Abstract
Microcystins (MCs) are hepatotoxic secondary metabolites produced by several genera of cyanobacteria. Human exposure routes include drinking contaminated water and consuming contaminated fish, vegetables, crops, and even food supplements that contain cyanotoxin. This study investigated the presence of MCs in a stream with a long history of use as a source of water for irrigation farming. To establish the risk of a lack of monitoring programs, we studied MCs contamination of irrigated vegetables. Toxin levels in the water were generally <0.05 μg L−1 in all the investigated stations. Total microcystin concentrations in spinach (0.306 μg Kg−1 upstream and 0.217 μg Kg−1 downstream), lettuce (0.085 μg Kg−1 upstream and 0.462 μg Kg−1 downstream), carrot (0.050 μg Kg−1 downstream and 0.116 μg Kg−1 downstream), cabbage (0.014 μg Kg−1 upstream and 0.031 μg Kg−1 downstream), tomatoes (0.233 μg Kg−1 upstream), and bitter leaf (0.460 μg Kg−1 upstream and 0.050 μg Kg−1 downstream) collected in March were higher than the levels detected in samples of the same vegetables collected in April. These results highlight Nigeria’s severe public health problem, especially in regions with long dry season spells. The level of MCs contamination of irrigated vegetables implies a potential for chronic exposure and associated health challenges.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cyanobacteria, also known as blue-green algae, are microorganisms and autotrophic organisms that are primary producers in aquatic systems; hence, they are categorized as algae (Picardo et al. 2019). Some strains have rich chemistry because they produce various bioactive compounds with varying properties (El Gamal 2010; Picardo et al. 2019). Meanwhile, other forms produce a wide range of toxic and potentially harmful compounds in the form of secondary metabolites known as cyanotoxins, which are regarded as one of the most important groups of natural toxins (Otten and Paerl 2015; Kimambo et al. 2019; Griffith and Gobler 2020).
Microcystins are hepatotoxins primarily found in marine and freshwater habitats worldwide (Liu et al. 2018; Wei et al. 2020). MCs are heat-stable and water-soluble cyclic peptides containing seven amino acids, and over 279 variants of the cyanotoxin have been identified to date (Bouaïcha et al. 2019). They are toxic to humans, domestic animals, livestock, and aquatic organisms because they are potent inhibitors of phosphatases of serine/threonine-protein 1 (PP1) and 2A (PP2A) as well as disruptors of intracellular homeostasis (Fontanillo and Köhn 2018; Massey et al. 2018; Zhao et al. 2021). The inhibition of these phosphatases disarranges the cell cytoskeleton, causes cell death, leads to intrahepatic hemorrhage, and sometimes kills exposed organisms (Carmichael 1992).
MCs are the most ubiquitous and commonly occurring cyanotoxins in freshwater systems (Pham and Utsumi 2018). Microcystins can get into the mammalian body when they consume contaminated water, food, and algal dietary supplements, through body contact, hemodialysis, and inhalation to a lesser extent (Massey et al. 2018). Due to MCs’ toxic consequences, the International Agency for Research on Cancer (IARC) has categorized this toxin as a possible carcinogen (Cancer 2010). To reduce and prevent MC health hazards, the World Health Organization (WHO) recommended a provisional 1 μg/L MC guideline for drinking water quality (WHO 1998). MCs are carcinogenic because they promote liver tumors (Duy et al. 2000; Falconer and Humpage 2005). Also, there is supporting evidence from China and elsewhere that MCs cause liver cancers in humans (Ueno et al. 1996; Humpage et al. 2000).
Plant irrigation with MCs-contaminated water is an important pathway for human exposure when contaminated food is consumed (Chen et al. 2004). The ability of MCs to bioaccumulate in plant and animal tissues is responsible for the risk involved in consuming contaminated food. Several laboratory and field studies show that several vegetables irrigated with MCs-contaminated water (dissolved MCs) and toxin-producing cyanobacteria strains (particulate MCs) bioaccumulate substantial amounts of this cyanotoxin (Crush et al. 2008; Hereman and do Carmo Bittencourt-Oliveira 2012). The bioaccumulation of MCs in plant and animal tissues led the World Health Organization (WHO) to recommend a maximum daily intake of 0.04 μg Kg−1 of body mass (WHO 1998).
Irrigation of vegetables in Northern Nigeria is usually done due to the uneven distribution and sometimes inadequate rainfall for vegetable production. The eutrophication and formation of cyanobacteria blooms in inland waters such as seasonal streams, rivers, and lakes make them unfit for irrigation purposes because of the frequent detection of cyanotoxins (Chia et al. 2009b, 2015). As there are no monitoring programs and management for cyanobacteria blooms and cyanotoxins in most developing countries, investigations highlight the presence, levels, and risk of contamination and consumption of irrigated crops (Peuthert et al. 2015; Cordeiro-Araújo et al. 2016; do Carmo Bittencourt-Oliveira et al. 2016). The investigated area and stream, Kwaru stream, represents a vital ecosystem type that has been poorly investigated. Most studies in Africa and other tropical regions focus on large water supply reservoirs, while less attention has been given to streams and rivers. Therefore, the study aimed to investigate the level of MCs in surface water and some irrigated vegetables at Kwaru stream, Hayin Dan-Mani, Kaduna, Nigeria. We investigated this ecosystem to demonstrate its importance and contributions to MCs as a human exposure pathway.
Materials and methods
Study area
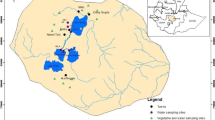
The study was carried out at Kwaru stream, Hayin Dan-Mani, Kaduna. The Kwaru stream is an urban stream that is impacted by many anthropogenic activities such as domestic solid waste dumping, wastewater from residential areas, and car washes, and it is an important source of drinking water for cattle and a variety of irrigated crops during the dry season. Kaduna is located in the northern Guinea savanna region of Nigeria, and it is characterized by two distinct seasons in the year: the dry and rainy seasons. The dry season runs from November to April and is characterized by cold and dry conditions, and the harmattan wind blows from the northeast toward the southwest. On the other hand, the rainy season, which runs from May to early October, is characterized by warm and humid conditions with the wind blowing from the southwest toward the northeast. The average monthly temperature of the region is between 26 and 34 °C (Bununu et al. 2015). Five sampling stations were selected along the irrigated stretch of Kwaru Stream based on the activities along the stream’s catchment. Station 1 (10°32′41.5″N, 007°24′26.5″E) is upstream and primarily receives run-off from the farm’s upstream stream. Station 2 (10°32′36.0″N, 007°24′25.5″E) is characterized by irrigation farming, dumping, and burning waste. Station 3 (10°32′21.6″N, 007°24′22.6″E) is depicted by dumping waste such as polythene bags, waste food, and cattle grazing and watering. The activities in station 4 (10°32′13.0″N, 007°24′16.4″E) include car and upholstery wash points, irrigation water pump sites, and open human and cattle defecation. At station 5 (10°32′15.5″N, 007°24′12.3″E), we found an inflow of wastewater from residential and agricultural areas.
Water and vegetable sample collection
Surface water from the Kwaru stream and vegetable samples were collected in March and April 2019, representing the driest months and the peak of the dry season. These months also coincide with when most irrigated vegetables attain harvest age and size. The samples of leafy vegetables collected included Amaranthus hybridus (spinach), Brassica oleracea (cabbage), Lactuca sativa (lettuce), Hibiscus cannabinus (kenaf), and Vernonia amygdalina (bitter leaf). Fruit vegetable samples included Daucus carota (carrot) and Lycopersicum esculentum (tomatoes). The samples were collected in triplicates upstream (stations 1, 2, and 3) and downstream (stations 4 and 5). Sample identification was authenticated at the Herbarium of the Department of Botany, Ahmadu Bello University, Zaria, Nigeria.
Cyanobacteria identification and quantification
The Prescott approach, which was adopted by American Public Health Association APHA (2005), Bartram and Rees (1999), and Chia et al. (2009a), was used to analyze cyanobacteria in the lab. The cyanobacteria biomass was determined using the Drop Count Technique (Bartram and Rees 1999). The number of cells in colonies and filaments was counted without separating them and expressed as the number of cells per mL (Chia et al. 2009a).
Analysis of physicochemical parameters
Physicochemical parameters such as water temperature, total dissolved solids, pH, electrical conductivity, water hardness, total alkalinity, dissolved oxygen, biological oxygen demand, chloride, nitrate-nitrogen, and phosphate-phosphorus were analyzed according to standard methods (American Public Health Association APHA 2005).
Microcystins analysis
Aliquots (5 ml) of water samples were frozen and thawed severally to break the cells (Chia and Kwaghe 2015). Following that, the cellular contents were extracted with 75% methanol (Chia and Kwaghe 2015). Next, vegetable samples were rinsed with tap water, followed by distilled water. Then, 2 g of each sample was cut with a razor blade, weighed, and homogenized using a mortar and pestle in 80% methanol to obtain a homogenous mixture (Díez-Quijada et al. 2018). The choice of 80% methanol was to extract the intracellular toxins (Turner et al. 2018). Finally, the homogenate was centrifuged at 2000 rpm (10 min), and the supernatant was extracted and preserved at −40 °C until ready for analysis.
Extracts were subjected to microcystin analysis using a 96-well Abraxis Microcystins-ADDA Enzyme-Linked Immunosorbent Assay (ELISA) kit, following the manufacturer’s instructions. The absorbance of the color reaction at the end of the ELISA procedure was read at 450 nm in a Bio-Rad iMark™ Microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA), and the concentrations expressed per cell quota of potential microcystin-producing species (Chia et al. 2019).
Data analyses
The statistical significance of changes in microcystins accumulated in vegetables and total microcystins in water was determined using analysis of variance (ANOVA). When significant differences existed, Tukey’s posthoc test was used to perform multiple comparisons. For all analyses, the significance level was set at p < 0.05. The total daily intake (TDI) limit of 0.04 g kg−1 body weight, determined by the World Health Organization, was used to estimate the risk of eating contaminated vegetables (WHO 2011). The first part of the analysis was based on the premise that an adult weighing 60 kg consumes at least 40 g of vegetables per day and has a microcystin content threshold of 60 μg kg−1 in vegetable tissues.
where MCs40g−1 is the concentration of MCs per 40g of vegetables, and BM is the body mass of an adult weighing at least 60 kg.
The monthly and seasonal risks of eating microcystin-contaminated vegetables from the study area were predicted using the hierarchical/multilevel modeling method. Equation (2) shows the model that was generated:
The vegetables are represented by the MCs. MCs represent the content of the ith observation from the jth group (stream location or month), β0 represent the overall mean or intercept, ßj represents the group-type mean, \({\sigma}_0^2\) represents within-group variance, and \({\sigma}_0^2\) represents between-group variance. The lmer function of the lme4 package (Bates 2010) of R was used to perform hierarchical modeling, with the restricted maximum likelihood (REML) set to FALSE. Analysis of variance with the ranova function of the jtools package of R was used to determine the significance of the various multilevel tests. Using cumulative distribution functions (CDF), the monthly and stream location risks of consuming contaminated vegetables from the analyzed water source were also demonstrated.
Using Pearson’s correlation coefficient and the R base stats function, the correlation between vegetable tissue-bound microcystins and cyanobacterial cell-bound microcystins was found. R (https://www.r-project.org) software for macOS was used for all statistical studies.
Results
Physicochemical conditions of the study area
A summary of the physicochemical characteristics of the investigated water body is given in Table 1. Overall, water temperature, TDS, sulfate, nitrate, and hardness were significantly different between the stations. Specifically, the water temperature was highest in station 1 and lowest in station 3. The changes in phosphate phosphorus were not significant, but nitrate-nitrogen levels were highest in stations 2 and 3 (p < 0.05). The first two stations (1 and 2) had the highest water hardness levels, while the lowest values of this parameter were found at station 5. Biochemical oxygen demand and DO were generally uniform across the investigated stations of the water body.
Cyanobacteria composition of the study area
Changes in phytoplankton composition and biomass across stations revealed that the potential microcystin species Anabaena flos-aquae, Microcystis flos-aquae, and Planktothrix spp. were the most abundant in most of the stations sampled (Fig. 1). To be specific, Planktothrix spp. had the highest biomass in stations 1, 2, and 5. The total biomass of this species exceeded 500,000 cells mL−1. A. flos-aquae was the dominant species in station 2 but was not recorded in the other stations. Other potential microcystin-producing species belonging to the genera Anabaena, Anacystis, Lyngbya, and Spirulina had very low biomass at all the investigated stations of the Kwaru stream.
Total microcystin concentrations in water
The total microcystin concentration in the water in the five different stations in March and April varied significantly (Fig. 2). The highest concentration was observed in April in station 4 (0.246 μg L−1), while the lowest was recorded in March (0.040 μg L−1) in station 2. There was no specific trend in the total MCs variation in March. Regardless of the sampling period or sampling station, MC concentrations were lower than 1.0 μg L−1, the maximum permissible level recommended by the WHO.
Total microcystin content of vegetables
On a general note, higher MC levels occurred in vegetables sampled in March than those collected in April (Fig. 3). Per sampling location, the levels of MCs bioaccumulated in the vegetables varied depending on the vegetable. In March upstream, the bitter leaf had the highest bioaccumulated microcystin concentration (0.460 μg Kg−1), while cabbage had the lowest (0.01 μg Kg−1). Downstream, lettuce (0.462 μg Kg−1) had the highest MCs concentration, while cabbage (0.030 μg Kg−1) had the lowest concentration. In April, the highest bioaccumulated microcystins level downstream occurred in spinach (0.217 μg Kg−1), while the lowest was found in cabbage (0.0007 μg Kg−1). In April, kenaf (0.021 μg Kg−1) had the highest upstream concentration, while the lowest was in cabbage (0.0017 μg Kg−1). There were no detectable levels of MCs in lettuce.
Total daily intake of microcystin-contaminated vegetables and cumulative distribution of tissue-bound microcystins
As MCs TDI calculations are based on bioaccumulated in edible parts of vegetables, higher TDIs were found in March than in April (Fig. 4). Spinach had the highest MCs TDI by an adult (TDIA) and a child (TDIC) throughout the present study (Fig. 4). Also, spinach, lettuce, tomatoes, bitter leaf, and carrots had TDIA and TDIC values greater than 0.04 μg Kg−1, the limit set by the WHO. Contrarily, in April, only spinach had TDIA and TDIC values greater than 0.04 μg Kg−1.
Total daily intake (TDI) of microcystins by an adult and a child following consumption of different vegetables irrigated with contaminated water from Kwaru stream, Kaduna. Values represent mean plus and minus standard deviation for n = 3. Values with asterisks are significantly different at p < 0.05
The CDFs of MCs in water showed marked variations in the risk of consuming untreated water from the stream (Fig. 5). The risk of drinking less than 0.1 μg L−1 MC-contaminated water was more than 75% across most stations (Fig. 5a). On the other hand, drinking water from station 4 posed the highest risk to humans and animals because more than 40% of the samples collected from the station had MC levels of more than 0.1 μg L−1. In line with the levels of MCs detected in water, the lowest risk of drinking contaminated water was in station 4. Furthermore, there was a 75% chance of consuming water containing more than 0.1 μg L−1 MC in April, whereas there was a 75% chance of consuming less than this amount in March (Fig. 5b).
The risk of consuming MC-contaminated vegetables was not significantly different in vegetables irrigated upstream and downstream (Fig. 6a). Regardless of the collection point of vegetables, the chance of consuming vegetables containing more than 0.1 μg g−1 MC was ca. 30%. Unlike the case of drinking MC-contaminated water, where the risk was higher in April, in terms of consuming contaminated vegetables, the risk was higher in March than in April (Fig. 6b). Specifically, there was a less than 10% chance of consuming vegetables containing more than 0.1 μg g−1 MC in April, whereas the reverse was observed in March. A more than 50% chance of consuming vegetables containing more than 0.1 μg g−1 MC was observed in March, indicating substantially different risk levels between the months. The CDFs of the investigated vegetables showed distinct differences, showing that risk differs between them (Fig. 6c). Specifically, consuming vegetables such as kenaf and cabbage posed the lowest risks, followed by bitter leaf and carrot. The vegetables with the highest risk of exposure to MC levels greater than 0.1 μg g−1 MC were spinach, tomatoes, and lettuce (Fig. 6c).
Discussion
Microcystins concentration in stream water
Most developed countries recognize the risk associated with the excessive proliferation of cyanobacteria and cyanotoxins, while the reverse is the case for most developing countries. Sadly, global climate change and eutrophication of aquatic ecosystems that promote the excessive proliferation of cyanobacteria and cyanotoxins are independent of the economic status of any country and continue to pose significant risks to humans and wildlife. Therefore, the increased occurrence of MCs in aquatic ecosystems and the contamination of irrigated crops pose currently unquantifiable health risks, particularly in developing countries due to a lack of data. The present study results demonstrate the need to generate data from tropical developing countries where conditions are highly suitable for the formation of harmful cyanobacterial blooms. The physicochemical characteristics of the Kwaru stream fell within the range of those reported for other water bodies in Nigeria and other tropical regions with a history of cyanobacterial blooms. We found detectable levels of MCs in the Kwaru stream with concentrations peaking at the end of the dry season, which is in line with generally reported changes in water quality, weather, and cyanotoxins content in Northern Nigeria (Chia et al. 2009b; Chia and Kwaghe 2015). Specifically, these changes could be attributed to increased daytime temperatures, reduced water levels, increased nutrients, and solar irradiation, favoring the proliferation of cyanobacteria and the production of cyanotoxins like microcystins (Mohamed et al. 2020; Chia et al. 2021). This hypothesis is supported by the presence of microcystin-producing cyanobacteria taxa such as Anabaena, Microcystis, and Planktothrix in the stream during the investigation. Similar amounts of microcystins (0.01–7.50 μg L−1) and the same microcystin-producing taxa were detected in freshwater and marine habitats in Nigeria by Chia et al. (2009a, b, 2021) and Kadiri et al. (2020).
There is a general water scarcity problem in several developing tropical countries, and northern Nigeria is no exception. This information is critical because people around the catchment of water bodies like Kwaru Stream drink the water without prior treatment. Fortunately, the detected levels of total MCs in the present study were lower than the recommended (WHO) guideline value of 1.00 μg L−1 for drinking water. However, there were marked differences between the risk of exposure to MCs dependent on the station and the month of water collection. These changes can be linked to the differences in anthropogenic activities along the stream's catchment.
Microcystin content in vegetables
The amount of MCs bioaccumulated in irrigated vegetables varied significantly. These differences are dependent on the levels of MCs in the environment, prevailing environmental conditions, species of vegetable, the nature and size of their leaves, and their physiological and biochemical adaptations to the presence of MCs (Chia et al. 2019). For example, higher evapotranspiration rates taking place on leaf surfaces than on other organs not directly exposed to solar irradiation result in substantial variations in the concentrations of MCs bioaccumulated by plants (Constable and Rawson 1980). The present study’s findings collaborate with those of Chia et al. (2019), who observed higher MC in the leaves of irrigated vegetables than in other plant parts. Also, Mohamed and Al Shehri (2009) and Chia et al. (2019) found the highest levels of MCs in Amaranthus hybridus (spinach) compared to other irrigated vegetables.
Furthermore, Crush et al. (2008) demonstrated that higher concentrations of MCs were detected in Lactiva sativa and Trifolium repens with broader leaves than in Brassica napus and Lolium perenne with smaller leaves. This contrasts with a study (Machado et al. 2017) that detected MC-LR in carrots at 5 μg kg−1 fresh weight, where the root is the edible portion. Finally, it is essential to note that fruits or vegetables like tomatoes had substantially high levels of MCs comparable to other broad-leaf vegetables, an indication that microcystin accumulation in fruits represents a significant exposure pathway.
In April, only spinach had a high MC concentration compared to other vegetables. The lower MCs’ bioaccumulated levels in all the vegetables in April negatively correlated with the highest MCs’ levels found in the water in the same month. This observation implies that when the length of irrigation, which leads to bioaccumulation of significant amounts in vegetable tissues, is not taken into account, having low MCs levels in water does not necessarily imply minimal risk. Previously, we showed that microcystin congeners vary in their bioaccumulation rates. The bioaccumulation and depuration of MC-LR depend on its saturation concentrations, irrigation duration, plant species, and exposure concentration (Cordeiro-Araújo et al. 2016; do Carmo Bittencourt-Oliveira et al. 2016). Also, the differences observed in the MC content of the investigated vegetables are not surprising as plants have different physiological, biochemical, and morphological adaptations that determine the rate at which they uptake and bioaccumulate MCs (Cordeiro-Araújo et al. 2016; do Carmo Bittencourt-Oliveira et al. 2016). Plants also differ in the rates at which they can excrete or depurate MCs and chemically detoxify MCs with antioxidant enzymes (Cordeiro-Araújo et al. 2016). This difference may also account for variations in bioaccumulated MCs in the investigated vegetables.
Total daily intake of MCs by humans (adults and children) and risk assessment
The concentration of MCs in vegetable tissues is usually directly proportional to the TDI by humans (Chia et al. 2019). Therefore, higher concentrations of MCs in vegetables resulted in higher TDI and risks for adults and children in the present study. A link between cumulative levels of microcystins and TDI has also been found in other crops, such as Phaseolus vulgaris, with values ranging from 0.125 to 0.391 μg Kg−1 body weight (Mohamed et al. 2022). Based on our findings, cabbage (March and April), lettuce (April), carrot (April), bitter leaf (April), tomatoes (April), and kenaf (April) posed no significant acute and chronic risk to humans since the TDI did not exceed the TDI limit set by WHO (0.04 μg per kg body weight per day). However, the TDI of MC in spinach in March and April and in lettuce, carrot, bitter leaf, and tomato in March exceeded the TDI limit. Thus, the risk of exposure to MCs is highest in March, as indicated by the CDF. While the risk may appear to be higher in March than in April, the chance of consuming high levels of MCs is dependent on the type of vegetable consumed. This outcome agrees with Chia et al. (2019)’s findings that irrigated vegetables in northern Nigeria have very high levels of microcystins. The result is also in line with previous studies indicating MCs accumulate differentially in various crops irrigated with water contaminated with blue-green algae or MCs (Cordeiro-Araújo et al. 2016; do Carmo Bittencourt-Oliveira et al. 2016; Machado et al. 2017). According to Wood et al. (2004), the WHO’s maximum daily intake of MC-LR is a suggested limit for healthy adults, meaning that children, the elderly, and sensitive/unhealthy people are more susceptible to the adverse effects of MC consumption. Thus, lower TDI than the WHO recommended levels may not necessarily mean lower risk, as vulnerable and unhealthy people may suffer severe ailments post-exposure to MC via this exposure route.
The human health risks from the TDI of MCs are plant-specific (Cordeiro-Araújo et al. 2016) and proportional to the degree and rate of bioaccumulation (Crush et al. 2008). Leafy vegetables, especially spinach and lettuce, showed the highest health risk among the various vegetables. Unfortunately, spinach and lettuce are among the most consumed vegetables worldwide, emphasizing the gravity of potential long-term health challenges (Zhang et al. 2010). Likewise, many studies have shown that MC-LR is resistant to heat, being stable at temperatures as high as 300 °C (Bruno et al. 2012).
Conclusion
Our study revealed that having low MCs levels in water does not necessarily translate to low risk when considerations are not given to the duration of irrigation that leads to bioaccumulation of substantial amounts in vegetable tissues. Specifically, total MCs in water during the months in all the stations were lower than the recommended (WHO) guideline value of 1.00 μg L−1 for drinking water. Also, the detected levels of MCs in irrigated vegetables were high, leading to higher TDIs than the 0.04 μg kg−1 body weight set by the WHO in March. This study demonstrates the need to develop management strategies for MCs in water bodies used for irrigation farming and the need to screen crops or vegetables harvested from farms before human consumption in developing tropical countries. In addition, consumer education must be encouraged and aggressively pursued to ensure they understand the consequences of MCs’ contamination of drinking water and irrigated vegetables.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
APHA (2005) Standard methods for the examination of water and wastewater. American Public Health Association, American Water Works Association, Washington, DC
Bartram J, Rees G (1999) Monitoring bathing waters: a practical guide to the design and implementation of assessments and monitoring programmes. CRC Press, Washington
Bates DM (2010) lme4: Mixed-effects modeling with R
Bouaïcha N, Miles CO, Beach DG et al (2019) Structural diversity, characterization and toxicology of microcystins. Toxins (Basel) 11:714
Bruno M, Gallo P, Messineo V, Melchiorre S (2012) Health risk associated with microcystin presence in the environment: the case of an Italian Lake (Lake Vico, Central Italy). Int J Env Prot 2:34–41
Bununu YA, Ludin ANM, Hosni N (2015) City profile: Kaduna. Cities 49:53–65
Cancer IA for R on (2010) Ingested nitrate and nitrite, and cyanobacterial peptide toxins. IARC monographs on the evaluation of carcinogenic risks to humans 94
Carmichael WW (1992) Cyanobacteria secondary metabolites—the cyanotoxins. J Appl Bacteriol 72:445–459
Chen J, Song L, Dai J et al (2004) Effects of microcystins on the growth and the activity of superoxide dismutase and peroxidase of rape (Brassica napus L.) and rice (Oryza sativa L.). Toxicon 43:393–400
Chia MA, Kwaghe MJ (2015) Microcystins contamination of surface water supply sources in Zaria-Nigeria. Environ Monit Assess 187:1–12
Chia AM, Abolude DS, Ladan Z et al (2009a) The presence of microcystins in aquatic ecosystems in Northern Nigeria: Zaria as a case study. Res J Environ Toxicol 3:170–178. https://doi.org/10.3923/rjet.2009.170.178
Chia AM, Oniye SJ, Ladan Z et al (2009b) A survey for the presence of microcystins in aquaculture ponds in Zaria, Northern-Nigeria: possible public health implication. Afr J Biotechnol 8:6282–6289. https://doi.org/10.5897/AJB09.1263
Chia MA, Cordeiro-Araujo MK, do Carmo Bittencourt-Oliveira M (2015) Growth and antioxidant response of Microcystis aeruginosa (Cyanobacteria) exposed to anatoxin-a. Harmful Algae 49:135–146
Chia MA, Auta ZZ, Esson AE et al (2019) Assessment of microcystin contamination of Amaranthus hybridus, Brassica oleracea, and Lactuca sativa sold in markets: a case study of Zaria, Nigeria. Environ Monit Assess 191:569. https://doi.org/10.1007/s10661-019-7725-4
Chia MA, Abdulwahab R, Ameh I et al (2021) Farmed tilapia as an exposure route to microcystins in Zaria-Nigeria: a seasonal investigation. Environ Pollut 271:116366
Constable GA, Rawson HM (1980) Effect of leaf position, expansion and age on photosynthesis, transpiration and water use efficiency of cotton. Aust J Plant Physiol 7:89–100
Cordeiro-Araújo MK, Chia MA, de Toledo Arruda-Neto JD et al (2016) Microcystin-LR bioaccumulation and depuration kinetics in lettuce and arugula: human health risk assessment. Sci Total Environ 566:1379–1386
Crush JR, Briggs LR, Sprosen JM, Nichols SN (2008) Effect of irrigation with lake water containing microcystins on microcystin content and growth of ryegrass, clover, rape, and lettuce. Environ Toxicol An Int J 23:246–252
Díez-Quijada L, Guzmán-Guillén R, Prieto Ortega AI et al (2018) New method for simultaneous determination of microcystins and cylindrospermopsin in vegetable matrices by SPE-UPLC-MS/MS. Toxins (Basel) 10:406
do Carmo Bittencourt-Oliveira M, Cordeiro-Araújo MK, Chia MA et al (2016) Lettuce irrigated with contaminated water: photosynthetic effects, antioxidative response and bioaccumulation of microcystin congeners. Ecotoxicol Environ Saf 128:83–90
Duy TN, Lam PKS, Shaw GR, Connell DW (2000) Toxicology and risk assessment of freshwater cyanobacterial (blue-green algal) toxins in water. Rev Environ Contam Toxicol 2000:113–185
El Gamal AA (2010) Biological importance of marine algae. Saudi Pharm J 18:1–25
Falconer IR, Humpage AR (2005) Health risk assessment of cyanobacterial (blue-green algal) toxins in drinking water. Int J Environ Res Public Health 2:43–50
Fontanillo M, Köhn M (2018) Microcystins: synthesis and structure–activity relationship studies toward PP1 and PP2A. Bioorg Med Chem 26:1118–1126
Griffith AW, Gobler CJ (2020) Harmful algal blooms: a climate change co-stressor in marine and freshwater ecosystems. Harmful Algae 91:101590
Hereman TC, do Carmo Bittencourt-Oliveira M (2012) Bioaccumulation of microcystins in lettuce. J Phycol 48:1535–1537
Humpage AR, Hardy SJ, Moore EJ et al (2000) Microcystins (cyanobacterial toxins) in drinking water enhance the growth of aberrant crypt foci in the mouse colon. J Toxicol Environ Heal Part A 61:155–165
Kadiri MO, Isagba S, Ogbebor JU et al (2020) The presence of microcystins in the coastal waters of Nigeria, from the Bights of Bonny and Benin, Gulf of Guinea. Environ Sci Pollut Res 27:35284–35293. https://doi.org/10.1007/s11356-020-09740-x
Kimambo ON, Gumbo JR, Chikoore H (2019) The occurrence of cyanobacteria blooms in freshwater ecosystems and their link with hydro-meteorological and environmental variations in Tanzania. Heliyon 5:e01312
Liu P, Wei J, Yang K et al (2018) Isolation, molecular identification, and characterization of a unique toxic cyanobacterium Microcystis sp. found in Hunan Province, China. J Toxicol Environ Heal part A 81:1142–1149
Machado J, Azevedo J, Freitas M et al (2017) Analysis of the use of microcystin-contaminated water in the growth and nutritional quality of the root-vegetable, Daucus carota. Environ Sci Pollut Res 24:752–764
Massey IY, Yang F, Ding Z et al (2018) Exposure routes and health effects of microcystins on animals and humans: a mini-review. Toxicon 151:156–162
Mohamed ZA, Al Shehri AM (2009) Microcystins in groundwater wells and their accumulation in vegetable plants irrigated with contaminated waters in Saudi Arabia. J Hazard Mater 172:310–315
Mohamed Z, Ahmed Z, Bakr A et al (2020) Detection of free and bound microcystins in tilapia fish from Egyptian fishpond farms and its related public health risk assessment. J Consum Prot Food Saf 15:37–47
Mohamed Z, Bakr A, Campos A et al (2022) Growth inhibition and microcystin accumulation in bush bean (Phaseolus vulgaris L.) plant irrigated with water containing toxic Chrooccocus minutus. Agric Water Manag 261:107381
Otten TG, Paerl HW (2015) Health effects of toxic cyanobacteria in US drinking and recreational waters: our current understanding and proposed direction. Curr Environ Health Rep 2:75–84
Peuthert A, Kun L, Baik S, Pflugmacher S (2015) Transfer of cyanobacterial toxins into edible plants via irrigation with lake water – a Chinese case study. Aperito J Aquat Mar Ecosys 1:1–7
Pham T-L, Utsumi M (2018) An overview of the accumulation of microcystins in aquatic ecosystems. J Environ Manag 213:520–529
Picardo M, Filatova D, Nunez O, Farré M (2019) Recent advances in the detection of natural toxins in freshwater environments. TrAC Trends Anal Chem 112:75–86
Turner AD, Dhanji-Rapkova M, O’Neill A et al (2018) Analysis of microcystins in cyanobacterial blooms from freshwater bodies in England. Toxins (Basel) 10:39
Ueno Y, Nagata S, Tsutsumi T et al (1996) Detection of microcystins, a blue-green algal hepatotoxin, in drinking water sampled in Haimen and Fusui, endemic areas of primary liver cancer in China, by highly sensitive immunoassay. Carcinogenesis 17:1317–1321. https://doi.org/10.1093/carcin/17.6.1317
Wei J, Xie X, Huang F et al (2020) Simultaneous Microcystis algicidal and microcystin synthesis inhibition by a red pigment prodigiosin. Environ Pollut 256:113444
WHO CT (1998) Microcystin-LR, Guidelines for drinking water quality. World Heal Organ Geneva, Geneva, pp 95–110
WHO G (2011) Guidelines for drinking-water quality. World Health Organ 216:303–304
Wood S, Briggs L, Sprosen J, Bloxham M, Ruck J, Wear B (2004) A report on microcystin levels in water, trout and freshwater mussels in Lakes Rotoiti and Rotoehu during 2003/2004. A Report for Environment Bay of Plenty
Zhang D, Xie P, Chen J (2010) Effects of temperature on the stability of microcystins in muscle of fish and its consequences for food safety. Bull Environ Contam Toxicol 84:202–207
Zhao S, Yuan C, Tuo X et al (2021) MCLR induces dysregulation of calcium homeostasis and endoplasmic reticulum stress resulting in apoptosis in Sertoli cells. Chemosphere 263:127868
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by Halima Abdullahi, Suwebat Ayanronke Akinyemi, Maria do Carmo Bittencourt-Oliveira, Yahuza Tanimu, and Mathias Ahii Chia. The first draft of the manuscript was written by Halima Abdullahi, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The study did not require ethical clearance because it does not involve human subjects.
Consent to participate
The study did not require consent to participate because it does not involve human subjects.
Consent for publication
The study was not a case study requiring consent to publish from subjects.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Vitor Vasconcelos
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abdullahi, H., Tanimu, Y., Akinyemi, S.A. et al. Assessment of microcystins in surface water and irrigated vegetables in Kwaru stream, Hayin Danmani, Kaduna-Nigeria. Environ Sci Pollut Res 29, 78303–78313 (2022). https://doi.org/10.1007/s11356-022-21381-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-21381-w