Abstract
This research aimed to investigate the influence of heavy metals on the reproductive health of rainbow trout (Oncorhynchus mykiss) captured from two locations, Verinag (S1) and Panzath (S2). Sixty (n. 60) mature rainbow trout samples (30 from each site) with body weights ranging from 400 to 650 g were collected from Verinag hatchery, which was less polluted (S1), and Panzath hatchery, which was more polluted (S2). The findings revealed significant differences between the two sites, S1 (less polluted) and S2 (more polluted), as well as significant variations within the tissues. Iron (Fe) and Zinc (Zn) were found to be highest in sampling water as well as in fish tissues sampled from S2 sites. Similarly, concerning S1 captured fishes, histopathological examination of testes from S2 captured fish was found. Testicular abnormalities that included disorganization of the seminiferous tubules, reduction in the number of germ cells (sperm cells, spermatozoa), vacuolization, and large empty areas in the seminiferous epithelium were found. In testicular cells, the frequency of apoptotic cells collected from S2 water increased significantly (P < 0.05). SOD, catalase, and glutathione peroxidase activity increased in S1 captured fishes but decreased in S2 captured fishes. MDA levels gradually increased in S2 captured fish, and the degree of heavy metal stress was positively correlated (P < 0.05). In male rainbow trout, testosterone and 11-ketotestosterone levels increased substantially in S1 captured fishes. While the fatty acids of testes in S2 fishes decreased with respect to S1 fishes. In conclusion, S2 captured fish suffered more damage due to heavy metals including cellular damage, apoptosis, oxidative damage, and altered steroid hormones when compared to fish from S1 waters.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metals are becoming one of the most serious environmental hazards due to their persistence, biological toxicity, non-degradability, and ability to infiltrate the food chain. Furthermore, heavy metal pollutants have been shown to react with some organic compounds, converting them into even more deadly metal–organic complex pollutants under specific conditions (Maceda-Veiga et al. 2013; Chowdhury et al. 2016). Heavy metals can be accumulated and concentrated to some extent by aquatic species. When heavy metal concentrations and toxicity levels surpass aquatic animals’ tolerance, they have significant toxic impacts on their related indicators or even essential aspects of life. Heavy metals such as copper (Cu), iron (Fe), and zinc (Zn) are necessary for fish metabolism (Schmitt et al. 2005; Has-Schon et al. 2007). The effects of different dietary zinc sources (mineral, nanoparticulate, and organic) onquantitative and qualitative semen rainbow trout (Oncorhynchus mykiss) was investigated (Kazemia et al. 2020). Various studies have shown that heavy metal accumulation in tissues is primarily influenced by metal concentrations in water and exposure time, while other environmental variables such as salinity, pH, hardness, and temperature also play a role in metal accumulation (Has-Schon et al. 2007). The ability of a fish to sustain itself is determined by its reproductive mechanism and fish exposed to contaminants, particularly heavy metals such as Cd, Fe, Pb, and Cu, may develop acute or chronic poisoning, affecting all biological processes, including reproduction (Zulfahmi et al. 2018).
The effects of environmental toxicants on fish are being studied using several methodologies; however, histology improves the quality of research by clearly showing cellular responses (Van Dyk et al. 2007). Histopathological evaluations of fish are a tool to reflective of the overall quality of the aquatic environment and can be linked to levels of pollutants such as heavy metal contamination. Fish develop pathological changes because of these pollutants. The histological examination of fish tissue is useful as a bio-assessment technique and may be used to identify biochemical and physiological changes induced by pollution exposure (Short and Meyers 2001). Histopathological changes in the African catfish liver following exposure to a pollutant have been reported in several studies (Karami et al. 2016). Antioxidants, both enzymatic and non-enzymatic, are essential defensive mechanisms that protect animals against environmental pro-oxidants by mitigating the severity of reactive oxygen species (Tabrez and Ahmad 2009). Reactive oxygen species, such as superoxide anion (O2−), hydroxyl radicals (OH), and hydrogen peroxide (H2O2), cause extensive cellular damage, including lipoperoxidation of polyunsaturated membrane lipids (Nishiyama et al. 1998). Potential biomarkers like antioxidant parameters such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase, and oxidative stress indices are widely employed as screening tools to determine the effects of environmental stress (Kutluyer et al. 2016). Fish are subject to stressors in their habitat. Antioxidants, both enzymatic and non-enzymatic, help the body maintain oxidative homeostasis and respond to oxidative stress. Reactive oxygen species (ROS) are slowed down or neutralized by these molecules. It has been revealed that sperm cells include antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPX). Antioxidants protect the lipid peroxidation sperm membrane by reducing oxidative stress (Cocchia et al. 2011). Antioxidant enzymes like SOD and CAT in the gonads experienced significant changes due to heavy metal accumulation. These enzymes may become inactive and exhibit inhibitory effects as a result of heavy metals exposure (El-Hak et al. 2022).
Steroid hormones are extremely vital and perform important roles in reproductive health (Kime et al. 1996; Rurangwa et al. 1998). Heavy metal contamination in the environment is known to alter the steroid hormone profile function in animals, including teleost fishes (Hontela 2005; Oliveira et al. 2008). Cadmium reported as an endocrine disruptor has been found to interfere with the production of steroids, eggs, and sperm in fish, as well as alter hormone synthesis in the testes (Vetillard and Bailhache 2005).
Because of their importance in fish metabolism, lipids, particularly fatty acid accumulation, are essential for reproduction and larval development throughout maturation (Ghaedi et al. 2016). Lipid and fatty acid fluctuations occur primarily in the ovaries, testis, and liver, likely to result in differences in gonadosomatic (GSI) and hepatosomatic indices (HSI) (Love 1980). Long-chain polyunsaturated fatty acids (LC-PUFAs) such as eicosapentaenoic (EPA), docosahexaenoic (DHA), and arachidonic (ARA) acids are essential for fish growth and function (Turkmen et al. 2017). Dietary lipids and fatty acids (FAs) are geared toward gonadogenesis throughout maturation, at the expense of somatic tissue investment. Saturated fatty acids (SFAs) are preferentially and mobilized away from muscle tissue during sexual development in farmed fish, according to prior findings (Cleveland et al. 2012; Manor et al. 2014; Fazio et al. 2021).
The Jhelum River is Kashmir’s major water source which flows through Kashmir. It is the main source of irrigation in the Kashmir valley. It is the primary source of the Verinag rainbow trout hatchery and is free of contamination. There is no anthropogenic activity on this river at the Verinag site, the Jhelum River has made a substantial contribution to Kashmir’s economic growth. The communities of fishermen rely heavily on this river for their livelihood; the river is well known for trout fish. In contradiction to it, community sewage, agricultural runoff, and effluent discharges pollute the Panzath spring. Unfortunately, the alarming increase in anthropogenic activity and effluent discharges in the Panzath spring has resulted in a significant decrease in water quality. Because fishes are more sensitive to changes in their environment, environmental contaminants or their metabolites can cause oxidative stress (Lopez-Lopez et al. 2011; Sattari et al. 2020; Müller et al. 2021). The rainbow trout (Oncorhynchus mykiss) is a well-evolved global fish and one of the most common commercial species (Abbate et al. 2020). The aim of the present study was to compare the histological, biochemical, and fatty acid levels of a representative species of rainbow trout collected from two locations, Verinag hatchery, least polluted (S1), and Panzath hatchery, more polluted (S2) Jammu and Kashmir (India). To our knowledge, this is the first investigation into how heavy metal exposure effects on reproduction of rainbow trout at the histological, biochemical, and fatty acid levels. The obtained information will offer novel insights on fish toxicity heavy metals.
Materials and methods
Fish and tissue collection
Rainbow trout (Oncorhynchus mykiss) is a commercially significant fish that has been designated as a pollution sentinel species because can provide information regarding the types, quantities, availability, and effects of environmental contaminants (Benedetto et al. 2016). Between August 2017 and April 2019, 60 adult rainbow trouts (30 from each site) with body weights ranging from 400 to 650 g were collected from two locations, Verinag hatchery, which was less polluted (S1), and Panzath hatchery, which was more polluted (S2) (Fig. 1). Immediately after capture, the fish were euthanized with a lethal dose of clove oil (0.20 mL of clove oil per 500 mL of water). The gonads were then removed and transported to the laboratory at 4 °C in 0.9% saline solution and subsequently processed for histological, biochemical, and fatty acid analyses.
Chemical analysis of water
Polyethylene bottles were used to collect samples from the S1 and S2 sites to test the presence of heavy metals; copper (Cu), lead (Pb), zinc (Zn), cadmium (Cd), arsenic (Ar), and iron (Fe) in the water using an atomic absorption spectrophotometer (Analyst 400, PerkinElmer, Inc. 940 Winter Street Waltham, MA 02,451 USA). After being treated with 5% nitric acid, the bottles underwent a complete deionized distilled water rinse. The water samples were then preserved by acidification with diluted hydrochloric acid (HCl, 37 percent, Merck, Germany). The solution was then put into a conical flask and evaporated to 20 ml by heating it to 110 °C on a hot plate. Then it was transferred into a pre-cleaned bottle for atomic adsorption spectrophotometer (AAS) examination. All analyses were done in triplicate.
Tissue sampling
Tissue samples from fish testis were dissected into two portions, one for histopathological studies and another for the determination of heavy metals in tissues. Fish tissues of (1 g) were placed into polyethylene tubes and then treated with 8 mL nitric acid and 4 mL perchloric acid. All the chemicals and standard solutions that were used in the present study were purchased from Merck (Darmstadt, Germany), and they were all analytical quality. The samples were digested at 100 °C with ultra-pure nitric acid and perchloric acid till the solution became clear. The tubed sample was allowed to boil until all tissues had been dissolved. After cooling and filtering, they were put in plastic bottles. The determination of heavy metals (Cu, Pb, Zn, Cd, Ar, and Fe) was measured using an atomic absorption spectrophotometer, (Analyst 400, PerkinElmer, Inc. 940 Winter Street Waltham, MA 02,451 USA). The International Atomic Agency’s standard reference materials (MA-A-2/TM) were used to verify the precision and accuracy (IAEA). All analyses were done in triplicate.
Histopathological analysis of testes
The testes (n = 20, 10 each from the site) were fixed and dehydrated in graded ethanol (Merck, Darmstadt, Germany) before being cleared in xylene (Merck, Darmstadt, Germany). The samples were then fixed in paraffin blocks and dissected using a rotary microtome (Weswox Optik Model MT-1090A 15,125, India, Haryana). Hematoxylin and Eosin/Toluidine blue were used to stain the sections. Under a light microscope (Olympus CX31, Japan), tissue sections were examined and photographed.
Apoptotic assay (acridine orange staining)
The method of Kalia and Bansal (2009) for acridine orange staining was followed. A droplet (50 µl) of acridine orange was used to stain around 10 µL of cell solution on a micro slide. Under a fluorescence microscope (Olympus LS, Japan) using 500–525 nm filters, the slides were examined. Cells that appeared green were normal, yellow/orange cells were pre-apoptotic, and red cells were apoptotic.
Biochemical analysis
Antioxidant enzymes, malondialdehyde (MDA), catalase, SOD, and glutathione peroxidase (GPx) were investigated using the following techniques.
Malondialdehyde (MDA)
In a test tube, 0.1 ml tissue homogenate was prepared by mixing with phosphate-buffered saline (PBS, pH 7.2), 0.1 ml Tris–HCl buffer, 0.1 ml FeSO4, and 0.1 ml ascorbic acid (Fisher Chemical™), and 0.6 ml dH2O (deionized water) (Merck, Darmstadt, Germany) was added, making the volume 1.0 ml (Santamaría et al. 1997). For 15 min, the reaction mixture was incubated at 37 °C. One milliliter of 2,4,6-trichloroanisole (TCA) and 2 ml of 2,4,6-tribromoanisole (TBA) (HiMedia) were added and thoroughly mixed in. The solution was heated in a boiling water bath for 15 min before being centrifuged at 1000 rpm for 10 min to remove the flocculent adduct. The pink color produced by Malondialdehyde (MDA) formation was measured using NanoPhotometer™ UV/Vis Spectrophotometer From Implen GmBH, Germany, at 532 nm against a blank.
Catalase
A total of 0.5 ml of testicular cell suspension was incubated for 30 min at 4 °C with 50 mM phosphate buffer saline for catalase estimation. NanoPhotometer™ UV/Vis Spectrophotometer from Implen GmBH, Germany, was used to record the absorbance at 240 nm for 3 min at an interval of 20 s following the addition of newly produced 6 mM H2O2 (Merck, Darmstadt, Germany). The measure of catalase activity was directly related to the reduction in H2O2 absorbance per unit time (Aebi 1984).
Superoxide dismutase
The method stated by Marklund and Marklund (1974) was followed to measure superoxide dismutase. SOD catalyzes the dismutation of the superoxide radical into (O2−) hydrogen peroxide H2O2 and elemental oxygen (O2) and therefore offers a vital defense against the superoxide radical’s toxicity.
Glutathione peroxidase (GPx)
The dithiobis nitrobenzoic acid procedure by Rotruck et al. (1973) was used to measure GPx activity, which is based on the interaction between residual glutathione after GPx action and 5,5′-dithio bis-(2-nitro benzoic acid) to create a complex that absorbs maximum at 412 nm.
Protein estimation
The total protein content was calculated using the Lowry et al. (1951) method, which used bovine serum albumin as a reference.
Extraction of lipid
The lipids from the fish (n = 20, 10 from each site; during the reproductive season) were extracted using Folch et al. (1957) method. In the concentration of 10 g of sodium sulfate anhydrous (Na2SO4) (Merck, Darmstadt, Germany), 5 g of tissue (testis) was crushed. The total lipids were obtained from paste using a 1:15 (w/v) ratio of chloroform: methanol (2:1 v/v) (Merck, Darmstadt, Germany). G-3 sintered funnel was used to filter the extract. The filtrate was placed in a separating tunnel and washed with 0.9% normal saline (5:1 v/v). The difference between the initial and final weights of the crucible yielded the total lipid content. The overall lipids then were dissolved in 5 mL of chloroform (Merck, Darmstadt, Germany), and stored at 4 °C before being quantified.
Fatty acid analysis
The gas chromatograph mass spectrometer (Shimadzu Mass Spectrometer-2010 series, Italia) was used to analyze the fatty acids. Boron trifluoride was used to carry out the transesterification reaction of fatty acids. Gas chromatography was used to analyze fatty acid m column GC ethyl esters (FAME) using a 30 m × 0.25 mm fused silica capillary column and a flame ionization detector (Lochmann and Gatlin 1993). The extracted lipids were subsequently analyzed by GC MS with injection temperatures of 280 °C, quadruple temperatures of 150 °C, helium flow rates of 1.5 ml/min, and ion source temperatures of 230 °C. The injection was carried out in a split-less manner with a volume of 1 μL. The device was set to a temperature of 90 °C and kept there for 5 min. Following that, the temperature was raised at a pace of 10 °C each minute. Electron ionization at 70 eV was used to produce mass spectra of chemicals in tissue samples, and the detector was set to scan from 60 to 800 atomic mass units (AMU). Fatty acids were analyzed by comparing the retention periods with those of known standards. Heptadecanoic acid (17:0) was used as an internal standard to measure fatty acids, and the results were presented as mg/g of gonad wet weight.
Hormonal analysis: plasma testosterone and 11-ketotestosterone assay
The blood samples (n = 20, 10 from each site) were collected from the caudal vein and centrifuged for 15 min at 3000 g, following which the serum was kept at − 20 °C until ELISA analysis. Plasma was separated by centrifugation at 3000 rpm for 5 min at 4 °C. ELISA immunoenzymatic kits were used to detect testosterone and 11-ketotestesterone levels. This was done with commercially available enzyme-linked immunosorbent test kits from Cayman Chemical Company, USA. Protocols of Cuisset et al. (1994) and Nash et al. (2000) were used to determine sex steroid hormones. Each sample contains 50 µl of plasma, which is used to extract hormones. To extract testosterone and 11-ketotestosterone from plasma, diethyl ether, ethyl acetate/hexane 50:50 (v:v), and methylene chloride were used. Testosterone and 11-ketotestosterone assay standards were developed. The microplate reader (Spectramax 190, Beckman Coullter, Canada) was used to measure sample and reference absorbance at a wavelength of 412 nm once the plate was set up.
Statistical analysis
Data were expressed as the mean ± standard deviation and analyzed with one-way ANOVA performed with SPSS 19.0 for Windows. Pearson’s correlation analysis was used to examine the relationship between the sex steroid hormones. Tukey’s test was used to compare the average gonad fatty-acid composition across maturation stages. The level of significance was P < 0.05.
Results
Heavy metal bioaccumulation
The concentrations of Cu, Pb, Zn, Cd, Ar, and Fe were quantified in sampling water as well as in the testis. The findings revealed significant differences between the water samples taken from S1 (less polluted) and S2 (more polluted) sites (Table 1), as well as significant variations within the tissues (Table 1). Metals in water as well as in fish testes sampled from both S1 and S2 sites decreased in the order of Fe > Zn > Pb > Cu > Cd > Ar (Table 2). Overall, metal levels in fish collected from S2 were higher significantly than in samples collected from the S1 site. The results of the analysis of variance revealed that the mean concentrations of heavy metals differed significantly between the S1 and S2 water and testis samples (P < 0.05).
Testicular histopathology
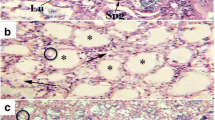
Histological analysis revealed that the fish testes captured from S1 waters had normal structure, as demonstrated by the well-organized distribution of cells in the seminiferous epithelium. The testes were comparable to those of other teleosts in terms of internal structure. Numerous germ cells at various stages of meiosis, differentiation, and maturation filled the germinal cysts. In the cysts, there were many primary spermatogonia (PSG), secondary spermatogonia (SSG), spermatocytes (SC) (Fig. 2a and b), and spermatozoa (SZ) (Fig. 2c) in the lobular lumen. Spermatogonia cells (SG) were round and deeply stained in Toluidine blue. Meiotic divisions produced the SCs from the SG. The nucleus of each SC was stained with hematoxylin. Toluidine blue was used to stain the spermatids (SPDs). They got reduced in size as they became mature, and chromatin became more evenly condensed. The SPDs were eventually converted into SZ, which are the smallest cells found in clusters in the testes. SZ were abundantly filled in the lumen of seminiferous lobules and stained strongly (Fig. 2c). While S2 fish were found to have testicular abnormalities. In comparison to S1 fishes, S2 fishes showed different alterations. Disorganization of seminiferous lobule structures, reduction in the number of germ cells (PSG, SSG, and SZ), vacuolization, and large empty areas in the seminiferous epithelium (Fig. 2d, e, and f) were among the abnormalities observed in S2 fishes. Significant differences were observed in the GSI values of the fishes collected from S1 and S2 sites (P < 0.05). S1 fishes had a GSI of 3.6%, whereas S2 fishes had a GSI of 3.4% (Fig. 3a).
Light micrograph of the testis of rainbow trout from S1 site depicting normal testicular structure with primary spermatogonia (PSG), secondary spermatogonia (SSG), spermatocyte (SC) (a, b); (arrowhead) organized seminiferous tubules with lumen filled with spermatozoa (SZ) (c). Light micrograph of the testis of rainbow trout from the S2 site depicting large empty spaces (double arrowhead), fewer germ cells (d), vacuolization (asterisk) (e); unorganized epithelium (triangle) (f)
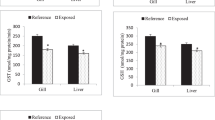
a The pattern of changes in GSI from S1 and S2 captured fishes. The values were expressed as mean ± SD (n = 20, 10 from each site). Asterisk value significantly higher than the ones from the other sites P < 0.05. b, c, d, e, f, g The pattern of changes in MDA, Catalase, SOD, and GPx from S1 and S2 captured fishes. The values were expressed as mean ± SD (n = 20, 10 from each site). Asterisk value significantly higher than the ones from the other sites P < 0.05. The pattern of changes in testosterone and 11-ketotestosterone from S1 and S2 captured fishes. The values were expressed as mean ± SD (n = 20, 10 from each site). Asterisk value significantly higher than the ones from the other sites P < 0.05
Apoptotic cell frequency
Changes in apoptotic cell frequency were analyzed. Acridine orange staining revealed testicular cells that fluoresced green, indicating that they were alive, whereas those that fluoresced orange and red were apoptotic. The frequency of apoptotic cells captured from S2 water increased significantly (P < 0.05) in testicular cells (Fig. 4c and d) as compared to S1 captured fish (Fig. 4a and b).
Enzymatic antioxidants
As shown in Fig. 3b, MDA levels were (0.33 ± 0.05 µmol/g wet tissue weight) in rainbow trout testis collected from S1 waters and gradually increased in S2 captured fish (0.48 ± 0.07 µmol/g wet tissue weight), which was strongly linked with the severity of heavy metal stress. Both CAT and SOD activity was recorded to be higher in the fish samples captured from S1 waters (0.86 and 0.63 U/mg protein) as compared to S2 fish (0.69 and 0.48 U/mg protein) respectively (Fig. 3c and d). Like the activity of SOD and CAT, the activity of GPx was higher in S1 as compared to S2 fish (Fig. 3e). There was a substantial reduction (P < 0.05) in SOD, CAT (U/mg protein), and GPx (µmole / mg protein) in response to increased heavy metal concentration in S1 waters.
Serum sex steroid levels
S1 and S2 fish have different testosterone and 11-ketotestosterone levels in their serum. Testosterone and 11-ketotestosterone in male rainbow trout followed the same trend as GSI. The amount of testosterone and 11-ketotestosterone is as follows: in S1 fish (0.64 and 0.41 ng/ml) and S2 fish (0.51 and 0.31 ng/ml) respectively (Fig. 3f and g). In male rainbow trout, testosterone and 11-ketotestosterone levels increased substantially in S1 fishes (P < 0.05).
Fatty acid composition
The fatty acid profile of rainbow trout testis is presented in Table 2. The fatty acid content of gonads varied depending on the gonadal stages of maturity. In S1 and S2 collected fishes, saturated fatty acids (SFA) account for 43.67 and 40.48%, respectively. S1 fish gonads had significantly more total monounsaturated fatty acids (MUFA) (P < 0.05). S1 and S2 fishes, respectively, have 11.21 and 8.61% MUFA. Polyunsaturated fatty acids (PUFA) account for most fatty acids in the testes. Docosahexaenoic acid (DHA) is the most abundant PUFA, accounting for about 45% of all PUFAs. Arachidonic acid (20:4, AA) and eicosapentaenoic acid (20:4, EPA) were two others important PUFAs that were higher. S1 and S2 captured fishes have 37.51 and 34.00% PUFAs, respectively.
Discussion
The present research is the first to focus on rainbow trout reproductive disruptions as well as various forms of gonadal changes under the influence of heavy metals. Heavy metal pollution in the environment has been identified as a major contributor to fish poisoning. Pollutants like heavy metals emitted by waste disposal, industries, or mining operations, are responsible for the decline in fisheries resources, and, as is known, these heavy metals cause bioaccumulation and biomagnification in the tissues of the aquatic organisms (Jarup 2003; Bakhshalizadeh et al. 2022). In the present study, the metal levels in testis tissues varied significantly depending on the metal content of the water. When compared to S1 sites, Fe, Zn, Pb, Cu, Cd, and Ar levels in the testis were the highest in S2. According to Varol et al. (2017), Fe has the highest mean content in rainbow trout (Oncorhynchus mykiss) raised in a dam reservoir on the Firat (Euphrates) river. In the samples, Zn was the second-most prevalent metal; these results are in support of the findings of the present study. According to Muinde et al. (2013), the trends for the accumulation of heavy metals in sediment and water samples from Lake Victoria were similar, that is, Zn > Pb > Cr > Cd; however, the trends for tilapia fish were Zn > Pb > Cd > Cr. In the present study, Fe was found to be higher in water samples as well as in fish testis. Heavy metals, such as Cd, accumulate in the gonadal fish tissue if reach up to 1000-fold higher than that in the surrounding water environment and become detrimental to reproduction (Dietrich et al. 2011). Heavy metal accumulation in the gonads is linked to decreased gamete quality, including decreasing sperm (Kocabaşet al. 2022) and ripe oocyte degeneration (Mansour et al. 2018). As a result, many fish reproduction mechanisms, such as seasonal hormonal cycles, spermatogenesis, and testis necrosis are affected (Kime 1999; Dietrich et al. 2010).
The heavy metals accumulated in different tissues affect cellular structure, physiology, and reproduction (Fatima and Usmani 2013). Many studies have shown that pollution, particularly heavy metals, can cause a reduction in the GSI as reported by Gerbron et al. (2014) in Rutilus rutilus which is in the support of the results of the present study. Heavy metals, industrial wastes, pesticides, and agricultural wastes have diverse histopathological effects on the reproductive tissues of fish gonads (Lye et al. 1998; Jaensson et al. 2007). These impacts may disrupt germ cell development and impair the capacity of fish to reproduce (Khillare et al. 2017; Mehanna 2005). Furthermore, spermatogenesis and lobular structures were impaired in the testis of the examined fish obtained from contaminated water, and sperm production was suppressed as reported by Jaensson et al. (2007) and Shobikhuliatul et al. (2013). In the present investigation, structural anomalies of testicular tissues were revealed in rainbow trout testis. The impact of contaminants on fish testes has been reported in previous studies (Sayed and Younes 2017; Heba and Mohamed 2019). The findings of the histological examination of the gonads of Clarias gariepinus also demonstrated that pollution interfered with gonad development (El-Hak et al. 2022). Heavy metal (Cd, Cu, Ar, Hg, and Pb) poisoning causes spermatogenesis inhibition, with large numbers of spermatogonia and spermatocytes but few spermatids and mature sperm, low activity, atrophy or necrosis of the interstitial cells, and changes in Sertoli cell structure (Kime 1995). Furthermore, bivalent ion competition (for example between Zn and Cd) might influence fish reproduction (Favier 1992).
To distinguish viable, early, and dead cells during apoptosis, a microscopic examination of cells was performed. Under a fluorescence microscope, three types of cells were identified: living cells (green), live apoptotic cells (yellow), and dead cells (red), suggesting that heavy metals influenced germ cell death. Heavy metal concentration increased the number of apoptotic cells, which corresponds to the findings of the histopathological alteration.
Reactive oxygen species (ROS) such as superoxide anion, hydrogen peroxide, and hydroxyl radical are known to cause oxidative stress, which is linked to testis damage (Shaikh et al. 1999; Stohs et al. 2000; Murugesan et al. 2005). The findings showed that heavy metal exposure considerably raised the amount of H2O2 in testes indicating that heavy metals such as Cd promoted the formation of reactive oxygen species (ROS). Antioxidant enzyme SOD is the cell’s initial line of defense against ROS, and it can defend against superoxide-induced oxidative damage by accelerating the conversion of the superoxide anion radical to molecular oxygen and hydrogen peroxide (Fridovich 1989). CAT is responsible for decreasing the damaging effects of H2O2 by turning it into water and oxygen. In the present research, S2 caught fish exhibited significantly lower Catalase and SOD activity in testes than S1 captured fish. (P < 0.05). Previous cites on this pathway have shown that excessive production of the superoxide anion radical or the presence of nitrites can inhibit catalase activity (Pandey et al. 2003; Arrillo and Melodia 1991). In addition, S2 fish showed a substantial increase in lipid peroxidation as compared to S1 fish. Similar investigations on fish species were reported from different contaminated areas, such as the Bizerte Lagoon (Ameur et al. 2012) and the Brazilian Pampa Biome (Nunes et al. 2015). The present results are inconsistent with those of Saliu and Bawa-Allah (2012), who found a decrease in SOD and CAT activity in lead-exposed Clarias gariepinus fish. Copper-treated freshwater teleost fish, Esomus danricus, showed decreased antioxidant activities of superoxide dismutase, catalase, and lipid peroxidation (Vutukuru et al. 2006).
In nature, trout usually consumes invertebrates from both terrestrial and aquatic communities (Fochetti et al. 2003). Dietary habits have a significant impact on lipid and protein levels (Oz, 2016). As a result, fatty acid content can vary greatly. In the present study, saturated and polyunsaturated fatty acids (PUFAs) made up most of the fatty acid reservoir in rainbow trout testis, followed by monounsaturated fatty acids (MUFAs). The main testicular fatty acids were 16:0, 18:0, 16:1, 18:1, 20:4 (AA), 20:5 (EPA), 22:5, and 22:6 (DHA), and all the fatty acids got decreased from S1 to S2 captured fish. The three main types of fatty acids found in Rhamdia quelen that were collected from the Rio Uruguay River were saturated (SFA = 35.5%), (MUFA = 28.1%), and (PUFA = 33.5%). DHA, palmitic acid, and AA were all found in large amounts (Anido et al. 2015). These results are in support to the findings of the present study. Fish exposed to Ni, Hg, or Ni plus Hg had relatively low levels of SFAs in their muscles (Senthamilselvan et al. 2016). In the present study, we also reported a decrease in SFAs in S2 captured fishes. Some desaturase enzymes may have been inhibited, resulting in a decrease in the amount of saturated fatty acids. This observation is supported by the fact that cadmium exposure reduced the activity of microsomal Δ9 desaturase as well as hepatic stearoyl-CoA desaturase (SCD) (Alvarez et al. 2007). PUFAs are regarded as essential for human and animal nutrition (Innis 2004). Saito et al. (1997) have reported that the fatty acid content of fish varies depending on the fishing site and is impacted by environmental factors and geographical conditions. Konar et al. (2010) also reported that the content of fatty acids like palmitoleic acid, linoleic acid, arachidonic acid, eicosapentaenoic acid, and docosapentaenoic acid (DPA) was less in cadmium-exposed muscle tissues as compared to without cadmium groups. The reduction in polyunsaturated fatty acids may be due to heavy metals stimulating the prostaglandin synthesis pathway. The alteration of metabolic pathways may be a major factor in the reduction of polyunsaturated fatty acid levels (Choi et al. 2002). The fatty acid composition and lipid levels of fish were impacted by the species, sex, degree of pollution, nutritional state, and seasonal fluctuation (Kitts et al. 2004).
In the present study, the concentrations of Testosterone and 11-ketotestosterone hormones in rainbow trout collected from S1 and S2 water were found to be significantly higher (P < 0.05) in fish captured in S1 waters (Fig. 3f and g). Hecker et al. (2002) reported that fish exposed to heavy metals had endocrine disruptor effects, resulting in a reduction in sex steroid hormone levels. Heavy metals, according to Salim (2015), can affect hormone production and function by inhibiting hormone synthesis, imitating natural hormones, and supplying receptors that limit hormone synthesis in cells. Especially, Cd ions have been shown to influence numerous mechanisms involved in fish reproduction, including seasonal hormonal cycles, spermatogenesis, and testis necrosis (Kime 1999; Mousa and Mousa 1999). Cadmium has also been shown to stimulate the production of gonad inhibitory hormone in the crab Uca pugilator (Rodriguez et al. 2000). Similarly, in male and female Japanese medaka (Oryzias latipes) exposed to Cd, alterations in circulating sex hormones have been observed (Foran et al. 2002; Tilton et al. 2003).
Conclusion
Heavy metal contamination not only increased the incidence of testicular deformities and apoptosis but also disrupted the normal steroidogenesis pattern in fish, resulting in impaired hormone production in male fish. The present study also found that the fish inhabiting the S2 site had higher levels of oxidative stress as measured by lipid peroxidation, as well as weaker antioxidant defense as measured by SOD, CAT, and GPx. Heavy metals have also been shown to affect the fatty acid components of the testis. As a result, this study indicated that the S2 was subjected to severe pollution, which had a significant impact on the fish population. In the context of biomonitoring, our findings might be used as a reference for fish ecotoxicological research in the polluted region in the future. The rainbow trout is a suitable bio-indicator of environmental pollution with heavy metals.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Change history
14 November 2022
A Correction to this paper has been published: https://doi.org/10.1007/s11356-022-24174-3
Abbreviations
- AA:
-
Arachidonic acid
- CAT:
-
Catalase
- DHA :
-
Docosahexaenoic acid
- EPA:
-
Eicosapentaenoic acid
- GPx:
-
Glutathione peroxidase
- GSI :
-
Gonadosomatic index
- MDA:
-
Malondialdehyde
- MUFA:
-
Monounsaturated fatty acids
- PSG :
-
Spermatogonia
- PUFA:
-
Polyunsaturated fatty acids
- ROS:
-
Reactive oxygen species
- SC :
-
Spermatocytes
- SFA:
-
Saturated fatty acids
- SG :
-
Spermatogonia cells
- SOD:
-
Superoxide dismutase
- SSG:
-
S econdary spermatogonia
- SZ:
-
S permatozoa
- TBA:
-
Thiobarbituric acid
References
Abbate F, Guerrera MC, Levanti M, Laurà R, Aragona M, Mhalhel K, Montalbano G, Germanà A (2020) Anatomical, histological and immunohistochemical study of the tongue in the rainbow trout (Oncorhynchus mykiss). Anat Histol Embryol 49:848–858. https://doi.org/10.1111/ahe.12593
Aebi H (1984) Catalase in vitro. Meth Enzymol 105:121–126. https://doi.org/10.1016/s0076-6879(84)05016-3
Alvarez SM, Gomez NN, Scardapane L, Fornes MW, Gimenez MS (2007) Effects of chronic exposure to cadmium on prostate lipids and morphology. Biometals 20:727–741. https://doi.org/10.1007/s10534-006-9036-9
Ameur WB, Lapuente J, Megdiche Y, Barhoumi B, Trabelsi S, Camps L, Serret J, López DR, Linares JG, Driss MR, Borràs M (2012) Oxidative stress, genotoxicity, and histopathology biomarker responses in mullet (Mugil cephalus) and sea bass (Dicentrarchus labrax) liver from Bizerte Lagoon (Tunisia). Mar Pollut Bull 64:241–325. https://doi.org/10.1016/j.marpolbul.2011.11.026
Benedetto A, Brizio P, Squadrone S, Scanzio T, Righetti M, Gasco L, Prearo M, Abete MC (2016) Oxidative stress related to chlorpyrifos exposure in rainbow trout: acute and medium term effects on genetic biomarkers. Pestic Biochem Physiol 129:63–69. https://doi.org/10.1016/j.pestbp.2015.10.019
Anido RV, Zaniboni-Filho E, Garcia AS, Baggio SR, Fracalossi DM (2015) Characterization of the ovary fatty acids composition of Rhamdia quelen (Quoy & Gaimard)(Teleostei: Siluriformes), throughout their reproductive cycle. Neotropical Ichthyol 13:453–460. https://doi.org/10.1590/1982-0224-20140139
Arrillo A, Melodia F (1991) Nitrite oxidation in Eisema foetida (Savigny): ecological implications. Funct Ecol 5:629–634. https://doi.org/10.2307/2389481
Bakhshalizadeh S, Liyafoyi AR, Saoca C, Piccione G, Cecchini S, Fazio F (2022) Nickel and cadmium tissue bioaccumulation and blood parameters in Chelon auratus and Mugil cephalus from Anzali free zone in the south Caspian Sea (Iran) and Faro Lake (Italy): A comparative analysis. J Trace Elem Med Biol 72:126999. https://doi.org/10.1016/j.jtemb.2022.126999
Choi JH, Change HW, Rhee SJ (2002) Effect of green tea catechin on arachidonic acid cascade in chronic cadmium-poisoned rats. Asia Pac J Clin Nutr 11:292–297. https://doi.org/10.1046/j.1440-6047.2002.00305.x
Chowdhury S, Mazumder MAJ, Al-Attas O, Husain T (2016) Heavy metals in drinking water: occurrences, implications, and future needs in developing countries. Sci Tot Environ 569–570:476–488. https://doi.org/10.1016/j.scitotenv.2016.06.166
Cleveland BM, Kenney PB, Manor ML, Weber GM (2012) Effects of feeding level and sexual maturation on carcass and fillet characteristics and indices of protein degradation in rainbow trout (Oncorhynchus mykiss). Aquaculture 338–341:228–236. https://doi.org/10.1016/j.aquaculture.2012.01.032
Cocchia N, Pasolini MP, Mancini R, Petrazzuolo O, Cristofaro I, Rosapane I, Sica A, Tortora G, Lorizio R, Paraggio G, Mancini A (2011) Effect of sod (superoxide dismutase) protein supplementation in semen extenders on motility, viability, acrosome status and ERK (extracellular signal-regulated kinase) protein phosphorylation of chilled stallion spermatozoa. Theriogenology. 15;75(7):1201–10. https://doi.org/10.1016/j.theriogenology.2010.11.031.
Cuisset B, Pradelles P, Kime DE, Kuhn ER, Babin P, Davail S, Le Menn F (1994) Enzyme immunoassay for 11-ketotestosterone using acetylcholinesterase as laberl: application to the measurement of 11-ketotestosterone in plasma of Siberian sturgeon. Comp Biochem Physiol 108:229–341. https://doi.org/10.1016/1367-8280(94)90035-3
Dietrich GJ, Dietrich M, Kowalski RK, Dobosz S, Karol H, Demianowicz W, Glogowski J (2010) Exposure of rainbow trout milt to mercury and cadmium alters sperm motility parameters and reproductive success. Aquatic Toxicol 97:277–284
Dietrich MA, Dietrich GJ, Hliwa P, Ciereszko A (2011) Carp transferrin can protect spermatozoa against toxic effects of cadmium ions. Comp Biochem Physiol C: Toxicol Pharmacol 153:422–429
El-Hak HNG, Ghobashy MA, Mansour FA, El-Shenawy NS, El-Din MIS (2022) Heavy metals and parasitological infection associated with oxidative stress and histopathological alteration in the Clarias gariepinus. Ecotoxicology 1-15. https://doi.org/10.1007/s10646-022-02569-9
Fatima M, Usmani N (2013) Histopathology and bioaccumulation of heavy metals (Cr, Ni and Pb) in fish (Channa striatus and Heteropneustes fossilis) tissue: a study for toxicity and ecological impacts. Pak J Biol Sci 16:412–420. https://doi.org/10.3923/pjbs.2013.412.420
Fazio F, Saoca C, Perillo L, Bakhshalizadeh S, Natale S, Piccione G, Spanò N (2021) Sex-related differences in hematological parameters in cultured striped bass (Walbaum, 1752). Iran J Fish Sci 20(6):1835–1840
Favier AE (1992) The role of zinc in reproduction hormonal mechanisms. Biol Trace Elem Res 32:363–382
Folch J, Lees M, Stanley GH (1957) A simple method for the isolation and purification of total lipids farm animal tissues. J Biol Chem 226:497–509. https://doi.org/10.1016/s0021-9258(18)64849-5
Fochetti R, Amici I, Argano R (2003) Seasonal changes and selectivity in the diet of brown trout in the River Nera (Central Italy). J Freshw Ecol 18(3):437–444. https://doi.org/10.1080/02705060.2003.9663979
Foran CM, Peterson BN, Benson H (2002) Influence of parental and developmental cadmium exposure on endocrine and reproductive function in Japanese medaka (Oryzia slatipes). Comp Biochem Physio C 133:345–354. https://doi.org/10.1016/s1532-0456(02)00128-x
Fridovich I (1989) Superoxide dismutases. An adaptation to a paramagnetic gas. J Biol Chem 264:7761–7764. https://doi.org/10.1016/S0021-9258(18)83102-7
Gerbron M, Geraldine P, Fernandes D, Rotchell JM, Porte C, Minier C (2014) Evidence of altered fertility in female Roach (Rutilus rutilus) from the River Seine (France). Environ Pollut 191:58–62. https://doi.org/10.1016/j.envpol.2014.04.015
Ghaedi A, Kabir MA, Hashim R (2016) Effect of lipid levels on reproductive performance of snakehead murrel, Channa striatus. Aquac Res 47:983–991. https://doi.org/10.1111/are.12557
Has-Schon E, Bogut I, Kralik G, Bogut S, Horvatic J, Cacic I (2007) Heavy metal concentration in fish tissues inhabiting waters of “Busko Blato” reservoar (Bosnia and Herzegovina). Environ Monit Assess 54:75–83. https://doi.org/10.1007/s10661-007-9627-0
Heba HA-K, Mohamed HM (2019) Bioaccumulation of heavy metals and physiological/histological changes in gonads of catfish (Clariasgariepinus) inhabiting Lake Maryout, Alexandria. Egypt. J Aquat Biol Fish 23:363–377. https://doi.org/10.21608/ejabf.2019.32036
Hecker M, Tyler CHR, Hoffman M, Maddix S, Karbe L (2002) Serum biomarkers in fish provide evidence for endocrine modulation in the Elbe River Germany. Environ Sci Technol 36:2311–2321. https://doi.org/10.1021/es010186h
Hontela A (2005) Adrenal toxicology: environmental pollutants and the HPI axis. In: Mommsen TP, Moon TW (eds) Biochemistry and molecular biology of fishes. Elsevier BV, Amsterdam, pp 331–363
Innis SM (2004) Polyunsaturated fatty acids in human milk: an essential role in infant development. Adv Exp Med Biol 554:27–43. https://doi.org/10.1007/978-1-4757-4242-8_5
Jaensson A, Scott AP, Moore A, Kylin H, Olsén KH (2007) Effects of a pyrethroid pesticide on endocrine responses to female odours and reproductive behavior in male parr of brown trout (Salmo trutta L.). Aquat Toxicol 81:1–9. https://doi.org/10.1016/j.aquatox.2006.10.011
Jarup L (2003) Hazards of heavy metal contamination. Br Med Bull 68(1):167–182
Kalia S, Bansal MP (2009) Regulation of apoptosis by Caspases under oxidative stress conditions in mice testicular cells: in vitro molecular mechanism. Mol Cell Biochem 322:43–52. https://doi.org/10.1007/s11010-008-9938-7
Karami A, Romano N, Hamzah H, Simpson SL, Yap CK (2016) Acute phenanthrene toxicity to juvenile diploid and triploid African catfish (Clarias gariepinus): molecular, biochemical, and histopathological alterations. Environ Pollut 212:155–165. https://doi.org/10.1016/j.envpol.2016.01.055
Kazemia E, Sourinejad I, Ghaedi A, Johari SA, Ghasemi Z (2020) Effect of different dietary zinc sources (mineral, nanoparticulate, and organic) on quantitative and qualitative semen attributes of rainbow trout (Oncorhynchus mykiss). Aquaculture 515:734529
Khillare K, Khillare YK, Wagh U (2017) Histological changes in gonads of fresh water fishes due to heavy metal pollution. World J Pharm Sci 6:601–609. https://doi.org/10.20959/wjpps20177-8743
Kime DE (1995) The effects of pollution on reproduction in fish. Rev Fish Biol Fish 5:52–96. https://doi.org/10.1007/BF01103366
Kime DE (1999) A strategy for assessing the effects of xenobiotics on fish reproduction. Sci Total Environ 225:3–11. https://doi.org/10.1016/s0048-9697(98)00328-3
Kime DE, Ebrahimi M, Nysten K, Roelants I, Moore HDM, Ollevier F (1996) Use of computer assisted sperm analysis (casa) for monitoring the effects of pollution on sperm quality of fish; application to effects of heavy metals. Aquat Toxicol 36:223–237. https://doi.org/10.1016/S0166-445X(96)00806-5
Kitts DD, Huynh MD, Hu C, Rites AWT (2004) Season variation in nutrient composition of Alaskan walleye Pollock. Can J Zool 82:1408–1415. https://doi.org/10.1139/z04-116
Kocabaş FK, Kocabaş M, Aksu Ö, Çakir Sahilli Y (2022) Ascorbic acid ameliorated the sperm quality of rainbow trout (Oncorhynchus mykiss) against arsenic toxicity: Impact on oxidative stress, fertility ability and embryo development. J Environ Sci Health C Toxicol Carcinog 40:119–132. https://doi.org/10.1080/26896583.2022.2060036
Konar V, Aydogmus C, Orun I, Kandemir S (2010) The effects of cadmium on fatty acid composition in the muscle and skin of Juvenile rainbow trout (Oncorhynchus mykiss, Walbaum 1792). J Anim Vet Adv 9:1191–1196. https://doi.org/10.3923/javaa.2010.1191.1196
Kutluyer F, Benzer F, Erişir M, Öğretmen F, İnanan BE (2016) The in vitro effect of cypermethrin on quality and oxidative stress indices of rainbow trout Oncorhynchus mykiss spermatozoa. Pestic Biochem Physiol 128:63–67
Lochmann RT, Gatlin DM III (1993) Essential fatty acid requirement of juvenile red drum (Sciaenops ocellatus). Fish Physiol Biochem 12:221–235. https://doi.org/10.1007/BF00004370
Lopez-Lopez E, Sedeno-Diaz JE, Soto C, Favari L (2011) Responses of antioxidant enzymes, lipid peroxidation, and Na+/K+-ATPase in liver of the fish Goodea atripinnis exposed to Lake Yuriria water. Fish Physiol Biochem 37:511–522. https://doi.org/10.1007/s10695-010-9453-0
Love RM (1980) The chemical biology of fishes. Academic Press, London
Lowry GM, Rosenbrough NH, Farr AI, Randall RJ (1951) Protein measurement with folin reagent. J Biol Chem 193:265–275. https://doi.org/10.1016/S0021-9258(19)52451-6
Lye CM, Frid CLJ, Gill ME (1998) Seasonal reproductive health of flounder Platichthys flesus exposed to sewage effluent. Mar Ecol Prog Ser 170:249–260. https://doi.org/10.3354/meps170249
Maceda-Veiga A, Monroy M, Navarro E, Viscor G, de Sostoa A (2013) Metal concentrations and pathological responses of wild native fish exposed to sewage discharge in a Mediterranean river. Sci Total Environ 449:9–19. https://doi.org/10.1016/j.scitotenv.2013.01.012
Manor ML, Weber GM, Cleveland BM, Kenneyb PB (2014) Effects of feeding level and sexual maturation on fatty acid composition of energy stores in diploid and triploid rainbow trout (Oncorhynchus mykiss). Aquaculture 418–419. https://doi.org/10.1016/j.aquaculture.2013.09.023
Mansour HAA, El-kady MAH, Almaaty AHA, Ramadan AM (2018) Effect of environmental pollution on gonads histology of the Nile Tilapia, Oreochromis niloticus from Lake Manzala, Egypt. Egypt J Aquat Biol Fish 22(Special Issue):563–572. https://doi.org/10.21608/EJABF.2018.28005
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–474. https://doi.org/10.1111/j.1432-1033.1974.tb03714.x
Mehanna SF (2005) Population dynamics and management of the Nile Tilapia (O. niloticus) in Wadi El-Raiyan Lakes. Egypt Afr J Biol Sci 1:79–88
Mousa SA, Mousa MA (1999) Immunocytochemical and histological studies on the hypophyseal–gonadal system in the freshwater Nile tilapia, Oreochromis niloticus (L.), during sexual maturation and spawning in different habitats. J Exp Zool 284:343–354. https://doi.org/10.1002/(sici)1097-010x(19990801)284:3%3c343::aid-jez12%3e3.0.co;2-v
Muinde VM, Nguu EK, Ogoyi DO, Shiundu PM (2013) Effects of heavy metal pollution on omega-3 polyunsaturated fatty acids levels in tilapia fish from Winam gulf of lake Victoria. Open Environ Eng J 6:22–31
Müller, AK; Markert, N; Leser, K; Kämpfer, D; Schiwy, S; Riegraf, C; Buchinger, S; Gan, L; Abdallah, AT; Denecke, B; Segner, H; Brinkmann, M; Crawford, SE; Hollert, H (2021) Bioavailability and impacts of estrogenic compounds from suspended sediment on rainbow trout (Oncorhynchus mykiss) Aquat Toxicol; 231–105719. https://doi.org/10.1016/j.aquatox.2020.105719
Murugesan P, Muthusamy T, Balasubramanian K, Arunakaran J (2005) Studies on the protective role of vitamin C and E against polychlorinated biphenyl (Aroclor 1254)-induced oxidative damage in Leydig cells. Free Radic Res 39:1259–1272. https://doi.org/10.1080/10715760500308154
Nash JP, Cuisset BD, Bhattacharyya S, Suter HC, Le Menn F, Kime DE (2000) An enzyme linked immunosorbant assay (ELISA) for testosterone, estradiol and 17,20β-dihydroxy- 4-pregnen-3-one using acetylcholinesterase as tracer: application to measurement of diel patterns in rainbow trout (Oncorhynchus mykiss). Fish Physiol Biochem 22:355–363. https://doi.org/10.1023/A:1007850014021
Nishiyama Y, Ikeda H, Haramaki N, Yoshida N, Imaizume T (1998) Oxidative stress is related to exercise intolerance in patients with heart failure. Am Heart J 135:115–120. https://doi.org/10.1016/s0002-8703(98)70351-5
Nunes M, da Silva FW, Costa-Silva D, Wallau G L, Posser T, Franco JL (2015) Assessment of water pollution signs in the Brazilian Pampa biome using stress biomarkers in fish (Astyanax sp.). J Ecosyst 1–7. https://doi.org/10.1155/2015/415293
Oliveira M, Serafim A, Bebianno MJ, Pacheco M, Santos MA (2008) European eel (Anguilla anguilla L) metallothionein, endocrine, metabolic, and genotoxic responses to copper exposure. Ecotox Environ Safe 70:20–26. https://doi.org/10.1016/j.ecoenv.2007.10.034
Oz M (2016) Nutrition and gender effect on body composition of rainbow trout (Oncorhynchus mykiss). Rev Aquac 1(1):20–25
Pandey S, Parvez S, Sayeed I, Haque R, Bin-Hafeez B, Raisuddin S (2003) Biomarkers of oxidative stress: a comparative study of river Yamuna fish Wallago attu (Bl. & Schn.). Sci Total Environ 309:105–115. https://doi.org/10.1016/S0048-9697(03)00006-8
Rodriguez EM, López Greco LS, Fingerman M (2000) Inhibition of ovarian growth by cadmium, in the fiddler crab Uca pugilator (Decapoda, Ocypodidae). Ecotoxicol Environ Saf 46:202–206. https://doi.org/10.1006/eesa.1999.1896
Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG (1973) Selenium: biochemical role as a component of glutathione peroxidase. Science 179:588–590. https://doi.org/10.1126/science.179.4073.588
Rurangwa E, Roelants I, Huyskens G, Ebrahimi M, Kime DE, Ollevier F (1998) The minimum acceptable spermatozoa to egg ratio for artificial insemination and the effects of heavy metal pollutants on sperm motility and fertilization ability. In the african catfish (Clarias gariepinus, Burchell 1822). J Fish Biol 53:402–413. https://doi.org/10.1111/j.1095-8649.1998.tb00989.x
Saito H, Ishihara K, Murase T (1997) The fatty acid composition in tuna (Bonito, Euthynnus pelamis) caught at three different localities from tropics to temperate. J Sci Food Agri 73:53–59. https://doi.org/10.1002/(SICI)1097-0010(199701)73:1%3c53::AID-JSFA707%3e3.0.CO;2-5
Salim, F (2016) Histopathological Effect of heavy metal on different organs of fresh water fish tissues from Garmat Ali River adjacent to Al- Najebyia Power Station. Kufa J Med Sci, 6:141–153. https://journal.uokufa.edu.iq/index.php/kjvs/article/view/4005
Saliu JK, Bawa-Allah KA (2012) Toxicological effects of lead and zinc on the antioxidant enzyme activities of post juvenile Clarias gariepinus. Res Environ 2:21–26. https://doi.org/10.5923/j.re.20120201.03.10.5923/j.re.20120201.03
Santamaría A, Santamaría D, Díaz-Muñoz M, Espinoza-González V, Ríos C (1997) Effects of N omega-nitro-L-arginine and L-arginine on quinolinic acid-induced lipid peroxidation. Toxicol Lett 93:117–124. https://doi.org/10.1016/s0378-4274(97)00082-9
Sattari M, Vajargah MF, Bibak M, Bakhshalizadeh S (2020) Relationship between trace element content in the brain of bony fish species and their food items in the southwest of the Caspian Sea due to anthropogenic activities. Avicenna J Environ Health Eng 7(2):78–85
Sayed AH, Younes HA (2017) Melano macrophage centers in Clarias gariepinus as an immunological biomarker for toxicity of silver nanoparticles. J Microsc Ultrastruct 5:97–104. https://doi.org/10.1016/j.jmau.2016.07.003
Schmitt CJ, Hinck JE, Blaze VS, Denslow ND, Dethloff G, Bartish TM, Coyle JJ (2005) Environmental contaminants and biomarker responses in fish from the riogrande and its US. Tributaries: Spatial and temporal trends. Sci Total Environ 350:161–193. https://doi.org/10.1016/j.scitotenv.2005.01.038
Senthamilselvan D, Chezhian A, Suresh E (2016) Synergistic effect of nickel and mercury on fatty acid composition in the muscle of fish Lates calcarifer. J Fish Aquat Sci 11:77–84. https://doi.org/10.3923/jfas.2016.77.84
Shaikh ZA, Vu TT, Zaman K (1999) Oxidative stress as a mechanism of chronic cadmium-induced hepatotoxicity and renal toxicity and protection by antioxidants. Toxicol Appl Pharmacol 154:256–263. https://doi.org/10.1006/taap.1998.8586
Shobikhuliatul JJ, Andayani S, Couteau J, Risjani Y, Minier C (2013) Some aspects of reproductive biology on the effect of pollution on the histopathology of gonads in Puntius javanicus from Mas River, Surabaya Indonesia. J Biol Life Sci 4:191. https://doi.org/10.5296/jbls.v4i2.3684
Short S, Meyers TR (2001) Histology in finfish. In: National Wild Fish Health Survey (ed) Laboratory Procedures Manual. 2nd edn , Alaska Fish and Game CF Division Olympia, Washington p.p.1–12
Stohs SJ, Bagchi D, Hassoun E, Bagchi M (2000) Oxidative mechanisms in the toxicity of chromium and cadmium ions. J Environ Pathol Toxicol Oncol 19:201–213. https://doi.org/10.1615/JEnvironPatholToxicolOncol.v20.i2.10
Tabrez S, Ahmad M (2009) Effect of wastewater intake on antioxidant and marker enzymes of tissue damage in rat tissues: implications for the use of biochemical markers. Food Chem Toxicol 47:2465–2478. https://doi.org/10.1016/j.fct.2009.07.004
Tilton SC, Foran CM, Benson WH (2003) Effects of cadmium on the reproductive axis of Japanese medaka (Oryzias latipes). Comp Biochem Physiol C 136:265–276. https://doi.org/10.1016/j.cca.2003.09.009
Turkmen S, Castro PL, Caballero MJ, Hernández-Cruz CW, Saleh R, Zamorano MJ, Regidor J, Izquierdo M (2017) Nutritional stimuli of gilthead seabream (Sparus aurata) larvae by dietary fatty acids: effects on larval performance, gene expression and neurogenesis. Aquac Res 48:202–213. https://doi.org/10.1111/are.12874
Van Dyk JC, Pieterse GM, Van Vuren JHJ (2007) Histological changes in the liver of Oreochromis mossambicus (Cichlidae) after exposure to cadmium and zinc. Ecotoxicol Envitron Saf 66:432–440. https://doi.org/10.1016/j.ecoenv.2005.10.012
Varol M, Kaya GK, Alp A (2017) Heavy metal and V concentrations in rainbow trout (Oncorhynchus mykiss) farmed in a dam reservoir on the Firat (Euphrates) River: risk-based consumption advisories. Sci Total Environ 599:1288–1296. https://doi.org/10.1016/j.scitotenv.2017.05.052
Vetillard A, Bailhache T (2005) Cadmium: an endocrine disrupter that affects gene expression in the liver and brain of juvenile rainbow trout. Biol Repr 72:119–126. https://doi.org/10.1095/biolreprod.104.029520
Vutukuru SS, Chintada S, Madhavi KR, Rao JV, Anjaneyulu Y (2006) Acute effects of copper on superoxide dismutase, catalase and lipid peroxidation in the freshwater teleost fish, Esomus danricus. J Fish Physiol Biochem 32:221–229. https://doi.org/10.1007/s10695-006-9004-x
Zulfahmi I, Muliari M, Akmal Y, Batubara AS (2018) Reproductive performance and gonad histopathology of female Nile tilapia (Oreochromis niloticus Linnaeus 1758) exposed to palm oil mill effluent. Egypt J Aquat Res 44:327–332. https://doi.org/10.1016/j.ejar.2018.09.003
Acknowledgements
The authors are thankful to the Chairman of the Department of Zoology, Kurukshetra University, Kurukshetra, for providing laboratory facilities.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by RAB. RAB and FF wrote the first draft of the manuscript. CS, OSK, CC, MCG, SNL, and FF edited and reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Bruno Nunes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Heavy metal damage in trout testis.
The original online version of this article was revised: The images of Figures 3 and 4 should be interchanged.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bhat, R.A., Saoca, C., Cravana, C. et al. Effects of heavy pollution in different water bodies on male rainbow trout (Oncorhynchus mykiss) reproductive health. Environ Sci Pollut Res 30, 23467–23479 (2023). https://doi.org/10.1007/s11356-022-23670-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-23670-w