Abstract
Polycyclic aromatic hydrocarbons (PAHs) are widespread toxic pollutants in the atmosphere and have attracted much attention for decades. In this study, we compared the health risks of PAHs based on different toxic equivalent factors (TEFs) in a heavily polluted area during heating and non-heating periods. We also pay attention to occupancy probability (OP) in different polluted areas. The results showed that there were big differences for calculations by different TEFs, and also by OP or not. Age groups except adults were all lower calculated by OP than not. The sensitivity analysis results on the incremental lifetime cancer risks (ILCR) for population groups by Monte Carlo simulation identified that the cancer slope factor extremely affected the health risk assessment in heating periods, followed by daily inhalation exposure levels. However, daily inhalation exposure levels have dominated the effect on the inhalation ILCR and then followed by the cancer slope factor in non-heating periods. The big differences by different calculations investigated that it is important to set up the correlations between the pollution level and health risks, especially for the longtime health assessment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are considered to be the largest contributors to lung cancer risks among the fine particulate (PM2.5) (Luo et al. 2015), and they have attracted worldwide public concern in the past decades for their detrimental effects on human health (Zhao et al. 2020; Chen et al. 2018; Yu et al. 2015; Park et al. 2006; Okona-Mensah et al. 2005). Studies reported that China accounted for about a fifth of global PAHs emissions in 2004 (Zhang and Tao 2009; Zhang et al. 2008), and they were widely dispersed in the atmosphere environment especially bound on the particulate. Several researchers have developed the characteristics of particle-bound PAHs in many Chinese cities (Wang et al. 2016; Li et al. 2015; Wei et al. 2015; Chen et al. 2014; Gao et al. 2013; Li et al. 2010; Wang et al. 2009a, 2008; Zhang et al. 2007; Feng et al. 2006). The combustion of solid fuels like coal combustion and biomass burning emits more pollutants in the winter heating periods and so the northern cities in China usually have higher pollutants than the southern cities, especially in the heating periods (Zhang et al. 2020a, b; Li et al. 2021; Gao et al. 2015a; Fan et al. 2014; Kong et al. 2010). One study has shown that the life expectancy for residents in northern China has been about 5.5 years lower than in southern China, likely because of the higher exposure to total suspended particulates in the air (Chen et al. 2013), which is also associated with the inhalation exposure of PAHs.
People’s actual exposure to these compounds is affected by many factors, such as the exposure environment, exposure time, and personal health status. Many models and calculations based on different parameters are used to assess the exposure risks of these compounds to human beings, and toxic equivalent factors (TEFs) are often used to calculate the cancer risks, and then compared with the standards set by the US Environmental Protection Agency (US EPA). A great deal of lung cancer risk assessments of PAHs have been conducted in urban or rural areas in different regions and cities in China (Wang et al. 2014; Xia et al. 2013; Zhang et al. 2012; Bai et al. 2009). These assessments indicated a high risk existed for the elderly and infants who are more sensitive to exposure to these pollutants. However, these reports were calculated by different single TEFs and methods, and limit data of the target region usually contains only single settings. Exposure to different microenvironments was not done in these studies, and up to this point, there has been no assessment of these calculations. Meanwhile, seldom researches considered the uncertainties of the assessment (Xia et al. 2013; Chen and Liao 2006).
The weather conditions in Xi’an, which is located on the Guanzhong Plain at the southern edge of the Loess Plateau, are not good for pollutants diffusion, with 45% no wind frequency in winter, an annual average wind speed of 1.7 m/s. It has about 1.5 million private cars and the main energy source was coal (about 70%). This results in Xi’an having seriously poor air quality. Our team has comprehensively detected the aerosol composition and source apportionment.
In short, in order to fully understand the differences among the health risks calculated by different TEFs, in this study, we compared the inhalation exposures and incremental lifetime cancer risks (ILCR) based on four different TEF calculation methods that were usually used. We used a probabilistic risk assessment to evaluate the carcinogenic risk from personal exposure to particle-bound PAHs in suburban and urban areas for specific age groups in Xi’an, where there is documented bad weather conditions, especially for fine particles (Wang et al. 2017; Gao et al. 2015b; Cao et al. 2009), and the characteristics and possible sources of organic compounds like PAHs, n-alkanes, and phthalates esters have been conducted here. However, a full understanding of inhalation exposure for PAHs in this area was unavailable. The uncertainties during the progress of the calculated health risks by model were also considered.
Material and methods
Sampling
In this study, PM2.5 samples were simultaneously collected at fifteen sites dispersed in Xi’an (see supporting materials Fig. S1): ten were placed in urban residential areas (URAs), two in suburban areas (SUAs), two in traffic corridors (TCs), and one in an industrial area (IA). These sampling sites distributed from the city center to the suburban, which including the relative light pollution sites to the heavy pollution sites. The distribution of the sampling sites was affected by many factors, like the noise of the sampler and the willing of the volunteer family. We try our best to cover the possible exposure environment in the atmosphere to assess the human being inhalation risks in this area. Our previous paper detailed the sampling sites (Wang et al. 2017), and see the supporting materials. The samplers were set outside the windows of urban residences and on the floor of the suburban residences, which was very similar to the indoor environment.
Chemical analyses and quality assurance and control
In this study, PAH analyses and quality assurance and control (QA/QC) can be found in our previous work (Wang et al. 2018). Briefly, we analyzed PAHs of samples through an in-injection port thermal desorption (TD) coupled with gas chromatography/mass spectrometry (GC/MS), using an Agilent 7890A GC/5975C MS system (Santa Clara, CA, USA). The in-injection port TD-GC/MS method and parameters were detailed in our previous works (Wang et al. 2018, 2016; Ho et al. 2011).
For the QA/QC, an Airmetrics PM2.5 Mini-Volume sampler (Springfield, OR, USA) was used for sample collection at a flow rate of 5 L/min, and samples were loaded on 47 mm quartz filters (QM/A®, Whatman Inc., U.K.) in this study. The samplers were calibrated routinely, and the variance for flow was approximately ± 2%. For chemical analysis, internal standards (IS), phenanthrene-d10 (C14D10) (98%, Aldrich, Milwaukee, WI, USA), chrysene-d12 (C18D12) (98%, Sigma-Aldrich, Bellefonte, PA, USA), and n-tetracosane-d50 (n-C24D50) (98%, Aldrich, Milwaukee, WI, USA) were added to each of the samples and blank filters, with blank analysis done each day for the instrument analysis assessment. Replicate samples were analyzed for each ten samples, and the relative standard deviations of them were from 1.7 to 14.8% in this study. The SRM 1649a urban dust (NIST, Gaithersburg, MD, USA) was applied here to correct PAH analysis. All data was calibrated using the mean value of the field blank samples.
Exposed population and occupancy probability
There was one hypothesis that the amount of indoor PAHs were estimated to be equal to the outdoor, even though outdoor air was usually dirtier than indoor air during heating periods and was opposite during non-heating periods. In this study, we compared the BaPeq, which calculated by using the TEF methods from Nisbet and Lagoy (1992), Yassaa et al. (2001), Collins et al. (1998), and USEPA (2010), which were used most frequently in previous studies. The TEFs of individual PAHs are summarized in Supporting Materials (Table S1). The corresponding BaPeq concentrations calculated basing on the TEFs from the four references was called BaPeq1, BaPeq2, BaPeq3, and BaPeq4.
The exposed populations were the general residential populations in the urban (URAs) and suburban areas (SUAs) in Xi’an, ranging in age from 4 to 70 years. For male and female categories, people were divided into four groups: children (4 to 10 years old), adolescents (11 to 17 years old), adults (18 to 60 years old), and seniors (61 to 70 years old). The body weight of each different population group was listed in Table 1 (Wang et al. 2009b).
Occupancy probability (OP) here was used to assess the probable time that a person spent in a specific setting, based on the route of people in Xi’an (Table 2). We assumed that urban and suburban residential environment was similar with the corresponding school environment for children and adolescents. Even though there are differences between different transportation ways and working environments, the traffic was assumed for their exposure when people went to school or work. And the industry was assumed as the work exposure for adults in both the URAs and SUAs. We divided five time intervals (j) for population exposure in one day based on daily routes, from 23:00 to 7:00, 7:00 to 9:00, 9:00 to 17:00, 17:00 to 19:00, and 19:00 to 23:00 as j = 1, 2, 3, 4, and 5, respectively, with j = 1 and 5 representing the resting time for the urban and suburban populations, j = 2 and 4 were the traffic time for each group, and j = 3 was the working time or school time. Considering different distributions for urban and suburban people, different OP values were set between the two categories. OP was assumed to follow uniform distribution during each period, and the daily basis OP at 24-h for urban (suburban), traffic, and industry areas was equal to 1. The corresponding inhalation rate (IR) was calculated based on the data from Wang et al. (2009b), where the IR1 and IR5 are for the rest time, IR2 and IR4 are for a small amount of physical exertion, and IR3 is for medium physical exertion. We compared the differences between the occupancy probability and only single setting exposures.
Health risk assessment model
There were two types of inhalation exposure calculations: one “divided” taking into consideration the OP and the other “non-divided” is without considering the OP. For the divided exposure method (DEM), the BaPeq, based on PAH levels in urban (suburban), traffic, and industrial areas were combined with the specific OP values for different age groups of male and female populations.
They were calculated for the daily inhalation levels of PAHs. It was calculated as:
where EI represents the daily inhalation levels of PAHs for urban and suburban residents with different age groups’ exposure at different environmental settings. OPij means population exposure probabilities in different settings i (i = 1, 2, and 3 represent urban or suburban, traffic, and industrial setting, respectively) in the jth time interval (j =1, 2, 3, 4, and 5). Ci represents the BaPeq levels in the environmental setting i (ng/m3). OTj shows the occupancy time of the population exposure during the jth time interval (h). IRj represents the inhalation rate for different groups during the jth time interval (m3/day), that j =1 and 5 represent the resting activity (IR1) while j =2, 3, and 4 for child, adolescent, and senior groups represent the active activity (IR2). But for adult, j =2 and 4 are the mild activity (IR2); j = 3 is the moderate activity (IR3).
For the non-divided exposure method (NDEM), the daily inhalation levels of PAHs were calculated by:
where Ci is the BaPeq concentrations in environmental setting i (ng/m3) (suburban or urban) and IR is the daily inhalation rate (m3/day) for different age groups.
Then ILCR was used to assess the inhalation risk for different groups of urban and suburban residents. The ILCR model has been widely applied for PAHs carcinogenic risk assessment. It is defined as:
where EI (ng/person/day) is the daily inhalation level; SF represents the cancer slope factor of BaP, a geometric mean of it was 3.14 (mg/kg/day)−1 and geometric standard deviation of 1.8 for inhalation exposure (Chen and Liao 2006; Collins et al. 1991), respectively; EF (day/year), the exposure frequency (252 days/year) (USEPA 2001), ED (year), the exposure duration (children, 7; adolescents, 7; adults, 43; seniors, 10), cf (mg/ng), a conversion factor (10−6), and AT (days), the lifespan of carcinogens (25,550 days) (USEPA 2001).
Uncertainty analysis
A Monte Carlo simulation was implemented to quantify the uncertainty during the calculation process and its impact on the estimation of expected risk. We performed independent runs at 1000, 2000, 4000, 5000, and 10,000 iterations with each parameter sampled independently from the appropriate distribution at the start to test the convergence and the stability of the numerical output. The result shows that 5000 iterations are sufficient to ensure the stability of results. A confidence interval for expected risk was determined on the basis of the 2.5th and 97.5th quartiles of the simulation outcomes.
Sensitivity analysis was used to identify the most significant parameters included in the uncertainty and variability analysis. And the Monte Carlo simulation and sensitivity analysis were implemented by Crystal Ball software (Version 2000.2, Decisioneering, Inc., and Denver, CO, USA). During simulations, the sensitivity of each variable relative to one another was assessed by calculating rank correlation coefficients between each input and output.
Results and discussion
Seasonal variation and characteristics of PAHs and BaPeq
The seasonal variations of PAHs in different environment settings are displayed in Fig. 1, and the detailed concentrations and characteristics are summarized in supporting materials (Table S2–S3). The total average Σ20PAHs in urban, suburban, traffic, and industry settings were 160.9, 268.5, 188.3, and 187.5 ng/m3 in heating periods, respectively, much higher than those in non-heating periods, which were 20.8, 37.7, 20.1, and 42.6 ng/m3, respectively. The SUAs had the highest concentrations, followed by the TCs, IA, and URAs during heating periods, whereas IA had the highest concentrations during non-heating periods, followed by SUAs, university, and URAs. This was due to the much higher PAHs that existed on June 11 in the IA, which reached 300.4 ng/m3, though there was no obvious weather condition change.
The PAH levels in Xi’an always stay at higher levels compared with other regions, like Guangzhou, Shanghai, Hong Kong, Qingdao, and Sanya (Xu et al. 2013; Cao et al. 2013; Guo et al. 2009; Wang et al. 2015). It has levels comparable with Beijing, Taiyuan, and Liaoning Province (Duan et al. 2012; Xia et al. 2010; Kong et al. 2010), where there are heavy pollutant emissions. But Xi’an has the opposite trend with suburban areas showing higher concentrations than in urban areas. The ratio between the SUAs and URAs was similar with 1.7 and 1.8, respectively, in heating and non-heating periods. It was also much higher than the other areas (Xia et al. 2013). This is due to the large amount of solid fuels, the incomplete combustion of coal, and biomass burning in this area that emit large amounts of pollutants, especially in heating periods. This might also cause that in heating periods the SUAs have the highest average ratio of ΣHMW-PAHs to the total PAHs: 86%, followed by TCs (81%), then IA and URAs (80%). However, in non-heating periods, the average ratio of ΣHMW-PAHs to the total PAHs in the IA and SUAs was 78% and 76%, followed by URAS (70%) and then the TCs (69%). In heating periods, SUAs had the highest mean concentration of BaP, 15.8 ng/m3, much higher than 9.1 ng/m3 in the URAs. It was about ten-fold the annual average standard of WHO and the European Union. It was also higher than the daily average standards in China (2.5 ng/m3); however, it was different in non-heating periods, in which the IA had the highest concentration of BaP (4.1 ng/m3), followed by SUAs (3.7 ng/m3), URAs (1.8 ng/m3), and the TCs which had the lowest levels of 1.3 ng/m3. It was similar with previous studies in that heating periods usually have basically higher concentrations than in other seasons (Xia et al. 2013; Zhou et al. 2005).
According to Fig. 2, the BaPeq has the same trends associated with PAHs, that in SUAs with 57.3 ng/m3 had the highest levels in heating periods, and in the IA with 11.5 ng/m3 had the highest in non-heating periods. From Table S2, it showed that there are big differences between the BaPeq calculated by different TEFs. The BaPeq4 had the highest values, followed by BaPeq1, BaPeq3, and BaPeq2. In heating periods, BaPeq1 accounted for 22.9% of total PAHs. BaPeq4 accounted for 44.7% of total PAHs. The ratio for BaPeq3 and BaPeq2 to total PAHs was 16.0% and 9.2%, respectively. In non-heating periods, relatively high values were obtained, for which BaPeq4 accounted for 53.3% of total PAHs, BaPeq1 accounted for 27.8%, and BaPeq3 and BaPeq2 accounted for 16.2% and 12.8%, respectively. Due to the lower percentage of high molecule PAHs species in non-heating periods, low molecule PAHs contributed more to BaPeq in non-heating periods then in heating periods.
Figure 3 investigates the kriging interpolation of BaPeq concentrations during heating and non-heating periods in Xi’an with different TEFs. In heating periods, there were no distribution differences between different BaPeq. In the northwest, people have the highest relative exposure, whereas residents living in the southeast showed a lower exposure. Some residential areas in the city center U1, U2, and U3 also showed higher relative exposure. These residential buildings are old, and there are lots of restaurants surrounding them. In the old city wall, cars’ speeds are limited which also might contribute to heavy pollution emissions, especially for the rush hour. Residents living in U8 had the lowest exposure and people living in U9 and U10 also had low exposures. During non-heating periods, BaPeq4 and BaPeq1 had the same trends, and BaPeq2 and BaPeq3 also had the same trends. All these are strongly associated with the different distributions of PAHs species. Based on BaPeq4 and BaPeq1, S1 had the highest exposure risk followed by I (industry), and S2 had relatively lower exposure risk compared with BaPeq2 and BaPeq3, where S2 has almost the highest exposure risks. It also showed that people in the western part of the city were suffering higher potential cancer risks than in other parts. People living in industrial areas showed almost highest exposures followed by S2. This is different with heating periods, that in industry it has obvious heavy characters. It also significantly displayed that in heating periods, there are higher health risks throughout the whole city compared with non-heating periods, indicating that it is crucial to assess the potential cancer risk for population in Xi’an.
Daily inhalation exposure of PAHs basing on occupancy probability
The daily inhalation exposure BaPeq concentration can be estimated from the Eqs.(1), and it can be associated with age group-specific OP values and IR values for different environmental settings. Eqs.(2) was used to calculate the daily inhalation exposure BaPeq concentrations based on the NDEM.
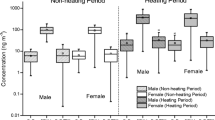
The probability distributions of inhalation exposure of PAHs for population groups were summarized in supplemental materials (Table S4–11). They were strongly correlated with the BaPeq. The highest daily inhalation exposure levels were obtained by calculating using BaPeq4, followed by BaPeq1, BaPeq3, and BaPeq2. In heating periods, the average of daily inhalation exposures of BaPeq4 for different age groups of both genders in SUAs from 508 to 2289 ng/day compared with 407.9 to 2029 ng/day in URAs. In non-heating periods, the inhalation exposure of BaPeq4 was from 91.0 to 488.1 ng/day for different age groups of both genders in SUAs and from 67.9 to 413.9 ng/day in URAs.
However, based on BaPeq2, the corresponding value was from 107.6 to 483.5 ng/day in SUAs and 82.3 to 417.7 ng/day in URAs during heating periods, but was from 23.1 to 131.3 ng/day in SUAs and 33.7 to 110.0 ng/day in URAs during non-heating periods. Those were big differences.
Regardless of using the DEM or NDEM or basing the exposure calculations on different TEFs, for both seasons, the inhalation exposure for each population group was higher in SUAs than in URAs, which was according with using the geometric mean concentration of BaPeq and PAHs. As for gender, males showed higher exposure levels than females in all age groups in the same seasons and settings for SUAs and URAs. This might be due to the fact that males have larger IR than females. The difference between genders is smallest for children, and the adult age group has the highest difference. This is also associated with the differences of the IRs for different groups. In doing the calculation, we did not take into consideration the exposure of adult females of time spent in the kitchen; the female adults might show higher exposure doses than male adults from the emissions of PAHs from cooking oil and food and the PAHs from wood and coal burning (Xia et al. 2013; Yoon et al. 2007; Zhang et al. 2009) in urban and especially in suburban areas. For the age groups in the two seasons and areas, the adults had the highest exposures in the two seasonal groups whether calculated by the DEM or NDEM. Adolescents and seniors had similar inhalation exposures for both male and female in SUAs and URAs, and children has the lowest exposure levels.
In order to assess the differences between the DEM and NDEM, independent samples t-test analysis was used and results are summarized in Table S12. For the different BaPeq, there were similar results. Except for the adult group, there were obvious differences for the other age and gender groups for SUAs during heating periods. For urban residents, only the senior group had similar exposures during heating periods. During non-heating periods, the adults and seniors exposures’ were similar for both genders in SUAs and the differences for male adolescents are also exiting. The adolescent and senior groups for both genders had similar exposures for urban residents. However, for BaPeq3, for suburban residents during non-heating periods, adolescents for both genders also have similar exposure levels.
Comparing the DEM and NDEM, almost all calculations done using the DEM are lower than NDEM (ratios are lower than 1) (Table 3), except for the adult of the divide categories for the four calculations. Only the ratio for the adults in the SUAs during heating periods by BaPeq3 is lower than 1, and the ratio for urban adult residents during non-heating periods is much higher than 1(2.64). The adolescent and senior groups in URAs during non-heating periods are higher than 1 by BaPeq2 and BaPeq3, which differs from BaPeq1 and BaPeq4. The DEM results for boys and girls are almost half of the NDEM for suburban residents for all four calculations, and the ratios are higher for URAs than SUAs. Meanwhile, the ratios calculated by BaPeq1 are similar with BaPeq4 for all groups during heating and non-heating periods.
There are limited reports concerning PAH inhalation exposure, and different reports have chosen different parameters. In Taiyuan, the median BaPeq concentrations of inhalation exposure ranged from 150 to 518 and 40.8 to 121.5 ng/day for different age groups in winter and summer (Xia et al. 2013). In urban areas in Tianjin, children and adult exposure in outdoor settings were 322 and 519 ng/day, respectively (Bai et al. 2009). Chen and Liao (2006) estimated the median BaPeq concentrations of inhalation exposure levels in Taiwan for adult, children, and infants were 1628, 1590, and 252 ng/day, respectively. The higher exposure might be due to the pollution sources samples from traffic, industry, and rural areas in Taiwan.
Inhalation risk assessment
In Fig. 4, the calculated ILCR by the DEM and NDEM for population groups of urban and suburban residents during heating and non-heating periods in Xi’an are summarized (also see Table S13–20). According to the USEPA (1980), a one in a million chance of additional human cancer over a 70-year lifetime (ILCR = 10−6) is the level of risk considered acceptable or inconsequential, whereas an ILCR equal or high than 10−4 is considered to be serious, and there is a high probability for such health problems (Xia et al. 2013; Asante-Duah 2002).
In this study, the sequence of ILCR calculated by different TEFs are ILCR4 > ILCR1 > ILCR3 > ILCR2. This exactly corresponds with the BaPeq values. The ratios of the DEM to the NDEM are also coincident with the BaPeq. For the NDEM, the ILCRs in decreasing order were adults, children, adolescents, and seniors for male, while seniors were a little higher than adolescents for females. This is because children have lower weights and larger inhalation rates than seniors, though the seniors have longer exposure years compared with children and adolescents. Suburban and urban areas have the same trends, but suburban areas were much higher than urban areas. Using the NDEM, the SUAs during heating periods all show that potential risks and ILCR are higher than 10−6 for four calculations, even though only 14.3% of adolescents and seniors, and 3.6% of children are lower than 10−6 by calculated BaPeq2. The ILCR values were from 3.55 × 10−6 to 7.64 × 10−5 for adults for all of the calculations, indicating that adults have the highest potential risks of all the age groups. For the NDEM, the URAs during heating periods all had ILCR4 values higher than 10−6. More than 94% of ILCR1 results for all groups were higher than 10−6, with all adults being higher than 10−6. These values were decreased to 34.5% for adolescents and seniors by ILCR2. Comparing heating periods, ILCRs were relatively low during non-heating periods, and again adults were shown to have the highest potential risks. ILCR values of adults based on BaPeq4 were all higher than 10−6, but the ratio decreased to 84.4% for adult males and 59.4% for adult females based on the BaPeq2 for suburban residents, 32.5% of adult males and 21.5% of adult females for urban residents during non-heating periods.
There were big differences between the DEM and the NDEM. The ILCR in decreasing order was adults, seniors, adolescents, and children for suburban and urban residents, and suburban groups were higher than the urban groups. The ILCR values for ILCR1 and ILCR4 were all higher than 10−6 for all groups in suburban areas during heating periods, and about 50% of children and adolescent, 80% of seniors were potentially at risk for the ILCR2 calculation. The ILCR values for adults were from 5.5 × 10−6 to 6.44 × 10−5 by four calculations. ILCR, calculated by BaP4 and BaP1 were all higher than 10−6 for urban residents during heating periods. About 17% of children and adolescents, 25% of seniors were higher than 10−6 for ILCR2, while all adults exceeded potential risk levels. During non-heating periods, almost all adults showed potential health risks for suburban and urban residents, and fewer than 20% of the children, adolescents, and seniors had potential risks.
All the ratios of DEM to the NDEM were from 0.48 to 2.64. The adult groups calculated by the DEM were higher than when calculated by the NDEM. Children, adolescents and seniors were lower when calculated by DEM as compared with the NDEM. Though there were little difference for genders, males showed higher ratios for children, adults, and seniors, while female adolescents were investigated higher ratios.
According to the census in Xi’an, there were 6.187 million population in urban areas and 2.400 million in rural at 2013. People who were between 0 to 14 years, accounted for 12.9% of the total population, those between the ages of 15 and 64 accounted for 78.6% of the total population, and those older than 65 were about 8.5% of the total population (Xi’an Statistical Yearbook 2015). Based on the DEM ILCR1 data in heating periods, there would be 3 persons 0–14 years old that have a potential risk for getting from lung cancer from a lifetime exposure at the pollution levels. And for people ages 15 to 64, there would be about 101 and 44 persons in urban and rural areas that would get lung cancer from a lifetime exposure at heating periods’ levels. For seniors older than 65, about 2 persons are at risk from a lifetime exposure given the heating periods’ levels, whereas by the NDEM, the corresponding age groups’ results were 5 persons (0–14 years), 109 persons (15–64 years), and 3 persons (>65 years), respectively. In non-heating periods, there were 19 persons by NDEM and 32 persons by the DEM from 15 to 64 years who were at risk by ILCR1 data. Based on ILCR2, there were no differences for the DEM and NDEM for the 0–14 year-olds and greater than 65 year-olds with 2 people who are at risk. For people from 15 to 64 years as calculated by the DEM, 59 persons in heating and 16 persons in non-heating periods were at risk, more than with the NDEM calculations which projected 45 persons in heating and 9 persons in non-heating periods. People from 15 to 64 years old had the highest risks, with 79 persons by the NDEM and 99 persons by the DEM in heating periods, 11 persons by the NDEM, and 22 persons by the DEM in non-heating periods. People from 0 to 14 years old and greater than 65 years old were similar with the other ILCR calculated. ILCR4 projected the greatest risks, with 212 persons from 15 to 64 years by the NDEM and 281 by the DEM in heating periods. Even in non-heating periods, there were still 38 and 59 persons with cancer risks by the NDEM and DEM, respectively.
Even though the calculations results for the children and seniors were lower than the adults and adolescents, they still had higher risks for their sensitive immunologic system. There were such big differences between the DEM and NDEM calculations, and by different BaPeq TEFs. More data and longtime assessment methods are needed.
Sensitivity analysis
The sensitivity analysis results on the ILCR for population groups in Xi’an are shown in Figs. 5 and 6 by the rank correlation by average, and each of the sensitivity analysis results from different ILCRs were summarized in Fig. S2–9. The cancer slope factor (SF) was the most important factor for all groups in heating periods, with from 0.589 to 0.828 by the NDEM and from 0.766 to 0.911 by the DEM, followed by the EI. The EI accounted for 0.085 to 0.178 for suburban and 0.114 to 0.380 for urban residents. SF showed a higher contribution using the DEM, while the EI displayed a higher contribution for the NDEM. But the contributions of SF and EI in urban areas showed big differences between the DEM and NDEM. The average contributions of SF for all age groups were 0.607 and 0.841 by the NDEM and DEM, respectively. There were no obvious differences for different age groups and genders.
During non-heating periods, EI was the most important factor: from 0.580 to 0.659 by the NDEM and from 0.534 to 0.862 by the DEM, followed by the SF. The contribution of EI was higher in the SUAs than in the URAs, while SF had the opposite trends. EI showed higher contributions by the DEM than by the NDEM.
There were no differences between genders for all age groups for urban and suburban residents. EI showed higher contributions for adults than the other age groups using the DEM. This was associated with the previous studies for PAH health risk assessment (Bai et al. 2009; Chen and Liao 2006).
In this study, the BW of child and adolescent also called important contributions. It has shown the impact of the parameters selected on the accuracy of the risk assessment. But now about the exposure parameters in China, usually cited from the foreign standards. It is urging to setting exposure parameters for people in China.
Conclusions
In this study, we compared health risk assessments by different TEFs, and compared calculations based on occupancy probability. The results showed that there were big differences among different calculation methods. About 49 persons would likely develop lung cancer from lifetime exposure at heating periods’ levels by ILCR2 (the lowest potential health risks), whereas about 225 people by ILCR4 (the highest potential health risks) by the NDEM during heating periods. The corresponding data were from 13 to 42 persons in non-heating periods. However, by the DEM, the results were 63 to 292 persons in heating and 20 to 63 persons in non-heating periods. The ILCR values were higher in heating than in non-heating periods, in suburban as compared with urban areas, for adults compared with other age groups, and for males compared with females. The cancer slope factor for BaP inhalation exposure was dominant contributions in heating periods, while the daily inhalation exposure level had dominant contributions for the ILCR risk assessment in non-heating periods. Such big differences showed that more data are needed to assess the potential risks of PAHs by TEFs and different exposure ways.
References
Asante-Duah K (2002) Public health risk assessment for human exposure to chemicals. Kluwer, Netherlands
Bai ZP, Hu YD, Yu H, Wu N, You Y (2009) Quantitative health risk assessment of inhalation exposure to polycyclic aromatic hydrocarbons on citizens in Tianjin, China. B Environ Contam Toxical 83:151–154
Cao JJ, Zhu CS, Chow JC, Watson JG, Han YM, Wang GH, Shen ZX, An ZS (2009) Black carbon relationships with emissions and meteorology in Xi'an, China. Atmos Res 94:194–202
Cao JJ, Zhu CS, Tie XX, Geng FH, Xu HM, Ho SSH, Wang GH, Han YM, Ho KF (2013) Characteristics and sources of carbonaceous aerosols from Shanghai, China. Atmos Chem Phys 13:803–817
Chen SC, Liao CM (2006) Health risk assessment on human exposed to environmental polycyclic aromatic hydrocarbons pollution sources. Sci Total Environ 366:112–123
Chen L, Hu G, Fan R, Lv Y, Dai Y, Xu Z (2018) Association of PAHs and BTEX exposure with lung function and respiratory symptoms among a nonoccupational population near the coal chemical industry in Northern China. Environ Int 120:480–488
Chen Y, Cao JJ, Zhao J, Xu HM, Arimoto R, Wang GH, Han YM, Shen ZX, Li GH (2014) n-Alkanes and polycyclic aromatic hydrocarbons in total suspended particulates from the southeastern Tibetan Plateau: Concentrations, seasonal variations, and sources. Sci Total Environ 470:9–18
Chen YY, Ebenstein A, Greenstone M, Li HB (2013) Evidence on the impact of sustained exposure to air pollution on life expectancy from China’s Huai River policy. Proc Natl Acad Sci U S A 110:12936–12941
Collins JF, Brown JP, Dawson SV, Marty MA (1991) Risk assessment for benzo[a]pyrene. Regul Toxicol Pharmacol 13:170–184
Collins JF, Brown JP, Alexeeff GV, Salmon AG (1998) Potency equivalency factors for some polycyclic aromatic hydrocarbons and polycyclic aromatic hydrocarbon derivatives. Regul Toxicol Pharmacol 28:45–54
Duan JC, Tan JH, Wang SL, Chai FH, He KB, Hao JM (2012) Roadside, urban, and rural comparison of size distribution characteristics of PAHs and carbonaceous components of Beijing, China. J Atmos Chem 69:337–349
Fan RF, Li JN, Chen LG, Xu ZC, He DC, Zhou YX, Zhu YY, Wei FS, Li JH (2014) Biomass fuels and coke plants are important sources of human exposure to polycyclic aromatic hydrocarbons, benzene and toluene. Environ Res 135:1–8
Feng JL, Hu M, Chan CK, Lau PS, Fang M, He LY, Tang XY (2006) A comparative study of the organic matter in PM2.5 from three Chinese megacities in three different climatic zones. Atmos Environ 40:3983–3994
Gao B, Guo H, Wang XM, Zhao XY, Ling ZH, Zhang Z, Liu TY (2013) Tracer-based source apportionment of polycyclic aromatic hydrocarbons in PM2.5 in Guangzhou, southern China, using positive matrix factorization (PMF). Environ Sci Pollut R 20:2398–2409
Gao B, Wang XM, Zhao XY, Ding X, Fu XX, Zhang YL, He QF, Zhang Z, Liu TY, Huang ZZ, Chen LG, Peng Y, Guo H (2015a) Source apportionment of atmospheric PAHs and their toxicity using PMF: Impact of gas/particle partitioning. Atmos Environ 103:114–120
Gao ML, Cao JJ, Seto E (2015b) A distributed network of low-cost continuous reading sensors to measure spatiotemporal variations of PM2.5 in Xi'an, China. Environ Pollut 199:56–65
Guo ZG, Lin T, Zhang G, Hu LM, Zheng M (2009) Ocurrence and sources of polycyclic aromatic hydrocarbons and n-alkanes in PM2.5 in the roadside environment of a major city in China. J Hazard Mater 170:888–894
Ho SSH, Chow JC, Watson JG, Ng LPT, Kwok Y, Ho KF, Cao JJ (2011) Precautions for in-injection port thermal desorption-gas chromatography/mass spectrometry (TD-GC/MS) as applied to aerosol filter samples. Atmos Environ 45:1491–1496
Indoor Air Quality Standards (GB/T 18883-2002 (1980) Ministry of Environmental Protection of China; http://www.zhb.gov.cn. USEPA Method 5 of 40 CFR Part 60. http://www.epa.gov/ttn/emc/methods/method5.html.
Kong SF, Ding XA, Bai ZP, Han B, Chen L, Shi JW, Li ZY (2010) A seasonal study of polycyclic aromatic hydrocarbons in PM2.5 and PM2.5-10 in five typical cities of Liaoning Province, China. J Hazard Mater 183:70–80
Li JY, Gao WK, Gao LM, Xiao Y, Zhang YM, Zhao SM, Liu Z, Liu ZR, Tang GQ, Ji DS, Hu B, Song T, He LY, Hu M, Wang YS (2021) Significant changes in autumn and winter aerosol composition and sources in Beijing from 2012 to 2018: Effects of clean air actions. Environ Pollut 268:115855
Li W, Wang C, Shen HZ, Su S, Shen GF, Huang Y, Zhang YC, Chen YC, Chen H, Lin N, Zhuo SJ, Zhong QR, Wang XL, Liu JF, Li BG, Liu WX, Tao S (2015) Concentrations and origins of nitro-polycyclic aromatic hydrocarbons and oxy-polycyclic aromatic hydrocarbons in ambient air in urban and rural areas in northern China. Environ Pollut 197:156–164
Li WF, Peng Y, Bai ZP (2010) Distributions and sources of n-alkanes in PM2.5 at urban, industrial and coastal sites in Tianjin, China. J Environ Sci-China 22:1551–1557
Liu SZ, Tao S, Liu WX, Dou H, Liu YN, Zhao JY, Little MG, Tian ZF, Wang JF, Wang LG, Gao Y (2008) Seasonal and spatial occurrence and distribution of atmospheric polycyclic aromatic hydrocarbons (PAHs) in rural and urban areas of the North Chinese Plain. Environ Pollut 156:651–656
Luo P, Bao LJ, Li SM, Zeng EY (2015) Size-dependent distribution and inhalation cancer risk of particle-bound polycyclic aromatic hydrocarbons at a typical e-waste recycling and an urban site. Environ Pollut 200:10–15
Nisbet ICT, Lagoy PK (1992) Toxic Equivalency Factors (TEFs) for Polycyclic Aromatic-Hydrocarbons (Pahs). Regul Toxicol Pharmacol 16:290–300
Okona-Mensah KB, Battershill J, Boobis A, Fielder R (2005) An approach to investigating the importance of high potency polycyclic aromatic hydrocarbons (PAHs) in the induction of lung cancer by air pollution. Food Chem Toxicol 43:1103–1116
Park SY, Lee SM, Ye SK, Yoon SH, Chung MH, Choi J (2006) Benzo[a]pyrene-induced DNA damage and P53 modulation in human hepatoma HepG2 cells for the identification of potential biomarkers for PAH monitoring and risk assessment. Toxicol Lett167: 27-33.
USEPA (2001) Risk assessment Guidance for superfund, volume I: human health evaluation manual (Part E, Supplemetal Guidance for dermal risk assessment), EPA/540/R/99/005. Washington DC, USA7 Office of Emerage and Remedial Response.
USEPA (2010) Development of a relative potency factor (RPF) approach for polycyclic aromatic hydrocarbon (PAH) mixtures. In Support of Summary Information on the Integrated Risk Information System (IRIS). External review draft. U.S. Environmental Protection Agency: Washington, DC
Wang GH, Kawamura K, Xie MJ, Hu SY, Gao S, Cao JJ, An ZS, Wang Z (2009a) Size-distributions of n-alkanes, PAHs and hopanes and their sources in the urban, mountain and marine atmospheres over East Asia. Atmos Chem Phys 9:8869–8882
Wang JZ, Dong ZB, Li XP, Gao ML, Ho SSH, Wang GH, Xiao S, Cao JJ (2018) Intra-urban levels, spatial variability, possible sources and health risks of PM2.5 bound phthalate esters in Xi’an. Aerosol Air Qual Res 18(2):485–496
Wang JZ, Cao JJ, Dong ZB, Guinot B, Gao ML, Huang RJ, Han YM, Huang Y, Ho SSH, Shen ZX (2017) Seasonal variation, spatial distribution and source apportionment for polycyclic aromatic hydrocarbons (PAHs) at nineteen communities in Xi’an, China: the effects of suburban scattered emissions in winter. Environ Pollut 231(2:1330–1343
Wang JZ, Ho SSH, Ma SH, Cao JJ, Dai WT, Liu SX, Shen ZX, Huang RJ, Wang GH, Han YM (2016) Characterization of PM2.5 in Guangzhou, China: use of organic markers for supporting source apportionment. Sci Total Environ 550:961–971
Wang JZ, Ho SSH, Cao JJ, Huang RJ, Zhou JM, Zhao YZ, Xu HM, Liu SX, Wang GH, Shen ZX, Han YM (2015) Characteristics and major sources of carbonaceous aerosols in PM2.5 from Sanya, China. Sci Total Environ 530:110–119
Wang XF, Cheng HX, Xu XB, Zhuang GM, Zhao CD (2008) A wintertime study of polycyclic aromatic hydrocarbons in PM2.5 and PM2.5-10 in Beijing: assessment of energy structure conversion. J Hazard Mater 157:47–56
Wang YH, Hu LF, Lu GH (2014) Health risk analysis of atmospheric polycyclic aromatic hydrocarbons in big cities of China. Ecotoxicology 23:584–588
Wang ZS, Duan XL, Liu P, Nie J, Huang N, Zhang JL (2009b) Human exposure factors of Chinese people in environmental health risk assessment. Res Environ Sci (in Chinese) 22:1164–1170
Wei C, Han YM, Bandowe BA, Cao JJ, Huang RJ, Ni HY, Tian J, Wilcke W (2015) Occurrence, gas/particle partitioning and carcinogenic risk of polycyclic aromatic hydrocarbons and their oxygen and nitrogen containing derivatives in Xi'an, central China. Sci Total Environ 505:814–822
Xi’an Statistical Yearbook 2015. http://www.xatj.gov.cn/ptl/def/def/2015/tjnj/indexch.htm
Xia ZH, Duan XL, Tao S, Qiu WX, Liu D, Wang YL, Wei SY, Wang B, Jiang QJ, Lu B, Song YX, Hu XX (2013) Pollution level, inhalation exposure and lung cancer risk of ambient atmospheric polycyclic aromatic hydrocarbons (PAHs) in Taiyuan, China. Environ Pollut 173:150–156
Xia ZH, Duan XL, Qiu WX, Liu D, Wang B, Tao S, Jiang QJ, Lu B, Song YX, Hu XX (2010) Health risk assessment on dietary exposure to polycyclic aromatic hydrocarbons (PAHs) in Taiyuan, China. Sci Total Environ 408:5331–5337
Xu HM, Tao J, Ho SSH, Ho KF, Cao JJ, Li N, Chow JC, Wang GH, Han YM, Zhang RJ, Watson JG, Zhang JQ (2013) Characteristics of fine particulate non-polar organic compounds in Guangzhou during the 16th Asian Games: effectiveness of air pollution controls. Atmos Environ 76:94–101
Yassaa N, Meklati BY, Cecinato A, Marino F (2001) Particulate n-alkanes, n-alkanoic acids and polycyclic aromatic hydrocarbons in the atmosphere of Algiers City Area. Atmos Environ 35:1843–1851
Yoon E, Park K, Lee H, Yang JH, Lee C (2007) Estimation of excess cancer risk on time-weighted lifetime average daily intake of PAHs from food ingestion. Hum Ecol Risk Assess 13:669–680
Yu YX, Li Q, Wang H, Wang B, Wang XL, Ren AG, Tao S (2015) Risk of human exposure to polycyclic aromatic hydrocarbons: a case study in Beijing, China. Environ Pollut 205:70–77
Zhang K, Zhang BZ, Li SM, Wong CS, Zeng EY (2012) Calculated respiratory exposure to indoor size-fractioned polycyclic aromatic hydrocarbons in an urban environment. Sci Total Environ 431:245–251
Zhang KY, Zhao CF, Fan H, Yang YK, Sun Y (2020a) Toward understanding the differences of PM2.5 characteristics among five China urban cities. Asia-Pacific J Atom Sci 56(4):493–502
Zhang LC, An J, Liu MY, Li ZW, Liu Y, Tao LX, Liu XT, Zhang F, Zheng DQ, Gao Q, Guo XH, Luo YX (2020b) Spatiotemporal variations and influencing factors of PM2.5 concentrations in Beijng. China Environ Pollut 262:114276
Zhang YQ, Wang Y, Tan PG (2007) Levels and statistical analysis of aerosol phase PAHs over Qingdao alongshore. J Zhejiang Univ-Sc A 8:1164–1169
Zhang YX, Dou H, Chang B, Wei ZC, Qiu WX, Liu SZ, Liu WX, Tao S (2008) Emission of polycyclic aromatic hydrocarbons from indoor straw burning and emission inventory updating in China. Environ Challenges Pacific Basin 1140:218–227
Zhang YX, Tao S (2009) Global atmospheric emission inventory of polycyclic aromatic hydrocarbons (PAHs) for 2004. Atmos Environ 43:812–819
Zhang YX, Tao S, Shen HZ, Ma JM (2009) Inhalation exposure to ambient polycyclic aromatic hydrocarbons and lung cancer risk of Chinese population. Proc Natl Acad Sci U S A 106:21063–21067
Zhao T, Yang LX, Huang Q, Zhang Y, Bie SJ, Li JS, Zhang W, Duan SF, Gao HL, Wang WX (2020) PM2.5-bound polycyclic aromatic hydrocarbons (PAHs) and their derivatives (nitrated-PAHs and oxygenated-PAHs) in a road tunnel located in Qingdao, China: Characteristics, sources and emission factors. Sci Total Environ 720. https://doi.org/10.1016/j.scitotenv.2020.137521
Zhou JB, Wang TG, Huang YB, Mao T, Zhong NN (2005) Size distribution of polycyclic aromatic hydrocarbons in urban and suburban sites of Beijing, China. Chemosphere 61:792–799
Data availability
All data generated or analyzed during the study are included in this published article and its supplementary files. The original data is available if reasonably request.
Funding
This study was supported by National Natural Science Foundation of China (41603126) and Natural Science Fundamental Research Plan of Shaanxi Province (2018JQ4004). It was also supported by two open funds from Key Laboratory of Aerosol Chemistry and Physics, institute of Earth Environment, CAS (KLACP2003) and Guangdong Provincial Key Laboratory of Utilization and Protection of Environmental Resources (KLEPRU-01). Jingzhi Wang also received financial support from China Scholarship Council (201906875007).
Author information
Authors and Affiliations
Contributions
Jingzhi Wang and Junji Cao designed this study. Yongming Han and Junji Cao provided sampling and analysis equipment. Zhibao Dong and Ge Ma provide experiments management and associates. Yumeng Wang, Zedong Wang, and Runyu Wang performed the compounds analysis. Xinxin Ding conducted the sample collection and chemical experiments. Yumeng Wang and Zedong Wang contributed to data analysis and writing assistance. Jingzhi Wang and Neil McPherson Donahue contributed for the literature search and revision of the paper. All authors commented and modify the paper.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Lotfi Aleya
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 2210 kb)
Rights and permissions
About this article
Cite this article
Wang, Y., Wang, Z., Wang, J. et al. Assessment of the inhalation exposure and incremental lifetime cancer risk of PM2.5 bounded polycyclic aromatic hydrocarbons (PAHs) by different toxic equivalent factors and occupancy probability, in the case of Xi’an. Environ Sci Pollut Res 29, 76378–76393 (2022). https://doi.org/10.1007/s11356-022-21061-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-21061-9