Abstract
2,4-Dinitrophenol (2,4-DNP) is a toxic compound that is widely used in many industrial and agricultural processes. This compound has low biodegradability in the environment due to its aromatic structure, and it is unsuccessfully eliminated by other chemical methods. Therefore, in this study, an integrated oxidation and reduction method was used to remove 2,4-DNP from the aqueous medium, in order to simultaneously use the benefits of oxidizing and reducing radicals in 2,4-DNP degradation. 2,4-DNP degradation was modeled by response surface methodology (RSM) and central composite design (CCD). According to the results obtained from RSM, the optimal values for the studied parameters were obtained at pH = 8.9, time = 25 min, ZnO dose = 0.78 g/L, SO3 = 1.89 mmolL−1 and 2,4-DNP concentration = 5 mg/L. Also, the removal efficiency with the integrated process was 3 to 4 times higher than the advanced oxidation or advanced reduction processes alone. Analysis of the data showed that at the time of the study, 2,4-DNP had been converted to linear hydrocarbons, and increased periods of time were required for complete mineralization. A decrease in the first-order model rate constant (kobs) and an increase in 2,4-DNP degradation rate (robs) were observed at higher DNP concentrations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitrophenols are aromatic organic compounds with nitro, hydroxyl and other groups. One of the derivatives of nitrophenol is 2,4-dinitrophenol (2,4-DNP), which is widely used in pesticides, solvents, plastics, pigment, dyes, textiles, resins, wood preservatives and petrochemicals (Bagal et al. 2013; Xiong et al. 2019). Due to the widespread use of nitrophenol compounds, some studies have stated its concentration in industrial effluent is around 1000 mg/L (Dehghani et al. 2011; Paisio et al. 2009). Therefore, this nitro aromatic compound can easily enter aquatic environments through industrial effluents or the widespread use of pesticides. 2,4-DNP is listed as a priority pollutant by EPA due to its high toxicity, and its concentration in receiving waters is reported to be less than 10 ng/L (Dadban et al. 2015).
Studies have shown that exposure to low concentrations of 2,4-DNP can damage the central nervous system, bone marrow, heart, and causes eczema, nausea, vertigo and inhibition of cell growth. Conventional treatment technologies such as biological treatment, conventional chemical treatment, sedimentation and adsorption were not designed to eliminate aromatic compounds such as 2,4-DNP. On the other hand, these methods, in addition to having significant costs, lead to incomplete degradation of nitrophenols and may create more toxic residuals (Daraei et al. 2010; Ramteke and Gogate 2015). Therefore, in most conventional treatment processes, the pollutant is not completely eliminated: they only change it from one phase to another, and it may be transformed into another pollutant (Yazdanbakhsh et al. 2018). Also, biological purification processes are not a suitable option for these nitroaromatic compounds due to the need for extended treatment times and the inability to degrade toxic organic matter and other non-biodegradable compounds (Khan et al. 2019; Völker et al. 2016). For a complete decomposition of 2,4-DNP, the simultaneous presence of oxidizing and reducing agents is essential, to reduce both –NO2 to –NH2 and to oxidize C–C and C–H bonds. The oxidation of the nitro group to NO3− also occurs (Jeong et al. 2010). Also, Jeong et al. (2010) reported that in combined advanced oxidation/reduction processes (AO/RPs), oxidizing radicals destroy contaminants through electron transfer, and by abducting or adding hydrogen to their double bonds. On the other hand, reducing radicals produce organic radicals and free ions by attacking the bonds of aromatic compounds. These organic radicals bind to the hydrogen atom in the solvent to destroy the target pollutant (Jeong et al. 2010; Khan et al. 2017).
Therefore, treatment technologies based on AO/RP scan be a suitable alternative for the mineralization of nitroaromatic compounds. In the AO/RPs, by the simultaneous production of oxidizing radicals such as •OH and strongly reducing agents such as hydrated electron (eaq−), sulfite radical anion (SO3•−) and activated hydrogen (H•), degradation and mineralization of organic pollutants are performed (Khan et al. 2017; Yu et al. 2013). The presence of oxidizing and reducing radicals in the environment leads to non-selective decomposition of the contaminant, higher reaction rate, generation of safer residuals, production of unstable and highly degradable compounds and low energy consumption (Trojanowicz et al. 2017; Azarpira et al. 2019). In order to generate oxidizing and reducing free radicals concurrently, photocatalytic and photochemical agents are needed to provide for the energy for the decomposition of ionic, covalent and hydrogen bonds, as well as for the electrolysis of water (González-Sarrías et al. 2017) in the photochemical reactor.
In order to produce oxidizing radicals, various types of semiconductor photocatalysts such as titanium dioxide (TiO2), zinc oxide (ZnO) can be used. In this study, ZnO was selected as photocatalyst and used to produce OH radicals, and also ultraviolet light (UVC254) was used as its activating agent. On the other hand, the production of reducing radicals SO3•−, H•, eaq− requires the addition of some reducing agents. Due to the high potential of sulfite in the reduction of pollutants, sodium sulfite was selected as a suitable agent for the production of highly capable reducing species. Activation of sulfite is also done by irradiating ultraviolet lamps (Jeong et al. 2010). The advantages of AO/RPs are to produce faster free radicals and electrons, shorter reaction time, almost complete destruction of the target pollutant, non-toxic residual production and highly degradable products. Furthermore, literature reviews have shown that the efficiency of AO/RPs in the degradation of pollutants is twice as much as the advanced oxidation process alone or the advanced reduction process alone (Deng and Ezyske 2011; Entezari et al. 2019).
In UV/ZnO/SO3 system and under oxygen-free conditions, destructive radicals are formed according to reactions 1–9 and can simultaneously oxidize and reduce 2,4-DNP (Ahuja 2018).
Photolysis reactions:
Photochemical reactions:
Photocatalysis reactions:
Combined oxidation/redaction reactions:
According to the above, AO/RPs based on UV/ZnO/SO3 can be a suitable alternative for degradation of 2,4-DNP due to its excellent efficiency in degrading aromatic pollutants, no hazardous residues, being harmless to the environment and simplicity of operation (Ahuja 2018). Also, this combined process is highly efficient for the decomposition of persistent aromatic pollutants by •OH. Therefore, the aim of this study was to investigate the performance, mechanism and factors affecting the degradation of 2,4-DNP by the AO/RPs based on UV/ZnO/SO3, the effect of interfering ions in water on this process, investigation of 2,4-DNP mineralization, determination of by-products of 2,4-DNP degradation, as well as the toxicity of these products.
Materials and methods
Materials
2,4-dinitrophenol (C6H4(NO)2, purity ≥ 98%, MW = 184.11), hydrochloric acid (HCl, 98%), sodium hydroxide (98%), sodium sulfite (Na2SO3, purity ≥ 97%) and ZnO (particle size ≤ 200 nm, purity ≥ 97%) were purchased from M/s Merck Co (Germany). Methanol (CH3OH) and Milli-Q ultra-pure water—HPLC grade (≥ 99.8%) were purchased from M/s Sigma-Aldrich.
UV photochemical reactor setup and experimental procedure
All experiments were performed in a 500-mL sealed glass cylindrical photochemical reactor. A low-pressure UVC lamp (Philips) with an emission wavelength of 253.4 nm and a power of 11 W was used as the radiation source. The UV lamp was inserted into a quartz sleeve and then dipped into a sealed photochemical reactor. To ensure safe operation, the photochemical reactor was covered with aluminum foil to both prevent excess light from entering and to reflect lost ultraviolet radiation, thus increasing efficiency. As UV radiation can raise the temperature of the solution in the photochemical reactor, a water pump was used to circulate the cooling water flow around the photochemical reactor body, and the temperature was kept in the range of 25 ± 2 °C. Before starting the experiments, the UV lamp was turned on and allowed to warm for 30 min to ensure proper operation in the desired range. A magnetic stirrer was used to homogenize the contents of the photochemical reactor. Also, a cooling fan was installed in the reactor to prevent the accumulation of excess heat. In order to create anaerobic conditions and eliminate the interference of dissolved oxygen (DO) as the acceptor electron, N2 gas was injected into the test solution, and the DO level in the photochemical reactor was minimized. A stock of 2,4-DNP solution (250 mg/L) was prepared daily, and then desired concentrations were made using this stock solution. Four hundred milliliters of the solution of 2,4-DNP with known concentration was introduced into the photochemical reactor under the same conditions (in terms of contaminant concentration, ZnO dose, sulfite dose). The solution pH was adjusted by 0.1 mol L−1 HCl and 0.1 mol L−1 NaOH to the desired values .After adding sodium sulfite and zinc oxide to the reaction solution, the pH of the solution was adjusted and considered as the initial pH. The advanced oxidation/reduction process began by turning on the UV lamp source. For this purpose, definite doses of ZnO and Na2SO3 were added to the sample containing 2,4-DNP and mixed using a magnetic stirrer at 300 rpm. After reaction time was over, sampling was performed, and the samples were filtered through a Whatman filter. To compare the performance of different processes, 2,4-DNP degradation experiments were performed using UV radiation alone, UV/SO3, UV/ZnO and UV/SO3/ZnO under the same reaction conditions. The effect of initial pH (3–11), 2,4-DNP concentration (1–50 mg/L), reaction time (1–20 min), sulfite (0.5–2.5 mmol L−1) and ZnO (0.1–0.9 g/L) on 2,4-DNP degradation was investigated by AO/RPs to find the optimum values. Also, the presence of scavengers and intermediate degradation products was discussed. In general, the oxidizing and reducing radicals produced by AO/RPs act non-selectively, and the water matrix contains ions that are scavengers of these free radicals; thus, they may interfere with 2,4-DNP degradation. Therefore, in this study, the role of some scavengers such as Cl−, NO3−, NO2− and HCO3− in AO/RPs was also investigated.
HPLC and GC-MS analysis
The extent of degradation, mineralization of 2,4-DNP and formation of intermediates products were determined using high-performance liquid chromatography (HPLC), total organic carbon analyzer (TOC) and GC-MS, respectively.
The residual concentration of 2,4-DNP has been analyzed by HPLC (Agilent 1100, USA) and a UV-Vis detector at a wavelength of 360 nm. The mobile phase contained a mixture of methanol and deionized water (70:30 ratio), and a flow rate of 1 mL/min was maintained. After removal of the sample catalyst, 60 μL of the samples was injected into the C18 column (4.6× 150 mm, 5 μm) (reverse phase) with a 100-μL Hamilton syringe, and the mobile phase of acetonitrile/phosphate buffer (flow rate 1 mL/min) was injected at room temperature.
TOC analyzer (model Shimadzu VCHS/CSN, Japan) was used to determine the amount of mineralization of samples.
A GC-MS (GC:7890A; Ms:5975C) device equipped with an HP-5 column and helium as a carrier gas was applied to study the intermediate degradation products of 2,4-DNP during the AO/RPs, at the 1–20 min reaction times. The injected sample volume was considered 1 μL.
Experimental design, modeling and optimization
In this study, the response surface method (RSM) based on CCD was used. RSM is a statistical and mathematical technique that is useful for optimization processes and can determine the optimum of several variables simultaneously with the least quantitative data and a suitable test design (Daraei et al. 2017). Five independent factors were examined for 2,4-DNP degradation: pH, 2,4-DNP concentration, time, sulfite concentration and ZnO dosage, coded as x1, x2, x3, x4 and x5 respectively, and the yield (Y) was obtained for these as the response-variables. In this method, five levels (−2, −1, 0, 1, 2) were considered as presented in Table 1. For designing and optimization of independent variables, the Minitab software package was used. A second-order polynomial equation (Eq. 10) was used to estimate the function coefficients and the theoretically predicted values of the response as follows:
where, Y = the predicted response, β0= regression coefficient for constant coefficient, βj= regression coefficient for linear terms, βjj= regression coefficient for square terms, βjk= regression coefficient for interaction terms, Xi, Xj2, Xj, Xk = level of independent variables.
As shown in Table 1, the number of experiments was performed for 5 levels and 5 variables, and 32 runs were obtained.
Kinetic model of TCS degradation and mineralization
In order to determine 2,4-DNP degradation mechanism using theAO/RP, Langmuir-Hinshelwood kinetics was used according to Eqs. (11)–(14).
where r0 represents the oxidation reaction rate (mg/L min), K′ is the reaction rate constant (mg/L min) and K is the adsorption constant (L/mg), respectively. Kobs is the first-order model rate constant (min−1) and obtained from the slope of the line plot ln (C/C0) versus time. C0 and C indicate the initial and final concentrations of 2,4-DNP (mg/L), respectively, and t refers to the reaction time. A negative sign in the equation indicates a reduction in the reactant concentration during chemical experiments. robs is 2,4-DNP degradation rate(Azizi et al. 2021).
Energy consumption estimate
AO/RPs, due to the use of ultraviolet lamps, ultrasonic waves, etc., are among the processes that work with electrical energy. Therefore, part of the costs related to the degradation reaction can be related to improper consumption of electricity. In order to reduce additional costs in these processes, estimating the amount of energy consumed per unit time can play an important role in reducing costs. In this regard, the International Union of Pure and Applied Chemistry (IUPAC) has provided an electric energy index per order (EEO) to compare the amount of energy consumed in UV-based technologies. The EEO parameter is calculated as the electrical energy input (in kilowatt-hours) to the photoreactor for degrading various pollutants per unit volume of the reactor. It is important to note that the values obtained for EEO are affected by various parameters such as the concentration, type and structure of the contaminant, as well as the quantity of auxiliary chemicals (such as ZnO and Na2SO3 ).
The performance of the photochemical reactor was also evaluated by its energy consumption rate. Equations (15–17) are used to calculate the EEO parameter (Behnajady et al. 2009).
where P, t and V represent the related power of UV lamp (kW), the irradiation time (min) and contaminated water volume in the photoreactor (L), respectively.
Results and discussion
Influence of the operational parameters on AO/RPs using UV/SO3/ZnO
In general, the results related to the effect of variables on the degradation efficiency are shown in Fig. 1a, b, c, d and e. The pH of the solution is an important parameter in the degradation process by AO/RPs, which can affect the efficiency of the process by reducing or increasing the concentration of reactive radicals. In the context of the influence of pH on AO/RPs, zero charge point (PZC) and acid dissociation constant (pKa) are important factors that must be considered. pKa is the acid dissociation of a solution and indicates the acidic strength of that solution. Zero charge point (PZC) is the pH where the sum of electrical charges is zero. This parameter affects the performance of catalysts in photocatalytic reactions. According to previous studies, at pHs lower than PZC, the ZnO surface will be positively charged due to the presence of H+ ions. In contrast, at higher pHs, the electric charge on the ZnO surface will be negative due to the presence of OH− ions. Therefore, by changing the pH of the solution, the adsorption-desorption properties of the 2,4-DNP at the ZnO surface can be controlled and improved AO/RPs efficiency. According to our results, in alkaline conditions the 2,4-DNP removal efficiency increased significantly. In AO/RPs, the degradation efficiency raised with increasing pH to 9 and then decreased with changing pH from 9 to 11. The proper performance of AO/RPs with UV/SO3/ZnO at alkaline pH can be described for the following reasons. In alkaline conditions, the concentration of negative hydroxide ions (OH−) is high, which leads to the increased formation of reactive •OH radicals. In contrast, in acidic conditions the concentration of hydrogen ions (H+) is high, and these ions act as a scavenger, thus removing the main reducing radicals such as SO3•−/eaq−, and thus the efficiency of AO/RPs according to the following equations.
Also, solution pH plays an important role in the generation of various sulfite species (H2SO3, HSO3− and SO32−). At alkaline pH, sulfite absorbs UV254 more strongly; thus, SO32− ion is the predominant species in the environment that is activated under UV radiation and can effectively produce SO3•−, •H and eaq−reducing radicals via Eqs. (3)–(5) (Entezari et al. 2019; Noorisepehr et al. 2020; Yanga et al. 2020).
According to the findings of Liu et al., pH can improve the degradation efficiency of AO/RPs by affecting the molar absorptivity of sulfite. At higher molar absorptivity values, the UV absorption rate increases, which leads to the production of more reducing radicals, and thus the 2,4-DNP removal efficiency increases. The decrease in efficiency at pH above 9 can be attributed to the presence of scavengers in abundance (Liu et al. 2013). On the other hand, with increasing the pH to 11, no significant increase in removal efficiency was observed (Fig. 1a). Some studies have attributed the low efficiency of the degradation at pH 11 to the protonation of sulfite, less eaq formation and capture reactive species by side reactions (sulfite). Also according to the findings of Milh et al. (2021), sulfite under acidic conditions in aqueous media forms the HSO3− ion, which is a precursor to •H. In contrast, under alkaline conditions, it appears in the form of SO32− ions, which can lead to the formation of •eaq−. Milh et al. (2021) reported that •eaq− is a stronger reducing agent than •H in destroying pollutants in the AO/RPs. Since the formation of •eaq− is much higher at alkaline pH, the degradation efficiency of contaminants will be higher under alkaline conditions. Many studies have reported the simultaneous presence of •eaq− and SO3•− radicals under alkaline conditions, both of which are strong reducing agents (Milh et al. 2021).
The effect of sulfite concentration and ZnO dose on 2,4-DNP degradation was presented in Fig. 1b. According to the results, the highest degradation of 2,4-DNP occurred in amounts of sulfite and ZnO 2 mM and 0.7 g/L respectively. In other words, increasing the sulfite concentration to 2 mM increased 2,4-DNP degradation (Fig. 1c). It seems that when the sulfite concentration increases in the presence of UV light, the rate of UV photon absorption increases, and therefore the rate of SO3•− formation also increases. However, the addition of more sulfite did not increase the 2,4-DNP degradation, meaning that the presence of higher sulfite amounts could absorb more UV light and interfere with ZnO activation and AO/RP function. Previous studies have reported that at high sulfite concentrations, the amount of reducing radicals increases and can act as a scavenger against oxidizing agents, and this side reaction reduces the AO/RP efficiency (Noorisepehr et al. 2020; Yanga et al. 2020; Liu et al. 2013). Milh et al. (2021) also reported that one of the reasons for the decrease in AO/RP performance at high sulfite concentrations could be the radical-radical interaction. Therefore in our study, sulfite at low concentrations had a better effect on 2,4-DNP degradation. It also seems that a higher dose of ZnO leads to the release of more zinc ions into the solution. Also, higher doses of ZnO absorb more UV rays and thus lead to more free radical production. Zinc ions can increase the rate of generation of reactive radicals and promote the removal efficiency of 2,4-DNP. According to the findings of Nuengmatcha et al. (2016), by increasing the ZnO dosage, the surface and more active sites of this catalyst are exposed to UV light to produce reactive radicals. However, with increasing the ZnO dosage from 0.7 to 0.9 g/L, the removal 2,4-DNP efficiency decreased slightly. Nuengmatcha et al. (2016) reported that the reason for the decrease in the AO/RPs performance at higher ZnO doses was the formation of turbidity due to zinc oxide particles and the lower impact of UV rays. Also at high doses, zinc oxide particles may accumulate, and thus the available active sites may be reduced. Our findings showed that the proper performance of AO/RPs with UV/SO3/ZnO requires a certain ratio of reducing and oxidizing agents such as SO3•−, eaq− and •OH, and these ratios vary depending on the type of contaminants, their bonds, and aromatic and aliphatic nature (Fig. 1d).
Fig. 1e showed that the 2,4-DNP degradation dropped with the increase of 2,4-DNP concentration from 5 to 45 mg/L. It seems that higher 2,4-DNP concentrations require more reducing and oxidizing radicals to degrade, and as the 2,4-DNP concentration increases, the concentration of these radicals in the reactor decreases. Therefore, further consumption of reducing and oxidizing agents and reducing them in high 2,4-DNP concentrations can reduce the performance of AO/RPs. In other words, the number of moles of 2,4-DNP is more than the number of moles of oxidizing and reducing radicals in solution. It is also considered in Fig. 1e that 2,4-DNP degradation rate raised with increasing the reaction time from 5 to 25 min. At high reaction times, more UV photons are absorbed by ZnO, and then the production of reactive radicals increases. As a result, more time is provided for the decomposition of 2,4-DNP molecules by these reactive radicals. On the other hand, since the ZnO surface can also act as an adsorbent, during long reaction times, the probability of collision between 2,4-DNP molecules and ZnO particles increases, and the rate of decomposition and adsorption of 2,4-DNP on the surface of this catalyst increases (Yanga et al. 2020; Liu et al. 2013; Milh et al. 2021).
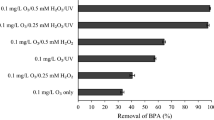
To compare the performance of different processes, 2,4-DNP degradation efficiency was determined using UV light, ZnO/SO3, UV/SO3, UV/ZnO and UV/SO3/ZnO under the same reaction conditions. According to the results of Figure 2, the degradation of 2,4-DNP by UV light alone was negligible. Also, ZnO/SO3, UV/SO3 and UV/ZnO processes were not able to decompose 2,4-DNP significantly. The inefficiency of these processes in 2,4-DNP degradation can be related to the low generation of oxidizing and reducing radicals in the photochemo reactor. Simultaneous application of UV/SO3/ZnO significantly increased the 2,4-DNP removal efficiency due to the simultaneous formation of SO3•−, eaq− and •OH radicals and their synergistic effects (Liu et al. 2013; Milh et al. 2021; Nuengmatcha et al. 2016).
Effect of scavengers on photo-degradation kinetics
Under the optimal conditions (pH = 9, 2,4-DNP concentration = 5 mg/L, SO3= 2 mM, ZnO dosage = 0.7g), the anions were added separately to the solution, and after 30 min the samples were analyzed. According to Fig. 3, in the presence of anions such as Cl−, NO3−, NO2−, HCO3− the 2,4-DNP degradation efficiency decreased approximately to 6.25%, 19.15%, 10.82% and 21.76%, respectively.
In general, the anions in the background water matrix can have a negative effect on the performance of AO/RPs by consuming and removing reactive radicals. These anions not only absorb reducing and oxidizing radicals but can also be adsorbed on the ZnO surface and reduce its activity. These anions can also reduce the UV absorption on ZnO and disrupt the production of oxidizing radicals formed by this process. On the other hand, the absorption of UV photons by water anions reduces the ionization of water molecules (Moussavi and Rezaei 2017). Studies have shown that nitrate is a scavenger for hydroxyl radicals, bicarbonate and chloride are scavengers of hydroxyl radicals and sulfite, and nitrite has been introduced as a scavenger of eaq−, HO• and H•. Some studies have also reported that anions in water matrix under UV irradiation formed anionic radicals that were less reactive than eaq− and •OH according to the following reactions (Penalver et al. 2013):
Therefore, in AO/RPs the reduction of •OH, eaq− , SO•3−, H•hydroxyl radicals by anions, organic compounds and other scavengers in water must be considered (Moussavi and Rezaei 2017).
Kinetic model of TCS degradation and mineralization and energy consumption estimate
As previously mentioned, due to the difficulty in measuring reactive radicals in the photoreactor, the 2,4-DNP degradation by AO/RPs was investigated using the pseudo-first-order model. For this purpose, reactions were performed at different 2,4-DNP concentrations (5–45 mg/L). According to the results from Table 2, a high regression coefficient (R2) (0.90) was obtained, which proved that 2,4-DNP degradation with AO/RPs followed the pseudo-first-order model. The results of this section also showed that there was a direct relationship between 2,4-DNP concentration and kobs and robs constants. Changes in the values of these constants with changes in 2,4-DNP concentrations are listed in Table 2. Decrease in the kobs values from 0.504 to 0.186 (min−1) and increase in the robs values from 2.19 to 9.48 (mgL−1 min−1) with the addition of 2,4-DNP concentration can be because, in more concentration on 2,4-DNP, pollutant molecules absorb more UV photons, and therefore, UV absorption by ZnO and sulfite is reduced, leading to poor activation of these two compounds and the formation of less reactive radicals (Moussavi and Rezaei 2017; Liu et al. 2016).
One of the important factors for choosing the appropriate method in water and wastewater treatment processes is the evaluation of energy consumption by that process. Because AO/RPs using UV lamps are associated with energy consumption, it is necessary to calculate energy costs and simple figures-of-merit based on electric energy consumption. In this study, EEO was calculated as the number of kWh of electrical energy required to degrade 2,4-DNP in 1 m3 of water, and the results were reported in Table 2. At high concentrations of 2,4-DNP, a decrease in degradation rate was associated with an increase in EEO. This showed that 2,4-DNP at higher concentrations requires more UV radiation for degradation (Daneshvar et al. 2005). According to Table 2, the values obtained for kobs constant and EEO were inversely correlated with each other, so that with increasing kobs, a decrease in EEO values was observed. The highest and lowest values of kobs and EEO were observed in low concentrations of 2,4-DNP. Therefore, it is inferred that high concentrations of 2,4-DNP prevent the proper and uniform penetration of UV light throughout the solution. Also, higher concentrations of 2,4-DNP produce more intermediate species, which by consuming free radicals can reduce the removal efficiency and kobs (Yazdanbakhsh et al. 2021).
TOC analysis was performed to estimate 2,4-DNP mineralization by AO/RPs based on UV/ZnO/SO3 at different times and under optimal conditions. Mineralization was investigated at 30, 60 and 90 min at 45 mg 2,4-DNP/L. According to the results obtained in Fig. 4, the amount of TOC decreased with increasing reaction time; in other words, 2,4-DNP mineralization increased with increasing reaction time. However, after 90 min, the mineralization was not complete. Therefore, reaction time is a critical factor in dinitrophenol degradation, and the conversion of 2,4-DNP to H2O and CO2 requires a longer time. Incomplete mineralization in the AORP process based on UV/SO3/ZnO proves that this process alone is not able to completely remove 2,4-DNP and its intermediates. Therefore, this method can be suggested as a pre-treatment for other treatment processes, including biological treatment.
Metabolites and pathways of 2,4-DNP photo-degradation
One of the essential steps in the degradation of contaminants with AO/RPs is to identify the favorable reaction sites of the contaminant for the oxidizing and reducing radicals. Mei et al. (2021) stated that the most active site for attacks of the reactive radicals is usually the highest orbital in the polluting molecule. In 2,4-DNP, these orbitals are located on O1, C1, C2, C3, C4 and C6, which in these situations, sensitive groups such as hydroxyl (−OH) and nitro (−NO2) are located.
In other words, the weak bonds of these groups are broken by reactive radicals and lead to complete mineralization or conversion of 2,4-DNP to other compounds. Various studies have shown that mineralization of aromatic compounds occurs by the attack of oxidizing and reducing radicals on the benzene ring, carbonyl groups, functional group and breaking the nitrogen-containing bonds. Accordingly, in the 2,4-DNP molecule, the highly oxidizing radical OH• was responsible for attacking the benzene ring, and SO•3/e−aq radicals were responsible for breaking the bond of the sensitive hydroxyl and nitro groups as well as the double bonds such as N=O. However, since e−aq has a high rate constant, double bond sites are more likely to be attacked by these reactive reducing electrons (Moussavi and Rezaei 2017).
In other words, one of the factors that facilitate the decomposition of aromatic compounds such as 2,4-DNP is the presence of functional groups on their rings. In the structure of 2,4-DNP, the functional groups are two nitro groups that have a high electronegativity and loss electrons in the presence of e−aq and are removed from the benzene ring. This reaction increases the rate of 2,4-dinitrophenol degradation by other reductive and oxidative species (Azizi et al. 2021).
Some studies have reported that in reduction reactions with sulfite, in addition to SO•3 radical, another SO•2 radical is also formed, and these radicals perform desulfurization, dechlorination and denitration. During the oxidation and reduction reactions of 2,4-DNP, intermediate products are also produced which are also attacked by OH•, SO•3, e−aq and H• radicals during sequential stages, which eventually leads to the decomposition of 2,4-DNP into compounds with less molecular mass (Yazdanbakhsh et al. 2021).
Briefly, at the beginning of the reaction, reducing and oxidizing radicals attack the functional groups and break them, and then, by attacking the benzene rings, they open the phenol structure and produce other by-products or linear hydrocarbons. If the reaction is complete, the linear hydrocarbons will eventually be converted to water and CO2[21]. Intermediates of 2,4-DNP degradation were detected by GC-MS. Since various factors are involved in 2,4-DNP degradation, by-products formed are summarized in Fig. 5.
Conclusion
In this study, in order to investigate the simultaneous effect of oxidizing and reducing radicals on 2,4-DNP degradation, sulfite and ZnO were used to produce OH•, SO•3, e−aq and H• radicals. Ultraviolet light was also used as a stimulant of these two compounds. Also, the efficiency of UV/SO3/ZnO process was compared with other processes which included UV, UV/ZnO, UV/SO3 and SO3/ZnO, and the results showed that the process involving UV/SO3/ZnO has a much higher efficiency in 2,4-DNP removal than other processes. The results showed that the highest 2,4-DNP removal efficiency (91%) with UV/SO3/ZnO process was obtained in 25 min, 2,4-DNP = 5 mg/L, ZnO dose = 0.7g/L and SO3 = 2mmol L−1. However, according to the results of TOC analysis, only 61% mineralization was performed at 45 mg 2,4-DNP/L. The EEO results showed that the energy consumption for 2,4-DNP degradation was optimal.
Data availability
Only the data described in this work is available, and there are no other links.
References
Ahuja S (2018) Advances in water purification techniques, 1st edn. Elsevier, Amsterdam
Azarpira H, Sadani M, Abtahi M, Vaezi N, Rezaei S, Atafar Z et al (2019) Photo-catalytic degradation of triclosan with UV/iodide/ZnO process: performance, kinetic, degradation pathway, energy consumption and toxicology. J Photochem Photobiol A Chem 371:423–432
Azizi S, Sarkhosh M, Asghar A, Mohseni M, Maaza M, Sadani M (2021) Degradation of codeine phosphate by simultaneous usage of eaq- and •OH radicals in photo-redox processes: influencing factors, energy consumption, kinetics, intermediate products and degradation pathways. Optik 243:167415
Bagal VM, Lele BJ, Gogate PR (2013) Removal of 2,4-dinitrophenol using hybrid methods based on ultrasound at an operating capacity of 7 L. Ultrason Sonochem 20(5):1217–1225
Behnajady MA, Vahid B, Modirshahla N, Shokri M (2009) Evaluation of electrical energy per order (EEO) with kinetic modeling on the removal of malachite green by US/UV/H2O2 process. Desalination 249:99–103
Dadban S, Sadeghi M, Shahryari A, Okhovat N, Bahrami F, Baneshi MM (2015) Heterogeneous catalytic ozonation of 2, 4-dinitrophenol in aqueous solution by magnetic carbonaceous nanocomposite: catalytic activity and mechanism. Deswater 57(43):20447–20456
Daneshvar N, Aleboyeh A, Khatae AR (2005) The evaluation of electrical energy per order (EEo) for photooxidative decolorization of four textile dye solutions by the kinetic model. Chemosphere 59:761–767
Daraei H, Manshouri M, Yazdanbakhsh AR (2010) Removal of phenol from aqueous solution using ostrich feathers ash. J Maz Univ Med Sci 20(79):81–87
Daraei H, Abdeltif A, Kamali H (2017) Assessment of phenol removal efficiency by synthesized zero iron nanoparticles and Fe powder using the response surface methodology. Iran J Chem Chem Eng 36:137–146
Dehghani M, Jaafari J, Alghasi A, Porkar G (2011) Using medium pressure ultraviolet reactor for removing azo dyes in textile wastewater treatment plant. World Appl Sci J 12(6):797–802
Deng Y, Ezyske CM (2011) Sulfate radical-advanced oxidation process (SR-AOP) for simultaneous removal of refractory organic contaminants and ammonia in landfill leachate. Water Res 45(18):6189–6194
Entezari M, Godini H, Sheikhmohammadi A, Esrafili A (2019) Enhanced degradation of polychlorinated biphenyls with simultaneous usage of reductive and oxidative agents over UV/sulfite/TiO2 process as a new approach of advanced oxidation/reduction processes. J Water Process Eng 32:100983
González-Sarrías A, Espín JC, Tomás-Barberán FA (2017) Non-extractable polyphenols produce gut microbiota metabolites that persist in circulation and show anti-inflammatory and free radical-scavenging effects. Trends Food Sci Technol 69:281–288
Jeong J, Song W, Cooper WJ, Jung J, Greaves J (2010) Degradation of tetracycline antibiotics: mechanisms and kinetic studies for advanced oxidation/reduction processes. Chemosphere 78(5):533–540
Khan S, He X, Khan JA, Khan HM, Boccelli DL, Dionysiou DD (2017) Kinetics and mechanism of sulfateradical- and hydroxyl radical-induced degradation of highly chlorinated pesticide lindane in UV/peroxymonosulfate system. Chem Eng J 318:135–142
Khan MF, Yu L, Achari G, Tay JH (2019) Degradation of sulfolane in aqueous media by integrating activated sludge and advanced oxidation process. Chemosphere 222:1–8
Liu X, Yoon S, Batchelor B, Wahab AA (2013) Degradation of vinyl chloride (VC) by the sulfite/UV advanced reduction process (ARP): effects of process variables and a kinetic model. Sci Total Environ 454–455:578–583
Liu X, Zhong J, Fang L, Wang L, Ye M, Shao Y, Li J, Zhang T (2016) Trichloroaceticacid reduction by an advanced reduction process based on carboxyl anion radical. Chem Eng J 303:56–63
Mei Q, Wei F, Han D, An Z, Sun J, Li M, Wei B, Xie J, He M (2021) Degradation mechanisms, kinetics and eco-toxicity assessment of 2,4-dinitrophenol by oxygen-containing free radicals in aqueous solution. Mol Phys 119(9):1886365
Milh H, Yu X, Cabooter D, Dewil R (2021) Degradation of ciprofloxacin using UV-based advanced removal processes: comparison of persulfate-based advanced oxidation and sulfite-based advanced reduction processes. Sci Total Environ 764:144510
Moussavi G, Rezaei M (2017) Exploring the advanced oxidation/reduction processes in the VUV photoreactor for dechlorination and mineralization of trichloroacetic acid: parametric experiments, degradation pathway and bioassessment. Chem Eng J 328:331–342
Noorisepehr M, Kakavandi B, Isaric AA, Ghanbari F, Dehghanifard E, Ghomie N, Kamrani F (2020) Sulfate radical-based oxidative degradation of acetaminophen over an efficient hybrid system: peroxydisulfate decomposed by ferroferric oxide nanocatalyst anchored on activated carbon and UV light. Sep Purif Technol 250:116950
Nuengmatcha P, Chanthai S, Mahachai R, Oh WC (2016) Sonocatalytic performance of ZnO/graphene/TiO2 nanocomposite for degradation of dye pollutants (methylene blue, texbrite BAC-L, texbrite BBU-L and texbrite NFW-L) under ultrasonic irradiation. DyesPigm 134:487–497
Paisio CE, Agostini E, González PS, Bertuzzi ML (2009) Lethal and teratogenic effects of phenol arenarum embryos. J Hazard Mater 167(1-3):64–68
Penalver JJL, Pacheco CVG, Polo MS, Utrilla JR (2013) Degradation of tetracyclines in different water matrices by advanced oxidation/reduction processes based on gamma radiation. J Chem Technol Biotechnol 88:1096–1108
Ramteke LP, Gogate PR (2015) Removal of ethylbenzene and p-nitrophenol using combined approach of advanced oxidation with biological oxidation based on the use of novel modified prepared activated sludge. Process Saf Environ Prot 95:146–158
Trojanowicz M, Bojanowska-Czajka A, Capodaglio AG (2017) Can radiation chemistry supply a highly efficient AO(R) P process for organics removal from drinking and waste water: a review. Environ Sci Pollut Res 24(25):20187–20208
Völker J, Castronovo S, Wick A, Ternes TA, Joss A, Oehlmann J, Wanger M (2016) Advancing biological wastewater treatment: extended anaerobic conditions enhance the removal of endocrine and dioxin-like activities. Environ Sci Technol 50(19):10606–10615
Xiong Z, Zhang H, Zhang W, Lai B, Yao G (2019) Removal of nitrophenols and their derivatives by chemical redox : a review. Chem Eng J 359:13–31
Yanga L, He L, Xue J, Ma Y, Shi Y, Wu L, Zhang Z (2020) UV/SO32−based advanced reduction processes of aqueous contaminants: current status and prospects. Chem Eng J 397:125412
Yazdanbakhsh A, Eslami A, Moussavi G, Rafiee M, Sheikhmohammadi A (2018) Photo-assisted degradation of 2,4,6-trichlorophenol by an advanced reduction process based on sulfite anion radical: degradation, dechlorination and mineralization. Chemosphere 191:156–165
Yazdanbakhsh A, Eslami A, Mahdipour F, Ghanbari F, Ghasemi M, Atamaleki A, Sharifi H, Lin KL (2021) Dye degradation in aqueous solution by dithionite/UV-C advanced reduction process (ARP): Kinetic study, dechlorination, degradation pathway and mechanism. J Photochem Photobiol A Chem 407:112995
Yu H, Nie E, Xu J, Yan S, Cooper WJ, Song W (2013) Degradation of diclofenac by advanced oxidation and reduction processes: kinetic studies, degradation pathways and toxicity assessments. Water Res 47(5):1909–19018
Acknowledgements
The authors would like to thank the Environmental Health Engineering Research Center, the Kerman University of Medical Sciences, for their scientific supports.
Funding
This work was supported by the vice-chancellor for Research and Technology of Kerman University of Medical Sciences (Grant No. 99000827) and the code of research ethics certificate IR.KMU.REC.1400.015.
Author information
Authors and Affiliations
Contributions
HD conceived, designed and performed the experiments, analyzed and interpreted the data, and wrote the paper; KT performed the bibliographic revision, carried out the comparison of results and collaborated in the discussion of results.; AM, JM, AM analyzed and interpreted the data and critically revised the work.
Corresponding author
Ethics declarations
Ethics approval
This work does not contain any studies with human participants or animals.
Consent to participate
All authors provided informed consent to participate in this research study.
Consent for publication
No applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Ricardo A. Torres-Palma
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 119 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Daraei, H., Mittal, A., Toolabian, K. et al. Study on the biodegradability improvement of 2,4 dinitrophenol in wastewater using advanced oxidation/reduction process with UV/SO3/ZnO. Environ Sci Pollut Res 30, 22273–22283 (2023). https://doi.org/10.1007/s11356-022-23231-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-23231-1