016/j.envpol.2018.09.044
Billsson K, Westerlund L, Tysklind M, Olsson PE (1998) Developmental disturbances caused by polychlorinated biphenyls in zebrafish (Brachydanio rerio). Mar Environ Res 46:461–464. https://doi.org/10.1016/S0141-1136(97)00041-X
Biro PA, Stamps JA (2010) Do consistent individual differences in metabolic rate promote consistent individual differences in behavior ? Trends Ecol Evol 25:653–659. https://doi.org/10.1016/j.tree.2010.08.003
Blanc M, Alfonso S, Bégout ML et al (2021) An environmentally relevant mixture of polychlorinated biphenyls (PCBs) and polybrominated diphenylethers (PBDEs) disrupts mitochondrial function, lipid metabolism and neurotransmission in the brain of exposed zebrafish and their unexposed F2 offspring. Sci Total Environ 754:142097. https://doi.org/10.1016/j.scitotenv.2020.142097
Breivik K, Sweetman A, Pacyna JM, Jones KC (2007) Towards a global historical emission inventory for selected PCB congeners - a mass balance approach. 3. An Update. Sci Total Environ 377:296–307. https://doi.org/10.1016/j.scitotenv.2007.02.026
Calò M, Alberghina D, Bitto A et al (2010) Estrogenic followed by anti-estrogenic effects of PCBs exposure in juvenil fish (Spaurus aurata). Food Chem Toxicol 48:2458–2463. https://doi.org/10.1016/j.fct.2010.06.013
Carvan MJ, Kalluvila TA, Klingler RH et al (2017) Mercury-induced epigenetic transgenerational inheritance of abnormal neurobehavior is correlated with sperm epimutations in zebrafish. PLoS One 12.https://doi.org/10.1371/journal.pone.0176155
Castanheira MF, Herrera M, Costas B et al (2013) Can we predict personality in fish? Searching for consistency over time and across contexts. PLoS ONE 8:1–9. https://doi.org/10.1371/journal.pone.0062037
Chapman BB, Morrell LJ, Krause J (2010) Unpredictability in food supply during early life influences boldness in fish. Behav Ecol 21:501–506. https://doi.org/10.1093/beheco/arq003
Chen L, Yu K, Huang C et al (2012) Prenatal transfer of polybrominated diphenyl ethers (PBDEs) results in developmental neurotoxicity in zebrafish larvae. Environ Sci Technol 46:9727–9734. https://doi.org/10.1021/es302119g
Chen L, Wang X, Zhang X et al (2017) Transgenerational endocrine disruption and neurotoxicity in zebrafish larvae after parental exposure to binary mixtures of decabromodiphenyl ether (BDE-209) and lead. Environ Pollut 230:96–106. https://doi.org/10.1016/j.envpol.2017.06.053
Conrad JL, Weinersmith KL, Brodin T et al (2011) Behavioural syndromes in fishes: a review with implications for ecology and fisheries management. J Fish Biol 78:395–435. https://doi.org/10.1111/j.1095-8649.2010.02874.x
Couderc M, Poirier L, Zalouk-Vergnoux A et al (2015) Occurrence of POPs and other persistent organic contaminants in the European eel (Anguilla anguilla) from the Loire estuary, France. Sci Total Environ 505:199–215. https://doi.org/10.1016/j.scitotenv.2014.09.053
Daouk T, Larcher T, Roupsard F et al (2011) Long-term food-exposure of zebrafish to PCB mixtures mimicking some environmental situations induces ovary pathology and impairs reproduction ability. Aquat Toxicol 105:270–278. https://doi.org/10.1016/j.aquatox.2011.06.021
Darnerud PO, Eriksen GS, Jóhannesson T et al (2001) Polybrominated diphenyl ethers: occurence, dietary exposure, and toxicology. Environ Health Perspect 109:49–68
De Wit CA (2002) An overview of brominated flame retardants in the environment
Dingemans MML, van den Berg M, Westerink RHS (2011) Neurotoxicity of brominated flame retardants: (In)direct effects of parent and hydroxylated polybrominated diphenyl ethers on the (Developing) nervous system. Environ Health Perspect 119:900–907
Dray S, Dufour A-B (2007) The ade4 package: implementing the duality diagram for ecologists. J Stat Softw 22:1–20
Dzieweczynski TL, Portrais KB, Stevens MA et al (2018) Risky business: changes in boldness behavior in male Siamese fighting fish, Betta splendens, following exposure to an antiandrogen. Environ Pollut 235:1015–1021. https://doi.org/10.1016/j.envpol.2018.01.029
Egan RJ, Bergner CL, Hart PC et al (2009) Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav Brain Res 205:38–44. https://doi.org/10.1016/j.bbr.2009.06.022.Understanding
Environment PUN (1999) Guidelines for the identification of PCBs and materials containing PCBs. First issue. Prepared by UNEP Chemicals
Geffroy B, Sadoul B, Bardonnet A (2015) Behavioural syndrome in juvenile eels and its ecological implications. Behaviour 152:147–166. https://doi.org/10.1163/1568539x-00003236
Geffroy B, Alfonso S, Sadoul B, Blumstein DT (2020) A world for reactive phenotypes. Front Convervation Sci 1:611919. https://doi.org/10.3389/fcosc.2020.611919
Glazer L, Hahn ME, Aluru N (2016) NeuroToxicology Delayed effects of developmental exposure to low levels of the aryl ( PCB126) on adult zebrafish behavior. Neurotoxicology 52:134–143. https://doi.org/10.1016/j.neuro.2015.11.012
Godin JGJ, Classon LJ, Abrahams MV (1988) Group vigilance and shoal size in a small characin fish. Behaviour 104:29–40. https://doi.org/10.1163/156853988X00584
Gonzalez ST, Remick D, Creton R, Colwill RM (2016) Effects of embryonic exposure to polychlorinated biphenyls ( PCBs ) on anxiety-related behaviors in larval zebra fi sh. Neurotoxicology 53:93–101. https://doi.org/10.1016/j.neuro.2015.12.018
Gray JS (2002) Biomagnification in marine systems: the perspective of an ecologist. Mar Pollut Bull 45:46–52. https://doi.org/10.1016/S0025-326X(01)00323-X
Hamilton TJ, Krook J, Szaszkiewicz J, Burggren W (2021) Shoaling, boldness, anxiety-like behavior and locomotion in zebrafish (Danio rerio) are altered by acute benzo[a]pyrene exposure. Sci Total Environ 774:145702. https://doi.org/10.1016/j.scitotenv.2021.145702
Hany J, Lilienthal H, Sarasin A et al (1999) Developmental exposure of rats to a reconstituted PCB mixture or aroclor 1254: effects on organ weights, aromatase activity, sex hormone levels, and sweet preference behavior. Toxicol Appl Pharmacol 158:231–243. https://doi.org/10.1006/taap.1999.8710
Herman JJ, Spencer HG, Donohue K, Sultan SE (2014) How stable “should” epigenetic modifications be? Insights from adaptive plasticity and bet hedging. Evolution (n y) 68:632–643. https://doi.org/10.1111/evo.12324
Holma-Suutari A, Ruokojärvi P, Komarov AA et al (2016) Biomonitoring of selected persistent organic pollutants (PCDD/Fs, PCBs and PBDEs) in Finnish and Russian terrestrial and aquatic animal species. Environ Sci Eur 28:1–10. https://doi.org/10.1186/s12302-016-0071-z
Horri K, Alfonso S, Cousin X et al (2018) Fish life-history traits are affected after chronic dietary exposure to an environmentally realistic marine mixture of PCBs and PBDEs. Sci Total Environ 610–611.https://doi.org/10.1016/j.scitotenv.2017.08.083
Horri K (2018) Effets d’une exposition alimentaire chronique à un mélange environnemental de PCB et PBDE sur les traits d’histoire de vie , la bioénergétique et la dynamique des populations de poissons. Ecotoxicologie. PhD dissertation, Normandie Université
Huntingford FA, Andrew G, Mackenzie S et al (2010) Coping strategies in a strongly schooling fish, the common carp Cyprinus carpio. J Fish Biol 76:1576–1591. https://doi.org/10.1111/j.1095-8649.2010.02582.x
Jensen S, Johnels AG, Olsson M, Otterlind G (1969) DDT and PCB in marine animals from Swedish waters. Nature 224:247–250. https://doi.org/10.1038/224488a0
Jones KC, de Voogt P (1999) Persistent organic pollutants (POPs): state of the science. 100:209–221. https://doi.org/10.1016/S0269-7491(99)00098-6
Jørgensen EH, Vijayan MM, Aluru N, Maule AG (2002) Fasting modifies Aroclor 1254 impact on plasma cortisol, glucose and lactate responses to a handling disturbance in Arctic charr. Comp Biochem Physiol - C Toxicol Pharmacol 132:235–245. https://doi.org/10.1016/S1532-0456(02)00069-8
Kalueff A V, Stewart AM, Gerlai R (2014) Zebrafish as an emerging model for studying complex brain disorders. 35:63–75.https://doi.org/10.1016/j.tips.2013.12.002.Zebrafish
Kannan N (2000) New types of persistent halogenated compounds. In: The Handbook of Environmental Chemistry Vol. 3 Part K. Springer, Berlin Heidelberg, Berlin, Heidelberg pp. 127–156
Kemmlein S, Herzke D, Law RJ (2009) Brominated flame retardants in the European chemicals policy of REACH-Regulation and determination in materials. J Chromatogr A 1216:320–333. https://doi.org/10.1016/j.chroma.2008.05.085
Khan MA, Lichtensteiger CA, Faroon O et al (2002) The hypothalamo-pituitary-thyroid (HPT) axis: a target of nonpersistent ortho-substituted PCB congeners. Toxicol Sci 65:52–61. https://doi.org/10.1093/toxsci/65.1.52
Klaminder J, Jonsson M, Leander J et al (2019) Less anxious salmon smolt become easy prey during downstream migration. Sci Total Environ 687:488–493. https://doi.org/10.1016/j.scitotenv.2019.05.488
Klocke C, Lein PJ (2020) Evidence implicating non-dioxin-like congeners as the key mediators of polychlorinated biphenyl (Pcb) developmental neurotoxicity. Int J Mol Sci 21.https://doi.org/10.3390/ijms21031013
Kodavanti PRS (2005) Neurotoxicity of persistent organic pollutants: possible mode(s) of action and further considerations. Dose-Response 3:dose-response.0. https://doi.org/10.2203/dose-response.003.03.002
Krause J, Ruxton GD (2002) Living in groups. Oxford University Press
Kumar A, Diricx M, Pong L et al (2020) Expression pattern of potential biomarker genes related to growth, ion regulation and stress in response to ammonia exposure, food deprivation and exercise in common carp ( Cyprinus carpio ). Aquat Toxicol 122–123:93–105. https://doi.org/10.1016/j.aquatox.2012.05.013
Law RJ, Allchin CR, de Boer J et al (2006) Levels and trends of brominated flame retardants in the European environment. Chemosphere 64:187–208. https://doi.org/10.1016/j.chemosphere.2005.12.007
Lawrence C (2007) The husbandry of zebrafish (Danio rerio): a review. Aquaculture 269:1–20. https://doi.org/10.1016/j.aquaculture.2007.04.077
Legler J, Brouwer A (2003) Are brominated flame retardants endocrine disruptors? Environ Int 29:879–885. https://doi.org/10.1016/S0160-4120(03)00104-1
Levin ED, Bencan Z, Cerutti DT (2007) Anxiolytic effects of nicotine in zebrafish. Physiol Behav 90:54–58. https://doi.org/10.1016/j.physbeh.2006.08.026
Lovato AK, Creton R, Colwill RM (2016) Effects of embryonic exposure to polychlorinated biphenyls (PCBs) on larval zebrafish behavior. Neurotoxicol Teratol 53:1–10. https://doi.org/10.1016/j.ntt.2015.11.002
Luo Q, Cai ZW, Wong MH (2007) Polybrominated diphenyl ethers in fish and sediment from river polluted by electronic waste. Sci Total Environ 383:115–127. https://doi.org/10.1016/j.scitotenv.2007.05.009
Macaulay LJ, Bailey JM, Levin ED, Stapleton HM (2015) Persisting effects of a PBDE metabolite, 6-OH-BDE-47, on larval and juvenile zebrafish swimming behavior. Neurotoxicol Teratol 52:119–126. https://doi.org/10.1016/j.ntt.2015.05.002
Martin JM, Bertram MG, Saaristo M et al (2019) Impact of the widespread pharmaceutical pollutant fluoxetine on behaviour and sperm traits in a freshwater fish. Sci Total Environ 650:1771–1778. https://doi.org/10.1016/j.scitotenv.2018.09.294
Maximino C, de Brito TM, da Silva Batista AW et al (2010) Measuring anxiety in zebrafish: a critical review. Behav Brain Res 214:157–171. https://doi.org/10.1016/j.bbr.2010.05.031
Mccarthy ID, Fuiman LA, Alvarez MC (2003) Aroclor 1254 affects growth and survival skills of Atlantic croaker. Mar Ecol Prog Ser 252:295–301
McFarland VA, Clarke JU (1989) Environmental occurrence, abundance, and potential toxicity of polychlorinated biphenyl congeners: considerations for a congener-specific analysis. Environ Health Perspect 81:225–239. https://doi.org/10.1289/ehp.8981225
Millot S, Cerqueira M, Castanheira MF et al (2014) Behavioural stress responses predict environmental perception in european sea bass (dicentrarchus labrax). PLoS ONE 9:1–7. https://doi.org/10.1371/journal.pone.0108800
Montiglio PO, Royauté R (2014) Contaminants as a neglected source of behavioural variation. Anim Behav 88:29–35. https://doi.org/10.1016/j.anbehav.2013.11.018
Mustafa A, Roman E, Winberg S (2019) Boldness in male and female zebrafish (Danio rerio) is dependent on strain and test. Front Behav Neurosci 13:00248. https://doi.org/10.3389/fnbeh.2019.00248
Orn S, Andersson PL, Forlin L et al (1998) Environmental contamination and toxicology the impact on reproduction of an orally administered mixture of selected PCBs in zebrafish (Danio rerio). Arch Environ Contam Toxicol Environ 57:52–57
OSPAR (2009) Background document on certain brominated flame retardants - In OSPAR Convention for the Protection of the Marine Environment of the North-East Atlantic, London.
OSPAR (2013) List of chemicals for priority action (revised 2013) (reference number 2004–12). In OSPAR Convention for the Protection of the Marine Environment of the North-East Atlantic, London
Otake T, Yoshinaga J, Enomoto T et al (2007) Thyroid hormone status of newborns in relation to in utero exposure to PCBs and hydroxylated PCB metabolites. Environ Res 105:240–246. https://doi.org/10.1016/j.envres.2007.03.010
Péan S, Daouk T, Vignet C et al (2013) Long-term dietary-exposure to non-coplanar PCBs induces behavioral disruptions in adult zebra fish and their offspring. Neurotoxicol Teratol 39:45–56. https://doi.org/10.1016/j.ntt.2013.07.001
Puzyn T, Mostrag A (2012) Organic pollutants ten years after the Stockholm convention environmental and analytical update, IntechOpen. https://doi.org/10.5772/1381
R Development Core Team (2021) R software. R A Lang. Environ. Stat. Comput. R Found. Stat. Comput. Vienna
Rahman F, Langford KH, Scrimshaw MD, Lester JN (2001) Polybrominated diphenyl ether (PBDE) flame retardants. Sci Total Environ 275:1–17. https://doi.org/10.1016/S0048-9697(01)00852-X
Réale D, Reader SM, Sol D et al (2007) Integrating animal temperament within ecology and evolution. Biol Rev 82:291–318
Réale D, Garant D, Humphries MM et al (2010) Personality and the emergence of the pace-of-life syndrome concept at the population level. Philos Trans r Soc B Biol Sci 365:4051–4063
Rey S, Boltana S, Vargas R et al (2013) Combining animal personalities with transcriptomics resolves individual variation within a wild-type zebrafish population and identifies underpinning molecular differences in brain function. Mol Ecol 22:6100–6115. https://doi.org/10.1111/mec.12556
Robinson CD, Webster L, Martínez-Gómez C et al (2017) Assessment of contaminant concentrations in sediments, fish and mussels sampled from the North Atlantic and European regional seas within the ICON project. Mar Environ Res 124:21–31. https://doi.org/10.1016/j.marenvres.2016.04.005
Rusiecki JA, Baccarelli A, Bollati V et al (2008) Global DNA hypomethylation is associated with high serum-persistent organic pollutants in Greenlandic inuit. Environ Health Perspect 116:1547–1552. https://doi.org/10.1289/ehp.11338
Saaristo M, Brodin T, Balshine S et al (2018) Direct and indirect effects of chemical contaminants on the behaviour, ecology and evolution of wildlife. Proceedings Biol Sci 285.https://doi.org/10.1098/rspb.2018.1297
Safe SH, Pallaroni L, Yoo K et al (2001) Toxicology of environmental estrogens. Reprod Fertil Dev 13:307–315
Sih A, Bell A, Johnson JC (2004) Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol Evol 19:372–378. https://doi.org/10.1016/j.tree.2004.04.009
Smith BR, Blumstein DT (2008) Fitness consequences of personality: a meta-analysis. Behav Ecol 19:448–455. https://doi.org/10.1093/beheco/arm144
Tanaka Y, Fujiwara M, Shindo A et al (2018) Aroclor 1254 and BDE-47 inhibit dopaminergic function manifesting as changes in locomotion behaviors in zebrafish embryos. Chemosphere 193:1207–1215. https://doi.org/10.1016/j.chemosphere.2017.11.138
Thörnqvist P, Mccarrick S, Ericsson M et al (2018) Bold zebrafish (Danio rerio) express higher levels of delta opioid and dopamine D2 receptors in the brain compared to shy fish. Behav Brain Res 0–1.https://doi.org/10.1016/j.bbr.2018.06.017
United Nations Environment Programme (2012) UNEP Annual Report 2012 - 1972–2012: Serving People and the Planet
Vignet C, Le Menach K, Lyphout L et al (2014) Chronic dietary exposure to pyrolytic and petrogenic mixtures of PAHs causes physiological disruption in zebrafish—part II: behavior. Environ Sci Pollut Res 21:13818–13832. https://doi.org/10.1007/s11356-014-2762-6
Wang X, Yang L, Wu Y et al (2015) The developmental neurotoxicity of polybrominated diphenyl ethers: effect of DE-71 on dopamine in zebrafish larvae. Environ Toxicol Chem 34:1119–1126. https://doi.org/10.1002/etc.2906
Wang X, Yang L, Wang Q et al (2016) The neurotoxicity of DE-71: effects on neural development and impairment of serotonergic signaling in zebrafish larvae. J Appl Toxicol 36:1605–1613. https://doi.org/10.1002/jat.3322
Wang F, Fang M, Hinton DE et al (2018) Increased coiling frequency linked to apoptosis in the brain and altered thyroid signaling in zebrafish embryos (Danio rerio) exposed to the PBDE metabolite 6-OH-BDE-47. Chemosphere 198:342–350. https://doi.org/10.1016/j.chemosphere.2018.01.081
Wang YP, Hong Q, Qin D ni et al (2012) Effects of embryonic exposure to polychlorinated biphenyls on zebrafish (Danio rerio) retinal development. J Appl Toxicol 32:186–193. https://doi.org/10.1002/jat.1650
Weidinger G, Wolke U, Köprunner M et al (1999) Identification of tissues and patterning events required for distinct steps in early migration of zebrafish primordial germ cells. Development 126:5295–5307
Wilson ADM, Whattam EM, Bennett R et al (2010) Behavioral correlations across activity, mating, exploration, aggression, and antipredator contexts in the European house cricket, Acheta domesticus. Behav Ecol Sociobiol 64:703–715. https://doi.org/10.1007/s00265-009-0888-1
Wilson V, Guenther A, Øverli Ø et al (2019) Future directions for personality research: contributing new insights to the understanding of animal behavior. Animals 9.https://doi.org/10.3390/ani9050240
Xin F, Susiarjo M, Bartolomei MS (2015) Multigenerational and transgenerational effects of endocrine disrupting chemicals: a role for altered epigenetic regulation? Semin Cell Dev Biol 43:66–75. https://doi.org/10.1016/j.semcdb.2015.05.008
Yang CY, Yu ML, Guo HR et al (2005) The endocrine and reproductive function of the female Yucheng adolescents prenatally exposed to PCBs/PCDFs. Chemosphere 61:355–360. https://doi.org/10.1016/j.chemosphere.2005.02.089
Yoon C, Kawakami K, Hopkins N (1997) Zebrafish vasa homologue RNA is localized to the cleavage planes of 2- and 4-cell-stage embryos and is expressed in the primordial germ cells. Development 124:3157–3165
Yu L, Lam JCW, Guo Y et al (2011) Parental transfer of polybrominated diphenyl ethers (PBDEs) and thyroid endocrine disruption in zebrafish. Environ Sci Technol 45:10652–10659. https://doi.org/10.1021/es2026592
Acknowledgements
We thank Christine Jarc, Lucette Joassard and Didier Leguay from Ifremer (LRH) for their valuable help in running this long-lasting experiment and Véronique Loizeau, Catherine Munschy, Nathalie Olivier, Karine Héas-Moisan and Xavier Philippon from Ifremer (LBCO) for their realization of PCB and PBDE analyses and interpretation. The authors thank Emilie Levavasseur (emilie.levavasseur@laposte.net) for the zebrafish drawing in Fig. 1. We also thank the three anonymous reviewers for helpful comments of the previous version of the manuscript.
Funding
This work was supported by the French National Research Agency, project Fish’N’POPs (ANR-13-CESA-020). SA received a PhD grant from Ifremer to conduct this research.
Author information
Authors and Affiliations
Contributions
Conceptualization: SA, XC and MLB. Data curation: SA, MB and XC. Formal analysis: SA and XC. Funding acquisition: XC and MLB. Investigation: SA, MB and XC. Methodology: SA, XC and MLB. Project administration: XC and MLB. Supervision: XC and MLB. Visualization: SA. Writing — original draft: SA.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted under the approval of the Animal Care Committee of Poitou–Charentes # 84 COMETHEA (France) under the project authorization number CE2012-23 and followed the recommendations of the Directive 2010/63/EU.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Bruno Nunes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alfonso, S., Blanc, M., Cousin, X. et al. Exposure of zebrafish to an environmental mixture of persistent organic pollutants triggers an increase in anxiety-like syndrome but does not affect boldness in unexposed offspring. Environ Sci Pollut Res 30, 21439–21452 (2023). https://doi.org/10.1007/s11356-022-23689-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-23689-z