Abstract
Varicella is a rising public health issue. Several studies have tried to quantify the relationships between meteorological factors and varicella incidence but with inconsistent results. We aim to investigate the impact of temperature and relative humidity on varicella, and to further explore the effect modification of these relationships. In this study, the data of varicella and meteorological factors from 2011 to 2019 in 21 cities of Guangdong Province, China were collected. Distributed lag nonlinear models (DLNM) were constructed to explore the relationship between meteorological factors (temperature and relative humidity) and varicella in each city, controlling in school terms, holidays, seasonality, long-term trends, and day of week. Multivariate meta-analysis was applied to pool the city-specific estimations. And the meta-regression was used to explore the effect modification for the spatial heterogeneity of city-specific meteorological factors and social factors (such as disposable income per capita, vaccination coverage, and so on) on varicella. The results indicated that the relationship between temperature and varicella in 21 cities appeared nonlinear with an inverted S-shaped. The relative risk peaked at 20.8 ℃ (RR = 1.42, 95% CI: 1.22, 1.65). The relative humidity-varicella relationship was approximately L-shaped, with a peaking risk at 69.5% relative humidity (RR = 1.25, 95% CI: 1.04, 1.50). The spatial heterogeneity of temperature-varicella relationships may be caused by income or varicella vaccination coverage. And varicella vaccination coverage may contribute to the spatial heterogeneity of the relative humidity-varicella relationship. The findings can help us deepen the understanding of the meteorological factors-varicella association and provide evidence for developing prevention strategy for varicella epidemic.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Varicella (or chickenpox) is an extremely contagious infectious disease, which is mainly caused in infants and children by the first infection of the ubiquitous varicella-zoster virus. Varicella is an important public health concern worldwide. The World Health Organization (WHO) estimated approximately 4.2 million varicella cases with severe complications in 2014, and approximately 4200 related deaths occurring per year in the world (WHO 2014). In China, the incidence and outbreak events of varicella have increased significantly in recent years, with an incidence of 70.14 per 100,000 people in 2019 (Dong et al. 2020).
Many factors may influence the varicella epidemics, such as vaccination (Pan et al. 2021) and personal contact (Marziano et al. 2018). And several studies have investigated the possible association between the occurrence of varicella infection and meteorological factors such as relative humidity and temperature (Critselis et al. 2012; Lu et al. 2020; Wang et al. 2018). Some studies have suggested that relative humidity is associated with varicella (Chan et al. 2014; Yang et al. 2016), whereas another study showed a weak link between relative humidity and varicella (Chen et al. 2017). It has been reported that the mean temperature has a considerable influence on the incidence of varicella and is closely related to the transition of varicella patterns (Sumi 2018). However, the results of different studies have been inconsistent. For instance, a study conducted in Lanzhou, China (Wang et al. 2018) demonstrated an approximately M-shaped nonlinear relationship between temperature and varicella; meanwhile, a study in Guangzhou, China, reported an inverted U-shaped nonlinear relationship between temperature and varicella (Lu et al. 2020).
Some infectious diseases, including malaria, measles, Japanese encephalitis, and mumps, exhibit heterogeneity in their spatial and temporal distributions (Tang et al. 2017; Wang et al. 2014; Yu et al. 2018; Zhang et al. 2012). Previous studies have reported that the transmission of varicella is affected by different geographic regions, climatic belts, population densities, and socio-economic statuses (such as vaccination coverage) (Daulagala and Noordeen 2018, Toi and Dwyer 2010, WHO 2016), and that these characteristics may contribute to the spatial heterogeneity of the effect of meteorological factors on varicella incidence. However, the heterogeneity of meteorological factors on varicella remains to be further explored. Previous studies have primarily been conducted in one city (Lu et al. 2020; Wang et al. 2018), which prevents the modeling and quantitative assessment of heterogeneous relationships. Thus, it is necessary to investigate the effect of spatial heterogeneity of meteorological factors on varicella and explore the effects of modification factors on heterogeneity.
In this study, based on the data from 21 cities in Guangdong Province in south China, we investigated the impact of temperature and relative humidity on varicella and further explored the effect modification for its spatial heterogeneity. The aim of the study was to provide results that would identify the potential predictive factors for the varicella epidemic, and therefore provided information to aid public health authorities.
Materials and methods
Study sites
Guangdong Province is located at the southern tip of the Chinese mainland coast. It ranges from 109° 39′ to 117° 19′ E longitude and 20° 13′ to 25° 31′ N latitude, comprising a total of 21 prefecture-level cities and an extremely high population of 126 million people as of 2020 (Guangdong Bureau of Statistics 2021). Socio-economic conditions vary among cities (Guangdong Bureau of Statistics 2021). Significant differences were observed in indicators such as the population density, disposable income per capita, and medical institutions. For example, the disposable income per capita of Shenzhen is 64,878 Chinese Yuan (CNY), which is approximately 2.8 times of Jieyang (21,821 CNY). Guangdong is located in a typical monsoon-influenced climate area, with a wet hot summer and a dry cold winter. The number of varicella cases in Guangdong ranks among the top three in China (Dong et al. 2020). Therefore, Guangdong is an ideal site to study the relationship between varicella and meteorological factors.
Varicella is not a notifiable infectious disease in China, but it was categorized as a surveillance infectious disease by the Guangdong Department of Health in 2005. Subsequently, all medical practitioners in both public and private sectors in Guangdong were required to report varicella cases online to the National Notifiable Infectious Diseases Reporting Information System (NNIDRIS), using a standardized form. The varicella vaccine is a voluntarily self-funded vaccine that has not been included in Expanded Program on Immunization in Guangdong Province. Various cities in the province have considerably different levels of varicella immunization (Zhu et al. 2016).
Based on the school calendar provided by the Department of Education of Guangdong Province and Guangzhou Municipality, schools are opened during autumn and spring, and closed during winter and summer. The summer vacation lasts from July to August for approximately seven weeks, while the winter vacation begins in February or March for about four weeks.
Data collection
Data of varicella and meteorological variables
Daily data series of varicella cases from January 1, 2011, to December 31, 2019, were collected from NNIDRIS. A clinical case of varicella should be reported to NNIDRIS within 24 h of diagnosis, using a standardized form. For this study, these daily counts of varicella clinical cases based on the date of symptom onset were then aggregated for each of the 21 cities in Guangdong.
Daily monitoring data of meteorological factors, including mean temperature, mean relative humidity, accumulated precipitation, and wind speed for the 21 selected cities from January 1, 2011, to December 31, 2019, were collected from the China Meteorological Data Sharing Service System (http://www.cma.gov.cn/), which was constructed by the Chinese National Meteorological Information Centre. The daily meteorological data for each city was calculated as the average of the monitoring stations in counties within the city administrative boundaries. For incomplete data, the missing values were imputed with the mean of the values before and after each missing value; meanwhile, the abnormal values of the meteorological variables were handled in the same way for the missing value. However, this was negligible due to the very small proportion of missing values (no meteorological variables with more than 0.4% missing values) and the high quality of monitoring data. For cities with no meteorological monitoring stations within their administrative boundaries, data from stations in adjacent cities closest to the city center was used (Luo et al. 2020).
City-specific characteristics
The climatic and social characteristics of each of the 21 cities in Guangdong Province were collected. The annual mean of the daily monitoring data of meteorological factors at each city/station was calculated to represent the climatic differences including temperature, relative humidity, precipitation, and wind speed. City-specific social characteristics were collected from the China City Statistical Yearbook, including geographical variables (latitude), demographic variables (population density), and socioeconomic variables, such as disposable income per capita, vaccination coverage, and medical institutions per 10,000 people.
Statistical analysis
We first fitted a DLNM for each of the 21 cities to explore the exposure–response relationship between meteorological factors and varicella incidence. Then, the single exposure–response relationships were pooled to determine the overall cumulative exposure–response using multivariate meta-analysis. Finally, meta-regression analysis was applied to explore the effect of medication factors on the spatial heterogeneity of the exposure–response relationship.
City-specific analysis
Given that multiple previous studies have suggested that the meteorological factors-varicella relationship can be delayed (Gao et al. 2020; Wu et al. 2016), a DLNM was incorporated. The DLNM was fitted to each of the 21 cities to relate the daily series of varicella counts to mean temperature or relative humidity.
The model for temperature or relative humidity can be specified as:
where \({\mu }_{t}\) is the expected number of varicella on date t; \({\beta }_{0}\) is the intercept; Exposuret,l is the cross-basis exposure (temperature or relative humidity) matrix of lag, varicella, and temperature (or relative humidity); ns indicates a smoother base on natural cubic splines; df is the degree of freedom; DOW is an indicator for day of week; Holiday is a binary variable for the public holiday; Time is an indicator for calendar time; Term was introduced as a binary variable for the school term; and β is the regression coefficient.
A quasi-Poisson regression model was adopted to allow for overdispersion. The bi-dimensional exposure-lag-response relationship between meteorological factors (temperature or relative humidity) and varicella was described through a cross-basis function (Gasparrini et al. 2010) using natural cubic splines for the exposure–response and lag-response relationships. The lag interval was determined by the incubation period of varicella infections (21 days) (Ayoade and Kumar 2021). The pooled estimates of the temperature-varicella relationship returned to 1 on day 21 (Supplementary Fig. 1). To control for unmeasured time-varying confounding, we used natural cubic splines of Time to remove long-term trends and seasonality. The df value was set according to previous studies (Xiao et al. 2017; Yang et al. 2020). We either incorporated relative humidity in the same lag range of temperature, or incorporated temperature in the same lag range of relative humidity. We also adjusted for the effect of the day of week (DOW), holidays (including national public holidays), and school terms as indicator variables. Relative risk (RR) with its 95% confidence interval (CI) was used to estimate the risk of meteorological factors on varicella. Our preliminary exploration revealed that there was a nonlinear relationship between meteorological variables and varicella. According to the previous studies (Lam et al. 2018; Ma et al. 2015), the value of a meteorological variable corresponding to the minimum RR for varicella was set as the reference value when estimating the varicella risk. In this study, the exposure–response relationship between temperature and varicella showed that the extreme high temperature corresponded to the lowest RR value. Thus, we set the reference temperature at 31 °C which was at the 99th percentile of temperature distribution (defined as the extreme high temperature) referring to Fang and Lin et al.'s studies (Fang et al. 2021; Lin et al. 2017). For the relationship between relative humidity and varicella, the relative humidity value of 52.5% which corresponded to the minimum RR was set as a reference value.
Multivariate meta-analysis and meta-regression analysis
A multivariate meta-analysis was applied to pool the city-specific estimations following the method of previous studies (Gasparrini and Armstrong 2013) to examine the overall meteorological factor-varicella relationship, and a meta-regression analysis was used to capture the potential effect modifiers of the relationship. The estimated city-specific meteorological factor-varicella relationship, represented by RR, was pooled using a multivariate meta-analysis to estimate the overall nonlinear relationship between meteorological variables and varicella.
To identify the potential modification factors of significant heterogeneity, meta-regression analyses modeling city-specific characteristics were performed (Supplementary Table 1, Supplementary Table 2). These included independent variables such as latitude, population density, disposable income per capita, medical institutions per 10,000 people, and the annual average of meteorological factors. First, we fitted a multivariate meta-regression model with an intercept only, allowing for heterogeneity being modeled through random effects (denoted as the intercept-only model). We then ran a single meta-predictor analysis by incorporating city-specific characteristics into the model separately (denoted as single meta-predictor models) and compared it to the intercept-only model. For the second-stage analysis, we used the maximum likelihood estimation to obtain estimates, and a Wald test to test the significance of meta-predictors and differences between models. Residual heterogeneity was assessed using multivariate extension of I2 statistics (Gasparrini et al. 2012).
The meta-predictor Wald test was selected for further exploration. The effect of these meta-predictors was displayed by predicting the averaged associations between meteorological factors and varicella for the 10th and 90th percentiles of their distribution, using the baseline reference of 31 °C for temperature and 52.5% for relative humidity.
The indicator of cities with P < 0.05, which are considered statistically significant for the Wald test, is a categorized proxy variable of potential effect modifiers that had the largest impact in their category and a more sensible interpretation.
Sensitivity analysis
To check the robustness of our estimates, sensitivity analyses were conducted by changing the meta-regression method, where the REML model and fixed-effect model were fitted (Supplementary Fig. 2).
All the implementations were accomplished using R software version 4.0.2. Specifically, exposure–response analysis, meta-analysis, meta-regression, and accompanying graphical presentation were carried out with dlnm (Gasparrini 2011), mvmeta (Gasparrini et al. 2012), and metafor packages. Statistical significance was set at P < 0.05, and all P values were 2-sided.
Results
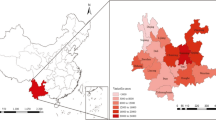
From 2011 to 2019, 617,898 varicella cases were reported in the 21 cities in Guangdong, with an annual average incidence of 6.33 per 10,000. Varicella incidence varies geographically, with most cases occurring in densely populated cities (Fig. 1a). The cities in the Pearl River Delta such as Shenzhen, Guangzhou, Foshan and Zhongshan had higher prevalence than that in other cities in Guangdong; that is, their respective incidences per 10,000 were 15.98, 13.85, 9.77, and 9.27 (Fig. 1b).
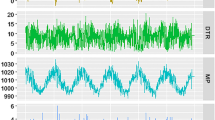
Figure 2 depicts the time series of daily varicella counts and weather conditions in Guangdong from 2011 to 2019. There was obvious seasonality of a bimodal pattern for varicella occurrence, especially during 2017–2019, with the first peak from April to June, and the second peak from November to January of the following year. We observed that high varicella incidence seems to be related to temperate temperatures and higher humidity (for instance, in April) and low temperature and low humidity (for instance, in December) (Fig. 2).
Figure 3 shows the exposure–response relationship between meteorological factors (temperature and relative humidity) and varicella. The overall pooled estimates suggested a nonlinear relationship between temperature and varicella, with an approximately inverted S-shape (Fig. 3a). We found the relative risk of varicella peaked at 20.8 °C (RR = 1.42, 95% CI: 1.22,1.65), with reference to the 99th percentile of temperature distribution. For relative humidity, the overall pooled estimates suggested a nonlinear relationship between relative humidity and varicella, with an approximately L-shaped upturned tail (Fig. 3b). The threshold relative humidity was found to be approximately 52.5%. The RR of relative humidity on varicella was 1.25 (95% CI: 1.04,1.50) when comparing 69.5% (corresponding to the maximum RR with significance) with 52.5% (Supplementary Table 3). The meteorological factor-varicella relationship curves differed across the 21 cities (Fig. 3).
Pooled overall curves of temperature-varicella relationship (a) and relative humidity-varicella relationship (b) based on 21 cities in Guangdong Province, China, 2011–2019 (solid blue lines are the reference value, the dotted blue lines are the value corresponded to the maximum relative risk (RR) with statistically significance)
Table 1 summarizes the results of the heterogeneity analysis. For temperature, the single meta-predictor models showed that disposable income per capita and varicella vaccination coverage could partially explain the heterogeneity. Similar modification effect was found between varicella vaccination coverage and relative humidity. We also found a borderline impact of medical institutions and annual average temperature. The modification effect of these characteristics on temperature was negligible compared to that of disposable income per capita, or varicella vaccination coverage. Demographic, socioeconomic (excluding vaccination coverage), and meteorological characteristics were not significant in explaining the heterogeneity of relative humidity.
We only displayed the modification effects of disposable income per capita and varicella vaccination coverage (p value of Wald test < 0.05) for temperature and varicella vaccination coverage for relative humidity (p value < 0.05) because they had the largest impact and had a more sensible interpretation. Disposable income per capita had the best ability to explain heterogeneity, with the related I2 statistic dropping from 39.6% (intercept-only model) to 36.4% (Table 1). We found that higher disposable income per capita and varicella vaccination coverage could elevate the risk of temperature or relative humidity on varicella (Fig. 4a, b). We found that the risk of relative humidity on varicella was higher in regions with higher vaccination coverage (Fig. 4c).
The modification of disposable income per capita and vaccination coverage on the temperature-varicella relationship (a, b) and vaccination coverage on relative humidity-varicella relationship (c). Predictions for the 10th (red dot-dashed line) and 90th (blue dashed line) percentiles of disposable income per capita and vaccination coverage of cities. Reference at 31 °C for temperature and 52.5% for relative humidity, respectively. The 95% CI of RR are reported as shaded area
From the results of the sensitivity analysis, we observed that the results based on the REML model and fixed-effect model were similar, and the exposure–response curve showed no significant change (Supplementary Fig. 1).
Discussion
Based on data from 21 cities in South China, we conducted a two-stage analysis to investigate the exposure–response relationship between temperature, relative humidity, and varicella, and further explored the spatial heterogeneity of the relationship. We observed a nonlinear exposure–response relationship between temperature, relative humidity, and varicella incidence. These relationships may be modified by socioeconomic factors such as income and vaccination coverage. To the best of our knowledge, this is the first study to explore the modification effect of the relationship between meteorological factors and varicella incidence.
We found a nonlinear relationship between temperature and varicella, which is approximately inverted S-shaped, with the highest RR being observed at around 21 °C and low RR at cold or high temperatures. This result is similar to that of studies conducted in Lanzhou (Wang et al. 2018) and Guangzhou (Lu et al. 2020). The authors reported a nonlinear temperature-varicella association of approximately M-shape or inverted U-shape. The mechanism underlying the relationship between temperature and varicella is not fully understood. One possible reason is that temperature affects both varicella-zoster viruses (VZV) in titer and in vivo activity because of the heat sensitivity of VZV (WHO 2016), and the virus may be affected at higher temperatures (Halloran et al. 2012). Another reason may be related to the school calendar (Bakker et al. 2021). In China, students would take summer and winter vacations when suffering from extremely hot and cold temperatures, which significantly decreases the exposure between those students, corresponding to fewer opportunities to be affected (Marziano et al. 2018). Nevertheless, the inverted S-shaped curve for temperature and varicella would be helpful for further exploration in the prediction of varicella outbreaks.
A nonlinear relationship between relative humidity and varicella was observed in this study. The relative risk of varicella increased when the relative humidity was > 52.5%. Several studies have demonstrated that relative humidity is positively associated with infectious diseases such as hand-foot-mouth disease (HFMD), which is partly similar to our findings (Bo et al. 2020; Wu et al. 2014). Both varicella and HFMD can be transmitted through close contact and mainly affect infants and children. The exact mechanism underlying the potential association between relative humidity and varicella and transmission is unknown. One hypothesis is that during days of higher humidity, children tend to stay indoors with air conditioning; this increases the chance for close contact with others, thus facilitating varicella transmission (Sun et al. 2011; Ueshiba et al. 2013). It is necessary to strictly implement a school registration system for absenteeism due to illness as well as conduct morning checks of students’ basic health conditions, including basic visible symptoms such as fever and pox rash, to aid the early detection of physical abnormalities and prevent disease spread.
Our study indicated that a high disposable income per capita could elevate the association between temperature and varicella. The modification effects of disposable income per capita may be attributed to its association with population density and socioeconomic development. Guangdong is a province with a large population, high population density, and high disposable income per capita, particularly in cities located in the Pearl River Delta. Previous studies have found that factors such as population density, nursery attendance, and socioeconomic development may also influence the epidemiology of VZV in areas where universal vaccination has not yet been implemented (Arat et al. 2019; Chan et al. 2018). Changes in the epidemiology of varicella infection, particularly in tropical regions, have been attributed to demographic changes, including increased population density and urbanization (Daulagala et al. 2017; Neiderud 2015). Seroprevalence rates of varicella infection have been observed to be higher among urban populations, regardless of age distribution (Li et al. 2019; Liyanage et al. 2007). Specifically, urban settings may enable enhanced social interaction and population mobility within and between communities, thus facilitating viral transmission. Such evidence was corroborated by findings and indicated that children with enhanced social interaction, such as those attending to nurseries, are more likely to contract varicella infections (Santermans et al. 2015). This shows the importance and difficulty of preventing the spread of the virus, as social interaction and population mobility are inevitable. To block the varicella epidemic and limit the size of outbreaks, pre-outbreak and post-outbreak varicella vaccination should be prioritized, along with self-home quarantine for the infected.
Districts with higher varicella vaccination coverage were associated with a higher RR, either in the temperature-varicella relationship or the relative humidity-varicella relationship. The mechanism underlying this phenomenon remains unknown. One possible reason might be the high population density. The Pearl River Delta region, which has a high level of varicella immunization, is the most economically developed and densely populated area in Guangdong Province. A high population density could facilitate the spread of varicella (Daulagala et al. 2017) because the residents living in dense areas have a higher probability of getting into close contact with others. Another reason might be that the vaccination coverage of varicella (Ni et al. 2018) has not reached the standard recommended by the WHO (WHO 2016). Vaccine coverage that remains < 80% over the long term is expected to shift varicella infections to the older age group of children in some settings, which may increase morbidity and mortality, despite a reduction in the total number of cases (WHO 2016). This suggests that more attention should be paid to varicella vaccination uptake to establish an immune barrier.
This study has a few limitations. First, being an observational ecological study, results should be interpreted at the population level and cannot be read as causal associations. Second, multiple factors may influence varicella epidemics, such as meteorological factors, vaccination coverage, socioeconomic factors, and host susceptibility (Lu et al. 2020). We were unable to account for all confounding factors, and the important confounder of vaccine coverage was only considered in the multivariate regression analysis and not in the model. These factors may have influenced our results to some extent. Third, vaccination policies and coverage varied across the 21 cities, which could have influenced the results.
Conclusion
Our study showed that there is a nonlinear relationship between temperature, relative humidity, and varicella. Medium temperature and high relative humidity would elevate the risk of varicella infection in South China. Disposable income per capita and varicella vaccination coverage may modify the relationship between meteorological factors and varicella. Our findings can help deepen our understanding of how meteorological factors impact varicella epidemics. Furthermore, this evidence can provide implications for related public health decisions, such as varicella prediction, based on meteorological factors.
Data availability
The data of daily meteorological variables from 2011 to 2019 can be accessed from the China Meteorological Data Sharing Service System (http://www.cma.gov.cn/), and city-specific socio-economic characteristics for 21 cities in Guangdong can be accessed from the official website of Guangdong Provincial Bureau of Statistics (http://stats.gd.gov.cn/gdtjnj/). The data of detailed surveillance generated during and/or analyzed during the current study are not publicly available due to the data management requirements but are available from the corresponding author on reasonable request.
References
Arat A, Burström B, Östberg V, Hjern A (2019) Social inequities in vaccination coverage among infants and pre-school children in Europe and Australia - a systematic review. BMC Public Health 19:290
Ayoade F, Kumar S (2021) Varicella Zoster, StatPearls. StatPearls Publishing LLC, Treasure Island FL
Bakker KM, Eisenberg MC, Woods R, Martinez ME (2021) Exploring the Seasonal Drivers of Varicella Zoster Virus Transmission and Reactivation. Am J Epidemiol 190:1814–1820
Bo Z, Ma Y, Chang Z, Zhang T, Liu F, Zhao X, Long L, Yi X, Xiao X, Li Z (2020) The spatial heterogeneity of the associations between relative humidity and pediatric hand, foot and mouth disease: Evidence from a nation-wide multicity study from mainland China. Sci Total Environ 707:136103
Chan DYW, Edmunds WJ, Chan HL, Chan V, Lam YCK, Thomas SL, van Hoek AJ, Flasche S (2018) The changing epidemiology of varicella and herpes zoster in Hong Kong before universal varicella vaccination in 2014. Epidemiol Infect 146:723–734
Chan JY, Lin HL, Tian LW (2014) Meteorological factors and El Nino Southern Oscillation are associated with paediatric varicella infections in Hong Kong, 2004–2010. Epidemiol Infect 142:1384–1392
Chen B, Sumi A, Wang L, Zhou W, Kobayashi N (2017) Role of meteorological conditions in reported chickenpox cases in Wuhan and Hong Kong, China. BMC Infect Dis 17:538
Critselis E, Nastos PT, Theodoridou K, Theodoridou M, Tsolia MN, Hadjichristodoulou C, Papaevangelou V (2012) Time trends in pediatric hospitalizations for varicella infection are associated with climatic changes: a 22-year retrospective study in a tertiary Greek referral center. PLoS One 7:e52016
Daulagala S, Noordeen F, Fara MMF, Rathnayake C, Gunawardana K (2017) Exposure rate of VZV among women attending antenatal care clinic in Sri Lanka - a cross sectional study. BMC Infect Dis 17:625
Daulagala S, Noordeen F (2018) Epidemiology and factors influencing varicella infections in tropical countries including Sri Lanka. Virusdisease 29:277–284
Dong P, Wang M, Liu Y (2020) Epidemiological characteristics of varicella in China, 2016–2019. Chinese J Vaccin Immunization 26:403–406
Fang J, Song J, Wu R, Xie Y, Xu X, Zeng Y, Zhu Y, Wang T, Yuan N, Xu H, Song X, Zhang Q, Xu B, Huang W (2021) Association between ambient temperature and childhood respiratory hospital visits in Beijing, China: a time-series study (2013–2017). Environ Sci Pollut Res Int 28:29445–29454
Gao Y, Niu Y, Sun W, Liu K, Liu X, Zhao N, Yue Y, Wu H, Meng F, Wang J, Wang X, Liu Q (2020) Climate factors driven typhus group rickettsiosis incidence dynamics in Xishuangbanna Dai autonomous prefecture of Yunnan province in China, 2005–2017. Environ Health : a Glob Access Sci Source 19:3
Gasparrini A, Armstrong B, Kenward MG (2010) Distributed lag non-linear models. Stat Med 29:2224–2234
Gasparrini A (2011) Distributed lag linear and non-linear models in R: the package dlnm. J Stat Softw 43:1–20
Gasparrini A, Armstrong B, Kenward MG (2012) Multivariate meta-analysis for non-linear and other multi-parameter associations. Stat Med 31:3821–3839
Gasparrini A, Armstrong B (2013) Reducing and meta-analysing estimates from distributed lag non-linear models. BMC Med Res Methodol 13:1
Guangdong Bureau of Statistics (2021) Guangdong Statistical Yearbook 2021. Guangdong Bureau of Statistics
Halloran SK, Wexler AS, Ristenpart WD (2012) A comprehensive breath plume model for disease transmission via expiratory aerosols. PLoS One 7:e37088–e37088
Lam HCY, Chan JCN, Luk AOY, Chan EYY, Goggins WB (2018) Short-term association between ambient temperature and acute myocardial infarction hospitalizations for diabetes mellitus patients: A time series study. PLoS Med 15:e1002612
Li XK, Gao XL, Li Y, Xu H, Fan CL (2019) Seroepidemiology of varicella among the healthy population aged 1–19 years in Harbin, China. Chin J Contemp Pediatr 21:203–207
Lin Z, Meng X, Chen R, Huang G, Ma X, Chen J, Huang M, Gui Y, Chu C, Liu F, Kan H (2017) Ambient air pollution, temperature and kawasaki disease in Shanghai, China. Chemosphere 186:817–822
Liyanage NP, Fernando S, Malavige GN, Mallikahewa R, Sivayogan S, Jiffry MT, Vitarana T (2007) Seroprevalence of varicella zoster virus infections in Colombo district, Sri Lanka. Indian J Med Sci 61:128–134
Lu JY, Zhang ZB, He Q, Ma XW, Yang ZC (2020) Association between climatic factors and varicella incidence in Guangzhou, Southern China, 2006–2018. Sci Total Environ 728:138777
Luo C, Ma Y, Liu Y, Lv Q, Yin F (2020) The burden of childhood hand-foot-mouth disease morbidity attributable to relative humidity: a multicity study in the Sichuan Basin, China. Sci Rep 10:19394
Ma W, Wang L, Lin H, Liu T, Zhang Y, Rutherford S, Luo Y, Zeng W, Wang X, Gu X, Chu C, Xiao J, Zhou M (2015) The temperature-mortality relationship in China: An analysis from 66 Chinese communities. Environ Res 137:72–77
Marziano V, Poletti P, Béraud G, Boëlle PY, Merler S, Colizza V (2018) Modeling the impact of changes in day-care contact patterns on the dynamics of varicella transmission in France between 1991 and 2015. PLoS Comput Biol 14:e1006334
Neiderud CJ (2015) How urbanization affects the epidemiology of emerging infectious diseases. Infect Ecol Epidemiol 5:27060
Ni L, Zheng X, Huang Y (2018) Modelling and predicting the long-term impact of varicella vaccine immunization Modern. Prev Med 45:449–453
Pan J, Wang Y, Cao L, Wang Y, Zhao Q, Tang S, Gong W, Guo L, Liu Z, Wen Z, Zheng B, Wang W (2021) Impact of immunization programs on 11 childhood vaccine-preventable diseases in China: 1950–2018. Innovation 2:100113
Santermans E, Goeyvaerts N, Melegaro A, Edmunds WJ, Faes C, Aerts M, Beutels P, Hens N (2015) The social contact hypothesis under the assumption of endemic equilibrium: Elucidating the transmission potential of VZV in Europe. Epidemics 11:14–23
Sumi A (2018) Role of temperature in reported chickenpox cases in northern European countries: Denmark and Finland. BMC research notes 11:377
Sun Y, Wang Z, Zhang Y, Sundell J (2011) In China, students in crowded dormitories with a low ventilation rate have more common colds: evidence for airborne transmission. PLoS One 6:e27140
Tang X, Geater A, McNeil E, Deng Q, Dong A, Zhong G (2017) Spatial, temporal and spatio-temporal clusters of measles incidence at the county level in Guangxi, China during 2004–2014: flexibly shaped scan statistics. BMC Infect Dis 17:243
Toi CS, Dwyer DE (2010) Prevalence of varicella-zoster virus genotypes in Australia characterized by high-resolution melt analysis and ORF22 gene analyses. J Med Microbiol 59:935–940
Ueshiba R, Abe K, Kingetsu M, Ito M (2013) Suggestion to school pharmacists to utilize absolute humidity parameter for maintaining air-conditioning. Yakugaku Zasshi : Journal of the Pharmaceutical Society of Japan 133:479–483
Wang JY, Li S, Ma HP, Dong JY, Wang YH, Zhang W, Zhang XY, Li P, Li SY (2018) Research on the relationship between the daily mean temperature and the daily cases of varicella during 2008–2016 in Lanzhou, China. Chin J Prev Med 52:842–848
Wang L, Hu W, Soares Magalhaes RJ, Bi P, Ding F, Sun H, Li S, Yin W, Wei L, Liu Q, Haque U, Sun Y, Huang L, Tong S, Clements AC, Zhang W, Li C (2014) The role of environmental factors in the spatial distribution of Japanese encephalitis in mainland China. Environ Int 73:1–9
WHO (2014) Varicella and herpes zoster vaccines: WHO position paper, June 2014. Wkly Epidemiol Rec 89:265–287
WHO (2016) Varicella and herpes zoster vaccines: WHO position paper, June 2014–Recommendations. Vaccine 34:198–199
Wu H, Wang H, Wang Q, Xin Q, Lin H (2014) The effect of meteorological factors on adolescent hand, foot, and mouth disease and associated effect modifiers. Glob Health Action 7:24664
Wu J, Cheng J, Xu Z, Zhao K, Zhao D, Xie M, Yang H, Wen L, Li K, Su H (2016) Nonlinear and Interactive Effects of Temperature and Humidity on Childhood Hand, Foot and Mouth Disease in Hefei, China. Pediatr Infect Dis J 35:1086–1091
Xiao X, Gasparrini A, Huang J, Liao Q, Liu F, Yin F, Yu H, Li X (2017) The exposure-response relationship between temperature and childhood hand, foot and mouth disease: A multicity study from mainland China. Environ Int 100:102–109
Yang F, Ma Y, Liu F, Zhao X, Fan C, Hu Y, Hu K, Chang Z, Xiao X (2020) Short-term effects of rainfall on childhood hand, foot and mouth disease and related spatial heterogeneity: evidence from 143 cities in mainland China. BMC Public Health 20:1528
Yang Y, Geng X, Liu X, Wang W, Zhang J (2016) Association between the incidence of varicella and meteorological conditions in Jinan, Eastern China, 2012–2014. BMC Infect Dis 16:179
Yu G, Yang R, Wei Y, Yu D, Zhai W, Cai J, Long B, Chen S, Tang J, Zhong G, Qin J (2018) Spatial, temporal, and spatiotemporal analysis of mumps in Guangxi Province, China, 2005–2016. BMC Infect Dis 18:360
Zhang Y, Liu QY, Luan RS, Liu XB, Zhou GC, Jiang JY, Li HS, Li ZF (2012) Spatial-temporal analysis of malaria and the effect of environmental factors on its incidence in Yongcheng, China, 2006–2010. BMC Public Health 12:544
Zhu Q, Wang X, Su W, Cui Y, Liang J, Wu C, Shao X-p, Zheng H-z (2016) Effectiveness of varicella vaccine based on several varicella outbreaks in Guangdong Province. South China J Prev Med 42:129–133
Acknowledgements
We are grateful for all people who diagnosed and submitted the varicella cases to the National Notifiable Infectious Disease Surveillance System. We thank the staffs at the hospitals, local health departments, and county-, district-, and prefecture-level Centers for Disease Control and Prevention for their valuable assistance in data collection.
Funding
This work was primarily supported by the National Key Research and Development Program of China (2018YFA0606200), Guangdong Medical Science and Technology Research Foundation (B2020105), the Key-Area Research and Development Program of Guangdong Province (2019B111103001), and the Science and Technology Program of Guangzhou (202102080554).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Conceived and designed the experiments: Limei Sun, Jianpeng Xiao. Analyzed the data: Yihan Li, Jianpeng Xiao. Contributed reagents/materials/analysis tools: Yihan Li, Jialing Li, Zhihua Zhu, Weilin Zeng, Qi Zhu, Zuhua Rong, Jianxiong Hu, Xing Li, Guanhao He, Jianguo Zhao, Lihua Yin, Yi Quan, Qian Zhang, Manman Li, Li Zhang, Yan Zhou, Tao Liu, Wenjun Ma, Siqing Zeng, Qing Chen, Limei Sun, Jianpeng Xiao. Wrote the paper: Yihan Li, Jianpeng Xiao, Weilin Zeng. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
The study was approved by the ethics committee of Guangdong Provincial Center for Disease Control and Prevention (No. W96-027E-201925).
Consent to participate
Not applicable.
Consent for publication
The authors have provided consent to publish this work.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Lotfi Aleya
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Y., Li, J., Zhu, Z. et al. Exposure–response relationship between temperature, relative humidity, and varicella: a multicity study in South China. Environ Sci Pollut Res 30, 7594–7604 (2023). https://doi.org/10.1007/s11356-022-22711-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-22711-8