Abstract
Bacteria play a vital role in ecological processes of soil contaminated by heavy metals. Here, soil sampling was carried out around a tailings pile contaminated to different degrees by cadmium (Cd), lead (Pb) and arsenic (As). The bacteria in the soil were cultured, separated and purified on Luria–Bertani medium, and the changes in bacterial communities in soils with different pollution levels were analysed with 16S rRNA sequencing. Bacillus pacificus strain MZ520364 was found to be highly tolerant to Cd, Pb and As, and single-metal and multimetal tolerance experiments were further conducted with this strain. The results obtained from alpha diversity and operational taxonomic unit (OTU) statistical analyses showed a significant difference in bacterial composition among soils with different metal pollution levels, and the highest bacterial diversity was found at the most severely polluted site. Evidence from variance partitioning analysis (VPA) and the Spearman correlation heatmap analysis showed that the leading factors affecting bacterial community composition were cation exchange content (CEC), pH, total Zn, total As, and available As concentrations in soil. Additionally, in the single-metal treatments, B. pacificus MZ520364 could tolerate 600 mg/L Cd2+, 1000 mg/L Pb2+ or 700 mg/L As3+. When Cd, Pb and As coexisted, the best growth of B. pacificus MZ520364 was present at 120 mg/L Cd2+, 200 mg/L Pb2+ and 150 mg/L As3+. The effect of Cd, Pb and As on the growth of the strain followed the order of Cd > As > Pb, and the heavy metal combination showed more toxicity than single metals. In summary, our results revealed the ecological impact of soil physicochemical properties on the diversity and richness of soil bacterial communities and suggested that B. pacificus MZ520364 may be used for the remediation of Cd–Pb–As co-contaminated soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Human activities in mining areas have caused serious heavy metal pollution in soil, especially of cadmium (Cd), lead (Pb), and arsenic (As) (Satapute et al. 2019). The diversity of soil bacteria is related to heavy metal pollution in the soil, and severe heavy metal pollution poses a major threat to the survival of soil bacteria and to public health (Huang et al. 2021; Li et al. 2017). It is crucial to study the structure of bacterial communities in a metal-contaminated soil ecosystem to evaluate the bacterial composition response in soil contaminated by heavy metals (Li et al. 2021; Ma et al. 2020).
Soil bacterial communities can drive the processes of matter cycling in soil, for example, promoting nutrient cycling and adjusting soil pH (Che et al. 2018; Luo et al. 2017). Many biogeochemical processes, such as chelation, coprecipitation and detoxification of heavy metals in soil, are also mediated by soil bacterial communities (Llado et al. 2017). Excessive heavy metals in the soil ecosystem have significant impacts on plant growth and bacterial community diversity (Boechat et al. 2017; Fan et al. 2018; Rahbari et al. 2021). Excessive heavy metal concentrations in soil exert an adverse effect on soil bacterial abundance and soil enzyme activities (Liu et al. 2020a, b; Sharma and Archana 2016; Shirokikh et al. 2015). The growth, metabolism and cell function of soil bacteria are also disturbed by heavy metals (Mesa-Marin et al. 2016; Rousk and Rousk 2018).
Zhao et al. (2020) proved that the composition of the soil microbial community has direct and indirect effects on the soil ecosystem, and it is important to determine the dominant bacterial communities that may contribute to specific processes in soil. Li et al. (2016) screened some tolerant bacteria, such as Serratia, Pseudomonas and Bacillus to examine the potential for remediating combined metal-contaminated soil. Ma et al. (2015) used hyperaccumulators and inoculation with tolerant bacterial species, including Bacillus pumilus E2S2, Bacillus sp. E1S2, Bacillus sp. E4S1, Achromobacter sp. E4L5 and Stenotrophomonas sp., to remediate metal-contaminated soil. In addition, many studies have demonstrated the obvious impact of heavy metals on the soil environment (Liang et al. 2021; Liu et al. 2015; Shen et al. 2019; Xu et al. 2017). However, it is not clear whether the community structure of soil bacteria will change with the increase in heavy metal pollution (Chen et al. 2020). Therefore, an in-depth study of soil pollution and soil microbial variations in mining areas is helpful for the subsequent remediation of metal-contaminated soil.
In this study, a tailings pile area in Datun town, Gejiu city, Yunnan Province, China, was selected as the study site, and soil sampling and investigation in the mining area were conducted. By analysing the relationship between soil physicochemical properties and soil bacterial communities, the changes in microbial communities in contaminated soil were studied, and bacteria tolerant to heavy metals (Cd, Pb and As) were screened out. The objectives of the present work were (1) to cultivate, isolate, purify and identify the strains that could tolerate heavy metals (Cd, Pb and As); (2) to reveal the response of soil bacterial communities to heavy metal pollution in soil and (3) to analyse the relationship between soil physicochemical properties and soil bacterial communities.
Materials and methods
Site description and sample collection
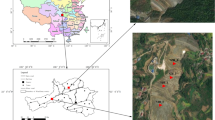
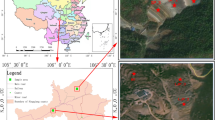
The study sites were located in Datun town, Gejiu city, Yunnan Province, China, which has a humid subtropical monsoon climate. The sites were abandoned farmlands beside a tin tailings pile, which caused serious heavy metal pollution to the local environment, particularly of Cd, Pb and As.
The soil samples were collected from three sites named A, B and C near the tin tailings pile with different levels of heavy metal contamination, and the geographical location of the sampling sites is shown in Fig. 1. Site A was near a mountain and the farthest away from the pollution source and could be considered to have relatively low metal pollution. Site B was located in the middle of the metal pollution gradients. Site C was the closest to the tin tailings pile and experienced the most serious metal pollution. The soil samples at each site were collected using a square 5-point sampling method at a depth of 5–10 cm. Specifically, a sampling point was in the centre of “5 points”, with the other four points on the diagonal line equal to the central sampling point. At each point, approximately 300 g of soil was weighed, and a total of 1.5 kg of soil at each site was collected and sealed in a sterilized paper bag. There were three repetitions at each site. The soil samples were immediately transported to the laboratory on dry ice.
Experimental design
Each soil sample was divided into three parts for further study. One part was used for culture experiment of soil bacteria, one part was air-dried to analyse the physicochemical properties of the soil, and the rest was stored at − 80 °C for DNA extraction and microbial community analysis by 16S rRNA technology. Within 24 h after sample collection, soil samples (10 g) with different metal contamination levels were weighed and placed into an Erlenmeyer flask containing 90 mL of sterile water. The sample was shaken for 20 min until evenly dispersed and left for 5 min. The soil sample dilution solution (0.1 mL) at each concentration was added to the sterilization plate, spread evenly with a glass coating rod, and then samples were placed in an incubator at a constant 37 °C for 24 h (Wang et al. 2016). The dominant strains in each soil sample were selected, streaked and separated in Luria–Bertani (LB) solid medium and then cultured at a constant 37 °C for 24 h, and the above operation was repeated until a single colony was produced. The purified strains were inoculated into liquid medium containing heavy metals (Cd, Pb and As) at different concentrations, and the strains tolerant to heavy metals were screened out.
The air-dried soil samples were sieved through a 100-mesh sieve for physicochemical property analysis. The sieved soil (10 g) was used for pH measurement (soil/water = 1:2.5, w/v). The soil was digested with aqua regia-HClO4, and the concentrations of Pb, Cd, Cu, Zn, etc., were determined by atomic absorption spectrometry (Varian AA240FS, USA) (Zheng et al. 2016); the concentration of As was measured by atomic fluorescence spectrometry (AFS, AF-610D) (Li et al. 2014). The sieved soil sample (5.00 g) was added to a 25-mL solution mixture containing 0.0050 M diethylenetriaminepentaacetic acid (DTPA), 0.10 M triethanolamine and 0.010 M CaCl2, shaken for 2 h, centrifuged at 3000 rpm for 5 min, extracted and then used to determine the available Cd (A-Cd), available Pb (A-Pb), available Cu (A-Cu), etc. (Liu et al. 2020a, b). In the case of available As (A-As), the sieved soil sample (2.50 g) was added to 25 mL of NaHCO3 (0.50 mol/L) as the extractant and then reduced by thiourea-ascorbic acid as described above (Huang et al. 2005).
The Nemerow pollution index (PNemerow) is the most commonly used index to evaluate multimetal pollution in soil. It comprehensively reflects the different degrees of pollution from various pollutants to the soil, and it highlights the effect of heavy pollution with high concentrations on the soil environment. The calculation of PNemerow is based on the following formula (Li et al. 2014):
Here, Ci is the measured metal concentration, mg/kg; Si is the risk screening values of heavy metals for soil contamination of agricultural land in China (GB15618-2018), which is shown in Table 1; and n is the number of heavy metals. PI ≤ 0.70: uncontaminated; 0.70 < PI ≤ 1.00: warning limit; 1.00 < PI ≤ 2.00: mild pollution; 2.00 < PI ≤ 3.00, moderate pollution; PI > 3.00: heavy pollution.
Structure and diversity of the bacterial community
High-throughput sequencing technology was used to determine the composition and types of bacteria in the soil. The soil samples were subjected to 16S rRNA sequencing by Guangzhou Gene-Denovo Biotechnology Co., Ltd. (Guangzhou City, China). The soil samples were sequenced by 16S rRNA, DADA2 was used to filter and correct the reads, and non-redundant reads and corresponding abundance information were output. The reads were spliced into tags. The primer sequences used in the PCR method were 341F: CCTACGGGNGGCWGCAG and 806R: GGACTACHVGGGTATCTAAT. The purified amplified products were connected to the sequencing adapter to construct a sequencing library, which was sequenced on an Illumina platform (Beckers et al. 2016; Callahan et al. 2016). A number of 16S rDNA sequence alignments were performed using Clustal W, and a phylogenetic analysis was constructed by the nearest neighbour joining method (Bjerketorp et al. 2021).
Heavy metal (Cd, Pb and As) tolerance of dominant bacteria
The strain’s tolerance to heavy metals (Cd, Pb and As) was determined experimentally. First, according to the results of preliminary experiments with high concentrations of Cd, Pb and As (0, 500, 1000 and 1500 mg/L), the strain was cultured in LB liquid medium (pH = 6.0) containing different concentrations of Cd2+ (0–1000 mg/L), Pb2+ (0–1000 mg/L) and As3+ (0–900 mg/L), respectively, with 4% inoculum. The strain was cultured in a rotary shaker at 25 °C and 150 rpm for 120 h, and the culture medium was collected at 6, 16, 22, 43, 63, 92 and 120 h, respectively. The OD600, defined as the optical density (OD) at 600 nm, was measured with an ultraviolet–visible spectrophotometer (Shanghai Meipuda UV-1600, Upadhyay et al. 2017).
According to the results of the single-metal experiment, an orthogonal experiment with three factors and four levels was further designed to study the tolerance of this strain under combined metal pollution conditions as follows: (1) factor A: Cd2+ (0, 120, 360 and 720 mg/L), (2) factor B: Pb2+ (0, 200, 500 and 900 mg/L) and (3) factor C: As3+ (0, 150, 450 and 800 mg/L). The strain culture and OD600 measurement procedures were the same as mentioned above.
Statistical analysis
The experimental data are expressed as the mean ± standard deviation (SD) of three repetitions for the same treatment. Principal component analysis (PCA) was used to determine the difference in bacterial composition among the three sampling sites based on the abundance of operational taxonomic units (OTUs). The program packages in R version 3.1.3 were used for the statistical analysis of all bacterial sequences (Shen et al. 2021). The α diversity index (Chao1, Ace, Shannon and Simpson index) and β diversity index were calculated, and quantitative insights into microbial ecology (QIIME, v1.80) were used for unweighted pair-group method with arithmetic mean (UPGMA) cluster analysis (Zhang et al. 2017). Variance partitioning analysis (VPA) and the Spearman correlation analysis were used to analyse the degree of influence of the environmental factors. The figures were drawn with Origin 2019. Statistical Product and Service Solutions (SPSS 20) were used for data statistics. The statistical significance level was accepted as P < 0.05 in the single-factor analysis of variance (ANOVA), followed by Tukey’s test for multicomparison of the averages.
Results
Soil physicochemical properties and heavy metal pollution index
The physicochemical properties and heavy metal contamination characteristics of the soil samples are listed in Fig. 2 and Table 2. The letters A, B and C indicate three soil sampling sites with different heavy metal concentrations. The soil pH values of sites A, B and C were 5.49 ± 0.17, 7.76 ± 0.12 and 7.72 ± 0.15, respectively. At sites A, B and C, Cd, Pb, As, Cu and Zn all had high concentrations, but the concentrations of Cr and Ni in the soil were low. The concentrations of organic matter (OM) and total potassium (TK) were high, but the concentrations of total nitrogen (TN) and total phosphorus (TP) were low. A high concentration of free iron oxide (Fed) was shown at the three sampling sites, varying from 34,256 to 36,749 mg/kg (Table 2). The percentage of available Cd (A-Cd, A: available) among the total Cd (T-Cd, T: total) of the soil was the highest at the three sampling sites, and the percentage of A-Pb in T-Pb was highest at sites C and A. It should be noted that the percentage of A-Cu in T-Cu was the highest at site C, followed by sites B and A (Fig. 2a). The available forms of other heavy metals accounted for only a small proportion of the total concentrations. Because the Nemerow index at sites A (3.86), B (60.31) and C (78.10) were all over 3, these sites were all heavily polluted, but the pollution degree varied (Fig. 2b).
Cultivation of bacteria in soil and heavy metal (Cd, Pb and As) tolerance of Bacillus pacificus MZ520364
LB solid medium was used to cultivate the bacteria in the soil samples. The total number of colonies in the soil samples of each site was measured by the plate spreading method and the mixed inverted plate method as follows: site C (5.08 × 106 CFU/g) > site B (4.80 × 105 CFU/g) > site A (2.06 × 105 CFU/g). After the isolation and purification of each single colony, a heavy metal (Cd, Pb and As)-tolerant bacterium, G-3, was screened from the soil samples. The results from 16S rRNA sequencing analysis showed that the 16S rRNA sequence of strain G-3 was 99.93% similar to that of Bacillus pacificus. The 16S rRNA sequence was compared with those of similar strains, and a phylogenetic tree was constructed (Fig. 3). Combined with morphological characteristics and molecular sequencing results, the strain G-3 was identified as Bacillus pacificus with the number MZ520364 in the National Center for Biotechnology Information (NCBI).
To determine the maximum tolerance of B. pacificus MZ520364 to heavy metals (Cd, Pb and As), the strain was cultured under different concentrations of Cd, Pb and As, and the change in OD600 within 120 h was measured (Fig. 4). B. pacificus MZ520364 could grow in medium containing 600 mg/L Cd2+, but it could not grow when the Cd2+ concentration exceeded 600 mg/L (Fig. 4a). The maximum concentrations of Pb2+ and As3+ that the strain could tolerate were 1000 mg/L and 700 mg/L, respectively (Fig. 4b, c). In summary, strain G-3 could tolerate 600 mg/L Cd2+, 1000 mg/L Pb2+ or 700 mg/L As3+ in single-metal treatments.
Orthogonal experiments of the Cd, Pb and As tolerance of B. pacificus MZ520364
Based on the results of the single-factor experiment, three factors and four levels were chosen for further orthogonal experiments. From the analysis of variance, Cd (P ≤ 0.001) and As (P ≤ 0.001) had an extremely significant effect on the growth of the strain, and Pb (0.01 < P < 0.05) also had a significant effect on the strain’s growth. According to the range analysis in Table S1, the importance of the three factors followed the order of Cd (1.38) > As (0.57) > Pb (0.33), indicating that the concentration of Cd had the greatest impact on the growth of B. pacificus MZ520364, followed by those of As and Pb. Because the three factors had a significant effect on the strain’s growth, multiple comparisons among the averages were made. According to Table S1, the changing trends among the different levels of each factor were K1A > K2A > K3A > K4A, K1B > K2B > K3B > K4B, and K1C > K2C > K3C > K4C. The results showed that when multiple heavy metals (Cd, Pb and As) coexisted, the best conditions for the growth of B. pacificus MZ520364 were A2B2C2, meaning that the concentrations of Cd2+, Pb2+ and As3+ were 120 mg/L, 200 mg/L and 150 mg/L, respectively. This result indicated that the toxicity of combined heavy metals was greater than that of a single heavy metal.
OTU statistics and abundance analysis
The total number of tags for all samples was 993,000, resulting in a total of 9490 OTUs (Table 3). The minimum number of OTUs was 601, and the maximum number of OTUs was 1520, with an average of 1060. The OTU Venn diagram is presented in Fig. 5a, in which the OTU number at the three sampling sites followed the order of site C (1480) > site B (1060) > site A (922). The number of OTUs shared by the three sampling sites was 529, accounting for 57.4%, 50.1% and 35.6% of the total OTUs at sites A, B and C, respectively. The results showed that there were many soil bacterial taxa shared by sites A, B and C, and the community composition of the three sampling sites was similar to a certain degree. In addition, sites B and C had more bacterial taxa than site A, indicating that more soil bacteria colonized sites B and C.
The OTU rank curves of the soil samples are presented in Fig. 5b. Because of the difference in the width of each species in the horizontal direction, there were certain differences in each species. In general, the OTU rank curves of the soil samples at the same site were more concentrated, but the differences among the sites were large, and the abundance of species was also different. According to the relative abundance of each OTU at each site, a principal component analysis (PCA) was performed, and the results are presented in Fig. 5c. The horizontal axis in Fig. 5c represents the first principal component, which contributed 71.7% to the sample difference, and the vertical axis represents the second principal component, which contributed 17.8% to the sample difference. The distances between sites A, B and C were relatively long, indicating that there were great differences in the bacterial compositions of the three sampling sites.
Alpha diversity and bacterial community analysis
Based on the results of OTUs, the alpha diversity index of the soil samples was calculated, and the results are presented in Table 3. The Chao1 index, Ace index and Shannon index of the three soil sites were in the order of site C > site B > site A, indicating the difference in bacterial abundance and diversity among the three soil sites. The Simpson index values of the three soil sites were 0.97 (A), 0.98 (B) and 0.99 (C), indicating that there was no significant difference in species abundance among these three sites. The difference in the alpha diversity index among the three sites showed that the Chao1 index and Ace index at the three sites were significantly different, while the Shannon and Simpson index values of sites B and C were not. This proved that the soil bacteria at the three soil sampling sites had a significant difference in diversity, abundance and uniformity. The Chao1 index and Shannon index rarefaction curves reflecting the sequencing amount of the sample covered all populations and indirectly reflected the richness and diversity of the sample species (Fig. S1). The Chao1 index (Fig. S1a) and the Shannon diversity index (Fig. S1b) at the three sampling sites followed the same order of site C > site B > site A. The rarefaction curves of all samples tended to be flat, so the amount of sequencing data was sufficient, and there were certain differences in the abundance and diversity of species at each soil sampling site (Fig. S1).
At the species level, the distribution of bacteria at each soil sampling site is shown in Fig. 6a. The bacterial populations at each site were very different. Approximately 90% of the bacteria at the three sites could not be identified at the species level, and the dominant bacterial populations at each site were not the same. Among the taxa that were identified at the species level, the dominant bacterial populations at site A were Paenibacillus amylolyticus (4.70%) and Acinetobacter johnsonii (2.05%), the dominant bacterial populations at site B were Acinetobacter johnsonii (0.36%) and Bacteriovorax sp. EPA (0.30%), and the dominant bacterial populations at site C were Acinetobacter johnsonii (0.46%) and Agromyces humatus (0.14%). The similarity of the representative sequences of the OTUs was up to 97%. Heatmap analysis of each site at the species level is shown in Fig. 6b. The dominant bacterial populations at site A, Paenibacillus amylolyticus and Acinetobacter johnsonii, were relatively closed in the cluster tree, indicating that they were closely related to each other.
Samples with similar beta diversity were clustered together, reflecting the similarity among soil sampling sites. The Bray–Curtis distance is a common indicator that reflects the differences between two communities, and the results are shown in Fig. S2a. The three soil sites were clustered together, indicating that the species composition and abundance within the sites were similar. The species composition and abundance between sites A and B were similar, as well as those between sites B and C. However, there were differences in species composition and abundance between sites A and C. The results from the ANOSIM are presented in Fig. S2b. The Bray–Curtis distance algorithm was used to analyse the similarity between different sites at the species level, which also confirmed the results of the Bray–Curtis distance algorithm in Fig. S2a used to analyse the sample distance heatmap at the species level.
Effects of soil physicochemical parameters on the bacterial community
To evaluate the effects of physicochemical variables (25 items) on the abundance and diversity of the bacterial community in the soil, the VPA and Spearman correlation heatmap were used to reflect the impact of environmental factors. The VPA regarding the contribution of environmental factors showed that CEC had the greatest impact on the composition of the soil bacterial community (40.58%), followed by the pH (40.16%), T-Zn (37.23%), T-As (37.16%) and A-As concentrations (31.62%, Fig. 7a). The Spearman correlation heatmap analysis was used to study the relationship between soil physicochemical parameters and the relative abundance of the main bacterial populations, and the results are presented in Fig. 7b. The abundance of Paenibacillus amylolyticus was negatively correlated with T-As (0.001 < P ≤ 0.01), A-Ni (0.001 < P ≤ 0.01), A-As (0.01 < P < 0.05), T-Ni (0.01 < P < 0.05), T-Cu (0.01 < P < 0.05), A-Cu (0.01 < P < 0.05), A-Zn (0.01 < P < 0.05), TK (0.01 < P < 0.05), A-N (0.01 < P < 0.05) and A-P (0.01 < P < 0.05) concentrations in soil and positively correlated with T-Cr (0.01 < P < 0.05). The abundance of Bacteriovorax sp. EPA was positively correlated with T-As (0.001 < P ≤ 0.01), A-As (0.001 < P ≤ 0.01), T-Cu (P ≤ 0.001), A-Cu (P ≤ 0.001), T-Ni (P ≤ 0.001), A-Ni (P ≤ 0.001), A-Zn (0.001 < P ≤ 0.01), TK (0.001 < P ≤ 0.01), A-N (P ≤ 0.001) and A-P (P ≤ 0.001) concentrations and pH (0.001 < P ≤ 0.01) in soil and negatively correlated with T-Cr (0.001 < P ≤ 0.01). The abundance of Agromyces humatus was positively correlated with T-Cd (0.01 < P < 0.05), A-Cd (0.001 < P ≤ 0.01), T-Pb (0.01 < P < 0.05), A-Pb (0.01 < P < 0.05), A-Cr (0.001 < P ≤ 0.01), T-Zn (0.001 < P ≤ 0.01), TP (0.001 < P ≤ 0.01), A–K (P ≤ 0.001) and OM concentrations (0.001 < P ≤ 0.01) in soil and negatively correlated with SiO2 (0.01 < P < 0.05). However, the abundance of Acinetobacter johnsonii was not significantly correlated with soil physicochemical parameters.
Influence of soil physicochemical parameters on bacterial communities. a Variance partitioning analysis (VPA) for contribution analysis of environmental factors; b Heatmap of the Spearman correlation on species combined with UPGMA cluster analysis between the relative abundance of bacteria species and sample physicochemical parameters. Negative correlation and positive correlation are represented by blue color and red color. *0.01 < P < 0.05; **0.001 < P ≤ 0.01; ***P ≤ 0.001
Discussion
From the bacterial culture experiment, we showed that the total number of colonies in the soil samples at each site was in the order of site C (5.08 × 106 CFU/g) > site B (4.80 × 105 CFU/g) > site A (2.06 × 105 CFU/g), which is consistent with the results of alpha diversity analysis (Table 3). The present study showed that bacteria could withstand and resist the environmental pressure caused by long-term heavy metal pollution (Shar et al. 2021). In this study, most of the soil bacteria at sites B and C, such as Bacillus sp. and Acinetobacter sp., were tolerant to heavy metal stress. Therefore, a heavy metal (Cd, Pb and As)-tolerant bacterium was screened from the soil and identified as B. pacificus by 16S rRNA sequencing. Some studies have shown that many species in the genus Bacillus have a strong tolerance to heavy metals, such as Bacillus (RS-2), Bacillus (RS-3), Bacillus (CIK-517) and Bacillus (CIK-519) (Ahmad et al. 2016; Kumar et al. 2016). The remarkable polymetallic tolerance and adaptability of Bacillus sp. have been well documented. Other studies have also shown that in a single heavy metal–amended solid medium, Bacillus (KP2265891) could tolerate 100 mg/L Cd2+ and 1600 mg/L Pb2+ (Jiang et al. 2017; Oladipo et al. 2018), and some Bacillus (KC200017) could even tolerate 2100 mg/L Pb2+ (Zhu et al. 2013). For As, Bacillus pacificus could tolerate 700 mg/L As3+ (Fig. 4; Table S1), which is similar to the results of Gong et al. (2008), who reported that B. pacificus could still grow at 1000 mg/L As3+. Based on the results of single heavy metal tolerance, we further verified that in the case of Cd2+ (120 mg/L), Pb2+ (200 mg/L) and As3+ (150 mg/L) combined pollution, B. pacificus MZ520364 still grew very well. The results revealed that the strain showed a very high tolerance to Cd2+, Pb2+ and As3+. The orthogonal experiment showed that the tolerance of B. pacificus MZ520364 to heavy metals followed the order of Cd (1.38) > As (0.57) > Pb (0.33), and the combined heavy metals were more toxic than the single heavy metals. It has been reported that Bacillus sp. can dissolve minerals, generate chelates and biocontrol compounds and enhance the phytoremediation of metal-contaminated environments; Bacillus sp. is not easily poisoned by heavy metals and has a high tolerance to heavy metal pollution (Ndeddy and Babalola 2016; Sarathambal et al. 2017).
Heavy metals are widely present in raw materials and products from mining, smelting and manufacturing industries, such as batteries (Cd) and phosphogypsum (Cd and Zn), which contribute to heavy metal pollution in the environment (Jiang et al. 2019). Long-term mine tailings storage and stockpiling have caused high Cd, Pb and As contamination in the study area, and the accumulation of tailings has resulted in poor soil homogeneity and varying heavy metal concentrations in the soil. Because total Cd and Pb concentrations cannot fully reflect the toxicity of the two elements, it is important to evaluate the ecological effects of A-Cd, A-Pb, A-As and other soil physicochemical properties on soil bacterial abundance and diversity (Shahid et al. 2016). The pH value is one of the most important factors affecting A-Cd and A-Pb in the soils studied, and it was negatively correlated with the A-Cd concentration by enhancing competition between Cd2+ and H+ for negative ion surfaces (Nejad et al. 2021). Because site A was located on a hillside away from the tailings pile, it was less contaminated, and the soil physicochemical properties tended to be similar to those of the other two sites. However, sites B and C were heavily polluted in comparison with site A and had a long-term accumulation of tailings. The changes in available heavy metal percentages (Fig. 2a) were also governed by human and microbial activities (Fierer 2017). Among the environmental factors, the highest contribution to soil bacterial abundance and diversity was 40.58% and was mainly from CEC as well as from the level of heavy metal contamination (Fig. 7a). CEC reflects the capacity of soil to retain nutrients and the buffering capacity, which greatly affect the physiological and biochemical activities of bacteria (Fierer 2017; Rao et al. 2016).
Soil bacterial communities play an important role in soil ecosystems and significantly affect soil fertility and the toxicity of heavy metals (Liu et al. 2018). However, there is still no concrete evidence on how soil bacteria can be used to improve soil fertility and how bacterial communities will respond to soil heavy metal pollution (Fierer 2017). 16S rRNA technology, a common tool for in-depth studies of soil bacterial community composition, can be used to analyse the relationship between bacterial communities and environmental factors in addition to bacterial diversity (Guo et al. 2017; Ming et al. 2021). Both competition and environmental factors influence the abundance, diversity and ecological function of bacterial communities (Bharam et al. 2018). The diversity and abundance of bacteria in the soil environment can be affected by heavy metals; however, resistant bacteria are not only able to adapt to heavy metal pollution but also increase their species abundance (Zhao et al. 2019). The study showed that the degree of heavy metal contamination was negatively correlated with microbial abundance in agricultural soils, and with more severe contamination, the diversity and abundance of microbial communities in the soil started to decrease (Huang et al. 2017). However, the results obtained from 16S rRNA sequencing showed that the diversity of bacteria was the lowest at site A (the lowest metal-contaminated site). Other sequencing results, such as those reflected in the Venn diagram (Fig. 5a) and OTU rank curve (Fig. 5b), also showed that the diversity and abundance of bacteria at sites B and C were higher than those at site A. According to the Spearman correlation heatmap (Fig. 7b), the results showed that the abundance of the dominant species Paenibacillus amylolyticus (4.70%) at site A was negatively correlated with various environmental factors (T-As, A-Ni, A-As, T-Ni, T-Cu, A-Cu, A-Zn, TK, A-N and A-P) in soil and only positively correlated with T-Cr (0.01 < P < 0.05). The abundance of the dominant species Bacteriovorax sp. EPA (0.30%) at site B was positively correlated with various environmental factors (T-As, A-As, T-Cu, A-Cu, T-Ni, A-Ni, A-Zn, TK, A-N, A-P and pH) in the soil and only negatively correlated with T-Cr (0.001 < P ≤ 0.01). The abundance of the dominant strain Agromyces humatus (0.14%) at site C was positively correlated with various environmental factors (T-Cd, A-Cd, T-Pb, A-Pb, A-Cr, T-Zn, TP, A–K and OM) in the soil and only negatively correlated with SiO2 (0.01 < P < 0.05). Therefore, the abundance of Bacteriovorax sp. EPA and Agromyces humatus increased when the level of contamination was higher. A range of soil physicochemical parameters, such as pH, TP and heavy metal (Cd, Pb and As) concentrations, as well as human activities, can affect bacterial abundance. These may be the reasons why a higher microbial abundance was present in the contaminated soils compared to those of site A in this study. The analysis of α-diversity (Shannon index and Simpson index) among the present three different soil sites (A, B and C) showed that there was no significant difference between the bacterial community diversity and the level of heavy metal pollution at the species level. It has been reported that certain heavy metals (e.g. Cd and Pb) can accelerate the metabolic processes of microorganisms and that their toxicity is dose dependent (Oladipo et al. 2018). In addition, the dose-dependent toxicity of heavy metals and differences in bacterial susceptibility may lead to different microbial communities (Mahbub et al. 2017). Many microorganisms can tolerate different levels of heavy metal toxicity through intracellular complexation, cell wall adsorption and extracellular precipitation (Kumar et al. 2020). Moreover, the combined effect of biostimulation, soil physicochemical parameters and heavy metal stress will also construct new microbial communities (Rojjanateeranaj et al. 2017). In summary, the soil microbial community composition was affected by the degree of heavy metal pollution at sites B and C in the tailings pile area, where heavy metal contamination was severe. Additionally, the dominant populations were Bacteriovorax sp. EPA and Agromyces humatus, which were positively correlated with the degree of heavy metal contamination.
Conclusion
According to soil physical and chemical properties, bacterial culture experiments and 16S rRNA analysis, our results showed that a large difference in the relative abundance of soil microorganisms was found at different levels of soil contamination, and that the differences in abundance and composition of the bacterial communities mainly resulted from soil CEC, pH value, T-Zn, T-As and A-As concentrations. Furthermore, two dominant populations identified by 16S rRNA technology, Bacteriovorax sp. EPA and Agromyces humatus, were positively correlated with the degree of heavy metal contamination. Finally, the bacterium Bacillus pacificus MZ520364 was identified as tolerant to Cd, Pb and As. The effect of Cd, Pb and As on the growth of the strain followed the order of Cd > As > Pb, and combined heavy metals showed more toxicity than did single heavy metals. These findings help provide a new perspective for the ecological assessment and management of metal-contaminated mining areas. Further research is needed to apply the screened Cd–Pb–Astolerant strain, and related mechanisms, for the bioremediation of heavy metal contamination in mining site soils.
Data availability
The datasets used and analysed during the current study are available from the corresponding author on reasonable request. All data generated or analysed during this study are included in this published article.
References
Ahmad I, Akhtar MJ, Asghar HN, Ghafoor U, Shahid M (2016) Differential effects of plant growth-promoting rhizobacteria on maize growth and cadmium uptake. J Plant Growth Regul 35(2):303–315. https://doi.org/10.1007/s00344-015-9534-5
Beckers B, Beeck MOD, Thijs S, Truyens S, Weyens N, Boerjan W, Vangronsveld J (2016) Performance of 16s rDNA primer pairs in the study of rhizosphere and endosphere bacterial microbiomes in metabarcoding studies. Front Microbiol 7:650. https://doi.org/10.3389/fmicb.2016.00650
Bharam M, Hildebrand F, Forslund SK, Anderson JL, Soudzilovskaia NA, Bodegom PM, Bengtsson-Palme J, Anslan S, Coelho LP, Harend H, Huerta-Cepas J, Medema MH, Maltz MR, Mundra S, Olsson PA, Pent M, Polme S, Sunagawa S, Ryberg M, Tedersoo L, Bork P (2018) Structure and function of the global topsoil microbiome. Nature 560(7717):233–237. https://doi.org/10.1038/s41586-018-0386-6
Bjerketorp J, Levenfors JJ, Nord C, Guss B, Oberg B, Broberg A (2021) Selective isolation of multidrug-resistant Pedobacter spp. producers of novel antibacterial peptides. Fron Microbiol 12:642829. https://doi.org/10.3389/fmicb.2021.642829
Boechat CL, Giovanella P, Amorim MB, Saccol EL, Oliveira Camargo FA (2017) Metal-resistant rhizobacteria isolates improve Mucuna deeringiana phytoextraction capacity in multi-metal contaminated soils from a gold mining area. Environ Sci Pollut Res 24:3063–3073. https://doi.org/10.1007/s11356-016-8103-1
Callahan BJ, Mcmurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP (2016) DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods 13(7):581–583. https://doi.org/10.1038/nmeth.3869
Che R, Qin J, Tahmasbian I, Wang F, Zhou S, Xu Z, Cui X (2018) Litter amendment rather than phosphorus could dramatically change inorganic nitrogen pools in a degraded grassland soil by affecting nitrogen-cycling microbes. Soil Biol Biochem 120:145–152. https://doi.org/10.1016/j.soilbio.2018.02.006
Chen QL, Ding J, Zhu D, Hu HW, Manuel DB, Ma YB, He JZ, Zhu YG (2020) Rare microbial taxa as the major drivers of ecosystem multifunctionality in long-term fertilized soils. Soil Biol Biochem 141:107686. https://doi.org/10.1016/j.soilbio.2019.107686
Fan M, Xiao X, Guo Y, Zhang J, Wang E, Chen W, Lin Y, Wei G (2018) Enhanced phytoremediation of Robiniapseudoacacia in heavy metal-contaminated soils with rhizobia and the associated bacterial community structure and function. Chemosphere 197:729–740. https://doi.org/10.1016/j.chemosphere.2018.01.102
Fierer N (2017) Embracing the unknown: disentangling the complexities of the soil microbiome. Nat Rev Microbiol 15(10):579–590. https://doi.org/10.1038/nrmicro.2017.87
Gong HZ, Lu CW, Li B, Jiang H, Gao S, Zhang XY, Sun GF (2008) Tolerance of selected microorganisms to arsenic. Chin J Public Health 24(2):210–211. https://doi.org/10.3321/j.issn:1001-0580.2008.02.034
Guo HH, Nasir M, Lv JL, Dai YC, Gao JK (2017) Understanding the variation of microbial community in heavy metals contaminated soil using high throughput sequencing. Ecotox Environ Safe 144:300–306. https://doi.org/10.1016/j.ecoenv.2017.06.048
Huang DL, Liu LS, Zeng GM, Xu P, Huang C, Deng LJ, Wang RZ, Wan J (2017) The effects of rice straw biochar on indigenous microbial community and enzymes activity in heavy metal contaminated sediment. Chemosphere 174:545–553. https://doi.org/10.1016/j.chemosphere.2017.01.130
Huang R, Cui XY, Luo XZ, Mao P, Zhuang P, Li YX, Li YW, Li ZA (2021) Effects of plant growth regulator and chelating agent on the phytoextraction of heavy metals by Pfaffiaglomerata and on the soil microbial community. Environ Pollut 283:117159. https://doi.org/10.1016/j.envpol.2021.117159
Huang RQ, Wang G, Tang RY, Liao SQ, Chen YH (2005) Extraction method for available arsenic in acid soils. J Agro-Environ Sci 24(3):610–615. https://doi.org/10.3321/j.issn:1672-2043.2005.03.041
Jiang B, Adebayo A, Jia JL, Xing Y, Deng SQ, Guo LM, Liang YT, Zhang DY (2019) Impacts of heavy metals and soil properties at a Nigerian e-waste site on soil microbial community. J Hazard Mater 362:187–195. https://doi.org/10.1016/j.jhazmat.2018.08.060
Jiang J, Pan CH, Xiao AP, Yang XA, Zhang GM (2017) Isolation identification and environmental adaptability of heavy-metal-resistant bacteria from ramie rhizosphere soil around mine refinery. 3 Biotech 7:5. https://doi.org/10.1007/s13205-017-0603-2
Kumar A, Subrahmanyam G, Mondal R, Cabral-Pinto MMS, Shabnam AA, Jigyasu DK, Malyan SK, Fagodiya RK, Khan SA, Yu ZG (2020) Bio-remediation approaches for alleviation of cadmium contamination in natural resources. Chemosphere 268:128855. https://doi.org/10.1016/j.chemosphere.2020.128855
Kumar KV, Jaiprakash C, Chander MP, Reesu R, Maile A, Lall C (2016) Heavy metal tolerant nonendosymbiont plant growth promoting Rhizobacteria associated with the roots of evergreen shrubs Casuarina equisetifolia. Recent Res Sci Technol 8:30–35. https://doi.org/10.19071/rrst.2016.v8.3086
Li CZ, Wang XF, Huang H, Wang L, Wei F, Zhang CL, Rong Q (2021) Effect of multiple heavy metals pollution to bacterial diversity and community structure in farmland soils. Hum Ecol Risk Assess 27(3):724–741. https://doi.org/10.1080/10807039.2020.1752143
Li X, Dong SW, Yao Y, Shi WJ, Wu MH, Xu H (2016) Inoculation of bacteria for the bioremediation of heavy metals contaminated soil by Agrocybeaegerita. RSC Adv 6(70):65816–65824. https://doi.org/10.1039/C6RA11767H
Li XQ, Meng DL, Li J, Yin WJ, Wu HQ, Liu XD, Cheng C, Xiao YH, Liu ZH, Yan ML (2017) Response of soil microbial communities and microbial interactions to long-term heavy metal contamination. Environ Pollut 231(1):908–917. https://doi.org/10.1016/j.envpol.2017.08.057
Li YY, Wang HB, Wang HJ, Yin F, Yang XY, Hu YJ (2014) Heavy metal pollution in vegetables grown in the vicinity of a multi-metal mining area in Gejiu China: total concentrations speciation analysis and health risk. Environ Sci Pollut R 21:12569–12582. https://doi.org/10.1007/s11356-014-3188-x
Liang ZT, Zhang WJ, Yang YS, Ma JC, Li SX, Wen Z (2021) Soil characteristics and microbial community response in rare earth mining areas in southern Jiangxi Province China. Environ Sci Pollut Res 28:56418–56431. https://doi.org/10.1007/s11356-021-14337-z
Liu HK, Wang C, Xie YL, Luo Y, Sheng MP, Xu F, Xu H (2020a) Ecological responses of soil microbial abundance and diversity to cadmium and soil properties in farmland around an enterprise-intensive region. J Hazard Mater 392:122478. https://doi.org/10.1016/j.jhazmat.2020.122478
Liu J, He XX, Liu XR, Chen WC, Zhou QX, Shu WS, Huang LN (2015) Ecological effects of combined pollution associated with e-waste recycling on the composition and diversity of soil microbial communities. Environ Sci Technol 49(11):6438–6447. https://doi.org/10.1021/es5049804
Liu P, Yang Y, Li M (2020b) Responses of soil and earthworm gut bacterial communities to heavy metal contamination. Environ Pollut 265:114921. https://doi.org/10.1016/j.envpol.2020.114921
Liu W, Zuo QQ, Zhao CC, Wang ST, Shi YP, Liang SX, Zhao C, Shen SG (2018) Effects of Bacillus subtilis and nanohydroxyapatite on the metal accumulation and microbial diversity of rapeseed (Brassica campestris L.) for the remediation of cadmium-contaminated soil. Environ Sci Pollut Res 25:25217–25226. https://doi.org/10.1007/s11356-018-2616-8
Llado S, Lopez-Mondejar R, Baldrian P (2017) Forest soil bacteria: diversity involvement in ecosystem processes and response to global change. Microbiol Mol Biol Rev 81(2):e00063. https://doi.org/10.1128/MMBR.00063-16
Luo L, Meng H, Gu JD (2017) Microbial extracellular enzymes in biogeochemical cycling of ecosystems. J Environ Manage 197:539–549. https://doi.org/10.1016/j.jenvman.2017.04.023
Ma Y, Oliveira RS, Nai FJ, Rajkumar M, Luo YM, Rocha I, Freitas H (2015) The hyperaccumulator Sedum plumbizincicola harbors metal-resistant endophytic bacteria that improve its phytoextraction capacity in multi-metal contaminated soil. J Environ Manage 156:62–69. https://doi.org/10.1016/j.jenvman.2015.03.024
Ma YJ, Wang YT, Chen Q, Li YS, Guo DC, Nie XH, Peng XW (2020) Assessment of heavy metal pollution and the effect on bacterial community in acidic and neutral soils. Ecol Indic 117:106626. https://doi.org/10.1016/j.ecolind.2020.106626
Mahbub KR, Krishnan K, Naidu R, Andrews S, Megharaj M (2017) Mercury toxicity to terrestrial biota. Ecol Indic 74:451–462. https://doi.org/10.1016/j.ecolind.2016.12.004
Mesa-Marin J, Naranjo EM, Pajuelo E, Caviedes MA, Rodriguez-Llorente ID (2016) Heavy metal pollution structures soil bacterial community dynamics in SW Spain polluted salt marshes. Water Air Soil Poll 227(12):466. https://doi.org/10.1007/s11270-016-3176-5
Ming HX, Fan JF, Liu JW, Su J, Wan ZY, Wang YT, Li DW, Li MF, Shi TT, Jin Y, Huang HL, Song JX (2021) Full-length 16S rRNA gene sequencing reveals spatiotemporal dynamics of bacterial community in a heavily polluted estuary China. Environ Pollut 275:116576. https://doi.org/10.1016/j.envpol.2021.116567
Ndeddy ARJ, Babalola OO (2016) Effect of bacterial inoculation of strains of Pseudomonas aeruginosa Alcaligenesfaecalis and Bacillus subtilis on germination growth and heavy metal (Cd, Cr, and Ni) uptake of Brassica juncea. Int J Phytoremediat 18(2):200–209. https://doi.org/10.1080/15226514.2015.1073671
Nejad ZD, Rezania S, Jung MC, Al-Ghamdi AA, Mustafa AMA, Elshikh MS (2021) Effects of fine fractions of soil organic semi-organic and inorganic amendments on the mitigation of heavy metal(loid)s leaching and bioavailability in a post-mining area. Chemosphere 271:145354. https://doi.org/10.1016/j.chemosphere.2021.129538
Oladipo OG, Ezeokoli OT, Maboeta MS, Bezuidenhout JJ, Tiedt LR, Jordaan A, Bezuidenhout CC (2018) Tolerance and growth kinetics of bacteria isolated from gold and gemstone mining sites in response to heavy metal concentrations. J Environ Manage 212:357–366. https://doi.org/10.1016/j.jenvman.2018.01.038
Rahbari A, Fatemi H, Esmaiel Pour B, Rizwan M, Soltani AA (2021) Lead (Pb)-resistant bacteria inhibit Pb accumulation in dill (Anethumgraveolens L.) by improving biochemical physiological and antioxidant enzyme response of plants. Environ Sci Pollut Res 28:5704–5713. https://doi.org/10.1007/s11356-020-10851-8
Rao S, Lu Y, Huang F, Cai YX, Cai KZ (2016) A review of researches on effects of biochars on soil microorganisms. J Ecol Rural Environ 32(1):53–59. https://doi.org/10.11934/j.issn.1673-4831.2016.01.010 (in Chinese)
Rojjanateeranaj P, Sangthong C, Prapagdee B (2017) Enhanced cadmium phytoremediation of Glycine max L. through bioaugmentation of cadmium-resistant bacteria assisted by biostimulation. Chemosphere 185:764–771. https://doi.org/10.1016/j.chemosphere.2017.07.074
Rousk J, Rousk K (2018) Responses of microbial tolerance to heavy metals along a century-old metal ore pollution gradient in a subarctic birch forest. Environ Pollut 240:297–305. https://doi.org/10.1016/j.envpol.2018.04.087
Sarathambal C, Khankhane PJ, Gharde Y, Kumar B, Varun M, Arun S (2017) The effect of plant growth promoting rhizobacteria on the growth physiology and Cd uptake of Arundodonax L. Int J Phytoremediat 19(4):360–370. https://doi.org/10.1080/15226514.2016.1225289
Satapute P, Paidi MK, Kurjogi M, Jogaiah S (2019) Physiological adaptation and spectral annotation of arsenic and cadmium heavy metal-resistant and susceptible strain Pseudomonas taiwanensis. Environ Pollut 251:555–563. https://doi.org/10.1016/j.envpol.2019.05.054
Shahid M, Dumat C, Khalid S, Niazi NK, Antunes PMC (2016) Cadmium bioavailability uptake toxicity and detoxification in soil-plant system. Rev Environ Contam T 241:74–137. https://doi.org/10.1007/398_2016_8
Shar S, Reith F, Ball AS, Shahsavari E (2021) Long-term impact of gold and platinum on microbial diversity in australian soils. Microb Ecol 81(4):977–989. https://doi.org/10.1007/s00248-020-01663-x
Sharma RK, Archana G (2016) Cadmium minimization in food crops by cadmium resistant plant growth promoting rhizobacteria. Appl Soil Ecol 107:66–78. https://doi.org/10.1016/j.apsoil.2016.05.009
Shen CC, He JZ, Ge Y (2021) Seasonal dynamics of soil microbial diversity and functions along elevations across the treeline. Sci Total Environ 794:148644. https://doi.org/10.1016/j.scitotenv.2021.148644
Shen ZJ, Xu DC, Li LL, Wang JJ, Shi XM (2019) Ecological and health risks of heavy metal on farmland soils of mining areas around Tongling City Anhui China. Environ Sci Pollut Res 26:15698–15709. https://doi.org/10.1007/s11356-019-04463-0
Shirokikh IG, Solovyeva ES, Ashihmina TJ (2015) Functional and structural features of streptomycete complexes isolated from soils with varying degrees of heavy-metal contamination. Contemp Probl Ecol 8(1):125–132. https://doi.org/10.1134/S1995425515010138
Upadhyay N, Vishwakarma K, Singh J, Mishra M, Kumar V, Rani R, Mishra RK, Chauhan DK, Tripathi DK, Sharma S (2017) Tolerance and reduction of chromium (VI) by Bacillus sp. MNU16 isolated from contaminated coal mining soil. Front Plant Sci 8:778. https://doi.org/10.3389/fpls.2017.00778
Wang L, Wang Z, Gao G, Huang J (2016) Identification and application of a probiotic strain functioning in both ammonia nitrogen removal and Vibrio parahaemolyticus antagonism. Prog Fishery Sci 37(3):78–84. https://doi.org/10.11758/yykxjz.20150519001
Xu DM, Yan B, Chen T, Chang L, Lin HZ, Xiao XM (2017) Contaminant characteristics and environmental risk assessment of heavy metals in the paddy soils from lead (Pb)-zinc (Zn) mining areas in Guangdong Province South China. Environ Sci Pollut Res 24:24387–24399. https://doi.org/10.1007/s11356-017-0052-9
Zhang JY, Ding X, Guan R, Zhu CM, Xu C, Zhu BC, Zhang H, Xiong ZP, Xue YG, Tu J, Lu ZH (2017) Evaluation of different 16S rRNA gene V regions for exploring bacterial diversity in a eutrophic freshwater lake. Sci Total Environ 618:1254–1267. https://doi.org/10.1016/j.scitotenv.2017.09.228
Zhao XQ, Huang J, Lu J, Sun Y (2019) Study on the influence of soil microbial community on the long-term heavy metal pollution of different land use types and depth layers in mine. Ecotox Environ Safe 170:218–226. https://doi.org/10.1016/j.ecoenv.2018.11.136
Zhao XQ, Sun Y, Huang J, Wang H, Tang D (2020) Effects of soil heavy metal pollution on microbial activities and community diversity in different land use types in mining areas. Environ Sci Pollut Res 27:20215–20226. https://doi.org/10.1007/s11356-020-08538-1
Zheng WH, Wang C, Zhang HY, Feng T, Wang FL, Yang L, Xiao Y, Hua ZY, Wang X (2016) Content monitoring and assessment of heavy metals in edible mushrooms from Urumqi municipalitu. Southwest China J Agric Sci 29(2):396–401. https://doi.org/10.16213/j.cnki.scjas.2016.02.034
Zhu J, Zhang ZD, Song SQ, Wang W, Gu MY, Xie YQ, Tang QY, Zhang LJ (2013) Diversity investigation of heavy metal resistant bacteria isolated from radiation-polluted soil. Environ Sci Technol 36(12):29–32. https://doi.org/10.3969/j.issn.1003-6504.2013.12.007 (inChinese)
Funding
The work was financially supported by the National Natural Science Foundation of China (Grant No.: 31960264).
Author information
Authors and Affiliations
Contributions
T Wang conducted the experiments, data analysis and draft writing. HB Wang conceived and designed the experiments and revised the manuscript. HJ Wang helped with field sampling. KP Feng drew the map of sampling sites. HY Li provided good suggestions for bacterial analysis. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Robert Duran
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, T., Wang, H., Feng, K. et al. Soil bacteria around a derelict tailings pile with different metal pollution gradients: community composition, metal tolerance and influencing factors. Environ Sci Pollut Res 29, 60616–60630 (2022). https://doi.org/10.1007/s11356-022-20142-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-20142-z