Abstract
Phthalates (PAEs) are common endocrine disrupting chemicals (EDCs) that disrupt fetal development. The present study aimed to evaluate the effects of single and coexposure to phthalates in early pregnancy on fetal growth restriction (FGR) by a nested case–control study based on the Guangxi Zhuang Birth Cohort (GZBC). Maternal serum concentrations of seven phthalates in 97 neonates with FGR and 291 matched controls were detected through gas chromatography–mass spectrometry (GC–MS). The associations between phthalates and FGR were analyzed using multiple logistic regression, weight quantile sum (WQS) regression, and Bayesian kernel machine regression (BKMR) models. We found that exposures to butyl-benzyl phthalate (BBP, ORadj = 1.849, 95% CI: 1.080–3.177, Padj = 0.025, Ptrend = 0.046), di (2-ethyl-hexyl) phthalate (DEHP, ORadj = 3.893, 95% CI: 1.305–11.910, Padj = 0.015, Ptrend = 0.098) and dimethyl phthalate (DMP, ORadj = 1.722, 95% CI: 1.089–2.725, Padj = 0.020, Ptrend = 0.002) were significantly positively associated with the risk of FGR, while mono-butyl phthalate (MBP) showed a significant negative association with FGR (ORhigh = 0.192, 95% CI: 0.036–0.795, Padj = 0.033, Ptrend = 0.035) only among girls. The WQS model identified that BBP, di(2-ethyl)phthalate (DEP), DMP, DEHP, di-n-butyl phthalate (DBP), and MBP were highly weighted in the association with FGR. The BKMR model supported the positive association between joint exposure to phthalates and the risk of FGR and identified no significant interaction between the seven phthalates. Overall, maternal exposure to BBP, DEHP, and DMP may cause adverse effects on FGR, especially with combined effects.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phthalates are common endocrine disrupting chemicals (EDCs) that can pass through the placenta, which are harmful to fetal development and cause cognitive impairment (Yang et al. 2019). Exposure to phthalates often comes from plasticizers for food packaging, children’s toys, decoration materials, personal care products, and some medical equipments (Ashrap et al. 2020). Maternal exposure to di(2-ethyl-hexyl)phthalate (DEHP), a widely used plasticizer, inhibits cell proliferation and reduces placental size (Sun et al. 2022). Serum levels of phthalate metabolites are valuable for the assessment of internal exposure. It is most common to measure urinary phthalate exposure because of the higher concentrations of phthalate metabolites in the urine and reduced contamination from parental diester phthalate or the subsequent formation of metabolites by enzymes present in the blood (Prevention CfDCa 2009). However, studies have reported moderate or strong correlations between urine and serum concentrations of phthalate metabolites, and both biological matrices can be used as valid indicators of phthalate exposure in humans (Frederiksen et al. 2010; Hines et al. 2009; Hogberg et al. 2008; Kato et al. 2004). Recent studies detecting phthalate metabolites in maternal blood, cord blood, first trimester urine, and amniotic fluid have suggested that phthalate exposure might be transferred from mother to baby (Wang et al. 2019).
Fetal growth restriction (FGR) is also known as intrauterine growth retardation (IUGR). Previous studies have observed that prenatal and gestational phthalate exposure could perturb fetal growth and disrupt later development. Maternal inflammation connected to oxidative stress causes FGR mediated through phthalates, which is a plausible mechanism underlying the association between phthalate exposure and FGR (Kamai et al. 2019). The impacts of phthalates on fetal and placental development through interrupting thyroid hormone signaling, lipid metabolism and transport, and reactive oxygen species were demonstrated (Martinez-Razo et al. 2021; Tang et al. 2020). Prenatal exposure to phthalates may cause premature birth, FGR, and growth retardation. Wonjong Lee et al. discovered harmful effects of dibutyl phthalate (DBP) in mouse-derived pluripotent neural progenitor cells (NPCs) and hippocampal neurogenesis in C57BL/6 mice, indicating that DBP exposure may cause learning and memory dysfunctions (Lee et al. 2019). In addition, in utero di-n-hexyl phthalate (DHP) and di-cyclohexyl phthalate (DCHP) exposure resulted in IUGR in rats, which was manifested by reduced fetal weight, delayed ossification, and placental disruption (Ahbab et al. 2017).
Investigations with human studies have reported the associations of phthalates with fetal growth, birth outcomes, and child development (Tsai et al. 2017). However, these findings are inconsistent. Several reasons may have contributed to the apparent heterogeneity of previous findings. First, the effects of exposure to a single phthalate are different. The Michigan Mother-Infant Pairs (MMIP) study found that mono (3-carboxypropyl) phthalate was significantly associated with higher birth weight and Fenton z scores (Goodrich et al. 2019), while a cohort study from the Wuhan Women and Children Medical and Healthcare Centre revealed the associations of prenatal exposure to DEHP with decreased fetal growth and decreased birth size, and the relationships between gestational DEHP exposure and increased postnatal weight gain rates (Li et al. 2021). Second, little is known about the association between first-trimester maternal exposure to phthalates and neonatal outcomes, with conflicting findings. Levels of mono(2-ethylhexyl) phthalate (MEHP), mono(2-ethyl-5-carboxypentyl) phthalate and DEHP in urine collected at approximately 10, 18, and 26 weeks of gestation were associated with increased odds of preterm birth (Ferguson et al. 2014), whereas Shoaff et al. observed no association between average phthalate metabolite concentrations in urine collected at approximately 16 and 26 weeks of gestation and birth size or gestational duration in the adjusted model (Shoaff et al. 2016). Third, although the effects of phthalates on the development of offspring have been widely discussed before, to the best of our knowledge, specific studies on the combined effects of simultaneous exposure to multiple phthalates on fetal health are lacking. The MMIP study (Goodrich et al. 2019) discussed the effects of widespread exposure to EDCs and metals on fetal health, but it did not consider the interaction between the studied substances of exposure. Human health risk assessments, without accounting for the combined effects of phthalate exposure, have potentially underestimated their true cumulative risks to fetal development (Tefre de Renzy-Martin et al. 2014).
Concentrations of phthalates were significantly higher in urban homes than in rural homes (Zhang et al. 2020). However, the phthalate exposure patterns of Chinese women might be different from those of women in other regions. Among the Chinese population, those living in industrial cities generally have higher levels of phthalates. For instance, the MEHP level of the population from Wuhan, Hubei (Zhang et al. 2019) (7.42 ng/mL in the winter, 5.01 ng/mL in the summer) is much higher than that of the population from Xiamen, Fujian (Li et al. 2019) (0.22 ng/mL). The levels of phthalates in people of different regions or different age groups vary greatly. The serum level of DMP among Guizhou children (Fu et al. 2021) is 31.62 ng/mL, which is closely related to exposures during pregnancy, infancy and schooling, while DMP level of adults from Shijiazhuang, Hebei, is 3.2 ng/mL (Zhang et al. 2018).
Given the limitations in previous studies, we conducted this nested case–control study. The present study aimed to investigate the relationships between single and combined exposure to phthalates and their metabolites during early pregnancy and the risk of FGR in the Chinese Zhuang population from Guangxi. This study has a certain guiding significance for medical health and personal protection during pregnancy.
Materials and methods
Study population
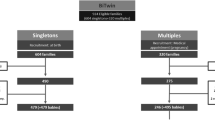
Volunteers were from the Guangxi Zhuang Birth Cohort (GZBC), a prospective and ongoing birth cohort in Guangxi, China, beginning in June 2015 (Liang et al. 2020). The Zhuang people are the most populous ethnic minority in China, mainly living in the Guangxi Zhuang Autonomous Region. Previous studies have shown potential differences in environmental exposures, such as exposure to perfluorohexane sulfonate, between Zhuang and Han populations (Liu et al. 2022). This study is a subset of the GZBC. The inclusion criteria were as follows: (1) Zhuang pregnant women; (2) women with a gestational age ≤ 22 weeks at the time of enrollment; (3) women with complete questionnaires; (4) women with available serum samples; and (5) women with a singleton pregnancy. Women who had a history/family history of congenital disease, those with a pregnancy history of birth defects/premature birth/stillbirth and those who took drugs 3 months before enrollment that may have teratogenic effects on fetuses were excluded. Ultimately, a total of 97 mothers who gave birth to infants with FGR were included as cases, and 291 mothers who gave birth to infants without FGR were matched with a ratio of 1:3 as controls by age at pregnancy. All the participants provided written consent. This project was approved by the Ethics Committee of Guangxi Medical University (No. 20140305–001).
Data collection
A face-to-face interview based on structured questionnaires was performed by professional interviewers to collect information including maternal age, ethnicity, gravidity, parity, occupation, regular exercise, cosmetic use during pregnancy, alcohol use during pregnancy, passive smoking during pregnancy, supplementation of folate, and stress. Moreover, maternal heights were measured during the first examination in the first trimester, and prepregnancy weights were self-reported. Maternal body mass index (BMI, in kg/m2) was calculated as weight (in kg) divided by the square of height (in m). Information at the time of the baby’s birth (including sex, birth weight, length and gestational age) was obtained from the Maternal and Child System. The determination of FGR was based on birth weight and gestational age according to the definition of FGR (Shrivastava and Master 2020), and fetuses with birth weights less than the 10th customized percentile or less than two standard deviations for that population reference (Capital Institute of et al. 2020) were diagnosed with FGR.
Blood sampling and serum phthalate measurements
A non-anticoagulant blood collection tube was used to collect 5 mL of peripheral venous blood from the pregnant women. The tubes were centrifuged for 10 min at 4000 rpm at 4 °C. Then, the serum and clots were separated into two layers and placed in two cryotubes, which were stored in a refrigerator at − 80 °C until subsequent processing.
Maternal serum samples were analyzed for 5 phthalate esters (BBP: butyl-benzyl phthalate, DBP: di-n-butyl phthalate, DEHP: di (2-ethyl-hexyl) phthalate, DEP: di (2-ethyl) phthalate, and DMP: dimethyl phthalate) and 2 phthalate metabolites (MBP: mono-butyl phthalate, and MEHP: mono-(2-ethylhexyl) phthalate). Phthalates and internal standard solutions (D4-DEP, D4-DBP, D4-BBP, D4-DEHP, and D4-DMP) were purchased from Sigma–Aldrich (St. Louis, MO, USA), AccuStandard Inc. (New Haven, CT, USA), Dr. Ehrenstorfer GmbH (Augsburg, Germany) and ANPEL (Shanghai, China). The purities of all the standards were ≥ 98%.
After being thawed overnight at 4 ℃, 0.5 mL of the serum sample was transferred to a 5 mL glass centrifuge tube. Then, 50 μL of 100 ng/mL internal standard solution and 1.5 mL of acetonitrile were spiked into the serum sample in sequence and vortexed for 1 min. After 10 min of ultrasound, the tubes were centrifuged for 10 min at 4000 rpm at 4 °C, and the supernatants were transferred to a clean glass tube. The above operations were repeated for the residue. Then, the supernatants were combined into one tube and placed into a vacuum drying oven to evaporate until dry. Later, 0.5 mL of 1 M sodium dihydrogen phosphate buffer (pH = 4.66) and 50 μL of 200 U/mL β-glucosidase solution were spiked. The mixtures were incubated at 37 ℃ for 90 min. Then, 2 mL of ethyl hexanoate was added to the incubated samples. Next, the mixtures were vortexed for 1 min, sonicated for 10 min, and centrifuged at 4000 rpm at 4 ℃ for 10 min. The supernatants were aspirated into clean tubes and redissolved in 1 mL of hexyl hydride:methyl tert-butyl ether (V/V, 19:1), which was repeated. Then, the same two mixtures were combined into one tube and placed into a vacuum drying oven to evaporate until dry. Subsequently, the dried extracts were mixed with 20 μL BSTFA (1% TMCS), and the volume was set with pyridine to 100 μL. After oscillating and mixing, derivatization was performed in a 30 ℃ water bath for 30 min.
Phthalates were separated and detected by a gas chromatograph-mass spectrometer (GC–MS, Thermo Fisher ISQ™LT, USA). The GC was equipped with an HP-5MS capillary column with a (5%-phenyl)-methylpolysiloxane phase (column length: 30 m, internal diameter: 250 µm, film thickness: 0.25 µm). The chromatographic conditions were set as follows: injector temperature: 260 °C; injection mode: splitless; carrier gas flow rate: 18.6 mL/min; oven program: start at 100 °C, hold for 1 min, then increase by 10 °C/min to 200 °C, hold for 4 min, and increase by 6 °C/min to 250 °C and hold for 1 min; and injection volume: 1 μL. The MS was operated in the electron impact mode, and its conditions were set as follows: ionization energy: 70 eV; MS source temperature: 300 °C; and transfer line temperature: 280 °C. The selected ion monitoring (SIM) mode was used to acquire data, and the solvent delay parameter was set to 7.50 min.
The internal standard method was used for quantification. The concentration standard curves were plotted by the least square method, and the limits of detection (LODs) were defined as a signal-to-noise ratio of 3 (for detailed information, see Supplementary Table S1). Moreover, recovery experiments were performed to evaluate the accuracy and precision of the method. The recoveries of phthalates were controlled between 80 and 120%. The intraday and interday coefficients of variation of blood samples with high, medium, and low concentrations were all controlled within 10%. The LODs for BBP, DBP, DEHP, DEP, MBP, MEHP, and DMP were 0.746 ng/mL, 0.145 ng/mL, 0.490 ng/mL, 1.120 ng/mL, 0.942 ng/mL, 0.720 ng/mL, and 0.497 ng/mL, respectively. The determined concentration below the LOD was calculated as the value of the LOD divided by \(\sqrt{2}\). The detection rates of all phthalates were above 50%.
Statistical analysis
Normally distributed continuous variables were compared using the independent samples t test, while nonnormally distributed continuous variables were compared by the Wilcoxon rank sum test. Categorical variables were analyzed using the chi-square test. Since the concentrations of phthalates in the maternal serum samples were nonnormally distributed, log10 transformation was used to transform the skewed data to approximately conform to normality when they were treated as continuous variables.
A logistic regression model was applied to assess the associations between the risk of FGR and early gestational phthalate exposure. The exposure levels of phthalates were tertiled as ordered categorical variables, except that the DMP level was binomial. We conducted a trend test using integer variables (ordinal values 0, 1, and 2 corresponding to the above categories) to examine the dose–response relationship in regression models. Furthermore, restricted cubic splines (RCSs) were used with 3 knots at the 10th, 50th, and 90th percentiles of the log10-transformed phthalate concentrations, with the reference value (OR = 1) set at the 50th percentile.
Afterward, we used a weight quantile sum (WQS) regression model, a nonparametric statistical analysis, to assess the combined impact of phthalate mixtures in relation to the studied health outcome. The analyses were run twice to test for associations between phthalates and FGR in either the positive or negative direction. With the effect parameter estimate (β1) constrained to be positive or negative, the concentrations of phthalates were tertiled and combined into a unidirectional weighted index, which represented the mixture effect of phthalates on the risk of FGR. After bootstrapping 10,000 times, 10,000 estimated weights for each phthalate, 10,000 mixture effect parameter estimates, and the associated standard errors, statistics, and P values were calculated. Phthalates with final weights greater than the cutoff (the inverse of the number of elements in the mixture) were considered to be significant for the WQS index, which was used to evaluate the correlation and significance of the mixture on FGR.
Then, we used the Bayesian kernel machine regression (BKMR) model with a probit link function (Bobb et al. 2015) to estimate the joint effects and interaction of the exposure to seven phthalates (BBP, DBP, DEHP, DEP, MBP, MEHP, and DMP) on FGR. Phthalates were log-transformed in the BKMR model. Based on Spearman correlation coefficients, we classified BBP into Group 1; DBP, DEHP, DEP, and DMP into Group 2; and MBP and MEHP into Group 3. By fitting hierarchical variable selection with 50,000 iterations, the group PIP for the three groups and the conditional posterior inclusion probability (Cond PIP) for individual phthalates were calculated and included in the final model. The PIP threshold was set to 0.5 as previously reported (Ashrap et al. 2020).
Statistical analysis was conducted in R 4.1.1 (Development Core Team) using the packages “gWQS”, “ggplot2”, “glmnet”, “corrplot”, “rms”, “psych”, and “bkmr”. Two-tailed P values less than 0.05 were considered statistically significant. All analyses were adjusted for potential confounders, including maternal height, prepregnancy weight, parity, stress, regular exercise, passive smoking during pregnancy, alcohol use during pregnancy, fetal sex, gestational age at birth, and mode of delivery.
Results
Demographic information
The demographic information of the subjects is summarized in Table 1. A total of 388 mother-infant pairs (97 cases vs. 291 controls) were included, of which 224 (57.73%) women were nulliparous and 164 (42.27%) women were multiparous. Maternal height and weight, folate supplementation during pregnancy, stress, fetal sex, and birth length and weight were significantly different between the cases and controls (all P values < 0.05).
Concentrations of phthalates in maternal serum samples
Table 2 shows the concentrations of the seven phthalates in the maternal serum samples. DEHP was detectable in all samples. BBP, DBP, DEP, MBP, MEHP, and DMP were detected in 82.5%, 99.0%, 87.6%, 81.2%, 86.9%, and 52.3% of all samples, respectively. DEHP (74.826 ng/mL) had the highest geometric mean concentration, while DMP (0.231 ng/mL) had the lowest.
Logistic regression analysis
Logistic regressions showed that maternal exposure to BBP (Ptrend = 0.046), DEP (ORhigh = 2.137, 95% CI: 1.028–4.542, P = 0.044), and DMP (ORhigh = 2.494, 95% CI: 1.414–4.411, P = 0.002, Ptrend = 0.002) was significantly positively associated with the risk of FGR, and all of these associations presented a significant trend in the P values (Table 3). Log-transformed BBP (ORadj = 1.849, 95% CI: 1.080–3.177, Padj = 0.025), DEHP (ORadj = 3.893, 95% CI: 1.305–11.910, Padj = 0.015), and DMP (ORadj = 1.722, 95% CI: 1.089–2.725, Padj = 0.020) were positively correlated with FGR. We did not find a statistical association between FGR and maternal exposure to MBP in the overall sample (all P > 0.05). When stratified by fetal sex, the positive effects of phthalates on FGR only existed for girls, which are shown in Supplementary Tables S2 and S3. MBP had a negative association with the risk of FGR, with a significant trend in the P values (ORhigh = 0.192, 95% CI: 0.036–0.795, P = 0.033, Ptrend = 0.035).
RCS analysis was conducted to detect the nonlinear correlations between the log10-transformed phthalate concentrations and the risk of FGR. The associations between phthalates and FGR were linear (all nonlinearity P values > 0.05), which are shown in Supplementary Figure S1.
WQS regression analysis
The WQS Index 1 and Index 2 were estimated in the positive and negative directions, respectively. The estimated weights for the seven phthalates are shown in Fig. 1. According to the WQS regression, BBP (0.338), DEP (0.263), DMP (0.209), DEHP (0.155), DBP (− 0.497) and MBP (− 0.430) were highly weighted in the association with FGR. WQS Index 1 had a significantly positive association with the risk of FGR (OR = 3.145, 95% CI: 1.769–5.726, P < 0.001), while WQS Index 2 was statistically negatively associated with the risk of FGR (OR = 0.529, 95% CI: 0.322–0.858, P = 0.011). Moreover, the two indices were combined in the adjusted logistic model and showed statistical significance (all P values < 0.001; Supplementary Table S4).
BKMR analysis
According to the Spearman correlation coefficients among the seven phthalates (Supplementary Figure S2), we divided the phthalates into three groups for the subsequent BKMR analysis. Supplementary Table S5 summarizes the probabilities of inclusion derived from the BKMR model for the three groups (Group PIP) and each phthalate (Cond PIP). The overall effect of the phthalate mixture was associated with FGR (Fig. 2). The risk of FGR was significantly increased when all the phthalates were higher than the 55th percentile (from the 55th to 75th percentile) compared with that when they were fixed at the 50th percentile. For each phthalate, when all of the other exposures were fixed at the 50th percentile, BBP, DEHP, DEP and DMP showed a positive relationship with the risk of FGR, while DBP and MBP showed an inverse relationship with the risk of FGR (Fig. 3). Furthermore, when the other exposures were fixed at their 50th percentile, we found no significant difference between certain phthalates and FGR, as the correlation curves were almost parallel, which indicated no interactions among phthalates (Supplementary Figure S3).
Discussion
For the first time, this nested case–control study performed on the region-specific Zhuang population in Guangxi, China, revealed significant associations of maternal exposure to phthalates with the risk of FGR. Several advanced analytical methods robustly support these findings, which fill the gap in understanding the effect of maternal phthalate coexposure on FGR. This study emphasized the importance of evaluating both single and combined effects of maternal phthalate mixtures on fetal outcomes using various statistical methods, which would be of great significance for maternal and child health. We found that maternal serum BBP, DEHP, DEP, and DMP had positive associations with the risk of FGR, and the effect of MBP was sex-specific, with a negative association with FGR in girls but not in boys. Previous studies have investigated the single effect of maternal exposure to phthalate substances on FGR. DEHP, DBP, BBP, and DEP influence the male gametes, reproduction and the offspring of exposed animals (Dobrzynska 2016).
DEHP, as a widely used plasticizer, has been studied repeatedly (Gao et al. 2017; Li et al. 2021; Maekawa et al. 2017; Santos et al. 2021; Shen et al. 2017). We found that a high concentration of DEHP was associated with an increased risk of FGR (ORadj = 3.83, 95% CI: 1.278–11.747, Padj = 0.017), which is consistent with a cohort in Rotterdam, the Netherlands (Santos et al. 2021). Susana Santos et al. indicated that higher maternal DEHP concentrations tended to be associated with lower fetal weight across gestation, and DEHP metabolites had correlations with growth retardation at birth, such as a smaller head circumference, a lower length at birth and being small for gestational age (Santos et al. 2021). A study with a birth cohort in Wuhan, China revealed sex-specific and trimester-specific relationships of DEHP exposure to neonatal development from the fetal to early childhood stages (Li et al. 2021). It found associations of DEHP in all three trimesters with fetal growth, birth weight, birth length, weight gain rates, and the baby’s BMI, especially the first-trimester DEHP concentration, which was negatively related to fetal growth in boys but positively related to birth length in girls and 24-month BMI in boys (Li et al. 2021). Another birth cohort in Boston observed consistent findings that urinary DEHP metabolites had significant inverse associations with estimated or actual fetal weight (Ferguson et al. 2016). In a DEHP-treated mouse model, the body weight and crown-rump length of both male and female fetuses were significantly decreased (Shen et al. 2017). The effect of DEHP exposure-induced fetal IUGR seems to be stage-specific, manifested as a marked reduction in fetal size at the late gestational stage and a slight reduction in fetal size at the middle stage (Shen et al. 2017). Inconsistent with the results of our findings, Maekawa et al. detected concentrations of chemicals including phthalates in maternal blood, cord blood, and amniotic fluid, but no significant differences were observed in the levels of DEHP and MEHP (Maekawa et al. 2017). We hypothesize that this is closely related to the concentration data conversion (log-transformed vs. raw), the sample size (388 vs. 145) and the region of the population (Chinese Zhuang vs. Japanese).
Based on cord blood measurements, phthalate levels such as DEHP, DEP, DBP, and BBP were thought to adversely affect fetal growth parameters by reducing the gestational period and leading to preterm delivery (Huang et al. 2014). BBP is commonly used for increasing flexibility and elasticity during the manufacturing of plastics. We found a positive correlation between BBP and FGR, and its developmental toxicity was previously confirmed in a zebrafish model, especially its toxicity to caudal development in zebrafish (Roy et al. 2017). Interestingly, high concentrations of DEP could increase the risk of FGR (ORhigh = 2.137, 95% CI: 1.028–4.542, P = 0.044), which, to the best of our knowledge, may be the first discovery concerning a single effect of exposure to DEP on FGR in humans. In the Wistar rat model, exposure to DEP during gestation and lactation was detrimental to the development of the small intestine (Setti Ahmed et al. 2018). Consistent with our study, a study in Guizhou, China, suggested that the negative effects of serum DMP on hepatic function were sex-related, which was more evident in girls than in boys (Fu et al. 2021).
In our study, a negative association of MBP (ORhigh = 0.192, 95% CI: 0.036–0.795, P = 0.033, Ptrend = 0.035) with FGR only existed among girls. A previous finding, discordant with ours, was that higher concentrations of MBP were associated with the risk of IUGR in an exposure–response relationship (Zhao et al. 2014). Physical exposure to higher levels of MEHP and MBP leads to lower birth weight and short birth length, modified by the paraoxonase-2 gene Ala148Gly (Xie et al. 2015) or LINE-1 gene methylation (Zhao et al. 2015). Concordant with our findings, studies among African American and white mothers from the US population reported an interaction of sex with MBP (P = 0.08), in which the associations were stronger for females than males (Bloom et al. 2021), and males were at higher risk of low birth weight than females with greater MBP concentrations (P = 0.002) (Bloom et al. 2019). Additionally, concurrent exposure to DBP and its MBP metabolite was associated with delayed pubarche in girls (Frederiksen et al. 2012). MBP was also associated with significant decreases in cholesterol (β = − 7.9 mg/dL, 95% CI: − 12.9– − 3.0), which may be a cardiometabolic biomarker (Vuong et al. 2021). Due to limited research on the protective effect of MBP on FGR in girls, further exploration of the influence of phthalates on fetal development is expected. A systematic review related to the effects of MBP on fetal or child development is required.
However, no significant results of MEHP were shown in our study. A previous study suggested that the effect of urinary MEHP exposure on IUGR seems to be more evident in male fetuses (Zhao et al. 2014), which may be due to the limited sample size of the study (Dumas-Mallet et al. 2017). In a mouse model, maternal exposure to 50 mg/kg/day of DBP induced earlier puberty and precocious development of the testis (Ma et al. 2021). We speculate that DBP may influence embryo maturation. A large number of studies have shown that lower doses of DBP cause more adverse effects than higher dose (Czubacka et al. 2021), which may explain why we did not find a significant relationship between maternal DBP exposure and FGR in a single exposure analysis.
Mammalian life stage sensitivity to phthalates appears to be most sensitive in the fetal stage but less so in the adult stage (Dobrzynska 2016). We found that maternal exposure to multiple phthalates in early pregnancy may increase the risk of FGR. Consistent with the logistic regression model, the WQS model showed that BBP, DEP, DMP, and DEHP were highly weighted in the positive association with FGR, while DBP and MBP were inversely associated with FGR in Zhuang mother-infant pairs. A prospective birth cohort in Anhui, China assessed the cumulative hazard index (HI) of combined DEP, DBP, dibenzyl phthalate (BBzP) and DEHP exposure and birth outcomes and observed an association between HIRfD and restricted fetal growth (e.g., decreased birth weight, birth length, and head and chest circumferences) (Gao et al. 2017). The HI value was inversely associated with head circumference (overall: β = − 0.10 cm, P = 0.020; girls: β = − 0.13 cm, P = 0.027), birth weight (girls: β = − 33.12 g, P = 0.036), and birth length (boys: β = − 0.17 cm, P = 0.041) (Gao et al. 2017). Consistently, our study found that cumulative coexposure to the seven phthalates generated an increased risk of FGR according to the BKMR model. Simultaneous exposure to DBP, DEHP, DEP, and DMP had the greatest impact on FGR, with a group PIP value of 0.888, followed by BBP, with a group PIP value of 0.751, indicating that they are the main factors that increase the risk of FGR. The National Institute of Environmental Health Sciences (NIEHS) has encouraged research to evaluate the effects of environmental chemical mixtures on health outcomes rather than on the traditional “one chemical at a time” approach (Birnbaum 2012). Standard regression, classification and prediction, and exposure–response surface estimation have been identified and classified by the NIEHS for analyzing chemical mixtures in epidemiological studies (Taylor et al. 2016). Consistent with the rules, our current study evaluated the effect of phthalate mixtures in multiple statistical methods, including logistic regression, RCS, WQS, and BKMR models. BKMR models provide graphical outputs to directly display the dose–response curve. However, the linear or nonlinear relationship of the dose–effect introduces additional complexities in the models, and the approximation functions are dependent on the specific data structures of the cohort, all of which led to challenges in making direct comparisons of the results from different cohorts (Zhuang et al. 2021). In the absence of other similar mixture studies with which to compare our results, the epidemiological explanations for the findings of the current mixture are based on FGR results from conventional single-pollutant analyses evaluating maternal preconception exposure to phthalates using multiple logistic regression models.
The strengths and limitations of this study should be noted. Our study had several strengths. First, our study used a variety of statistical methods to estimate the combined exposure of phthalates on the risk of FGR, which could be used to analyze the effects of phthalates on developmental outcomes in a more comprehensive manner. Moreover, the cases and controls in this study were matched by the age of the pregnant mothers, which could reduce the confounding bias. Our study also had limitations. This study only included pregnant women of Zhuang nationality in Guangxi, China, so the generalization of the results of this study must be performed with caution. Even though serum levels can be good internal biomarkers for detecting exposure to phthalates, comparisons with other types of samples, such as plasma, urine, or amniotic fluid samples, are still difficult according to the current approaches. In addition, the metabolism of phthalates is related to methylation or polymorphisms, which encouraged us to comprehensively explore the complex interactions of genes and the environment on adverse pregnancy outcomes. Finally, given the exploratory nature of the study, we detected phthalates and their metabolites at a gestational week ≤ 22 weeks, and we did not further estimate the effects of cumulative exposure to phthalates at different stages of pregnancy on fetal development.
Conclusion
In conclusion, elevated BBP, DEHP, DEP, and DMP concentrations were positively associated with a high risk of FGR, while DBP and MBP had inverse relationships with the risk of FGR. Coexposure to phthalates in early pregnancy increases the risk of FGR. Our findings indicate the risks of phthalates to maternal and child health, which provides new evidence for public health.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Ahbab MA, Guven C, Kockaya EA, Barlas N (2017) Comparative developmental toxicity evaluation of di- n-hexyl phthalate and dicyclohexyl phthalate in rats. Toxicol Ind Health 33:696–716
Ashrap P, Watkins DJ, Mukherjee B, Boss J, Richards MJ, Rosario Z, Velez-Vega CM, Alshawabkeh A, Cordero JF, Meeker JD (2020) Maternal blood metal and metalloid concentrations in association with birth outcomes in Northern Puerto Rico. Environ Int 138:105606
Birnbaum LS (2012) NIEHS’s new strategic plan. Environ Health Perspect 120:a298
Bloom MS, Wenzel AG, Brock JW, Kucklick JR, Wineland RJ, Cruze L, Unal ER, Yucel RM, Jiyessova A, Newman RB (2019) Racial disparity in maternal phthalates exposure; association with racial disparity in fetal growth and birth outcomes. Environ Int 127:473–486
Bloom MS, Valachovic EL, Begum TF, Kucklick JR, Brock JW, Wenzel AG, Wineland RJ, Cruze L, Unal ER, Newman RB (2021) Association between gestational phthalate exposure and newborn head circumference; impacts by race and sex. Environ Res 195:110763
Bobb JF, Valeri L, Claus Henn B, Christiani DC, Wright RO, Mazumdar M, Godleski JJ, Coull BA (2015) Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics 16:493–508
Capital Institute of P, Coordinating Study Group of Nine Cities on the Physical G, Development of C (2020) Growth standard curves of birth weight, length and head circumference of Chinese newborns of different gestation. Zhonghua Er Ke Za Zhi 58:738–746
Czubacka E, Czerczak S, Kupczewska-Dobecka MM (2021) The overview of current evidence on the reproductive toxicity of dibutyl phthalate. Int J Occup Med Environ Health 34:15–37
Dobrzynska MM (2016) Phthalates - widespread occurrence and the effect on male gametes. Part 2. The effects of phthalates on male gametes and on the offspring. Rocz Panstw Zakl Hig 67:209–221
Dumas-Mallet E, Button KS, Boraud T, Gonon F, Munafo MR (2017) Low statistical power in biomedical science: a review of three human research domains. R Soc Open Sci 4:160254
Ferguson KK, McElrath TF, Meeker JD (2014) Environmental phthalate exposure and preterm birth. JAMA Pediatr 168:61–67
Ferguson KK, Meeker JD, Cantonwine DE, Chen YH, Mukherjee B, McElrath TF (2016) Urinary phthalate metabolite and bisphenol A associations with ultrasound and delivery indices of fetal growth. Environ Int 94:531–537
Frederiksen H, Jorgensen N, Andersson AM (2010) Correlations between phthalate metabolites in urine, serum, and seminal plasma from young Danish men determined by isotope dilution liquid chromatography tandem mass spectrometry. J Anal Toxicol 34:400–410
Frederiksen H, Sorensen K, Mouritsen A, Aksglaede L, Hagen CP, Petersen JH, Skakkebaek NE, Andersson AM, Juul A (2012) High urinary phthalate concentration associated with delayed pubarche in girls. Int J Androl 35:216–226
Fu X, He J, Zheng D, Yang X, Wang P, Tuo F, Wang L, Li S, Xu J, Yu J (2021) Association of endocrine disrupting chemicals levels in serum, environmental risk factors, and hepatic function among 5- to 14-year-old children. Toxicology 153011. https://doi.org/10.1016/j.tox.2021.153011
Gao H, Xu YY, Huang K, Ge X, Zhang YW, Yao HY, Xu YQ, Yan SQ, Jin ZX, Sheng J, Zhu P, Hao JH, Tao FB (2017) Cumulative risk assessment of phthalates associated with birth outcomes in pregnant Chinese women: a prospective cohort study. Environ Pollut 222:549–556
Goodrich JM, Ingle ME, Domino SE, Treadwell MC, Dolinoy DC, Burant C, Meeker JD, Padmanabhan V (2019) First trimester maternal exposures to endocrine disrupting chemicals and metals and fetal size in the Michigan Mother-Infant Pairs study. J Dev Orig Health Dis 10:447–458
Hines EP, Calafat AM, Silva MJ, Mendola P, Fenton SE (2009) Concentrations of phthalate metabolites in milk, urine, saliva, and serum of lactating North Carolina women. Environ Health Perspect 117:86–92
Hogberg J, Hanberg A, Berglund M, Skerfving S, Remberger M, Calafat AM, Filipsson AF, Jansson B, Johansson N, Appelgren M, Hakansson H (2008) Phthalate diesters and their metabolites in human breast milk, blood or serum, and urine as biomarkers of exposure in vulnerable populations. Environ Health Perspect 116:334–339
Huang Y, Li J, Garcia JM, Lin H, Wang Y, Yan P, Wang L, Tan Y, Luo J, Qiu Z, Chen JA, Shu W (2014) Phthalate levels in cord blood are associated with preterm delivery and fetal growth parameters in Chinese women. PLoS One 9:e87430
Kamai EM, McElrath TF, Ferguson KK (2019) Fetal growth in environmental epidemiology: mechanisms, limitations, and a review of associations with biomarkers of non-persistent chemical exposures during pregnancy. Environ Health 18:43
Kato K, Silva MJ, Reidy JA, Hurtz D 3rd, Malek NA, Needham LL, Nakazawa H, Barr DB, Calafat AM (2004) Mono(2-ethyl-5-hydroxyhexyl) phthalate and mono-(2-ethyl-5-oxohexyl) phthalate as biomarkers for human exposure assessment to di-(2-ethylhexyl) phthalate. Environ Health Perspect 112:327–330
Lee W, Cho JH, Lee Y, Lee S, Kim DH, Ha S, Kondo Y, Ishigami A, Chung HY, Lee J (2019) Dibutyl phthalate impairs neural progenitor cell proliferation and hippocampal neurogenesis. Food Chem Toxicol 129:239–248
Li J, Qian X, Zhou Y, Li Y, Xu S, Xia W, Cai Z (2021) Trimester-specific and sex-specific effects of prenatal exposure to di(2-ethylhexyl) phthalate on fetal growth, birth size, and early-childhood growth: a longitudinal prospective cohort study. Sci Total Environ 777:146146
Li X, Liu L, Wang H, Zhang X, Xiao T, Shen H (2019) Phthalate exposure and cumulative risk in a Chinese newborn population. Environ Sci Pollut Res Int 26:7763–7771
Liang J, Liu S, Liu T, Yang C, Wu Y, Jennifer Tan HJ, Wei B, Ma X, Feng B, Jiang Q, Huang D, Qiu X (2020) Association of prenatal exposure to bisphenols and birth size in Zhuang ethnic newborns. Chemosphere 252:126422
Liu B, Wei B, Mo M, Song Y, Tang C, Tang P, Guo X, Tan C, Liu S, Huang D, Qiu X (2022) Exposure to perfluoroalkyl substances in early pregnancy and the risk of hypertensive disorders of pregnancy: a nested case-control study in Guangxi. China. Chemosphere 288:132468
Ma T, Zhou Y, Xia Y, Jin H, Wang B, Wu J, Ding J, Wang J, Yang F, Han X, Li D (2021) Environmentally relevant perinatal exposure to DBP disturbs testicular development and puberty onset in male mice. Toxicology 459:152860
Maekawa R, Ito R, Iwasaki Y, Saito K, Akutsu K, Takatori S, Ishii R, Kondo F, Arai Y, Ohgane J, Shiota K, Makino T, Sugino N (2017) Evidence of exposure to chemicals and heavy metals during pregnancy in Japanese women. Reprod Med Biol 16:337–348
Martinez-Razo LD, Martinez-Ibarra A, Vazquez-Martinez ER, Cerbon M (2021) The impact of di-(2-ethylhexyl) phthalate and mono(2-ethylhexyl) phthalate in placental development, function, and pathophysiology. Environ Int 146:106228
Prevention CfDCa (2009) Fourth national report on human exposure to environmental chemicals. Centers for Disease Control and Prevention. https://www.cdc.gov/exposurereport/index.html
Roy NM, Zambrzycka E, Santangelo J (2017) Butyl benzyl phthalate (BBP) induces caudal defects during embryonic development. Environ Toxicol Pharmacol 56:129–135
Santos S, Sol CM, van Zwol-Janssens C, Philips EM, Asimakopoulos AG, Martinez-Moral MP, Kannan K, Jaddoe VWV, Trasande L (2021) Maternal phthalate urine concentrations, fetal growth and adverse birth outcomes. A population-based prospective cohort study. Environ Int 151:106443
Setti Ahmed K, Kharoubi O, Aoues AEK, Bouchekara M, Khaladi B, Taleb M (2018) Effect of gestational and lactational exposure to DEHP, DINP, and DEP on intestinal morphology, disaccharidases, and alkaline phosphatase in rats during postnatal development. Am J Perinatol 35:1251–1259
Shen R, Zhao LL, Yu Z, Zhang C, Chen YH, Wang H, Zhang ZH, Xu DX (2017) Maternal di-(2-ethylhexyl) phthalate exposure during pregnancy causes fetal growth restriction in a stage-specific but gender-independent manner. Reprod Toxicol 67:117–124
Shoaff JR, Romano ME, Yolton K, Lanphear BP, Calafat AM, Braun JM (2016) Prenatal phthalate exposure and infant size at birth and gestational duration. Environ Res 150:52–58
Shrivastava D, Master A (2020) Fetal Growth Restriction. J Obstet Gynaecol India 70:103–110
Sun CC, Zhao S, Chu LL, Zhang SY, Li YL, Sun MF, Wang QN, Huang Y, Zhang J, Wang H, Gao L, Xu DX, Zhang SC, Xu T, Zhao LL (2022) Di (2-ethyl-hexyl) phthalate disrupts placental growth in a dual blocking mode. J Hazard Mater 421:126815
Tang ZR, Xu XL, Deng SL, Lian ZX, Yu K (2020) Oestrogenic endocrine disruptors in the placenta and the fetus. Int J Mol Sci 21(4). https://doi.org/10.3390/ijms21041519
Taylor KW, Joubert BR, Braun JM, Dilworth C, Gennings C, Hauser R, Heindel JJ, Rider CV, Webster TF, Carlin DJ (2016) Statistical approaches for assessing health effects of environmental chemical mixtures in epidemiology: lessons from an innovative workshop. Environ Health Perspect 124:A227–A229
Tefre de Renzy-Martin K, Frederiksen H, Christensen JS, Boye Kyhl H, Andersson AM, Husby S, Barington T, Main KM, Jensen TK (2014) Current exposure of 200 pregnant Danish women to phthalates, parabens and phenols. Reproduction 147:443–453
Tsai MS, Chen MH, Lin CC, Ng S, Hsieh CJ, Liu CY, Hsieh WS, Chen PC (2017) Children’s environmental health based on birth cohort studies of Asia. Sci Total Environ 609:396–409
Vuong AM, Braun JM, Sjodin A, Calafat AM, Yolton K, Lanphear BP, Chen A (2021) Exposure to endocrine disrupting chemicals (EDCs) and cardiometabolic indices during pregnancy: the HOME study. Environ Int 156:106747
Wang Y, Zhu H, Kannan K (2019) A review of biomonitoring of phthalate exposures. Toxics 7(2). https://doi.org/10.3390/toxics7020021
Xie C, Jin R, Zhao Y, Lin L, Li L, Chen J, Zhang Y (2015) Paraoxonase 2 gene polymorphisms and prenatal phthalates’ exposure in Chinese newborns. Environ Res 140:354–359
Yang C, Song G, Lim W (2019) A mechanism for the effect of endocrine disrupting chemicals on placentation. Chemosphere 231:326–336
Zhang J, Yin W, Li P, Hu C, Wang L, Li T, Gao E, Hou J, Wang G, Wang X, Wang L, Yu Z, Yuan J (2019) Interaction between diet- and exercise-lifestyle and phthalates exposure on sex hormone levels. J Hazard Mater 369:290–298
Zhang Q, Sun Y, Zhang Q, Hou J, Wang P, Kong X, Sundell J (2020) Phthalate exposure in Chinese homes and its association with household consumer products. Sci Total Environ 719:136965
Zhang SH, Shen YX, Li L, Fan TT, Wang Y, Wei N (2018) Phthalate exposure and high blood pressure in adults: a cross-sectional study in China. Environ Sci Pollut Res Int 25:15934–15942
Zhao Y, Chen L, Li LX, Xie CM, Li D, Shi HJ, Zhang YH (2014) Gender-specific relationship between prenatal exposure to phthalates and intrauterine growth restriction. Pediatr Res 76:401–408
Zhao Y, Shi HJ, Xie CM, Chen J, Laue H, Zhang YH (2015) Prenatal phthalate exposure, infant growth, and global DNA methylation of human placenta. Environ Mol Mutagen 56:286–292
Zhuang LH, Chen A, Braun JM, Lanphear BP, Hu JMY, Yolton K, McCandless LC (2021) Effects of gestational exposures to chemical mixtures on birth weight using Bayesian factor analysis in the Health Outcome and Measures of Environment (HOME) Study. Environ Epidemiol 5:e159
Acknowledgements
We sincerely thank all the participants and all staffs for their supports and cooperation. We also appreciate the Guangxi Colleges and Universities Key Laboratory of Prevention and Control of Highly Prevalent Diseases, and Academician Dongxin Lin workstation for providing the experimental environment.
Funding
This work was supported by the Guangxi Key Research Program (Grant number AB17195012) and the National Natural Science Foundation of China (Grant number 81860587).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Xiaojing Guo: Methodology, Formal analysis, Writing- original draft, Visualization. Yonghong Sheng: Methodology, Investigation, Data curation, Validation. Bihu Liu: Methodology, Validation, Investigation. Peng Tang: Validation, Investigation. Runfeng Liu: Validation, Investigation. Li Wu: Investigation. Jiehua Chen: Investigation. Dongping Huang: Methodology, Writing—review & editing, Supervision. Shun Liu: Methodology, Writing—review & editing, Supervision. Xiaoqiang Qiu: Project administration, Resources, Data curation, Writing—review & editing, Supervision, Funding acquisition. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All the participants had provided written consents. This project was approved by the ethics committee of Guangxi Medical University (No. 20140305–001).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Lotfi Aleya
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Guo, X., Sheng, Y., Liu, B. et al. Exposure to phthalates in early pregnancy and the risk of fetal growth restriction: a nested case–control study in a Zhuang Chinese population. Environ Sci Pollut Res 29, 57318–57329 (2022). https://doi.org/10.1007/s11356-022-19919-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-19919-z