Abstract
The present work aims to investigate the effects of various additives on the stability of graphene nanoplatelet (GnP)–based nanofluid phase change material (NFPCM) for cold thermal energy storage (CTES). The NFPCMs are prepared by dispersing six different types of surfactants (anionic, cationic, and non-ionic types) in deionized (DI) water at a mass ratio of 1:0.5 GnP to surfactant. NFPCMs can be found to be stable with a suitable surfactant even after 30 days using zeta-potential distribution, UV–vis absorption, visual inspection, and sedimentation tests at low temperature. The maximum enhancement in thermal conductivity of 8.3% and 48.3% is recorded in both liquid and solid states for the NFPCM with gum arabic (GA) respectively. The viscosity was enhanced by the dispersion of non-ionic surfactants, where the anionic surfactant (sodium dodecylbenzene sulfonate (SDBS)) NFPCM had a 29.9% lower augmentation compared to DI water. Furthermore, differential scanning calorimetry (DSC) results demonstrate that the phase change properties of the NFPCM are significantly affected depending on the surfactant type. The maximum phase change enthalpy is lowered (10.6%) in the Tween 80 NFPCM as compared to the base PCM. The long-term stability with the highest thermal transport property of the NFPCM storage unit integrated with the chiller is capable of achieving environmental pollution remediation by minimising the time it takes to charge the PCM.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
At present, global electricity consumption is increasing dramatically and is 25–30% with the use of air conditioning, refrigeration, and heat pump equipment (Yang et al. 2021; Sidney et al. 2021; Dhivagar et al. 2021a). The cold thermal energy storage (CTES) system has shown a promising solution using a phase change material (PCM) in various applications that can absorb, store, and release large amounts of energy during phase transition (Prabakaran et al. 2020; Dhivagar et al. 2021b). Water/ice is predominantly used as a storage material for CTES applications due to its potential advantages over other PCMs. However, the higher supercooling and low thermal transport behaviour hinder their practical applications (Zahir et al. 2019; Murugan et al. 2018). In recent years, the enhancing characteristics of PCM have gained much attention in improving the performance of storage systems. The addition of nano-sized material has been proven to be a viable option to improve the heat transfer behaviour of the storage material (Kim et al. 2020; Prabakaran et al. 2019; Dhivagar and Kannan 2022). Thus, the optimization of storage medium (base PCM) with dispersed nanoparticles is referred to as nanofluid phase change material (NFPCM) or nano-enhanced phase change material (NEPCM). Although the thermophysical properties of base PCM have been improved, they are not widely used due to their poor stability. Therefore, several studies have been reported to develop a stable NFPCM and improve the thermophysical properties of the base PCM. At present, carbon-based materials are attractive nano-additives, mainly due to their high thermal conductivity (up to about 5000 W m−1 K−1). Harish et al. (2017) reported that GnP represents 223% and 39% thermal conductivity improvement and better performance in solid and liquid states for GnP composite PCM. Sathishkumar et al. (2016) explored the performance of the 0.3–1.2% mass concentration range of GnP-water NFPCM and found 11.7% and 56% increases in thermal conductivity of NFPCM in liquid and solid states due to its large specific surface area with high thermal conductivity. Prabakaran et al. (2019) studied nanocomposites of GnP with OM08 and reported 45.69% and 102.17% higher thermal conductivity for 0.5 vol% of GnP in liquid and solid states. As a result, GnP exhibits better performance than MWCNT and SMWCNT nanomaterials due to its higher aspect ratio and better thermal boundary conductance (Kumar et al. 2016; Dhivagar et al. 2021c; Xian et al. 2020). Therefore, GnP as a nanoadditive was chosen for the present work to enhance the thermal performance of the CTES system. However, the inclusion of GnP into DI water easily aggregates due to their high Van der Waals forces and surface energy (Sarsam et al. 2016). Steric stabilization is ensured by surfactants and has some advantages over electrostatic stabilization because suspensions for electrical stabilization may require considerable acidity or base, which can have a negative impact on the reliability of the equipment (Demirkır and Erturk 2020). Therefore, surfactant-treated GnP must be prepared to improve properties in order to provide better performance in the transition process.
Among the various preparation techniques, the surfactant-treated nanofluid is considered to be an effective, simple, and cost-effective method for reducing sedimentation and increasing the stability of nanomaterials in aqueous dispersion. The use of surfactant with water converts the hydrophobic nature of nanoparticles to hydrophilic and increases the wettability (Mingzheng et al. 2012; Ganesh Kumar et al. 2021). As a result, appropriate surfactant selection (Fernandez-Merino et al. 2012) and efficient coating create an electrostatic repulsion and compensate for the van der Waals attractions. Thus, it has improved suspension stability to a greater extent and influences the thermophysical property (Ganeshkumar et al. 2017). In different circumstances, the role of the surfactant in stability, the thermophysical properties, and the phase change behaviour in an aqueous solution are different. Xuan et al. (2013) reported that surfactants have a great impact on the thermophysical properties and thermal performance of nanofluids. Chandrasekaran et al. (2014) indicated that the addition of CTAB had a significant influence on supercooling, about 59.4% in DI water, and no change in liquid sensible cooling and onset of freezing. Jia et al. (2014) reported that the addition of TiO2 nanoparticles reduced the supercooling rate of DI water by 11.5% and the dispersed surfactants (SDS) in TiO2 by 19.1%. It was concluded that the surfactants influenced the supercooling degree of TiO2 nanofluids primarily by modifying the contact angle of TiO2 nano-aggregates suspended in them. However, the most significant feature in facilitating the nucleation of the base PCM is the selection of suitable surfactants. (Morimoto et al. 2019).
Numerous studies have been reported on the effects of various surfactants on the nano-fluid stability and thermophysical properties of DI water-graphene. Borode et al. (2021) studied the effects of SDBS, SDS, GA, and Tween 80 on the dispersibility of GnP in an aqueous solution at various surfactant-GnP ratios. They noted that better stabilization was achieved for SDBS-GnP nanofluids at a ratio of 1:2, and that using Tween 80 would greatly enhance thermal conductivity and the use of GA would greatly increase the viscosity of water. Kim et al. (2018) examined the surfactant for graphene and CNT in the aqueous solutions, and observed that SDBS showed good stability. Nanofluids with SDS had better thermal conductivity than nanofluids with other surfactant dispersants. Nazari et al. (2019) evaluated the effect of various surfactants dispersed on aquatic media with GnP and the excellent performance of CTAB inhibiting surfactant stability. Mingzheng et al. (2012) indicated that the thermal conductivity ratios of surfactant solutions are stable to a certain mass concentration and that ionic surfactants are more sensitive to temperature than non-ionic surfactants (PVP). Therefore, commonly used surfactants such as sodium dodecyl sulphate (SDS), sodium dodecylbenzene sulfonate (SDBS), cetyltrimethylammonium bromide (CTAB), Tween 80, PVP (polyvinylpyrrolidone), and gum arabic (GA) have been identified.
Recent literature shows interest among researchers in analysing the features of surfactants for the preparation of stable nanofluids. However, the studies are limited to the influence of surfactants in DI water owing to its thermal performance and energy storage capacity. In addition to that, the sedimentation time and thermal properties of NFPCM with various types of surfactants have not been addressed. The long-term stability of NFPCMs with different surfactants needs to be analysed using different measurement techniques. The thermal transport properties like viscosity and thermal conductivity of PCMs need to be analysed in different atmospheric conditions. The influence of surfactants on the phase change properties of the NFPCMs should be studied at a low heat flow rate to examine the nucleation behaviour. The present study aims to investigate the thermal performance of DI water and GnP-based NFPCMs with suitable surfactants, which include two anionic surfactants (SDS and SDBS), one cationic surfactant (CTAB), and two nonanoic surfactants (Tween 80, PVP, and GA). In addition to that, the long-term stability of NFPCMs with different surfactants are analysed using the zeta potential distribution method, UV–vis absorbance, visual inspection, and a study on the settlement of nanomaterials at low temperature. Further, the best combination of NFPCM with suitable surfactant is reported for the performance enhancement of cold thermal energy storage systems.
Materials and methods
Materials
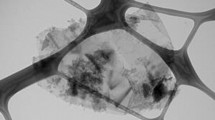
As a base PCM, DI water is preferred due to its favourable physical properties during phase transitions. The surfactants used in the present study, such as CTAB (cetyltrimethylammonium bromide), SDBS (sodium dodecylbenzene sulfonate), SDS (sodium dodecyl sulphate), Tween 80 (polysorbate), PVP (polyvinylpyrrolidone), and GA (gum arabic), are procured from Sisco Pvt., Ltd., India. A nanoadditive is used as graphene nanoplatelets (GnP) and imported from Cheap Tubes Inc., USA. It has an average surface area of 500–700 m2 g−1, an average thickness of 8–15 nm, X and Y dimensions of > 2 nm, purity of 97%, and the TEM (transmission electron microscope) image is depicted in Fig. 1.
Preparation of NFPCM
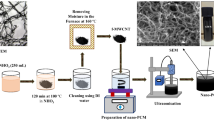
The well-known two-step method is applied for the preparation of all the PCM samples. The mass of the surfactant and nanoparticle is measured with a semi-micro weighing balance (Shimadzu–AUW220D model) with an accuracy of 0.01 mg. A critical micelle concentration (CMC) value is used to find the optimal mass concentration of surfactant with the inclusion of nanomaterial (Karimi et al. 2015). The UV–visible spectrophotometer is used to measure the absorbance of GnPs to examine the CMC value. The CMC values of various surfactants, such as CTAB, SDBS, SDS, Tween 80, PVP, and GA, were determined and the values are obtained in the range of 0.18 to 0.26 wt.% of surfactants for 0.50 wt.% of GnP-DI water mixtures. An account of constant mass concentration of 0.25 wt.% was taken for the surfactants to the limit quantity of nanofluid preparation samples and comparative study. Initially, the surfactant samples (SF) are prepared independently of the mixture with DI water in a 200-ml (class B type) standard volumetric flask and stirred for 30 min. Further, ultrasonication is done for 40 min in a water bath for the homogenous mixture. Next, the nanofluid samples are prepared individually at GnP mass concentrations of 0.5 wt.% to evaluate the stability of nanofluids. The nanoparticles are dispersed into surfactant solutions using magnetic stirring for about 40 min. Finally, the dispersant achieves a homogeneous mixture in the solution by a probe sonification for 60 min in steps of 10-min intervals to maintain a constant temperature. The prepared samples are listed in Table 1. There is no visual sedimentation after 30 min, and it is evident that the prepared PCM samples are used for further analysis. HR-SEM (high-resolution scanning electron microscopy) images are presented for some samples in Fig. 2a, b. According to the figure, the nanoparticles are dispersed in DI water with surfactants and have a smaller dimension, and are not folded.
Characterization of NFPCM
The stability of NFPCM is an important key factor for evaluating the thermal characteristics and enhancing the performance of the storage system. Zeta potential and particle size distribution are measured using a Zetasizer instrument (Malvern Zetasizer ZS) to study the dispersion stability of NFPCM samples. The ELS (electrophoretic light scattering) technique is used to measure the diffusion of GnP nanoparticles. Zeta potential measurements refer to the degree of repulsion between adjacent particles of the same charge in NFPCM dispersions (Sarsam et al. 2016). DLS (dynamic light scattering) techniques are used to determine particle size distribution in stabilized NFPCM samples. The measurements are carried out after the preparation and 30 days at room temperature to study the sedimentation rate. The average value is taken from three repeated records of measurement, and the standard accuracy of the instruments is 2%. Quantitative characterization of the stability can be obtained by the light measurements of absorbance of a suspension. A UV–vis spectrophotometer (Shimadzu UV 3600 Plus) is used to determine the stability, operating in the wavelength range of 200 to 800 nm. The samples are diluted in distilled water to allow sufficient light transmission through them. Each sample measurement is taken after preparation and over a time period. Furthermore, the visual sedimentation method is used to observe the stability of NFPCM samples at room temperature and at low temperature.
A viscometer (Brookfield DV-II + Pro) is used to measure the dynamic viscosity of PCM samples with a shear rate of 20 to 264 s−1. The repeated measurement is carried out to ensure the Newtonian flow behaviour of NFPCM samples at different shear rates. A TEMPOS portable device (Meter Group, Inc., USA) is used to measure the thermal conductivity of PCM, which uses the transient line heat source method. It has a probe for this measurement, consisting of a KS-3-type needle with a heater and temperature sensor inside. The experimental setup is fabricated especially to measure the thermal conductivity at specified temperature conditions. The major influencing thermal transport property of thermal conductivity is measured in both liquid and solid states at 25 to − 15 °C for all PCM samples. The schematic layout of the experimental arrangement is depicted in Fig. 3. The arrangement includes a well-insulated stainless-steel container with a constant temperature bath, a sample container, and a sensor probe. The probe needle is inserted vertically into a sample container without touching the wall surface. Each measurement is carried out in a sample container to reach equilibrium temperature with bath fluid, and a measurement is taken five times for accuracy and repeatability.
The influence of phase change properties on stabilized NFPCM by the addition of various surfactants is analysed using a differential scanning calorimeter (DSC, 214 Polyma, NETZSCH) in terms of specific heat, latent heat (phase change enthalpy), onset, end, and peak temperature during the heating (melting)-cooling (freezing) process. The calibration of the DSC instrument and the measurement procedure are referred to Sathishkumar and Cheralathan (2022a). A measuring sample of 20-32mg is sealed in an encapsulated aluminium pan, and a standard empty aluminium pan is utilised simultaneously as a reference sample. The measurement tests are carried out at a heat flow rate of 5 K min−1 over a temperature range of 30 to − 30 °C under a nitrogen gas atmosphere at a 60-mL min−1 gas flow rate. The power response and temperature range of DSC are well-calibrated by the high-purity (99.9%) iridium metal.
Uncertainty analysis
The uncertainty measurement of thermal conductivity and DSC are calculated using a standard procedure governed by Moffat (1988). Taking X as the experimental parameter with the least count, and ΔX as the deviation from the measured values, it can be expressed as shown in Eq. (1). The thermal conductivity measurement precision is ± 5%, and the uncertainty can be calculated as shown in Eq. (2). The value is found to be ± 8.2% and with a deviation of 0.01 W m−1 K−1. The precision value of ± 0.05 to ± 0.2% for latent heat by the manufacture of DSC measurement (214 Polyma-NETZSCH) and the measurement values showed good consistency with the standard PCM properties. The DSC measurement (214 Polyma-NETZSCH) values showed good consistency with standard PCM properties ranging from ± 0.05 to ± 0.2% for latent heat. The weight of the various materials is measured with an accuracy of 0.001 g using a mercury-balanced semi-micro weighing device. The accuracy of instruments and sensors used for the experiments is listed in Table 2.
Results and discussion
NFPCM stability
The stability of the DI water-GnP-based NFPCM with various surfactants is investigated through zeta potential, particle size distribution, UV–vis absorbance, visual observation, and low temperature stability. The constant mass ratio of 0.5:1 surfactant to nanomaterial is used for the comparative analysis in order to determine which surfactant is most effective.
Zeta potential analysis is widely used to establish the dispersion behaviour of nanofluids with an indication of the charge present on the surface of the particles. The zeta potential value is directly related to the stability of NFPCM, which is a measure of high repulsive forces between nanoparticles (Sundaram and Kalaisselvane 2021). The stability of the dispersion is determined by the repulsion that arises between particles with the same charge. The zeta potential stability ranges are > ± 30 mV (which is higher stability), = ± 30 mV (which is moderate stability), and < ± 30 mV (which is lower stability) (Kazemi et al. 2020). Figure 4 presents the absolute zeta potential value of NFPCM samples after preparation and 30 days of preparation. It is observed that the cationic surfactant sample is positively charged whereas the anionic and nonionic surfactant samples are negatively charged. Figure 5a, b show the zeta potential distribution results of NF1 and NF2 samples by total counts, which is exemplified graphically in the Zetasizer version. It can be seen that the dispersion of GnPs is stable for all the samples after preparation, except for the sample without surfactant in DI water. After 30 days, SDBS-GnP NFPCM has the highest value of − 35.9 mV, followed by GA- and SDS-GnP NFPCM with values of − 33.9 and − 30 mV, respectively, which has a stable dispersion. The Tween 80, PVP, and CTAB NFPCM samples can be found to have the lowest values of − 15.2, − 19, and 21.7 mV, respectively, indicating that they are unstable according to measured zeta potential value criteria. Thus, the results clearly show that the NFPCM samples with various surfactants exhibit moderate to good stability with minor sediments over time. In addition, the deviation in the zeta potential value is compared after preparation and after 30 days, which decreased to very small values of 5.8% and 10% for GA and SDBS-GnP, respectively. This indicates that the GA-NFPCM sample yielded good stability results after 30 days of preparation.
The NFPCMs’ physical stability and rheological behaviour are highly influenced by particle size distribution. The particle size distributions of GnP suspensions with different surfactant samples are analysed after preparation and 30 days later. A lower value indicates better stability, while a higher value leads to collisions, which result in agglomeration (Ilyas et al. 2020). Figure 6 illustrates the variation in average particle size distribution of various NFPCM samples over time. It can be found that the SDBS, CTAB, and GA-GnP have the smallest particle size diameter, and Tween 80 and PVP-GnP have the largest particle size diameter. Results indicate that the average particle size does not exceed the micron scale for all NFPCM after 30 days of preparation, and it is evident that the surfactants hold the stabilisation of NFPCM by zeta potential distribution. The particle size distribution profile of NF1 and NF2 samples by intensity is exemplified graphically as shown in Fig. 7a, b.
The UV–vis absorbance is a convenient method to characterise the stability of NFPCM. The light absorbance spectra with a wavelength of ~ 266 nm are used to characterise NFPCM samples with various surfactant mass concentrations of 0.25 wt.%. Figure 8a depicts the UV–vis spectrophotometry images of different non-covalently functionalized GnPs dispersed in DI water after preparation. The peak absorbance of GnPs indicates the better dispersion stability of NFPCM (Kazemi et al. 2020). It can be seen that GA has the highest single peak absorbance, followed by SDBS, Tween 80, SDS, CTAB, and PVP. Moreover, the UV–vis absorbance is used to evaluate the long-term dispersion stability of GnP NFPCM without and with a mixture of various surfactants after 30 days as presented in Fig. 8b. It can be evident that the surfactants used with a GnP NFPCM have better dispersion than the NFPCM without surfactant. Overall, the SDBS-and GA-NFPCM samples recorded the best homogeneity of suspension after preparation, and GA-GnP showed the highest stabilisation rate by zeta potential distribution and UV–vis absorbance after 30 days in DI water.
Further, the sedimentation rate is analysed by visual observation after 15 days of preparation for all NFPCM samples. Figure 9 shows a photographic view of samples taken over a 15-day period. It is seen that the NFPCM samples without surfactant exhibit poor stability over time compared to NFPCM samples with surfactant. Therefore, the addition of surfactants can improve the stability of nanofluids, and remarkably, GA and SDBS mixtures demonstrate lower sedimentation after 30 days. In this experiment, NFPCMs are cooled to − 7 °C in a constant bath tank in order to analyse their suspension stability at a lower temperature. At low temperatures, NFPCM with surfactants does not precipitate at the bottom of the bottle, and unexpectedly, NFPCM without surfactants agglomerates at the bottom and top of the bottle after changing phase from solid to liquid, as illustrated in Fig. 10.
Thermal characteristics
Figure 11 shows the comparison of DI water thermal conductivity measurement with ASHRAE standards (Sathishkumar et al. 2016). It can be shown that there is good agreement with standard data and a deviation error of ± 6% and ± 11.5% for liquid and solid states, respectively. The sensors used to measure the thermal conductivity of ice are calibrated on solid samples at a room temperature of 25 °C (± 1 °C) using a standard solid specimen (METER Group, Inc., USA). The enhancement in thermal conductivity is estimated as \(\left(\frac{{k}_{NF}-{k}_{BF}}{{k}_{BF}}\right)\), for various surfactant dispersed in 0.5 wt.% GnP NFPCM samples with respect to temperature. Figure 12 shows the thermal conductivity enhancements of NFPCM samples compared to DI water. The ionic and cationic surfactants are shown to have a small influence on a thermal conductivity value compared to DI water, whereas the non-ionic surfactants lower the thermal conductivity value. It is mainly dependent on the length of the alkyl chain, and the result is in good agreement with the previous report of water-based nanofluids with different surfactant solutions (Mingzheng et al. 2012). From the results, thermal conductivity enhancement was noticed in the order of GA, SDBS, SDS, CTAB, PVP, and Tween 80 (NF6, NF2, NF3, NF1, NF5, and NF4) in both solid and liquid states compared to base PCM. Generally, the thermal conductivity of base PCM is enhanced with optimization of dispersion behaviour and the inclusion of high-conductive materials. Comparing the results, the maximum thermal conductivity enhancement of 48.3% in solid state (at − 10 °C) and 8.3% in liquid state (at 25 °C) is recorded for GA-GnP NFPCM. This is due to the stabilisation of GnP in DI water; the addition of a suitable surfactant prevents agglomeration and enhances the active surface area, which helps to increase the thermal conductivity. The thermal conductivity is enhanced anomalously in their solid state due to the presence of stable high-conductivity GnPs that offer negligible thermal resistance at the interface with solid ice. It is evident that the UV–vis spectrophotometer and zeta potential values have good agreement with respect to surfactants. Moreover, the results showed that thermal conductivity was significantly enhanced by selecting the right surfactant, the nanoparticle concentration ratio, and the size or type of nanoparticle (Xian et al. 2020).
The viscosity measurement of DI water compared to the ASHRAE standard (Xian et al. 2020) and the average deviation in the values found to be 1.72% are shown in Fig. 13. The measurement of viscosity of NFPCMs depicts that they follows Newtonian behaviour (shear stress range of 1.5–1.92 N/m2) in the entire range of shear rate. Figure 14 shows the viscosity enhancements of NFPCMs over DI water with a shear rate of 100 s−1 at 25 °C. The result shows that GA-NFPCM has the highest viscosity, followed by Tween 80-, PVP-, SDS-, CTAB-, and SDBS-NFPCM, when compared to DI water. It can be seen that the enhancement of viscosity variation occurs with the addition of a type of surfactant. The NFPCM prepared with a non-ionic surfactant increases the viscosity of DI water as compared with anionic surfactant. This is due to the influence of the alkyl chain length of the surfactant molecules intertwined with DI water. Similar trends have been reported by the researchers (Sarsam et al. 2016; Borode et al. 2021). In contrast, the GA-GnP NFPCM caused a maximum enhancement of 47% and a minimum of 29.9% SDBS-GnP NFPCM, which has the highest stability. A dispersion of surfactant increases the viscosity of nanofluids used in fluid flow applications, which causes the pressure loss and a large increase in the pumping power. However, the increase in viscosity of base PCM and its higher colloidal stability emphasize the use of nanofluids as a storage medium in CTES systems. However, the long-term stability and high thermal transport properties of nanofluids provide better performance in storage applications. In the present study, GA-GnPs NFPCM can be considered the most effective one among other surfactant dispersions for cold thermal energy storage.
DSC analysis
The thermal energy storage behaviour of PCM is measured using a DSC (differential scanning calorimetry) instrument, and the DSC thermograms of PCM samples are depicted in Fig. 15a, b. The phase transition properties of PCMs in terms of latent heat, onset temperature, peak temperature, and end temperature, obtained by DSC, are listed in Table 3. The latent heat is measured by the area of the peak curve, and it is obtained 346.2 and 297 J g−1 for pure PCM during a heating and cooling process at 5 K min−1 heat flow rate. It can be seen that a single peak occurred for all the samples during the heating and cooling processes, and there is not much variation in the curve during heating compared to cooling. The latent heat value decreased for all PCM samples compared to pure PCM due to the addition of surfactant/nanomaterials and the mass of the PCM present in the test crucible (Wu et al. 2020). The latent heat values are affected by the phase change temperature during the heating and cooling processes. The latent heat value decreased for all PCM samples compared to pure PCM due to the addition of surfactant/nanomaterials and the mass of the PCM present in the test crucible (Wu et al. 2020). The latent heat values of NEPCMs during freezing and melting operations may vary by up to 10% due to differences in phase transition temperature, sample mass measured, and heat flow rates. It is recorded that the dispersion of surfactants lowers the phase change enthalpy of 8.3%, 8.2%, 7.3%, 6.3%, 5.4%, and 1% for Tween 80, PVP, SDS, GA, SDBS, and CTAB-NFPCM, respectively, during freezing at a cooling rate of 5 K min−1. It is due to the surfactant’s having an intermolecular bond with water molecules that lowers base PCM energy storage density. The results are in good agreement with a previous report obtained by Sathishkumar and Cheralathan (2022a). The onset freezing of water takes place at − 18.4 °C, due to the crystallisation of water, which depends on the sample mass, cooling rate, and surface characteristics of encapsulation (Vikram et al. 2019). The advancement of onset temperature significantly altered to − 19.3 °C, − 18 °C, and − 19 °C during freezing with the addition of GnP for the NF1, NF2, and NF4 samples, respectively. It can indicate that the higher nucleation action of stable impurities present in the water causes the advancement of onset freezing to behave (Kumaresan et al. 2021). As expected, the supercooling is reduced for all the samples with the presence of surfactants, where a higher reduction occurs for NFPCM samples due to faster crystallisation growth. Therefore, the present results demonstrate a type of surfactant with GnP sample performance during the phase transition process.
The specific heat is measured using the Cp ratio method to determine the variation of heat capacity during phase transition. The reference sample of sapphire (diameter of 4 mm) is used for the evaluation of specific heat. As shown in Fig. 16, the variation of specific heat is measured over a temperature range of 25 to 25 °C. Due to the phase transition of water at a 5 K min−1 heating rate, a strong peak curve is observed for all samples in the range of − 3.5 to 22 °C. In the solid region, the variation of specific heat is very small, whereas in the liquid region, the specific heat increases with the addition of GnP with respect to a temperature. The variation of specific heat is 2.2 to 3.62 J g−1 K−1 in the temperature range of − 25 to − 1 °C and 4.7 to 4.283 J g−1 K−1 in the range of 16 to 25 °C during change of solid and liquid phases of DI water, respectively. The specific heat of NFPCM lowered with the addition of surfactant and GnP in the base fluid. During phase transition, the specific heat varies extremely due to the higher thermal expansion of liquids for small temperature variations (Sathishkumar and Cheralathan 2022b). In comparison, the strong peak lowers with the addition of surfactants and GnP in the order of NF2, NF5, NF3, NF4, NF1, and NF6 compared to the DI water. In other words, a strong peak showed a higher specific heat capacity with the phase transition of DI water than the NFPCM. The results of specific heat curves indicated that the type of surfactant mixture of NFPCM had good potential for cold thermal energy storage.
Conclusions
In the present study, the dispersion stability, thermophysical properties, and phase change properties of DI water GnP-based NFPCM have been investigated using six different surfactants (CTAB, SDS, SDBS, Tween 80, PVP, and GA). The addition of all types of surfactants has improved the stability of GnPs dispersed in the DI water after preparation compared to NFPCM without surfactants. The long-term stability of NFPCMs shows that the dispersion of GA and SDBS has minimal deviation (5.8% and 10%, respectively) between the first day of preparation and after 30 days. The non-ionic type of PVP and Tween 80 were found to have poor stability by the results of zeta potential and UV–vis spectrophotometry. It is found that GA-GnP NFPCM exhibits superior performance by various stability analyses over time. The addition of the surfactants significantly influences the thermal conductivity, in which the GA-NFPCM sample showing maximum improvements of 48.3% and 8.3% in both liquid and solid states, respectively. The viscosity of nonionic samples are having highviscosity as compared to the DI water, and the anionic surfactants do not increase it significantly, which is due to the variation of alkyl chain length. The DSC analysis shows that the phase change properties of NFPCM have been influenced by the addition of surfactants due to its interfacial binding with water molecules. The latent heat of stabilised GA, SDBS, and CTAB NFPCM samples is not significantly affected during freezing. It can be concluded that GA can be a great surfactant to be a stable NFPCM over time by altering small changes in latent heat and enhancing thermophysical properties, which exhibits the best performance in the storage unit. Furthermore, the long-term stability of NFPCMs can be achieved by the dispersion of suitable additives in the base PCM, which facilitates energy-saving potential in CTES systems.
Data availability
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- h:
-
Heating
- c:
-
Cooling
- k:
-
Thermal conductivity
- S:
-
Uncertainty
- DI:
-
Deionized
- BF:
-
Base fluid
- NF:
-
Nano fluid
- SF:
-
Surfactant fluid
- GA:
-
Gum arabic
- TEM:
-
Transmission electron microscope
- DLS:
-
Dynamic light scattering
- ELS:
-
Electrophoretic light scattering
- PCM:
-
Phase change material
- HTF:
-
Heat transfer fluid
- CMC:
-
Critical micelle concentration
- DSC:
-
Differential scanning calorimetry
- CNT:
-
Carbon nanotubes
- GnP:
-
Graphene nanoplatelet
- SDS:
-
Sodium dodecyl sulphate
- PVP:
-
Polyvinylpyrrolidone
- SDBS:
-
Sodium dodecylbenzene sulfonate
- CTAB:
-
Cetyltrimethylammonium bromide
- CTES:
-
Cold thermal energy storage
- LHTES:
-
Latent heat thermal energy storage
- HRSEM:
-
High resolution scanning electron microscopy
- MWCNT:
-
Multi-wall carbon nanotubes
- NFPCM:
-
Nanofluid phase change material
- NEPCM:
-
Nano enhanced phase change material
References
Borode AO, Ahmed NA, Olubambi PA et al (2021) Effect of various surfactants on the viscosity, thermal and electrical conductivity of graphene nanoplatelets Nanofluid. Int J Thermophys 42:1–15. https://doi.org/10.1007/s10765-021-02914-w
Chandrasekaran P, Cheralathan M, Kumaresan V, Velraj R (2014) Enhanced heat transfer characteristics of water based copper oxide nanofluid PCM (phase change material) in a spherical capsule during solidification for energy efficient cool thermal storage system. Energy 72:636–642. https://doi.org/10.1016/j.energy.2014.05.089
Demirkır C, Erturk H (2020) Rheological and thermal characterization of graphene-water nanofluids: hysteresis phenomenon. Int J Heat Mass Transf 149:3–11. https://doi.org/10.1016/j.ijheatmasstransfer.2019.119113
Dhivagar R, Kannan KG (2022) Thermodynamic and economic analysis of heat pump-assisted solar still using paraffin wax as phase change material. Environ Sci Pollut Res 29:3131–3140. https://doi.org/10.1007/s11356-021-17183-1
Dhivagar R, Mohanraj M, Belyayev Y (2021a) Performance analysis of crushed gravel sand heat storage and biomass evaporator-assisted single slope solar still. Environ Sci Pollut Res 28:65610–65620. https://doi.org/10.1007/s11356-021-15487-w
Dhivagar R, Mohanraj M, Deepanraj B, Murugan VS (2021b) Assessment of single slope solar still using block and disc magnets via productivity, economic, and enviro-economic perspectives: a comparative study. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-021-15565-z
Dhivagar R, Mohanraj M, Raj P, Gopidesi RK (2021c) Thermodynamic analysis of single slope solar still using graphite plates and block magnets at seasonal climatic conditions. Water Sci Technol 84:2635–2651. https://doi.org/10.2166/wst.2021.156
Fernandez-Merino MJ, Paredes JI, Villar-Rodil S et al (2012) Investigating the influence of surfactants on the stabilization of aqueous reduced graphene oxide dispersions and the characteristics of their composite films. Carbon N Y 50:3184–3194. https://doi.org/10.1016/j.carbon.2011.10.039
Ganesh Kumar P, Sakthivadivel D, Thangapandian N et al (2021) Effects of ultasonication and surfactant on the thermal and electrical conductivity of water – solar glycol mixture based Al2O3 nanofluids for solar-thermal applications. Sustainable Energy Technol Assess 47:101371. https://doi.org/10.1016/j.seta.2021.101371
Ganeshkumar P, Kumaresan V, Velraj R (2017) Stability, viscosity, thermal conductivity, and electrical conductivity enhancement of multi-walled carbon nanotube nanofluid using gum arabic. Fullerenes, Nanotubes, Carbon Nanostruct 25(4):230–240. https://doi.org/10.1080/1536383X.2017.1283615
Harish S, Orejon D, Takata Y, Kohno M (2017) Enhanced thermal conductivity of phase change nanocomposite in solid and liquid state with various carbon nano inclusions. Appl Therm Eng 114:1240–1246. https://doi.org/10.1016/j.applthermaleng.2016.10.109
Ilyas SU, Ridha S, Abdul Kareem FA (2020) Dispersion stability and surface tension of SDS-Stabilized saline nanofluids with graphene nanoplatelets. Colloids Surf A Physicochem Eng Asp 592:124584. https://doi.org/10.1016/j.colsurfa.2020.124584
Jia L, Peng L, Chen Y et al (2014) Improving the supercooling degree of titanium dioxide nanofluids with sodium dodecylsulfate. Appl Energy 124:248–255. https://doi.org/10.1016/j.apenergy.2014.03.019
Karimi MA, Mozaheb MA, Hatefi-Mehrjardi A et al (2015) A new simple method for determining the critical micelle concentration of surfactants using surface plasmon resonance of silver nanoparticles. J Anal Sci Technol 6:4–11. https://doi.org/10.1186/s40543-015-0077-y
Kazemi I, Sefid M, Afrand M (2020) A novel comparative experimental study on rheological behavior of mono & hybrid nanofluids concerned graphene and silica nano-powders: characterization, stability and viscosity measurements. Powder Technol 366:216–229. https://doi.org/10.1016/j.powtec.2020.02.010
Kim S, Tserengombo B, Choi SH et al (2018) Experimental investigation of dispersion characteristics and thermal conductivity of various surfactants on carbon based nanomaterial. Int Commun Heat Mass Transf 91:95–102. https://doi.org/10.1016/j.icheatmasstransfer.2017.12.011
Kim SC, Prabakaran R, Sakthivadivel D et al (2020) Thermal transport properties of carbon-assisted phase change nanocomposite. Fullerenes, Nanotubes, Carbon Nanostruct 28:925–933. https://doi.org/10.1080/1536383X.2020.1786814
Kumar PG, Kumaresan V, Velraj R (2016) Experimental investigation on thermophysical properties of solar glycol dispersed with multi-walled carbon nanotubes. Fullerenes, Nanotubes, Carbon Nanostruct 24:641–652. https://doi.org/10.1080/1536383X.2016.1219852
Kumaresan V, Raghavan KS, Ponrajan Vikram M, Iyyappan J (2021) Role of graphitized mesoporous carbon on solidification and melting characteristics of water for cool thermal storage. Fullerenes, Nanotubes, Carbon Nanostruct 29:890–898. https://doi.org/10.1080/1536383X.2021.1910811
Mingzheng Z, Guodong X, Jian L et al (2012) Analysis of factors influencing thermal conductivity and viscosity in different kinds of surfactant solutions. Exp Therm Fluid Sci 36:22–29. https://doi.org/10.1016/j.expthermflusci.2011.07.014
Moffat RJ (1988) Describing the uncertainties in experimental results. Exp Therm Fluid Sci 1:3–17. https://doi.org/10.1016/0894-1777(88)90043-X
Morimoto T, Kawana Y, Saegusa K, Kumano H (2019) Supercooling characteristics of phase change material particles within phase change emulsions. Int J Refrig 99:1–7. https://doi.org/10.1016/j.ijrefrig.2018.11.039
Murugan P, Ganesh Kumar P, Kumaresan V et al (2018) Thermal energy storage behaviour of nanoparticle enhanced PCM during freezing and melting. Phase Transitions 91:254–270. https://doi.org/10.1080/01411594.2017.1372760
Nazari B, Ranjbar Z, Hashjin RR, et al (2019) Dispersing graphene in aqueous media: investigating the effect of different surfactants. Colloids Surfaces A Physicochem Eng Asp 582. https://doi.org/10.1016/j.colsurfa.2019.123870
Prabakaran R, Sidney S, Lal DM, et al (2019) Solidification of graphene-assisted phase change nanocomposites inside a sphere for cold storage applications. Energies 12(18):3473. https://doi.org/10.3390/en12183473
Prabakaran R, Prasanna Naveen Kumar J, Mohan Lal D et al (2020) Constrained melting of graphene-based phase change nanocomposites inside a sphere. J Therm Anal Calorim 139:941–952. https://doi.org/10.1007/s10973-019-08458-4
Sarsam WS, Amiri A, Kazi SN, Badarudin A (2016) Stability and thermophysical properties of non-covalently functionalized graphene nanoplatelets nanofluids. Energy Convers Manag 116:101–111. https://doi.org/10.1016/j.enconman.2016.02.082
Sathishkumar A, Cheralathan M (2022a) Influence of thermal transport properties of NEPCM for cool thermal energy storage system. J Therm Anal Calorim 147:367–378. https://doi.org/10.1007/s10973-020-10339-0
Sathishkumar A, Cheralathan M (2022b) Effect of active multi-walled carbon nanotubes (MWCNT) on the energy storage density of DI water for cool thermal storage system. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-022-18779-x
Sathishkumar A, Kumaresan V, Velraj R (2016) Solidification characteristics of water based graphene nanofluid PCM in a spherical capsule for cool thermal energy storage applications. Int J Refrig 66:73–83. https://doi.org/10.1016/j.ijrefrig.2016.01.014
Sidney S, Prabakaran R, Kim SC, Dhasan ML (2021) A novel solar-powered milk cooling refrigeration unit with cold thermal energy storage for rural application. Environ Sci Pollut Res 29(11):16346–16370. https://doi.org/10.1007/s11356-021-16852-5
Sundaram P, Kalaisselvane A (2021) Effect of different additives on freezing characteristics and stability of GnP-aqueous-based PCM for cold thermal storage. J Therm Anal Calorim. https://doi.org/10.1007/s10973-021-11056-y
Vikram MP, Kumaresan V, Christopher S, Velraj R (2019) Experimental studies on solidification and subcooling characteristics of water-based phase change material (PCM) in a spherical encapsulation for cool thermal energy storage applications. Int J Refrig 100:454–462. https://doi.org/10.1016/j.ijrefrig.2018.11.025
Wu W, Wang X, Xia M et al (2020) A novel composite PCM for seasonal thermal energy storage of solar water heating system. Renew Energy 161:457–469. https://doi.org/10.1016/j.renene.2020.06.147
Xian HW, Sidik NAC, Saidur R (2020) Impact of different surfactants and ultrasonication time on the stability and thermophysical properties of hybrid nanofluids. Int Commun Heat Mass Transf 110:104389. https://doi.org/10.1016/j.icheatmasstransfer.2019.104389
Xuan Y, Li Q, Tie P (2013) The effect of surfactants on heat transfer feature of nanofluids. Exp Therm Fluid Sci 46:259–262. https://doi.org/10.1016/j.expthermflusci.2012.12.004
Yang L, Villalobos U, Akhmetov B, et al (2021) A comprehensive review on sub-zero temperature cold thermal energy storage materials, technologies, and applications: state of the art and recent developments. Appl. Energy 288:116555. https://doi.org/10.1016/j.apenergy.2021.116555
Zahir MH, Mohamed SA, Saidur R, Al-Sulaiman FA (2019) Supercooling of phase-change materials and the techniques used to mitigate the phenomenon. Appl Energy 240:793–817. https://doi.org/10.1016/j.apenergy.2019.02.045
Acknowledgements
The authors wish to thank the Department of Mechanical Engineering, Puducherry Technological University (Erstwhile Pondicherry Engineering College), Puducherry, for the support and encouragement in carrying out this research work. The authors would also like to thank the Department of Mechanical Engineering, SRM Institute of Science and Technology, Kattankulathur, Chennai, for providing the facilities (DSC, TEMPOS, and UV-Vis spectrophotometer) to carry out the research work.
Author information
Authors and Affiliations
Contributions
Experimentation, formal analysis, writing—original draft preparation, P. Sundaram; writing—review and editing, P. Sundaram and A. Kalaisselvane; supervision, A. Kalaisselvane.
Corresponding author
Ethics declarations
Ethics approval
The authors attest that this paper has not been published elsewhere, the work has not been submitted simultaneously for publication elsewhere and the results presented in this work are true and not manipulated.
Consent to participate
All the individual participants involved in the study have received informed consent.
Consent for publication
The participants have consented to the submission of the study to the journal.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: George Z. Kyzas
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Palanichamy, S., Athiimoulam, K. Influence of various additives on stability and phase change characteristics of DI water-GnP-based NFPCM for cold thermal energy storage systems. Environ Sci Pollut Res 29, 66935–66949 (2022). https://doi.org/10.1007/s11356-022-20419-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-20419-3