Abstract
Increasing breast density is a risk factor for breast cancer. Geographic variations in breast density may be due to differences in lifestyle and diet, as well as environmental factors such as air pollution exposure. However, these environmental contributors have not been established. In this study, we evaluated an association between air pollution and mammographic breast density. The study population for this study was postmenopausal women who had undergone screening mammography at the Center for Preventive Medicine, St. Luke’s International Hospital, from April 2004 to September 2018. Individual mammography results were obtained from electronic charts. The ambient air pollution (PM2.5) density of the locations of interest, namely, the patients’ residential areas during the study period, was obtained. The mean PM2.5 exposure levels for 1, 3, 5, and 7 years were determined. A generalized estimating equations model was used to examine the association between air pollution density and dense breast. A total of 44,280 mammography results were included in this study, and 29,135 were classified in the non-dense breast group and 15,145 in the dense breast group. There was a 3% increase in the odds of having dense breasts after 1 year (OR = 1.027, 95% confidence interval (CI) 1.019–1.034) and 3 years of PM2.5 exposure (OR = 1.029, 95% CI 1.022–1.036). This further increased to 4% at 5-year exposure (OR = 1.044, 95% CI 1.037–1.052) and 5% at 7-year exposure (OR = 1.053, 95% CI 1.044–1.063). The risk for dense breasts increased if the factors of smoking, family history of breast and/or ovarian cancer, and history of childbirth were present.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Breast density is the proportion of epithelial and/or fibroglandular tissue to fatty tissue in the breast. High breast density may convey an increased intrinsic risk of developing cancer since it is a radiographic manifestation of higher fibroglandular breast tissue composition (Heller et al. 2018). A meta-analysis suggests an association between increased breast density and increased risk of breast cancer (McCormack and dos Santos Silva 2006).
Breast density is affected by several endogenous and exogenous factors, namely, age, body mass index (BMI), parity, use of hormonal therapy, menopausal status, genetic background, and personal or family history of breast cancer (Dorgan et al. 2013; Ramón y Cajal et al. 2015; Heller et al. 2018). There have also been reports of geographic variations in breast density between urban and rural areas, with higher breast density in women living in urban areas (Yaghjyan et al. 2017). Geographic variations in breast density may be due to differences in lifestyle and diet, as well as environmental factors such as air pollution exposure. However, the environmental contributors have not been established.
PM2.5 is defined as a particulate matter of less than 2.5 µm in diameter. It consists of a mixture of chemical components such as ammonium, elemental carbon, organic carbon matter, nitrate, silicon, sodium, and sulfate, among others (Bell et al. 2007). Several studies have shown the harmful effects of PM2.5 and its association with adverse health effects. Particulate matter in air pollution is classified as a group I carcinogen by the World Health Organization International Agency for Research on Cancer (WHO–IARC).
The studies of Caucasian populations suggested an association between PM2.5 and the incidence of breast cancer (Andersen et al. 2017; Villeneuve et al. 2018; White et al. 2019; Cheng et al. 2019). The mechanism of this association has not been elucidated, but one of the possible pathways is considered to be an intermediate effect of PM2.5 on an increase in breast density, thereby increasing the incidence of breast cancer. However, there are only a few studies on the association between PM2.5 and breast density. One of these studies, conducted by DuPre et al. on a Caucasian study population in the USA, provided inconclusive results (DuPre et al. 2017). Another study conducted in the USA, however, showed that women with extremely dense breasts had a higher mean PM2.5 exposure than women with fatty breasts (Yaghjyan et al. 2017). More evidence on this association is needed, but such studies have not yet been conducted in Japan or other Asian study populations.
The objective of this study was to determine the association between PM2.5 exposure and mammographic breast density among postmenopausal women who had undergone screening mammography in Tokyo, Japan.
Methods
Study population
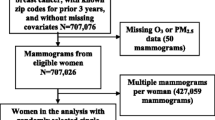
The study population of this study was postmenopausal women who had undergone screening mammography at the Center for Preventive Medicine, St. Luke’s International Hospital from April 2004 to September 2018. We limited our study population to postmenopausal women because statistical adjustment for the effects of physiological hormones on breast density in premenopausal women is not feasible. The exclusion criteria were premenopausal status, personal history of breast cancer, and missing data on covariates or PM2.5. The study was approved by the Institutional Review Board of St. Luke’s International University (No. 19-R038).
PM2.5 exposure assessment
In Japan, an individual can choose a medical institute for his/her annual health examination including mammography regardless of location. Also, the Center for Preventive Medicine at St. Luke’s International Hospital caters to employees’ annual health examinations of many organizations including private companies. This enabled a variety of addresses among individuals and subsequently various levels of exposure to PM2.5.
Data on the concentration of PM2.5 at the locations of interest, namely, the patients’ residential areas during the study period, were obtained from the official website of the Bureau of Environment of the Tokyo Metropolitan Government. The monitoring station nearest to the patient’s residence based on the postal code was determined using the Quantum Geographic Information System (QGIS) program. The mean levels of exposure to PM2.5 for 1, 3, 5, and 7 years were determined by counting the corresponding number of days backward from the day of the mammography examination and calculating the mean PM2.5 levels of that specific period.
Breast density assessment
Screening mammography examinations at the Center of Preventive Medicine are performed using full-field digital mammography images. The images and findings are interpreted and reported by doctors certified to read mammographic images. When there are discrepancies between the readers, the senior radiologist decides before the report is finalized. As part of the final report, the mammographic breast density is classified into one of the 4 classifications designated by the American College of Radiology’s Breast Imaging-Reporting and Data System (BI-RADS): almost entirely fatty, scattered fibroglandular density, heterogeneously dense, and extremely dense.
Covariates
The data on covariates were obtained from the answers to questionnaires filled out and submitted by the individuals at every visit for an annual health check-up. The data entries were processed through Optical Character Recognition (OCR) and pooled in the database (St. Luke’s Health Check-up Database: SLHCD). We used this database to obtain the study population’s demographic information, as well as the medical and personal histories.
Statistical analyses
A logistic marginal model for longitudinal data was used to examine the association between air pollution density and categorical data on mammographic breast density. Subjects who had almost entirely fatty or scattered fibroglandular density on mammography were included in the non-dense breast group, while those with heterogeneously or extremely dense categories were included in the dense breast group. We controlled for the following covariates: age, BMI, smoking, use of hormone replacement therapy, parity, age at menopause, and family history of breast and/or ovarian cancer. Subgroup analyses were done to compare the associations among smokers versus non-smokers, nulliparous versus parous women, and those with a family history of breast and/or ovarian cancer versus those without a family history.
Results
The patient demographics of the 44,280 mammography results included in this study are presented in Table 1: 29,135 were classified in the non-dense breast group and 15,145 in the dense breast group based on the mammographic density classification. The mean age of the women when the mammography was performed was 62 years in the non-dense breast group and 59 years in the dense breast group. Both groups had similar characteristics in terms of mean BMI, family history, and smoking status. There were more nulliparous women in the dense breast group (46.90%) compared to the non-dense breast group (25.75%). The majority of the subjects lived in urban areas and were non-smokers. The mean concentration of PM2.5 was similar in the non-dense breast and dense breast groups. The PM2.5 levels were noted to increase with a longer duration of exposure. The mean, median, and mode values of the daily PM2.5 exposure categorized by the duration of exposure are summarized in Table 2. The mean annual exposure was 17.39 µg/m3, greater than the Japanese annual standard (15.0 µg/m3) and the World Health Organization guideline (5 µg/m3). Ambient PM2.5 concentrations have gradually decreased over the last decades in Japan, which was reflected in slightly increasing PM2.5 concentrations with longer exposure time.
There was a 3% increase in the odds of having dense breasts after 1 year (OR = 1.027, 95% CI [1.019, 1.034]) and 3 years of PM2.5 exposure (OR = 1.029, 95% CI [1.022, 1.036]), and it further increased to 4% at 5-year exposure (OR = 1.044, 95% CI [1.037, 1.052]) and 5% at 7-year exposure (OR = 1.053, 95% CI [1.044, 1.063]) (Table 3).
For subgroup analysis, we found that the subgroups of individuals with a smoking history and a family history of breast or ovarian cancer had higher odds of having dense breasts, while the odds were lower in the subgroup of nulliparous individuals (Table 4). The odds of having dense breasts were higher at 4% among smokers at 1 year of exposure to PM2.5 (OR = 1.035, 95% CI [1.016, 1.054]) as compared to 3% among non-smokers (OR = 1.025, 95% CI [1.017, 1.033]). The odds were 5% at 5 years of exposure (OR = 1.05, 95% CI [1.032, 1.068]) and 6% at 7 years of exposure (OR = 1.056, 95% CI [1.034, 1.078]) among smokers, while the non-smokers’ odds were lower at 4% (OR = 1.043, 95% CI [1.035, 1.052]) and 5% (OR = 1.053, 95% CI [1.043, 1.064]) after 5 years and 7 years of PM2.5 exposure, respectively. A positive family history of breast or ovarian cancer had a higher modifying effect on the effect of PM2.5 on the odds of having dense breasts at 4% (OR = 1.039, 95% CI [1.012, 1.067]) at 1-year exposure, 5% (OR = 1.046, 95% CI [1.019, 1.074]) at 3-year exposure, 6% (OR = 1.062, 95% CI [1.037, 1.087]) at 5-year exposure, and 9% (OR = 1.091, 95% CI [1.059, 1.124]) at 7-year of exposure to PM2.5. Those without a family history had lower odds of having dense breasts at 3% (OR = 1.025, 95% CI [1.017, 1.033]) at 1-year exposure, 3% (OR = 1.027, 95% CI [1.020, 1.034]) at 3-year exposure, 4% (OR = 1.042, 95% CI [1.034, 1.050]) at 5-year exposure, and 5% (OR = 1.049, 95% CI [1.039, 1.059]) at 7-year exposure to PM2.5. For the subgroup analysis on parity, a history of childbirth had a higher modifying effect on the odds of having dense breasts after exposure to PM2.5 compared to those who had not given birth. The odds were higher at 3% among those with a history of childbirth at 1 year (OR = 1.027, 95% CI [1.018, 1.037]) and 3 years of exposure (OR = 1.030, 95% CI [1.020, 1.039]) and 5% at 5 years (OR = 1.046, 95% CI [1.037, 1.056]) and 7 years of exposure to PM2.5 (OR = 1.053, 95% CI [1.041, 1.065]). The odds were lower among those without a history of childbirth at 2% at 1-year (OR = 1.015, 95% CI [1.003, 1.027]) and 3-year exposure (OR = 1.018, 95% CI [1.006, 1.030]), 3% increase at 5-year exposure (OR = 1.030, 95% CI [1.017, 1.042]), and 4% increase at 7-year exposure to PM2.5 (OR = 1.049, 95% CI [1.039, 1.059]).
Discussion
In this study, we investigated the association between PM2.5 and mammographic breast density in a large Asian population. As result, we found an association between breast density and PM2.5, with an increasing trend as the exposure duration increased.
The results of this study were consistent with the previous study (Yaghjyan et al. 2017). On the contrary, a large-scale cohort study did not show any association between PM2.5 and breast density (DuPre et al. 2017). Both studies did not have an analysis of the duration of exposure to PM2.5.
Air pollution is ubiquitous, and everyone is exposed to it daily in different locations at various amounts. PM2.5 is believed to be the most relevant component of air pollution in terms of its health effects, which include cancer (Hamra et al. 2014). A study by Buschini et al. showed that the PM2.5 fraction of airborne particulate matter generally includes the highest concentration of DNA-damaging compounds (Buschini et al. 2001). Since PM2.5 can penetrate the human body easily due to its small size, its carcinogenic effect, probably through DNA damage, may initially be manifested as an increase in breast density. Increased duration of exposure to higher amounts of PM2.5 may therefore cause an increase in mammographic breast density.
The subgroup analyses conducted in this study were based on the risk factors associated with the incidence of breast cancer according to previous studies. Although there had been two previous studies on the association between PM2.5 and breast density in Caucasian study populations and these risk factors were considered as covariates in the statistical analyses, subgroup analyses were not performed. The results of our study showed that there was a higher increase in the risk of having dense breasts among smokers, individuals with a family history of breast and ovarian cancer, and those with a history of childbirth. Smoking and family history of breast and/or ovarian cancer were consistent with the previously demonstrated positive effect of these risk factors on the incidence of breast cancer. Parity or history of childbirth, on the other hand, had the opposite effect of increasing the odds of having dense breasts despite its decreasing effect on the risk of developing breast cancer.
Dense breast is known to be heritable (Boyd et al. 2002). Our study found that the group of individuals with a family history of breast and/or ovarian cancer had higher odds of having dense breasts compared to those without a family history. This may imply that those with a family history may be more susceptible to the effects of PM2.5, and further studies are warranted to elucidate the mechanism to identify preventive measures.
A study by Nakajima showed that nulliparity is associated with dense breasts, while parity, regardless of infant feeding method, induces atrophic changes in the breast tissue (Nakajima et al. 2020). Our study, though, showed that the group of individuals without a history of childbirth had a lower increase in the odds of having dense breasts compared to those with a history of childbirth. Further studies are necessary to provide a possible explanation for this inconsistency.
In this study, we analyzed a large sample of an Asian population. Most of the individuals who visited the institution for health check-ups, and were included in the study database, made repeated visits over several years. This made it possible for us to statistically analyze the data by using the logistic marginal model. These points are therefore the strengths of our study.
Our study also had limitations. Missing data and erroneous data were inevitable because the data for this study were obtained from a pooled database of answers on hand-written questionnaires that had been converted to electronic records through an Optical Character Recognition (OCR) system.
We used the residential address of the study participants to measure exposure to PM2.5. However, information on the duration of residence and moving was not available. Therefore, we assumed that the used address would represent the address for the past 1 year. Taking into account old ages in our study population, however, we speculate that the likelihood of moving in our study population is relatively low. According to the statistical agency of Japan, older adults move much less frequently than younger adults (Japan Statistics Bureau. 2022).
The values of PM2.5 exposure used in this study were surrogate measurements taken at the nearest monitoring stations to the individual’s residential area, and these values are not the actual exposure. Due to this non-differential bias, the effect estimate appears to be underestimated. However, this may not be a concern, because the effects of PM2.5 were not exaggerated rather, despite the underestimation through a surrogate measurement, a positive association was established.
Conclusion
In this study, we examined an association between PM2.5 exposure and mammographic breast density in an Asian population. We found a positive association between PM2.5 and mammographic breast density, and the strength of association increased with the increasing duration of exposure. We focused on breast density, which is considered a possible precursor to breast cancer. Hence, our study serves as a means to elucidate an intermediate association between exposure to PM2.5 and breast cancer occurrence.
Data availability
Individual patient data are not available to maintain confidentiality.
References
Andersen et al (2017) Long-Term exposure to ambient air pollution and incidence of postmenopausal breast cancer in 15 european cohorts within the ESCAPE project. Environ Health Perspect 125(10):107005. https://doi.org/10.1289/EHP1742
Bell ML et al (2007) Spatial and temporal variation in PM(2.5) chemical composition in the United States for health effects studies. Environ Health Perspect 115(7):989–995. https://doi.org/10.1289/ehp.9621
Boyd NF et al (1999) Mammographic densities and risk of breast cancer among subjects with a family history of this disease. JNCI: J Natl Cancer Inst 91(16):1404–1408. https://doi.org/10.1093/jnci/91.16.1404
Boyd NF et al (2002) Heritability of mammographic density, a risk factor for breast cancer. N Engl J Med 347(12):886–894. https://doi.org/10.1056/NEJMoa013390
Buschini A et al (2001) Urban airborne particulate: genotoxicity evaluation of different size fractions by mutagenesis tests on microorganisms and comet assay. Chemosphere 44(8):1723–1736. https://doi.org/10.1016/s0045-6535(00)00550-6
Cheng et al (2019) Association between ambient air pollution and breast cancer risk: The Multiethnic Cohort Study. Int J Cancer 146(3):699–711. https://doi.org/10.1002/ijc.32308
Dorgan JF et al (2013) Menstrual and reproductive characteristics and breast density in young women. Cancer Causes Control: CCC 24(11):1973–1983. https://doi.org/10.1007/s10552-013-0273-2
DuPre NC et al (2017) Residential particulate matter and distance to roadways in relation to mammographic density: results from the Nurses Health Studies. Breast Cancer Res 19(1):124. https://doi.org/10.1186/s13058-017-0915-5
Hamra GB et al (2014) Outdoor particulate matter exposure and lung cancer: a systematic review and meta-analysis. Environ Health Perspect 122(9):906–911. https://doi.org/10.1289/ehp/1408092
Heller SL, et al (2018) Hormonal effects on breast density, fibroglandular tissue, and background parenchymal enhancement. Radiographics : a review publication of the Radiological Society of North America, Inc 38(4):983–996. https://doi.org/10.1148/rg.2018180035
Japan Statistics Bureau (2022) Movements between prefectures by age in 2019. Available at: https://www.stat.go.jp/info/today/155.html. Accessed 2 Nov 2022
McCormack VA, dos Santos Silva I (2006) Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prevent A Publication Am Assoc Cancer Res Cosponsored Am Soc Preventive Oncol 15(6):1159–1169. https://doi.org/10.1158/1055-9965.EPI-06-0034
Nakajima E et al (2020) Association of parity and infant feeding method with breast density on mammography. Acad Radiol 27(2):e24–e26. https://doi.org/10.1016/j.acra.2019.03.020
Ramón y Cajal T et al (2015) Mammographic density and breast cancer in women from high risk families. Breast Cancer Res 17(1):93. https://doi.org/10.1186/s13058-015-0604-1
Villenueve et al (2018) Residential exposure to fine particulate matter air pollution and incident breast cancer in a cohort of Canadian women. Environmental Epidemiology 5:e021, 1–8
White et al (2019) Air pollution, clustering of particulate matter components, and breast cancer in the sister study: A U.S.-Wide Cohort. Environ Health Perspect 127(10):107002–1–107002–9
Yaghjyan L et al (2017) Association between air pollution and mammographic breast density in the Breast Cancer Surveilance Consortium. Breast Cancer Res 19(1):36. https://doi.org/10.1186/s13058-017-0828-3
Author information
Authors and Affiliations
Contributions
Conceptualization: ML and RK; methodology: ML, HY, TK, HT, and RK; formal analysis and investigation: ML, HY, TK, HT, and RK; writing—original draft preparation: RK; writing—review and editing: ML and RK.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the Research Ethics Board at St. Luke’s International Hospital.
Consent to participate
Written consents were acquired by study participants.
Consent for publication
All authors agreed with the publication of the content.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Lotfi Aleya
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kotake, R., Yamauchi, H., Kimura, T. et al. An association between mammographic breast density and fine particulate matter among postmenopausal women. Environ Sci Pollut Res 30, 25953–25958 (2023). https://doi.org/10.1007/s11356-022-23529-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-23529-0