Abstract
Cyanobacteria are among the beneficial and environmentally friendly natural candidates used in the biosynthesis of nanoparticles, with their ability to accumulate heavy metals from their environment, thanks to their biologically active compounds. In the current study, an aqueous extract of Oscillatoria princeps fresh biomass was used for the green synthesis of AgNPs. UV–vis spectrum, Fourier transforms infrared, scanning electron microscopy, and energy-dispersive spectroscopy were used to validate and characterize biosynthesized of OSC-AgNPs. The biosynthesis of AgNPs was visually verified in terms of the change in the color of the AgNO3 solution from yellowish brown to brown colors from 72 h onwards. An absorption peak of approximately 420 nm was detected in the UV–vis spectrum, corresponding to the plasmon resonance of AgNPs. FT-IR analysis showed the presence of free amino groups in addition to sulfur-containing amino acid derivatives that act as stabilizing agents. SEM images detected the roughly spherical shape of OSC-AgNPs with an average size of 38 nm. The pathogens tested were all susceptible to OSC-AgNPs showing varying antimicrobial effects on pathogenic microorganisms. E. coli and C. albicans displayed the maximum susceptibility, with zones of inhibition of 14.6 and 13.8 mm at 3-mM concentration, respectively, while B. cereus had the lowest zone of inhibition (10.6 mm) at 3-mM OSC-AgN03 concentration. In conclusion, AgNPs synthesized from Oscillatoria princeps inhibit biofilm formation, suggesting that AgNPs may be a promising candidate for the prevention and treatment of biofilm-associated infections caused by bacteria and yeasts.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nanotechnology is an emerging field that involves the synthesis, characterization, and development of various nanomaterials that have important roles in everyday life (Hamida et al. 2020). Nanotechnology is the creation of structures with improved or completely new physical, chemical, and biological properties by working at the level of atoms and molecules in units per billion. The term “nano” refers to any particle or material that has a size of at least one nanoscale (0.1–100 nm). Compared with large particles of the same materials, nanomaterials exhibit superior and novel properties depending on size (Jeon et al. 2021). Nanomaterials are known to have unique properties such as chemical reactivity, magnetism, electrical conductivity, optical effects, and physical strength. These properties of nanomaterials are a result of their small size (Adebayo-Tayo et al. 2019). There are thousands of commercial products based on nanotechnology, and this technology is expected to become a global business in the near future (Sharma et al. 2016).
Materials at the nanoscale are lighter, more robust, programmable materials, and require less material use in manufacturing and less energy in the production phase. Nanotechnological applications such as obtaining clean water, thanks to nanofilters that can clean sediments, chemical wastes, charged particles, bacteria, and other pathogens such as viruses, contribute to the sustainable environment. In the field of nanomedicine, nanoscale materials and nanoelectronic biosensors are used for various purposes such as diagnosing, monitoring, treating, and preventing diseases (Nikalje 2015). Today, it is anticipated that the ability to use nanotechnology will enable food companies to design and produce cheaper, safer, and more durable, and more nutritious products.
Silver nanoparticles (AgNPs) can interact with microorganism surfaces due to their large surface area and small size and are therefore used as antimicrobial agents (Durán et al. 2016). The use of silver metal by humanity as an antibacterial agent against microorganisms that cause various infectious diseases goes back to ancient times. The oxidation of silver ions is a slow process under normal conditions, and silver salts have been proposed to overcome this. However, the use of sparingly soluble silver salts leads to a slower release of silver ions, thus lower antibacterial effects. When silver ions are converted to AgNPs by green synthesis methods, their antimicrobial activity increases significantly, while their toxicity decreases (Kambale et al. 2020). Gold NPs (AuNPs) are in high demand for various applications due to their low toxicity, high photothermal, and photoacoustic activity (Lenartowicz et al. 2017). Other biogenic NPs such as copper oxide NPs, zinc oxide NPs, and selenium NPs have been reported in various studies to act as potent anticancer agents (Hamida et al. 2020).
Recently, the synthesis of nanoparticles has been focused on using minimal or negligible toxic substances that do not harm the environment and pose no risk to human health. At this point, green nanotechnology has emerged as a research area that includes environmentally friendly and energy-efficient approaches for the synthesis of inorganic nanoparticles (Jeffryes et al. 2015). Biological systems such as bacteria, viruses, algae, yeast, fungi, and plants are widely used in green nanotechnological methods (Mittal et al. 2013). The increasing interest in the use of biological materials in the synthesis of nanoparticles has led researchers to use algae species in this process.
Algae are of particular interest due to their unique diversity, high productivity, biologically active substances, and technical advances in their cultivation. Microalgae and Cyanobacteria are the primary producers of aquatic ecosystems, although they are among the primitive life forms on earth. They are considered renewable bioenergy sources due to their photosynthetic abilities. Today, they are discussed from the perspective of sustainability in many areas, from wastewater treatment to biodiesel production (Mahana et al. 2021). Algae are a group of organisms that can be found in a variety of habitats, from freshwater ecosystems to salty waters, from low temperatures to high temperatures, such as spring waters, under low light intensity and high pressure, such as lakes and seas (Bleeke et al. 2014; Varshney et al. 2015). They are a wide variety of autotrophic unicellular or multicellular organisms that can be cataloged as microalgae and macroalgae. Various algae species from Chlorophyceae, Phaeophyceae, Cyanophyceae, and Rhodophyceae have been reported to synthesize metallic NPs such as gold, silver, palladium, and ferrihydrite. Nanoparticles synthesized from algae cultures are relatively convenient and easy to use, with advantages such as less toxicity, less risk to the environment, and lower temperature synthesis (Sharma et al. 2016). Green synthesized AgNPs have been reported to have effective antibacterial potency and low cytotoxicity, suggesting their use in biomedical and pharmacological applications (Adebayo-Tayo et al. 2019; Bishoyi et al. 2021). Different studies have demonstrated the capacity of different Cyanobacterial strains to form different types of NPs, mainly metallic NPs (Ag-NPs, Au-NPs) and metal oxide NPs (ZnO NPs, CuO NPs). Lengke et al. (2007) performed intracellular and extracellular biosyntheses of AgNPs by the filamentous cyanobacterium Plectonema boryanum, and Tsibakhashvili et al. (2011) reported the synthesis of extracellular AgNPs using Arthrospira (Spirulina) platensis.
Most of the antibiotics used today to treat bacterial infections have become ineffective due to the emergence and spread of antibiotic-resistant bacterial strains. Therefore, researchers focused on finding new agents and alternative antibiotics that are effective against bacterial pathogens and looking for new mechanisms to fight infections (Tang et al. 2020). Recently, one of these alternatives is the quorum-quenching mechanism, which consists of inhibiting the quorum sensing messaging between the microorganisms, and has emerged as a promising topic (Tabbouche et al. 2017). Quorum sensing (QS) is a cell-to-cell communication mechanism between microorganisms, in which the expression of several genes, often associated with virulence factors and biofilm formation, is very important for their life and growth as a population. QS is controlled over the production and detection of signal molecules in a population density–dependent manner (Tang, et al. 2020). By means of quorum sensing molecules, the division of microorganisms, nutrition, biofilm, formation of spores, pigment production, and bacterial movements are controlled. Quorum sensing also affects bacterial virulence factors. In this case, it suggests that quorum quenching can prevent the emergence of resistance among bacteria (Tabbouche et al. 2017). Based on these facts, the search for herbal and other natural products for new therapeutic agents has increased in recent years. Anti-quorum sensing activities of phlorotannins from seaweed Hizikia fusiforme were investigated by Tang et al. (2020). As a result of the research, they stated that phlorotannins from H. fusiforme have anti-quorum sensing activities and potential in this regard.
Biofilms are film-like complexes that occur on biotic and abiotic surfaces and are sessile bacterial communities embedded in a self-synthesizing exopolysaccharide matrix. Bacteria in biofilms show different characteristics from free-living cells, such as different physiology and high resistance to antibiotics, which makes them a chronic and persistent source of infection. Therefore, there is a need to investigate potential anti-QS and antibiofilm compounds from natural sources to prevent bacterial infections due to increased multidrug resistance among pathogens (Sridevi et al. 2019). Bacteria within a biofilm can be up to 1000 times more resistant to antibiotics and persist after treatment with antibiotics (López and Soto 2020). In order to fight biofilms, new molecules that can be active against biofilm formation and diagnostic tools that can be used in practice are needed; in this context, algae are one of the natural products that emerged as a result of the searches in this area. In the study carried out by Gayatri et al. (2019), the antibiofilm effect of Chlorella powder was investigated and proved to have the potential to inhibit the pathogenic activity of Pseudomonas aeruginosa in biofilm formation by providing a new alternative to traditional antimicrobial agents.
In this study, AgNPs were synthesized with Oscillatoria princeps isolated from freshwater sources. The characterization of the synthesized AgNPs was performed by visual, UV–vis spectroscopy, Fourier transforms infrared spectrum (FT-IR), scanning electron microscopy (SEM), and energy-dispersive spectroscopic analysis (EDS). In addition, the antimicrobial activity of silver nanoparticles against pathogenic microorganisms were investigated. Although the studies carried out to date have defined the compounds produced by microalgae and Cyanobacteria species with antimicrobial activity and their effects, the studies on the anti-quorum sensing activity and antibiofilm activities of the extracts produced by Cyanobacteria are very limited. Anti-quorum sensing activity assay, violaceum quantitative evaluation, and biofilm inhibition assay were also studied this research using OSC-AgNPs obtained from O. princeps extract. Another important output of this study is the management of algal resources and bringing them to the industry in order to provide a balance between the sustainable management of the environment and natural resources and the long-term economic, social, and environmental development of these resources.
Materials and methods
Isolation, morpho‑taxonomy, and maintenance of Oscillatoria princeps
Cyanobacteria samples (Oscillatoria princeps) were collected and isolated using the micromanipulation technique from freshwater reservoirs from Ankara (Turkey) (latitude 39.92077 and longitude 32.85411). The morphological structure of the isolated strain was determined under a light microscope, and shape, structure, color, and individual filaments were measured. Morphological confirmation was done using the identification keys. O. princeps’s molecular characterization was carried out using polymerase chain reaction (PCR) and Fourier transform infrared (FT-IR) spectroscopy. DNA was extracted and purified from O. princeps using the method described by Singh et al. (2011). To check the accuracy and integrity of the extract, DNAs were taken on 1% agarose gel, and Qiagen QIAquick PCR cleaning kit was used to remove contaminants. Polymerase chain reaction (PCR) was used to determine the quality of the extracted genomic DNA template, and for Roche Expand high-fidelity PCR kit was used for 16 s primers (27F, 1492R) for PCR analysis of the species. DNA was measured with (http://www.nanodrop.com/Productnd2000overview.aspx) and sent to GATC Biotech for single sequence reading of all samples (http://www.gatc-biotech.com/en/index.html). Subsequent sequence analysis was performed by BLAST (Fig. 1). FT-IR analysis were performed at the Kırşehir Ahi Evran University Central Laboratory, Kırşehir (Turkey), using a Thermo Scientific Nicolet 6700 model FT-IR spectrometer. The stock cultures were inoculated into 50 ml of Erlenmeyer flask into liquid BG-11 medium at pH 6.8 and made axenic using the different combinations of antibiotics and allowed under appropriate conditions. Later, it was added to the Culture Collection (AEU-CCA) at Kırşehir Ahi Evran University and was kept under protection by giving the code number (CCA01Os02).
The cultures used in this study were cultivated as 270 ml medium + 30 ml culture at pH 6.8. The light source (Philips cool daylight, 50 µmol m−2 s−1) was applied to the cultures with a period of 16 L:8 D, and cultivation was performed at 22–25 °C.
Biosynthesis of silver nanoparticles utilizing fresh algal biomass
The extract to be used for silver nanoparticle synthesis was prepared from fresh O. princeps biomass. When O. princeps passed the “growth phase” approximately 4–6 weeks, the cells were centrifuged at 10,000 rpm for 10 min and then washed several times with distilled water to remove the trace metals from the surface of the Cyanobacteria biomass. O. princeps biomass was harvested by filtration. The fresh biomass (at least 2 g) was boiled at 100 °C for 20 min. After, the O. princeps extract was cooled and centrifuged at 5000 rpm for 10 min (Ahamad et al. 2021; Aziz et al. 2014). The obtained supernatant was stored at 4 °C until used in experiments. Ten milliliters of extracts of cultures were suspended in 90 ml of 1 mM, 2 mM, and 3 mM aqueous AgNO3 and incubated at room temperature. The solution was maintained in the dark condition to avoid auto-oxidation of silver. All the conditions of the experiments were monitored during the course of the study. Water with the addition of AgNO3 was used as a control. In this study, silver nanoparticles obtained from O. princeps extracts were labelled as OSC-AgNPs.
Characterization of synthesized silver nanoparticles
The bio-reduction of Ag+ ions were monitored by using UV–Vis spectrophotometer (Thermo Scientific Spectrophotometer Genesys 10S) in a 1-cm path length quartz cuvette having a spectral range of 200 and 800 nm. UV–Vis measurements were taken at 24-, 48-, and 72-h intervals. All measurements were performed in triplicate at a constant temperature of 25 ± 1 °C to confirm the synthesis of NPs. AgNPs were subjected to FT-IR spectrum analysis to detect biomolecules responsible for reducing Ag+ ions and identify whether the biomolecules were stabilizing and reducing agents. FT-IR analysis were performed at the Kırşehir Ahi Evran University Central Laboratory, Kırşehir (Turkey), using a Thermo Scientific Nicolet 6700 model FT-IR spectrometer. The spectrum was recorded in the range of 4000–500 cm−1 at a resolution of 4 cm−1. The sample has been also characterized for the development of silver nanoparticles by using scanning electron microscopy (SEM) and energy-dispersive spectroscopy (EDS) (FEI Model Quanta FEG 450) investigation. The size, shape, and morphology of OSC-AgNPs were characterized using scanning electron microscopy (SEM). SEM and EDS were performed at the Yozgat Bozok University Science and Technology Application and Research Center, Yozgat (Turkey).
Antimicrobial property
To study the antimicrobial effect of synthesized AgNPs from O. princeps (OSC-AgNPs), agar well diffusion method was used against gram-negative bacteria Aeromonas hydrophila (ATCC 7966), Klebsiella pneumoniae (ATCC 13,883), Vibrio anguillarum (ATCC 43,312), Pseudomonas aeruginosa (ATCC 27,853), and Escherichia coli (ATCC 25,922); gram-positive bacteria Staphylococcus aureus (ATCC 29,213), Bacillus subtilis (ATCC 6633), Bacillus cereus (709 Roma), and Enterococcus faecalis (ATCC 29,212); and yeast Candida albicans (ATCC 10,231).
In peptone water, the isolate was cultivated overnight. Mueller–Hinton agar (Lab M Ltd., UK) plates were seeded with the isolate’s 18-h-old culture. A sterile cork-borer with a diameter of 7 mm was used to cut uniform wells on the dried agar plate. The biosynthesized OSC-AgNPs were poured into each well at a volume of 75 µl. As negative controls, AgNO3 solution (1 mM, 2 mM, and 3 mM), and O. princeps extract were used in corresponding wells. The inoculation plates were incubated for 24 h at 37 °C. Plates were examined after incubation by measuring the diameter of inhibition zones (mm) (DIZ) surrounding the wells. Positive DIZ (mm) of more than 5 mm were evaluated (Prabhu et al. 2014; NCCLS 2003).
Antimicrobial effects of synthesized AgNPs and minimum inhibitory concentration (MIC) values were found and tested. Minimum inhibitory concentrations (MIC) for OSC-AgNPs against bacteria and yeast strains were examined in accordance with NCCLS guideline (NCCLS 2000). MIC values were determined spectrophotometrically in 96-well microtiter plates according to the microdilution broth method. Mueller–Hinton broth was used in the suspension of bacteria (0.5 McFarland), in solutions of OSC-AgNPs to be tested (1, 2, and 3 mM), and in MIC testing. For the antimicrobial effect test, 1000 μg/mL stock solutions of the synthesized OSC-AgNPs were prepared. Bacteria and yeast stock cultures were prepared to approximately 106 cfu/ml of bacteria and yeast prepared according to the McFarland 0.5 turbidity standard. The AgNPs were transferred to 96-well plates in two-fold serial dilutions with nutrient broth (500, 250, 125, 62.5, 31.2, 15.6, and 7.8 μg/mL). Then, in each well, a microbial inoculum prepared in the same nutrient medium was inoculated. Plates were incubated for 24-48 hours at 37°C.
Anti-quorum sensing activity assay
The anti-quorum activity of OSC-AgNPs on violacein production by Chromobacterium violaceum ATCC 12,472 was tested by an agar diffusion method using LB (Luria–Bertani) agar plates. A 100-μl volume of freshly grown C. violaceum (1 × 106) culture was spread onto the LB agar surface by a swap and 7-mm-diameter wells were punched into the agar using cork-borer. Then, the wells were filled with 75 μL of 1, 2, and 3 mM OSC-AgNPs extracts. Plates were observed after incubation at 30 °C for 24–48 h. Opaque zones of bacterial growth around the wells were indicative of QSI activity. The plates determined the inhibition of pigment production around the well. The formation of a clear halo around the disc and the formation of bacterial growth inhibition were evaluated as positive (McLean et al. 1997).
Violaceum quantitative evaluation
Anti-quorum sensing activity was tested against Chromobacterium violaceum ATCC 12,472. For this purpose, the extracts of AgNPs were first determined on 96-well plate as mentioned before, then OSC-AgNPs were tested for pigment production inhibition. Briefly, an 18-h (1 × 108 CFU/mL) bacterial culture of C. violaceum (ATCC 12,472) was suspended in LB broth with both absence and presence of the OSC-AgNPs diluted two-fold, and incubated at 30 °C for 24 h. To begin, 200 µl of treated and untreated cultures are placed in an Eppendorf tube and lysed by adding 200 µl of 10% SDS (sodium dodecyl sulfate), vortexing for 5 s, and incubating at room temperature for 5 min. Also, 900 μL water-saturated butanol (50 ml n-butanol mixed with 10 ml of distilled water) is added to cell lysate, vortexed for 5 s, and centrifuged at 13,000 g for 5 min. The upper (butanol) phase containing violaceum was collected and the absorbance is read at 580 nm in spectrophotometer (Jasco V-730-JAPAN). Decrease in pigment production in the presence of AgNP extracts is measured as: Percent inhibition = OD of control-OD of treated /OD of control × 100 (Khan et al. 2009).
Antibiofilm activity of OSC-AgNPs
The antibiofilm activities of OSC-AgNPs were determined by inoculating clinical pathogens S. aureus (ATCC 29,213), B. cereus (709 Roma), P. aeruginosa (ATCC 27,853), and E. coli (ATCC 25,922) in a 96-well microtiter plate containing 100-μL concentrations of extract. The OSC-AgNPs were separately added to all bacteria and incubated at 37 °C for 24 h. The planktonic cells were removed, and the tubes were washed with sterile water. Crystal violet (0.1%) of 125 μL was added to all the wells and incubated for 30 min at room temperature. After 30 min, the stain was removed and washed with sterile distilled water to remove unbound crystal violet stain. Finally, the adhered biofilm bound crystal violet was eluted in absolute ethanol (200 μL), and the absorbance was measured at 595 nm (Schillaci et al. 2013).
Results
Morphological features of O. princeps
Blue-green algae (Cyanobacteria) are microscopic and oxygen-producing prokaryotes, and common in aquatic environments, along with some terrestrial species. The genus Oscillatoria is a blue-green alga belonging to the Cyanobacteria phylum. Oscillatoria genus with blue-green mat appearance first grow at the bottom of freshwater reservoirs and then are released on the water surface (Guiry and Guiry 2021). The genus Oscillatoria is one of the dominant blue-green algae (Cyanobacteria) growing in a variety of habitats, and thallus consists of a single trichome. Long filamentous cells are discoid in shape and do not require heterocysts because they are surrounded by cell walls. It has many gas vesicles to optimize its position in the water (Castenholz and Waterbury 1989; Rani et al. 2016). Oscillatoria princeps Vaucher ex Gomont belongs to Cyanobacteria phylum. Mühlsteinová et al. (2018) defined the morphological structure of O. princeps as “Trichomes blue-green, highly motile, not constricted at the cross-walls, narrowing toward the often bent ends, 24–36 µm wide in the central area of the trichome and 14–27 (30) µm wide at the ends. Apical cells nearly hemispherical and often yellowish (together with up to five adjacent cells). Granulation never located at the cross-walls, but fine to larger granules randomly dispersed throughout cells. Cells 2–9 µm long, new cell walls form from the outside of the trichome often before the previous division is finished. Cell wall colourless and thick, necridic cells present, no sheath or calyptra observed” (Mühlsteinová et al. 2018). In this study, the cultures produced in the batch culture system were free-floating as a thick mass on the surface of the culture medium, some of which were attached to the glass walls of the container. O. princeps cultures were dark blue-green in color due to the dominance of phycobilins, i.e., phycocyanin and phycoerythrin pigment, and individual filaments were blue-green to olive green in color (Fig. 1).
Genetic characterization of O. princeps
The 16 s sequencing results were returned in FASTA format as shown in Fig. 1. The results confirmed the molecular identification of O. princeps by 99%. The DNA was measured on a nanodrop (http://www.nanodrop.com/Productnd2000overview.aspx), and the data are presented in Fig. 1.
Characterization of the nanoparticle biosynthesis
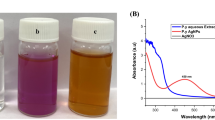
During the bio-reduction of AgNPs from silver ions, silver nanoparticle formation was visually identified by color change. The reaction mixture of AgNO3 and O. princeps extract turned from yellowish green to brown, indicating the formation of silver nanoparticles (Fig. 2). In this study, the formation of AgNPs was monitored by UV–Vis absorption spectra at 24-, 48- and 72-h time intervals and at 200 to 800 nm (Fig. 3). The color change observed in the formation of biosynthesized silver nanoparticles was due to the stimulation of the surface plasmon resonance (SPR) of the nanoparticles in the reaction mixture, and a peak was noted at the spectral range of 420 nm. It was clearly seen that the silver surface gave a plasmon resonance band following the formation of AgNPs and the intensity reached its maximum afterwards.
FT-IR analyses of AgNPs from O. princeps extract showed that bioactive groups surround biogenic silver nanoparticles. The absorption spectrum showed 6 clear bands in the wavelength range 4000–500 cm−1; these bands were assigned on the basis of standards and published FT-IR spectra (Sigee et al. 2002). As seen in Fig. 4, the FT-IR spectrum of OSC-AgNPs had six peaks at 3224 cm−1, 2952 cm−1, 2824 cm−1, 1631 cm−1, 1361 cm−1, and 1104 cm−1. FT-IR spectra of OSC-AgNPs showed bands for the presence of amino, carboxylic, hydroxyl, and carbonyl groups. Band distributions were predicted as residual water (-OH; band 1), lipid (-CH2; bands 2, 3 and 5), amide (protein; bands 1, 4 and 5), nucleic acid (> P = O; band 6), and starch (-C-O; band 6) (Table 1). The peaks at 3224 cm−1 (stretching amide A) and 1631 cm−1 (amide I band) indicated that amides on the AgNPs surface might have contained peptides and proteins found in the cell extract. In the obtained AgNPs spectra, it showed a strong broad absorption peak in the 3224 cm−1 band. This band is characteristic of the O–H stretching vibration of polysaccharide and the N–H stretching vibration of proteins. The band at 1631 cm−1 was assigned to the N–H bending stretching vibration of the amine groups of the proteins.
Scanning electron microscopy (SEM) was used to investigate the presence, morphological characterization, and size of AgNPs biosynthesized from the fresh extract of O. princeps. The particle size and distribution of the OSC-AgNPs were estimated under SEM visualization using ImageJ software. SEM images detected the roughly spherical shape of OSC-AgNPs with an average size of 38 nm in diameter was found to be well dispersed in solution and deposited on cell surfaces (Fig. 5). It has been reported that pH is an important factor in many physical parameters to prevent aggregation and agglomeration of nanometals. At lower pH, the reducing power of various functional groups is naturally less due to the higher H+ concentration. With an increase in pH, the reducing power of functional groups will also increase. This increase will allow the stability of the NPs and prevent their aggregation. The hydrophobic and hydrophilic interactions of the intramolecular force can prevent the agglomeration of metal nanoparticles (Sharma et al. 2016). This explains the agglomeration observed in AgNPs obtained from O. princeps extract. Synthesis of NPs obtained by using algae can occur extracellularly or intracellularly, depending on the type of algae and the dose used. It was concluded that the extracellular formation of metallic NPs is due to the activity of reducing agents present in the algal aqueous phase. These agents are polysaccharides, reducing sugars, peptides, proteins, pigments or other reducing factors that can reduce metal ions and precipitate metallic ions as nanoparticles (Mahdavi et al. 2013; Abdel-Raouf et al. 2013). In this study, AgNPs were deposited on the cell surface of O. princeps extract which was demonstrated by SEM observations (Fig. 5). EDS showed the presence of traces of nitrogen and oxygen and the formation of high amounts of silver particles. The presence of elemental silver signal was confirmed in the EDS analysis of AgNPs obtained from O. princeps (Fig. 6).
Determination of antimicrobial activity of synthesized with OSC-AgNPs
Effects of antimicrobial activity of the OSC-AgNPs using with diameter of inhibition zones (mm) (DIZ) and minimal inhibitory concentration (MIC) were investigated. Antimicrobial activity of OSC-AgNP formation obtained after adding the cell extract of O. princeps strains to 1 mM, 2 mM, and 3 mM AgNO3 solution was performed using the well diffusion method. The antimicrobial activity of OSC-AgNPs were investigated against gram-negative bacteria (A. hydrophila ATCC 7966, K. pneumoniae ATCC 13,883, V. anguillarum ATCC 43,312, P. aeruginosa ATCC 27,853, E. coli ATCC 25,922), gram-positive bacteria (S. aureus 29,213, B. subtilis ATCC 6633, B. cereus 709 Roma, E. faecalis ATCC 29,212), and yeast C. albicans ATCC 10,231. The mean values of three replicates of the diameter of inhibition zones (mm) around each well loaded with OSC-AgNPs solutions are shown in Table 2.
When a general evaluation of the diameters of the inhibition zones were made, only B. cereus among pathogenic microorganisms measured 11.8 mm DIZ at 1 mM, which was found to be higher than the 2-mM and 3-mM concentrations. For other pathogenic microorganisms, DIZs detected at 1 mM and 2 mM were found to be close to each other, and DIZ at 3 mM was found to be high in all of them. E. coli and C. albicans displayed the maximum susceptibility at 48 h, with zones of inhibition of 14.6 mm and 13.8 mm at 3-mM concentration, respectively, while B. cereus had the lowest zone of inhibition (10.6 mm) at 3-mM AgN03 concentration. It was clearly seen that E. coli was more sensitive to all concentrations of OSC-AgNPs than to other pathogens. Inhibition zones formed by pure AgNO3 were detected between 12 and 17 mm. Nutrient broth was used as a negative control for both the bacterial strains and did not show any zone of inhibition around the well of OSC-AgNPs.
Effects of antimicrobial activity of the OSC-AgNPs (all with minimal inhibitory concentration) (MIC) are shown in Table 2. The MIC values were found in tested microorganisms between 0.78 and 1.56 μg/ml by microdilution method synthesized OSC-AgNPs. As shown in Table 2, the highest activity of OSC-AgNPs were recorded against A. hydrophila, K. pneumoniae, V. anguillarum, P. aeruginosa, E. coli, S. aureus, and C. albicans with MIC value of 0.78 µg/ml, and the lowest activity was recorded against B. subtilis, B. cereus, and E. faecalis with MIC 1.56 µg/ml at 1-mM concentration. On the other hand, MIC values of P. aeruginosa, E. coli, and S. aureus were determined as 1.56 µg/ml at 3-mM concentration.
The antibiofilm activity of OSC-AgNPs
This present study was carried out to find the antibiofilm activity of OSC-AgNPs against the pathogenic bacteria S. aureus ATCC 29,213, B. cereus 709 Roma, P. aeruginosa ATCC 27,853, and E. coli ATCC 25,922, and the results are given in Table 3.
Determination of anti-quorum sensing activity of OSC-AgNPs on C. violaceum (ATCC 12,472)
In this study, the anti-QS properties of OSC-AgNPs were tested by agar diffusion method on LB agar plates using C. violaceum ATCC 12,472 strain. In the well diffusion test, AgNPs obtained from O. princeps were loaded into the wells drilled on the petri dish. In order to check that the obtained OSC-AgNPs have anti-QS activity, it was followed whether opaque zones, defined as inhibition zones, were formed around the wells. Then, these zones’ diameters were measured in millimeters. Significant inhibition of pigment production of OSC-AgNPs screened against C. violaceum ATCC 12,472 strain was detected at 1-mM, 2-mM, and 3-mM concentration and 18.8-mm, 20.2-mm and 20.4-mm isolates, respectively (Table 4, and Fig. 7). If the obtained OSC-AgNPs did not have anti-QS activity, a purple coloration would be seen around the discs since violacein production would not be inhibited. To confirm that violacein inhibition was dependent on OSC-AgNP, unpigmented bacteria in the anti-QS regions were re-grown on fresh LB agar without OSC-AgNP. These bacteria continued to produce pigment after removal of OSC-AgNP. OSC-AgNPs exhibited concentration-dependent inhibitory activity, and all concentrations tested showed a significant reduction in violacein content. Violacein production was reduced by 72.1% and 63.3% at 2% and 4% extract, respectively.
Discussion
Antibiotic resistance is among the most important topics on the global agenda in general, due to its effects on public health and economic burden. Rapidly increasing antibiotic resistance rates have an impact on global health, sustainable development, global economy, trade, and stability of countries. In recent years, in order to find solutions to such global problems, it is necessary to use the resources of the biosphere in the most efficient and sustainable framework and to reveal new resources. The secondary metabolites of Cyanobacteria show strong biological activities such as antiviral, antibacterial, antifungal, antitumoral, and anti-inflammatory activities that are useful for therapeutic purposes. In this study, the production of nanoparticles, by green synthesis using Cyanobacteria, O. princeps, and their sustainability as antimicrobial agents to solve a global problem was analyzed.
Following the synthesis of nanoparticles, chromatic changes in the reaction mixture are used as a visual cue for the synthesis of nanoparticles from metals. The browning of the reaction mixture is an indication of silver nanoparticle (AgNP) formation. In this study, it was observed that the color intensity increased with time from light green to brown from the start of the reaction to 72 h of incubation. UV–Vis spectroscopy is a technique used to confirm the formation and stability of nanoparticles. Based on the optical properties of the nanoparticles, it serves as a method for determining the reduction of silver nitrate to silver nanoparticles in an aqueous solution (He et al. 2002). Due to surface plasmon resonance (SPR), NPs show remarkable optical properties and are monitored using absorption spectroscopy in the UV–visible spectral region (190–1100 nm) (Focsan et al. 2011). Mulvaney (1996) and Mukherjee et al. (2001) stated that a SPR absorption band arises due to the combined vibrations of free electrons of metal NPs in resonance with the light wave, which depends on the aspect ratio, size, shape and dielectric constant of the NPs. The nanometals showed remarkable spectral properties relative to the surface plasmon resonance (SPR) due to the reciprocal vibrations of the light wave and free- electron resonance affected by each size and shape of the synthesized NPs. A single SPR peak was observed from the UV–Vis spectrum of AgNPs synthesized from O. princeps extract, suggesting that the resulting AgNPs were spherical in shape. This result is consistent with previous work that demonstrated the synthesis of silver nanoparticles from the Oscillatoria genus and recorded the plasmon resonance peak at 420 nm. Adebayo-Tayo et al. (2019) and Bishoyi et al. (2021) reported that during the synthesis of silver nanoparticles on Oscillatoria, color change was observed in the mixture in the same direction and SPR was detected at 420 nm. It is hypothesized that the mechanism behind the synthesis of AgNPs is used for the reduction of silver ions by NADH-dependent nitrate reductase enzymes (Dağlıoğlu and Öztürk 2019). Mahdieh et al. (2012) have suggested that a two-step mechanism is involved in which the aqueous metal ion is first adhered to the surface of algal cells as a result of electrostatic attraction between the positively charged metal ions and the negatively charged carboxylate ions present. This process is thought to be followed by the reduction of ions to metal NPs owing to the secretion of cellular reductase by cells. Hamouda et al. (2019) reported that silver nitrate concentration, concentration of bio-reducing agents, temperature, type of the metals, duration of the exposure, and pH are important parameters that affect the properties of NPs by controlling their size, morphology, and shape.
Polysaccharides, peptides, and pigments, a set of biomolecules found in algal extracts, are responsible for the reduction of metals. Proteins and sulfated polysaccharides mainly play a role in stabilizing and capping metal nanoparticles in aqueous solutions (Kannan et al. 2013). FT-IR spectroscopy is used as a method in nanoparticle synthesis studies to reveal the reducing agents responsible for the reduction, stabilization, and closure of metal nanoparticles. In some studies, various functional groups such as − C = O − , − NH2 − , and − SH − groups attached to the surface of biosynthesized nanometals have been characterized using FT-IR (Jena et al. 2013). FT-IR spectra show protein as possible biomolecule for reduction of biosynthesized Ag nanoparticles. Hamida et al. (2020) reported that the presence of amide bonds of proteins and the hydroxyl group of polysaccharides indicate that proteins and polysaccharides that surround silver may be responsible for the reduction of silver ions to AgNPs. According to the FT-IR spectra, it can be said as a result of the findings of previous studies that the main biomolecules responsible for the bio-reduction and stabilization processes are proteins and polysaccharides (Bishoyi et al. 2021; Hamida et al. 2020; Jena et al. 2014). In our study, the data obtained from the FT-IR spectra of OSC-AgNPs show that it is supported by previous studies.
Oscillatoria genus with antimicrobial properties, its phytochemical compositions have great potential for green synthesis of AgNPs against microbial diseases. Oscillatoriaceae species produce more than 300 forms of bioactive metabolites that cover almost all biological activities. The most commonly found secondary metabolites are phenolic compounds, alkaloids, fatty acids, pigments and pigment derivatives, linear and cyclic peptides, terpenoids, and N-glycosides (Demay et al. 2019). These molecules mediate the green synthesis of AgNPs at the molecular level and thus contribute to their antimicrobial activity. As indicated in Table 2, although the pathogens tested were all susceptible to OSC-AgNPs, different concentrations of OSC-AgNPs showed varying antimicrobial effects on pathogenic microorganisms. It was concluded that the OSC-AgNPs synthesized in this study showed similar antimicrobial activity to the results obtained from Cyanobacterial extracts, which supports the previously reported data. Adebayo-Tayo et al. (2019) reported that O. princeps ether extract had effective antibacterial activity against the test bacterial pathogens with a zone of inhibition ranging from 1 to 21 mm and OsSNPs exhibited strong antibiofilm activity. Bishoy et al. (2021) indicated that O. princeps extract synthesized AgNPs had antibacterial activities against MDR strains of MRSA, S. pyogenes, and E. coli with the inhibitory zone sizes, 14–16 mm, as recorded from agar well diffusion method, while MIC values were 100, 80, and 60 μg/ml. Hamouda et al. (2019) also revealed that synthesized Ag-NPs from Oscillatoria limnetica fresh aqueous extract showed antibacterial activity against E. coli and B. cereus. As a result of the studies, AgNPs’ selectivity against bacterial cells has been proven and no antimicrobial resistance cases have been reported so far. Since one of the mechanisms of action of AgNPs is through the destruction of bacterial cells, they also inhibit the ability of bacteria to mutate. All this makes AgNPs great weapons for the clinical management of microbial diseases (Kambale et al. 2020).
Liao et al. (2019) reported that the antimicrobial effects of AgNPs also depend on nanoparticle properties, including size and shape. These physicochemical properties of AgNPs support different mechanisms that allow them to interact, pass through cell walls or membranes, and directly affect intracellular components (Bruna et al. 2021). In this study, the size of AgNPs obtained from O. princeps was determined as an average size of 38 nm and roughly spherical in shape. The relatively small size of this detected nanoparticle increased the surface area and increased the bacterial cell wall interactions and membrane permeability. Antibacterial effects of AgNPs against gram-positive and gram-negative bacteria have been shown in some studies. However, AgNPs’ exact mechanism of inhibitory growth or bactericidal activities have not yet been fully elucidated (Yin et al. 2020; Bruna et al. 2021). Gram-positive bacteria have a very thick cell wall containing many peptidoglycan layers, while gram-negative bacteria have a single peptidoglycan layer. This structure of the cell wall of gram-positive bacteria acts as a barrier for the penetration of Ag+ ions into the cytoplasm. In gram-negative bacteria, however, Ag + ions can easily damage the cell wall (Liao et al. 2019). Remarkably, in this study, AgNPs synthesized using O. princes were found to be more active against the tested gram-negative bacteria than gram-positive bacteria. This situation is consistent with previous studies stating that gram-negative bacteria are generally more prone to Ag+ invasion than gram-positive bacteria (Rónavári et al. 2017; Bapat et al. 2018; Liao et al. 2019). AgNPs attached to the cell surface can accumulate in the pits formed in the cell wall and cause denaturation of the cell membrane. AgNPs enter the bacterial cytoplasm, and they interact with enzymes and amino acids, which can impair cell function. These disruptions that consist inside the bacterial cell may occur as the formation of reactive oxygen species and the destruction of deoxyribonucleic acid (DNA) (Rónavári et al. 2017; Yin et al. 2020; Liao et al. 2019). Chernousova and Epple (2013) stated that another mechanism of action on cellular toxicity may be due to silver ions from AgNPs dispersed in aqueous solutions. Adhesion of these silver ions to the cell wall and cytoplasmic membrane can increase the permeability of the cytoplasmic membrane and inhibit respiratory enzymes. It can also prevent the synthesis of proteins by denaturing ribosomes in the cytoplasm (Yin et al. 2020).
Biofilm is an architectural colony of microorganisms consisting of microbial cells adherent to each other and to a static surface (living or nonliving) within the matrix of extracellular polymeric substances they produce. These structures are generally pathogenic in nature, and approximately 65% of all bacterial infections detected in humans are associated with quorum sensing (QS)–mediated bacterial biofilms (Jamal et al. 2018). QS-controlled virulence factors and biofilm formation are vital for the development of chronic diseases of pathogenic microorganisms. Bacteria in biofilms are highly resistant due to their different physiology and show different properties against antibiotics (Sridevi et al. 2019). Algae have recently been nominated as a natural resource in the search for potential anti-QS and antibiofilm compounds from natural sources. Oscillatoria species show antioxidant, antimicrobial, and antibiofilm properties, thanks to their valuable metabolites (Xin et al. 2017), but studies on the antibiofilm activity of extracts and molecules produced by Oscillatoria species are quite limited (López and Soto 2020). In this study, the antibiofilm activity of the biosynthesized silver nanoparticles showed a reduction in the biofilm formation by all test pathogens that were used, thus implying that the biosynthesized silver nanoparticles were active against bacterial biofilm. The highest inhibition was against P. aeruginosa, while the lowest inhibition was against B. cereus. The extract of AgNPs of O. princeps has shown the highest in biofilm inhibition activity (75.0%) for P. aeruginosa and (65.0%) S. aureus. Adebayo-Tayo et al. (2019) studied on the biosynthesis of silver nanoparticles containing biosynthesized Oscillatoria sp. The results of this research showed the biosynthesized OsSNPs to have strong antibacterial activity and showed strong antibiofilm activity against all the pathogens used in the study. They reported that Citrobacter sp. showed the lowest biofilm inhibition and P. aeruginosa showed the highest inhibition. The antibacterial and antibiofilm activities of the crude extracts of 32 microalgae species from different classes were investigated by Lauritano et al. (2016). Among these species, the genus Leptocylindrus showed strong antibiofilm activity by inhibiting 90% of S. epidermidis when grown under stress conditions caused by nitrogen starvation. Sridevi et al. (2019) determined the antibiofilm activity of methanolic extracts of C. vulgaris against P. aeruginosa and S. aureus. They stated that the amounts of this extract at different concentrations showed significant reductions in biofilm formation in P. aeruginosa and S. aureus. There are similarities between the results obtained from this study and the values presented in the literature, and it is thought that some differences in the results are due to the biomaterial and microorganisms used.
The mechanism called quorum sensing (QS) is the communication of microorganisms with each other by means of diffusible chemical molecules called autoinducers and using a cell-to-cell signaling mechanism. QS only constitutes a general language for cross-talk between microorganisms of the same species, even between other prokaryotic or eukaryotic organisms (Jayaraman and Wood 2008). Some bacterial species communicate with each other during biofilm formation using quorum sensing (QS). Anti-QS activity is defined as inhibition of the QS mechanism and can be demonstrated using various biomonitor species such as Chromobacterium violaceum bacteria. Chromobacterium violaceum can be used as a tool for testing anti-QS effective substances (Çepni and Gürel 2011). In this study, the size of these zones varies depending on the degree of inhibition. All concentrations of AgNPs (1 mM, 2 mM, 3 mM) from O. princeps showed colony formation, an indication of anti-QS capacity against C. violaceum strain. Various studies have been conducted to investigate the anti-quorum sensing efficiencies of different natural sources, as the quorum quenching mechanism is considered a promising alternative to classical antibiotics. Szabó et al. (2010) demonstrated the QS inhibition potential of essential oils of various medicinal plants. In the study by Tabbouche et al. (2017), three wild mushroom’s extracts were tested for their antimicrobial and anti-quorum sensing activities. The results revealed that Amanita rubescens and Lactarius sp. extracts showed anti-quorum sensing activity against Chromobacterium violaceum. The antimicrobial and anti-quorum sensing (QS) activities of phlorotannins from seaweed Hizikia fusiforme were evaluated by Tang et al. (2020). Phlorotannins inhibited the QS activity of Chromobacterium violaceum 12,472 by inhibiting purple pigment production while exhibiting antimicrobial activity against selected bacterial pathogens.
Conclusion
Recent studies have revealed that AgNPs are high-value nanomaterials with unique properties and extensive applications in various fields of pharmaceutical sciences. AgNPs are used as antimicrobial agents in the production of medical devices such as prosthetic implants and nanocatheters, and there are antimicrobial bandages and household antiseptic sprays designed and developed from AgNPs. AgNPs represent powerful alternatives to conventional antimicrobial therapy. AgNPs have many of the criteria that new antimicrobial technologies must meet in order to be effective, such as antimicrobial performance, rapid action, and low cytotoxicity.
Commercial applications of AgNPs synthesized by Cyanobacteria offer one more step towards “green synthesis” in a clean, non-toxic, environmentally friendly method. AgNPs obtained through green synthesis lead to a reduction in environmental pollution as well as the protection of natural and non-renewable resources and are more suitable for cosmetic use as they are less allergenic. In this research, it has been shown that the aqueous extract of O. princeps isolated from freshwater sources has been biosynthesized in an environmentally friendly way by the biological protocol. The characterization of AgNPs was performed using UV–Vis spectrophotometer and FT-IR, and the presence, morphological characterization, and size of biosynthesized AgNPs were determined by scanning electron microscope (SEM). It has been demonstrated by the tests that the obtained OSC-AgNPs have strong antimicrobial, anti-QS, and antibiofilm activity against the test pathogens. As a result, AgNPs have structural properties that can be included in applications in the medical, cosmetic, and pharmaceutical industries, making them a promising nanomaterial.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author in reasonable request.
References
Abdel-Raouf N, Al-Enazi NM, Ibraheem IBM (2013) Green biosynthesis of gold nanoparticles using Galaxaura elongata and characterization of their antibacterial activity. Arab J Chem. https://doi.org/10.1016/j.arabjc.2013.11.044
Adebayo-Tayo B, Salaam A, Ajibade A (2019) Green synthesis of silver nanoparticle using Oscillatoria sp. extract, its antibacterial, antibiofilm potential and cytotoxicity activity. Heliyon https://doi.org/10.1016/j.heliyon.2019.e02502
Ahamad I, Aziz N, Zaki A et al (2021) Synthesis and characterization of silver nanoparticles using Anabaena variabilis as a potential antimicrobial agent. J Appl Phycol. https://doi.org/10.1007/s10811-020-02323-w
Aziz N, Fatma T, Varma A, Prasad R (2014) Biogenic synthesis of silver nanoparticles using scenedesmus abundans and evaluation of their antibacterial activity. Hindawi Publishing Corporation Journal of Nanoparticles. https://doi.org/10.1155/2014/689419
Bapat RA, Chaubal TV, Joshi CP et al (2018) An overview of application of silver nanoparticles for biomaterials in dentistry. Mater Sci Eng C. https://doi.org/10.1016/j.msec.2018.05.069
Bishoyi AK, Sahoo CR, Sahoo AP, Padhy RN (2021) Bio-synthesis of silver nanoparticles with the brackish water blue-green alga Oscillatoria princeps and antibacterial assessment. Appl Nanosci. https://doi.org/10.1007/s13204-020-01593-7
Bleeke F, Rwehumbiza VM, Winckelmann D, Klöck G (2014) Isolation and characterization of new temperature tolerant microalgal strains for biomass production. Energies. https://doi.org/10.3390/en7127847
Bruna T, Maldonado-Bravo F, Jara P, Caro N (2021) Silver nanoparticles and their antibacterial applications. Int J Mol Sci. https://doi.org/10.3390/ijms22137202
Castenholz RW, Waterbury JB (1989) Oxygenic photosynthetic bacteria, group É. Cyanobacteria. In: Staley, J. N. et al. (eds) Bergey’s Manual of systematic bacteriology. Williams and Wilkins Co., Baltimore
Chernousova S, Epple M (2013) Silver as antibacterial agent: ion, nanoparticle, and metal. Angew Chem Int Ed. https://doi.org/10.1002/anie.201205923
Çepni E, Gürel F (2011) Anti quorum sensing compounds obtained from plants and their potential in developing new drugs. Türk Mikrobiyol Cem Derg. https://doi.org/10.5222/TMCD.2011.131
Dağlıoğlu Y, Öztürk BY (2019) A novel intracellular synthesis of silver nanoparticles using Desmodesmus sp. (Scenedesmaceae): diferent methods of pigment change. Rendiconti Lincei. Scienze Fisiche e Naturali https://doi.org/10.1007/s12210-019-00822-8
Demay J, Bernard C, Reinhardt A, Marie B (2019) Natural products from cyanobacteria: focus on beneficial activities. Mar Drugs. https://doi.org/10.3390/md17060320
Durán N, Durán M, De Jesus MB, Seabra AB, Fávaro WJ, Nakazato G (2016) Silver nanoparticles: a new view on mechanistic aspects on antimicrobial activity. Nanomedicine. https://doi.org/10.1016/j.nano.2015.11.016
Focsan M, Ardelean II, Craciun C, Astilean S (2011) Interplay nanoparticle biosynthesis and metabolic activity of cyanobacterium Synechocystis sp. PCC 6803 between gold. Nanotechnology https://doi.org/10.1088/0957-4484/22/48/485101
Gayatri KV, Soundhari C, Pavithra BP (2019) biofilm inhibitory effect of Chlorella extracts on Pseudomonas aeruginosa. IJPSR https://doi.org/10.13040/IJPSR.0975-8232.10(4).1966-71
Guiry MD, Guiry GM (2021) AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. https://www.algaebase.org; searched on 06 December 2021.
Hamida RS, Ali MA, Redhwan A, Bin-Meferij MM (2020) Cyanobacteria – a promising platform in green nanotechnology: a review on nanoparticles fabrication and their prospective applications. Int J Nanomed. https://doi.org/10.2147/IJN.S256134
Hamouda RA, Hussein MH, Abo-elmagd RA, Bawazir SS (2019) Synthesis and biological characterization of silver nanoparticles derived from the cyanobacterium Oscillatoria limnetica. Sci Rep. https://doi.org/10.1038/s41598-019-49444-y
He R, Qian X, Yin J (2002) Preparation of polychrome silver nanoparticles in different solvent. J Mater Chem 1:3783–3786
Jamal M, Ahmad W, Andleeb S et al (2018) Bacterial biofilm and associated infections. J Chin Med Assoc. https://doi.org/10.1016/j.jcma.2017.07.012
Jayaraman A, Wood TK (2008) Bacterial quorum sensing: signals, circuits, and implications for biofilms and disease. Annu Rev Biomed Eng. https://doi.org/10.1146/annurev.bioeng.10.061807.160536
Jeffryes C, Agathos SN, Rorrer G (2015) Biogenic nanomaterials from photosynthetic microorganisms. Cur Opin Biotechnol. https://doi.org/10.1016/j.copbio.2014.10.005
Jena J, Pradhan N, Dash BP, Sukla LB, Panda PK (2013) Biosynthesis and characterization of silver nanoparticles using microalga Chlorococcum humicola and its antibacterial activity. Int J Nanomater Bios 3:1–8
Jena J, Pradhan N et al (2014) Microalga Scenedesmus sp.: a potential low-cost green machine for silver nanoparticle synthesis. J Microbiol Biotechnol. https://doi.org/10.4014/jmb.1306.06014
Jeon MS, Han SI et al (2021) Rapid green synthesis of silver nanoparticles using sulfated polysaccharides originating from Porphyridium cruentum UTEX 161: evaluation of antibacterial and catalytic activities. J Appl Phycol. https://doi.org/10.1007/s10811-021-02540-x
Kambale EK, Nkanga CI et al (2020) Green synthesis of antimicrobial silver nanoparticles using aqueous leaf extracts from three Congolese plant species (Brillantaisia patula, Crossopteryx febrifuga and Senna siamea). Heliyon https://doi.org/10.1016/j.heliyon.2020.e04493
Kannan RRR, Stirk WA, Staden JV (2013) Synthesis of silver nanoparticles using the seaweed Codium capitatum P.C. Silva (Chlorophyceae). S Afr J Sci Bot. https://doi.org/10.1016/j.sajb.2013.01.003
Khan MS, Zahin M, Hasan S, Husain FM, Ahmad I (2009) Inhibition of quorum sensing regulated bacterial functions by plant essential oils with special reference to clove oil. Lett Appl Microbiol. https://doi.org/10.1111/j.1472-765X.2009.02666.x
Lauritano C, Andersen JH et al (2016) Bioactivity screening of microalgae for antioxidant, anti-inflammatory, anticancer, anti-diabetes, and antibacterial activities. Front Mar Sci. https://doi.org/10.3389/fmars.2016.00068
Lenartowicz M, Marek PH, Madura ID, Lipok J (2017) Formation of variously shaped gold nanoparticles by Anabaena laxa. J Cluster Sci. https://doi.org/10.1007/s10876-017-1275-0
Lengke MF, Fleet ME, Southam G (2007) Biosynthesis of silver nanoparticles by filamentous cyanobacteria from a silver (I) nitrate complex. Langmuir. https://doi.org/10.1021/la0613124
Liao C, Li Y, Tjong SC (2019) Bactericidal and cytotoxic properties of silver nanoparticles. Int J Mol Sci. https://doi.org/10.3390/ijms20020449
López Y, Soto SM (2020) The usefulness of microalgae compounds for preventing biofilm infections. Antibiotics. https://doi.org/10.3390/antibiotics9010009
Mahana A, Guliy OI, Mehta SK (2021) Accumulation and cellular toxicity of engineered metallic nanoparticle in freshwater microalgae: Current status and future challenges. Ecotoxicol Environ Saf. https://doi.org/10.1016/j.ecoenv.2020.111662
Mahdavi M, Namvar F, Ahmad MB, Mohamad R (2013) Green biosynthesis and characterization of magnetic iron oxide (Fe3O4) nanoparticles using seaweed (Sargassum muticum) aqueous extract. Molecules. https://doi.org/10.3390/molecules18055954
Mahdieh M, Zolanvari A, Azimee A (2012) Green biosynthesis of silver nanoparticles by Spirulina platensis. Sci Iran. https://doi.org/10.1016/j.scient.2012.01.010
McLean KH, Winson MK et al (1997) Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acyl homoserine lactones. Microbiology 143:3703–3711
Mittal AK, Chisti Y, Banerjee UC (2013) Synthesis of metallic nanoparticles using plant extracts. Biotechnol Adv. https://doi.org/10.1016/j.biotechadv.2013.01.003
Mukherjee P, Ahmad A et al (2001) Fungus mediated synthesis of silver nanoparticles and their immobilization in the mycelial matrix: a novel biological approach to nanoparticle synthesis. Nano Lett. https://doi.org/10.1021/nl0155274
Mulvaney P (1996) Surface plasmon spectroscopy of nanosized metal particles. Langmuir. https://doi.org/10.1021/la9502711
Mühlsteinová R, Hauer T, De Lay P, Pietrasiak N (2018) Seeking the true Oscillatoria: a quest for a reliable phylogenetic and taxonomic reference point. Preslia (Prague). https://doi.org/10.23855/preslia.2018.151
Nikalje AP (2015) Nanotechnology and its Applications in Medicine. Med Chem. https://doi.org/10.4172/2161-0444.1000247
NCCLS (2003). Performance Standards for Antimicrobial Susceptibility Testing: 13th Informational Supplement (Disk Diffusion Supplemental Tables). NCCLS document M100-S13 (M2), supplement to NCCLS document M2-A8 (disk diffusion).
NCCLS (2000). Performance Standards for Antimicrobial Susceptibility Testing: 10th Informational Supplement (Aerobic Dilution, MIC Testing Supplemental Tables. NCCLS document M100-S10 (M7), supplement to NCCLS document M7-A5 (MIC testing).
Prabhu SS, Mohan RK, Sanhita P (2014) Production of bacteriocin and biosynthesis of silver nanoparticles by lactic acid bacteria isolated from yoghurt and its antibacterial activity. Scrut Int Res J Microbiol Biotechnol 1(3):7–14
Rani VU, Peruma UE, Palanivel S (2016) Morphology and taxonomy of Oscillatoria princeps Vaucher ex gomont (Oscillatoriales, Oscillatoriaceae). Indian Journal of Education and Information Management 5(1):2277–5374
Rónavári A, Kovács D et al (2017) Biological activity of green-synthesized silver nanoparticles depends on the applied natural extracts: a comprehensive study. Int J Nanomedicine. https://doi.org/10.2147/IJN.S122842
Schillaci D, Cusimano MG, Cunsolo V et al (2013) Immune mediators of sea-cucumber Holothuria tubulosa (Echinodermata) as source of novel antimicrobial and anti-staphylococcal biofilm agents. AMB Express. https://doi.org/10.1186/2191-0855-3-35
Sharma A, Sharma S, Sharma K et al (2016) Algae as crucial organisms in advancing nanotechnology: a systematic review. J Appl Phycol.https://doi.org/10.1007/s10811-015-0715-1
Sigee DC, Dean A, Levado E, Tobin MJ (2002) Fourier-transform infrared spectroscopy of Pediastrum duplex characterization of a micro-population isolated from a eutrophic lake. Eur J Phycol. https://doi.org/10.1017/S0967026201003444
Singh SP, Rastogi RP, Häder D-P, Sinha RP (2011) An improved method for genomic DNA extraction from cyanobacteria. World J Microbiol Biotechnol. https://doi.org/10.1007/s11274-010-0571-8
Sridevi N, Dhanusha V, Rajeswari M, Santhi N (2019) An in-vitro antibiofilm activity of Chlorella vulgaris. Asian J. Pharm. Clin. Res. https://doi.org/10.22159/ajpcr.2019.v12i18.34144
Szabó MA, Varga GZ, Hohmann J et al (2010) Inhibition of quorum-sensing signals by essential oils. Phytother Res. https://doi.org/10.1002/ptr.3010
Tabbouche SA, Gürgen A et al (2017) Antimicrobial and anti-quorum sensing activity of some wild mushrooms collected from Turkey. MSU J. of Sci., https://doi.org/10.18586/msufbd.347692
Tsibakhashvili NY, Kirkesali EI et al (2011) Microbial synthesis of silver nanoparticles by Streptomyces glaucus and Spirulina platensis. J Comput Theor Nanosci. https://doi.org/10.1166/asl.2011.1915
Tang J, Wang W, Chu W (2020) Antimicrobial and anti-quorum sensing activities of phlorotannins from seaweed (Hizikia fusiforme). Front Cell Infect Microbiol. https://doi.org/10.3389/fcimb.2020.586750
Varshney P, Mikulic P et al (2015) Extremophilic micro-algae and their potential contribution in biotechnology. Bioresour Technol.https://doi.org/10.1016/j.biortech.2014.11.040
Xin Y, Lu Y, Lee YY et al (2017) Producing designer oils in industrial microalgae by rational modulation of co-evolving type-2 diacylglycerol acyltransferases. Mol Plant. https://doi.org/10.1016/j.molp.2017.10.011
Yin IX, Zhang J et al (2020) The Antibacterial mechanism of silver nanoparticles and its application in dentistry. Int J Nanomed. https://doi.org/10.2147/IJN.S246764
Author information
Authors and Affiliations
Contributions
DY, İAE, and BE designed the experiments; DY, İAE, and BE performed the experiments; DY, İAE, and BE analyzed the data; DY, İAE, and BE prepared the draft; DY, İAE, and BE supervised for the present investigation. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors have consent for publication.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Vitor Vasconcelos
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yalçın, D., Erkaya, İ.A. & Erdem, B. Antimicrobial, antibiofilm potential, and anti-quorum sensing activity of silver nanoparticles synthesized from Cyanobacteria Oscillatoria princeps. Environ Sci Pollut Res 29, 89738–89752 (2022). https://doi.org/10.1007/s11356-022-22068-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-22068-y