Abstract
Carbon dioxide (CO2) emission to the atmosphere is the prime cause of certain environmental issues like global warming and climate change, in the present day scenario. Capturing CO2 from various stationary industrial emission sources is one of the initial steps to control the aforementioned problems. For this concern, a variety of resources, such as liquid absorbents, solid adsorbents, and membranes, have been utilized for CO2 capturing from various emission sources. Focused on membrane-based CO2 capture, polymeric membranes with composite structure (polymeric composite membrane) offer a better performance in CO2 capturing process than other membranes, due to the composite structure it offers higher gas flux and less material usage, thus facile to use high performed expensive material for membrane fabrication and achieved good efficacy in CO2 capture. This compressive review delivers the utilization of different polymeric composite membranes in CO2 capturing applications. Further, the types of polymeric materials used and the different physicochemical modifications of those membrane materials and their CO2 capturing ability are briefly discussed in the text. In conclusion, the current status and possible perspective ways to improve the CO2 capture process in industrial CO2 gas separation applications are described in this review.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Peoples are concerned about environmental and ecosystem protection to identify the possible solution to mitigate certain environmental issues like global warming and climate change, which is mainly triggered by the emission of greenhouse gases into the atmosphere (Yamasaki 2003). Carbon dioxide is a primary anthropogenic greenhouse gas for the aforementioned problems and the maximum amount persistent constituent among the greenhouse gases in the atmosphere (Yang et al. 2008). Nearly 60% and above the global warming effect is attributed to anthropogenic CO2 emissions (Yamasaki 2003). According to the International Panel on Climate Change Sixth Assessment Report (IPCC AR6) statement, in the year 2100, the amount of atmospheric CO2 may be reached up to 600 ppm and the effects increase the mean global temperature around 1.9 °C excess than normal and the causes increase the mean sea level about 3.5 m IPCC (2021). Hence, the IPCC stated, the global greenhouse gas (GHG) emissions must be reduced up to 50 to 80% by the year 2050; this is to be a possible solution to avoid dramatic consequences of global warming IPCC (2021).
The exhaust gas coming from the combustion of fossil fuels and various industrial processes (thermal power plant, steel, cement, and chemical) is the major contributor source of CO2 emission to the atmosphere (Merkel et al. 2010). In worldwide energy production, fossil fuels contribute more than 75% and above (Songolzadeh et al. 2014). The use of alternate energy sources, such as non-fossil fuels (renewable energy such as solar energy, wind energy) or gaseous fuels (e.g., biogas, syngas, and landfill gas) rather than fossil fuel, is the solution to minimize the CO2 emission to the atmosphere (Kapoor et al. 2019). The former cases (non-fossil fuel) cannot meet the required energy fed by fossil fuels (about 1000 terawatt-hours (TWh)) World Energy Outlook (2021). In the latter case, the problem twisted by the presence of CO2 in gaseous fuels reduces the calorific value and fuel efficiency; further, its acidic nature makes corrosion to the pipeline during transportation (Scholes et al. 2012). Therefore, considering the energy and environmental aspect, capturing CO2 from emission sources (flue gases) and gaseous fuel is the solution to tackle the aforementioned problems (Yamasaki 2003; Zhang et al. 2013). In literature, the capture of CO2 from various gas mixtures furnished by various technologies, such as amine absorption, solid adsorption, chemical looping, cryogenic, membranes, gas hydration, and chemical oxidation (Olajire 2010; Yeo et al. 2012; Thomas et al. 2016; Kapoor et al. 2019). Amongst membrane gas separation processes much attractive to the scientific communities in terms of succeeding advantages such as eco-friendliness, minimum energy consumption, operation compact, lesser capital cost, unit size compact, and energy efficiency (Brunetti et al. 2010; Scholes et al. 2012; Yeo et al. 2012).
In the past few decades, the membrane-based CO2 gas separation has proven there substantial grownup, breakthroughs, and benefits over the other technology used in this field (Ramasubramanian and Ho 2011). There is a lot of variety of membranes (polymeric, inorganic and mixed matrix (MMMs)) identified and verified their effectiveness in membrane CO2 gas separation processes in both academia and industrial point of view (Cong et al. 2007; Scholes et al. 2008; Yampolskii 2012; Baker and Low 2014; Rezakazemi et al. 2015). Among these, polymeric membranes with composite structure (polymeric composite membrane) substantially stand by in commercial purposes, due to their structural configuration making a lot of welfares, that is higher gas flux, more mechanical stability, and easy to form different membrane modules. Further, the independent collection of support and selective layer material facile to use variety of polymer material for membrane making as well as lower polymer material consumption facile to highly performed more expensive polymer material usage for the membrane making (Scholes et al. 2008; Yampolskii 2012; Dai et al. 2016). Over the long period, polymeric membranes with composite structure are extensively grownup for membrane CO2 gas separation application, because of their process ability, material availability, flexibility to forming any modules (flat sheet (F), tubular (T), hollow fiber (HF), spiral wound (SW)), reproducibility, and inexpensive processing ability (Sridhar et al. 2007a; Yampolskii 2012).
Up to now a large number of academic reports are found from the field of polymeric composite membranes in CO2 gas separation, while combined reviews are not yet found. From this concern, a comprehensive literature summary on various types of polymeric composite membranes, such as rubbery, glassy, co-polymers, facilitated and hydrogel polymers, and composite membranes in different CO2 gas separation applications (e.g., CO2/CH4, CO2/N2, and CO2/H2), are discussed in this review. Further, the advancement of these composite membranes to overcome the negativity that persisted in the gas permeation process is also discussed. Finally, this review summarized the current perspectives and future direction in the field of the polymeric composite membrane in CO2 gas separation.
Progress of polymeric composite membrane in carbon dioxide capture
Typically, the membrane gas separation application permeability (P) and selectivity (α) are the first two main factors that describe the performance of a membrane, and they are directly linked with the application point of view in which the productivity and purity of gas component (Amooghin et al. 2017). Polymeric membranes have some intrinsic trade-off relation between permeability and selectivity (i.e., selectivity increases, permeability decreases, and vice versa) in gas separation application (Freeman 1999). Lloyd M. Robeson has suggested that the trade-off between permeability and selectivity possesses a superior limit that provides an example of an upper bound diagram (Robeson 2008). Most of the polymeric membranes reported in the literature are occupied the place below this upper bound (Orme et al. 2001; Bum et al. 2010; Tomé et al. 2015; Mannan et al. 2016; Scofield et al. 2016; Nikolaeva et al. 2018; Zhuang et al. 2018; Sasikumar et al. 2021). If the polymeric membranes may be to overcome this upper bound, they have higher permeability with the same selectivity or same permeability with higher selectivity (Dilshad et al. 2017). This can be achieved by changing their material properties or reducing the barrier thickness or else combined to both (Rafiq et al. 2016). Then the researchers discovered that the membrane of composite form with functionalization of polymeric materials may fulfill the aforementioned criteria (Dai et al. 2016; Alqaheem et al. 2017; Maheswari and Palanivelu 2017; Bei et al. 2021). Over the decades, a lot of polymeric composite membranes have been produced with different polymeric materials (cellulose acetate (CA) and its derivatives (Shieh and Chung 2000; Mao et al. 2011; Ahmad et al. 2014; Shankar and Kandasamy 2019), polysulfones (PSf) (Scholes et al. 2010a; Rafiq et al. 2012), polycarbonates (PC) (Hellums et al. 1989; Iqbal et al. 2008), polyethylene oxides (PEO) (Suzuki et al. 1998; Wang et al. 2014; Lee and Kang 2019), perfluoropolymers (PFP)(Jansen et al. 2006; Scholes et al. 2015; Fang et al. 2016), natural or synthetic rubbers (Zhuang et al. 2018; Tseng et al. 2019), polypropylene (PP) (Teramae and Kumazawa 2007; Blinova et al. 2012), polyamines (PA) (Matsuyama et al. 1996; Kim et al. 2004; Shen et al. 2015; Chen et al. 2016), polyimides (PI) (Marek et al. 1996; Hillock and Koros 2007; Harra et al. 2019), co-polymers (Sridhar et al. 2007c; Ji et al. 2009; Chen et al. 2015), etc.) by various researchers’ extensively in CO2 gas separation application and they are observed remarkable results in lab scale and industrial scale (Alqaheem et al. 2017; Brinkmann et al. 2017).
Generally, in membrane CO2 gas separation, polymeric composite membranes are categorized by two types based on their gas transport mechanism (Wang et al. 2016). They are as follows:
-
i.
Solution-diffusion polymeric composite membranes
-
ii.
Facilitated transport polymeric composite membranes
Further, they are sub-divided by various types based on their nature of material usage in selective layer formation (Scholes et al. 2008; Du et al. 2012; Mannan et al. 2013). The schematic view of various polymeric composite membranes in CO2 gas separation is shown in Fig. 1. The detailed progress of different polymeric composite membranes is discussed in a further section.
Solution-diffusion polymeric composite membranes
The solution-diffusion polymeric composite membranes is well documented in gas separation application over the long period (Wang et al. 2016). In solution-diffusion polymeric composite membranes, the gas transportation is subject to solubility (S) and diffusivity (D) (i.e., P = DS) of gas molecules through the polymer selective layer, whereas solubility and diffusivity of gas molecules depend on the internal characteristic (chemical functionality) and nature (free volume, elasticity, etc.) of polymer selective layer materials (Freeman 1999; Rafiq et al. 2016). Based on the nature of polymeric material used in selective layer formation, the solution-diffusion polymeric composite membranes can be categorized into three types, i.e., rubbery polymeric composite membrane, glassy polymeric composite membranes, and co-polymeric composite membrane (Mannan et al. 2013). All these three different composite membranes experienced different CO2 gas separation efficiency. The gas separation performances of rubbery polymers more predominate on solubility bases, while diffusion bases in glassy polymers and both together in co-polymers (Wang et al. 2016). Generally, rubbery polymers have high permeability and low selectivity, while vice versa for glassy polymers and moderate for co-polymer (Sainan et al. 2013; Chen et al. 2015). However, this inherent trade-off performance of solution-diffusion polymeric composite membrane could not meet with effective CO2 gas separation. Further, the trade-off relation to solution-diffusion polymeric composite membrane overcome by the physicochemical modification of this polymeric material with different approaches, such as cross-linking (Liu et al. 2003; Hillock and Koros 2007; Chen et al. 2014), blending (Hosseinia et al. 2010; Zhu et al. 2015; Kim et al. 2016a, b, c), or doping (Reid et al. 2002; Xomeritakis et al. 2009; Lin et al. 2015) of other polymer and inorganic particles, surface functionalization (Xu and Coleman 1999; Kim et al. 2016a, b, c), etc. Some studies experienced enhanced results on that process (Fu et al. 2014; Halim et al. 2014; Xin et al. 2015; Nikolaeva et al. 2018). The following section of this review summarized the research progress of rubber, glassy, and co-polymeric composite membranes for CO2 gas separation.
The rubbery polymeric composite membrane in CO2 gas separation
Poly(dimethylsiloxane) (PDMS) is the foremost used rubbery polymeric material for gas separation application, because of its good physicochemical characteristic, i.e., mechanical and thermal stability, less aging effect, low cost, and ease of processing (Strathman et al. 1986; Wu et al. 2006; Saedi et al. 2014; Kim et al. 2016a, b, c). In 1986 Strathman and co-workers assessed the first PDMS-based polymeric composite membrane in gas separation application (Strathman et al. 1986). Later several studies (Table 1) produced the various polymer and inorganic materials supported (cellulose acetate (Wu et al. 2006), polysulfone (Li et al. 2013b; Pakizeh et al. 2013), polyvinylidene fluoride (PVDF) (Choi et al. 2010), polytetrafluoroethylene (PTFE) (Jia et al. 1991), polyimide (PI) (Qin et al. 2005), polyethersulfone (PES) (Sadrzadeh et al. 2009; Madaeni et al. 2013; Saedi et al. 2014), polyacrylonitrile (PAN) (Duval et al. 1993; Achalpurkar et al. 2007; Zhao et al. 2015a; Kim et al. 2016a, b, c), porous SS metal (PSSM) (Scholes et al. 2010b), Al2O3 ceramic (Sainan et al. 2013), and silica nitrate (Si3N4) (Firpo et al. 2015)) and PDMS composite membranes and examined their CO2 gas separation efficiency in various gas mixture in various aspects.
For example, Merkel et al. (2000) and Peter and Peinemann (2009) reported the detailed gas transport properties of various gas mixtures in PDMS/NMS-based composite membranes. Similarly, Firpo et al. (2015) reported the thickness dependence gas permeability variability of PDMS/Si3N4-based membrane. Madaeni et al. (2013) studied the effect of the coating method on the gas separation performance of the PDMS/PES composite membrane.
From reported literature (Table 1), it is clear that the composite membrane made with rubbery polymers exhibited the higher gas permeability and lower gas pair selectivity in most cases (Fritsch et al. 1993; Achalpurkar et al. 2007; Sainan et al. 2013). Generally, rubbery polymers possess high chain mobility and poor size sieving ability, thus indicating the overall selectivity based on the solubility of gas molecules. Though the gas solubility should not be altered significantly, the diffusivity of gas molecules is controlled by modifying the polymer architecture with help of physio-chemical treatment. Some studies experienced the enhancement of gas pair selectivity in rubbery polymeric composite membranes after some physicochemical treatment such as cross-linking (Qin et al. 2005; Pakizeh et al. 2013), surface functionalization (Oh and Zurawsky 1996; Achalpurkar et al. 2007; Hong et al. 2017), blending of polymer (Scofield et al. 2015), and inorganic particles (Duval et al. 1993; Hussain and König 2012; Sun et al. 2016). For example, Fritsch et al. (1993) examined the non-silicon material grafting enhancing the gas permeation properties (gas pair (CO2/N2) selectivity) of silicon rubbery membrane. Similarly, surface modification of PDMS membrane with plasma polymers increases the performance of bare membrane which was examined by Oh and Zurawsky (1996). In other work, Sainan et al. (2013) and Pakizeh et al. (2013) reported that the cross-linking of PDMS membrane increased the gas pair (CO2/N2 and CO2/H2) selectivity with respect to cross-linking content. Similarly, Scofield et al. (2015) and Hong et al. (2017) reported that the –NH2 group functionalization and blending or grafting of PEG polymer into PDMS membranes increase the gas pair (CO2/N2) selectivity from 9.8 (unmodified PDMS membranes) to 22 and 19, respectively.
On the other hand, the researchers observed overall gas separation performance of these modified membranes exceeds the Robeson trade line (Wang et al. 2016). Further, some studies explore the incorporation of the various inorganic particles (Zeolite 4A (Alavi et al. 2017), SiO2 (Fritsch et al. 1993), ZIF-5 nanoparticles (Hussain and König 2012), GO (Alavi et al. 2017), and carbon particles (Duval et al. 1993)) into rubbery PDMS polymer matrix which enhanced the gas separation performance in terms of permeability or selectivity. Some other works (Jia et al. 1991; Saedi et al. 2014; Kim et al. 2016a, b, c) also found a similar phenomenon in the rubbery polymeric composite membrane by chemical modification. Even though the physicochemical modifications improved the gas separation performance (selectivity) of rubbery polymeric composite membranes, still this improvement is not met for the required effective CO2 gas separation membrane criteria (> 100 selectivity) (Merkel et al. 2010). Further, the idea of the researcher to use this rubbery polymer materials as a gutter layer/productive layer formation in composite membranes production in CO2 gas separation and many studies obtained an encouraging result (both membrane formation and gas separation performance) (Li et al. 2013a; Fu et al. 2014; Kim et al. 2016a, b, c; Scofield et al. 2016; Selyanchyn et al. 2018).
Glassy polymeric composite membrane in CO2 gas separation
In industrial CO2 gas separation, glassy polymers are step forwarded than rubbery polymers, because they have a good balancing between permeability and selectivity along with good mechanical stability (Kim and Lee 2013; Adewole and Sultan 2019). A number of glassy polymeric composite membranes and their CO2 gas separation performance are compiled in Table 2. In listed most of the glassy polymeric composite membranes display in well CO2 permeability and CO2/light gas selectivity. From the beginning, the common glassy polymeric materials of cellulose acetates (Lu et al. 2016), cellulose nitrates (CN) (Shieh and Chung 2000), polysulfones (Pakizeh et al. 2013; Mannan et al. 2016), polyamides (Petersen and Peinemann 1997), polyimides (Park et al. 2003; Weigelt et al. 2019), polycarbonates (Hellums et al. 1989), polyacetylenes (PAc)(Nakagawa et al. 1988), etc. are utilized as polymer selective layer material in glassy polymeric solution-diffusion membranes. The advancement in this field leads to different kinds of glassy polymeric materials i.e., some porous organic polymers (Kim and Lee 2013), such as thermally rearranged (TR) polymers (Berchtold et al. 2012; Kim and Lee 2014; Brunetti et al. 2017; Kim et al. 2018; Lee et al. 2020), polymers of intrinsic microporosity (PIMs) (Lasseuguette et al. 2014; Sekizkardes et al. 2016, 2018), porous organic framework (hyperbranched porous polymer (HBPPs) (Taniguchi et al. 2017) (Peter et al. 2009), metal–organic frameworks (MOFs) (Sabouni et al. 2014; Fu et al. 2016; Kim et al. 2016a; Mohamed et al. 2020), and conjugated microporous polymers (CMPs) (Lindemann et al. 2014), was utilized as polymer selective layer material in CO2 gas separation which obtained amazing results, i.e., gas separation performance of these membrane materials goes to beyond the Robeson upper bound. Further, the continuity of this field researcher demonstrated the consequence of gas separation efficiency of the glassy polymeric membrane with the preparation method. Numerous studies (Lundy and Cabasso 1989; Chern and Wu 1997; Petersen and Peinemann 1997; Zhao et al. 2006; Teramae and Kumazawa 2007; Li et al. 2013a) explore the gas separation efficiency of glassy polymeric composite membranes improved by using different preparation methods (spin coating (SP), interfacial polymerization (IP), plasma polymerization (PP), lamination (L), co-extrusion (Co-ex), etc.). For example, Chern and Wu (1997) identified the composite membranes (polyamide membranes) with interfacial polymerization process display a better gas separation performance than some other methods (solution coating (SC) or film casting (FC), pouring (P)), because of the composite membrane produced from interfacial polymerization process has an ultra-thin selective layer thickness range from 1 µm to few nano-meter scales. Moreover, the reaction condition and reagent concentration provided the well-defined structural backbone on the polymer selective layer, which ensures the maximum gas transportation and better gas separation efficiency in membrane fabricated at IP method. Similarly, Teramae and Kumazawa (2007) and Lundy and Cabasso (1989) demonstrated that the higher gas separation efficiency contains an advanced ultra-thin (less than a 100 nm) polymer selective layer developed by plasma polymerization and lamination process.
Though the glassy polymeric materials step forward to better CO2 gas separation, the problem arises in form of CO2 plasticization and quick physical aging during the gas transportation (Zhou et al. 2003). There are several ways to suppress the CO2 plasticization and aging of glassy polymeric membranes like thermal treatment (Scholes et al. 2010a; Hernández-Martínez et al. 2018), cross-linking (Liu et al. 2003; Zhou et al. 2003; Hillock and Koros 2007), introducing additional functional groups, and blending of other polymers materials (Rhim et al. 2000; Hamad et al. 2001; Reid et al. 2002; Kapantaidakis et al. 2003; Hosseinia et al. 2010; Fernández-barquín et al. 2016; Kim et al. 2016a, b, c; Bei et al. 2021). In the meantime, they also considered a solution for improving the membrane CO2 gas separation performance. For example, Scholes et al. (2010a) study the CO2 plasticization effect of PSf/PTFE composite membrane at four different temperatures. It is observed that the plasticization potential of CO2 decreases with increase temperature. Hillock and Koros (2007) developed the chemically cross-linked polyimide membrane for CO2/CH4 gas separation. The cross-linked membrane exhibited greater plasticization resistance against CO2; moreover, they have shown significantly higher gas separation efficiency than the un-cross-linked membrane.
Likewise, Hosseinia et al. (2010) observed that the blending of poly(benzimidazole) (PBI) into polyimide composite membrane exhibited good resistance toward CO2-induced plasticization and physical aging. Another studies with surface functionalization of sulfonated polyphenylene oxide (S-PPO) composite membranes with different metal atoms (monovalent (Li+, Na+, K+), divalent (Mg2+, Ba2+, Ca2+), and trivalent (Al3+)) proved that the metal atom surface modification enhanced the gas separation performance and stability (Rhim et al. 2000) and (Hamad et al. 2001). Similarly, Reid et al. (2002) reported the poly(3-(2-acetoxyethyl)thiophene) (P3AcET)-based composite membrane enriched the gas separation performance and stability on surface modification with acid and base catalytic treatment. In present days, polymeric materials are modified with sterically hindered compounds facile to reduce gas-induced plasticization and physical aging (He et al. 2015).
The physicochemical modification of glassy polymer provided some solution to the CO2 plasticization and physical aging, but the gas permeability (gas transportation) further reduced, which makes several glassy polymeric composite membranes step backward for effective CO2 separation (Scholes et al. 2008). Hence, new approaches are needed to improve the gas transportation of glassy polymeric membranes. The gas transport in glassy polymeric composite membranes is influenced by the number of polymer properties, such as polarity, free volume content, crystallites, average molecular weight, glass transition temperature (Tg), degree of crystallization, degree of polymerization, interchain spacing, chain orientation, composition, and defects (Marek et al. 1996; Orlov et al. 2003; Park et al. 2003; Sridhar et al. 2007b; Yampolskii 2012; Taniguchi et al. 2017; Polevaya et al. 2019). For example, the material contains a crystalline domain which adds a tortuosity factor to gas diffusion as a resulting difficulty to gas transport through this material (Yasuda and Tsai 1974). Similarly, the polymer contains highly polar groups like ether oxygen, acetates, carbonates, and nitriles to increase the solubility of polar gases (CO2 and SO2) (Hamad et al. 2001)(Park et al. 2003). In other words, polymer side-chain functionalization creates a high free volume polymer matrix, which contributes more selective gas diffusion to the polymer matrix (Polevaya et al. 2019). On the other hand, the gas diffusivity is related to polymer chain flexibility, and the polymer chain flexibility is directly linked with the Tg value of the polymer matrix (Yampolskii 2012). From the above statements, it was observed that the negative performance of the glassy polymeric membranes changed by changing the polymer properties chemically or physically. Polevaya et al. (2019) demonstrate the chemical modification of glassy polymer of poly(1-trimethylsilyl-1-propyne) (PTMSP) side chain with ionic liquids produce the well-defined structural CO2-selective polymer network, which is more specific to.
CO2 gas transport rather than N2 and CH4. Moreover, the membrane stability is enhanced with ionic liquid side-chain functionalization. Similarly, Park et al. (2003) reported that the different polar groups (hydroxyl and carboxyl contains diamines of 2,2-bis(3-amino-4-hydroxyphenyl)hexafluoropropane (BAPAF), 2,4-diaminophenol dihydrochloride (DAP), 3,5-aminobenzoic acid (DABA)), functionalization of 6FDA (2,2-bis(3,4-dicarboxyphenyl)hexafluoropropane dianhydride)-based polyimides, and composite membrane amend the gas separation performance with respect to the different polar groups. Marek et al. (1996) stated interfacial polymerization with different monomer precursor combinations makes ultra-thin polyimide composite membrane composed of various chain flexibilities, packing density, and fractional free volume. The chain flexibility, packing density, and fractional free volume play a major role in gas transportation, which was varied with respect to the side-chain length of the monomer precursor. It has been reported that the lower side-chain precursor produce a more ridged polyimide network on the support substrate, which makes less gas transport and more gas pair selectivity, whereas in the case of long side-chain precursor more flexible to form extensive chain packing and facilitate more gas transport and favor higher gas permeability.
Many researchers considered various aspects to improve the gas separation performance of the glassy polymeric composite membrane by blending of polymers and inorganic particle into polymer matrix which display some positive results. Further developments focused on hybrid membranes (glassy polymers with inorganic particles or other polymers) for effective CO2 gas separation process (Basu et al. 2010b; Hosseinia et al. 2010; Sadeghi et al. 2011; Koschine et al. 2015; Friebe et al. 2016; Kim et al. 2016a, b, c, 2018; Mei et al. 2020). Friebe et al. (2016) prepared the NH2-MIL-125 MOF and Matrimid polymer composed of mixed matrix thin film composite membrane (MM-TFCM) for H2/CO2 separation expressed the gas separation efficiency at the higher temperature of 150 °C. Moreover, the gas separation performance of the MM-TFC membrane exceeded the H2/CO2 Robeson trade limit. Besides, free NH2 groups presented on pore system major contributor to the better gas separation performance. Another work by Awad and Aljundi (2018) prepared a carbide-derived-carbon filler (CDC) incorporating polyamide/PSf composite membrane by interfacial polymerization process with piperazine (PIP) and isophthaloyl chloride (IPC) precursors. The prepared membrane improved the gas separation performance with respect to the increment of CDC conc. In addition, the layer-by-layer (LB) deposition of the IP process declines the gas permeance and upturns the gas pair selectivity. Likewise, the pressure and temperature studies also revealed the better gas separation efficiency of the prepared MM-TFC membrane.
Further, the gas separation performance of glassy polymeric MM-TFC membranes is well footprinted in literature (Awad and Aljundi 2018; Hosseinia et al. 2010; Friebe et al. 2016; Mei et al. 2020), but the problems arise in the form of interfacial vide between glassy polymers and inorganic particles declined the gas separation performance (gas pair selectivity), which might be the reason to the poor adhesive nature of most inorganic particles into glassy polymers. Some studies have proven that the physicochemical modification of the filler particles (filler particles surface modifications with appropriated functional groups) improves the adhesive between polymer and filler particle, consequence of non-appearance of interfacial vide, and enhancement in gas separation efficiency (Khan et al. 2012; Kinoshita et al. 2017; Aliyev et al. 2018; Fauzan et al. 2020; Kanehashi et al. 2015). Khan et al. (2012) demonstrated that the MWCNTs + PIM-1-based MM-TFC membrane enhances the CO2 solubility selectivity and good interfacial adhesive between polymer and filler particles by on PEG functionalization of MWCNTs surface. Kinoshita et al. (2017) reported that the polyhedral oligomeric silsesquioxane (POSS) composed of PIM-1-based molecular sieving MM-TFC membrane possess superior gas separation for H2/CO2 mixture and well-defined interfacial interaction between polymer and POSS nanoparticles by amine and nitro functionality modification of POSS particle. Moreover, the additional functionalization of POSS particle retards the physical aging phenomenon of the MM-TFC membrane. Likewise, some other studies (Aliyev et al. 2018; Chung et al. 1999; Fauzan et al. 2020; Kanehashi et al. 2015; Kim et al. 2003, 2016a, b, c; Kusakabe et al. 1996; Sanchez-Lainez et al. 2018a, b; Suzuki et al. 1998) also demonstrated and obtained the moderate results on that physicochemical modification of polymer and filler particle in the glassy polymeric composite membrane, but still upgrading needs composite membranes with glassy polymer for real industrial CO2 gas separation application (Lillepärg et al. 2014).
Co-polymeric composite membrane in CO2 gas separation
Further to the growing concern of solution-diffusion polymeric composite membrane in CO2 gas separation, the researchers establish new aspects by using co-polymers as a polymer selective layer (Blume and Pinnau 1990; Reid et al. 2002; Liu et al. 2004). Blume and Pinnau (1990) patented the first co-polymer-based composite membrane in gas separation in 1990. While using co-polymer as selective layer material, the inherent property of copolymers such as both rubbery soft segment and glassy hard segment make an ultra-thin film and well-defined structural backbone for better gas transport pathway on hard support. This contributes to the superior gas separation performance (permeability and selectivity) via both solubility and diffusivity bases. Another advantage of co-polymers is that it exhibits desirable mechanical and thermal stability over a long period (Liu et al. 2004). These aspects were motivating the researchers to make several co-polymeric composite membranes in CO2 gas separation and thus strengthened the field of the co-polymer composite membrane in the gas separation process (Liu et al. 2004, 2005; Sridhar et al. 2007c; Yave et al. 2009, 2010b; Ji et al. 2010; Ren et al. 2012; Khalilinejad et al. 2015; Karunakaran et al. 2017). The currently existing various copolymers composed of composite membranes and their gas separation performance are provided in Table 3. It is clear that most of the copolymer used in selective layer formation was the soft or rubbery segment that encompasses ether-oxygen groups and hard segments designed by amine groups (Liu et al. 2004). These ether-oxygen and amine groups are more CO2-philic in nature and trigged the CO2 gas molecular transport more and specifically. This is an important key point for using copolymer for membrane CO2 gas separation (Sridhar et al. 2007c). In literature, these functionality bases are generally named as PEBAX (Marcq et al. 2005; Zhao et al. 2008; Sridhar et al. 2007c). Liu et al. (2004, 2005) prepared a composite membrane composed of PEBAX 2533/PSf and PEBAX 2533/PEI thin-film hollow fiber composite membranes that show remarkable performance in CO2/N2 gas separation. Similarly, Sridhar and co-workers (Sridhar et al. 2007a) prepared another kind of PEBAX (PEBAX 1657)/PVDF composite membrane for CO2/CH4 separation. The performance of PEBAX 1657/PEI and PEBAX 3533/PEI thin-film hollow fiber composite membranes for sour gas separation (SO2/CO2/N2) was also examined by other research groups (Kim et al., 2013a, b and Ren et al. 2012). The membrane shows stable and vestal gas separation performance at even the presence of other impurity gases. Similarly, some other studies (Car et al. 2008; Ahmadpour et al. 2014; Khalilinejad et al. 2015; Scofield et al. 2015) also found that the PEBAX composed of co-polymeric composite membrane offers better performance in CO2 gas separation.
Apart from PEBAX co-polymer, Yave and co-workers (Yave et al. 2010a) prepared a polyethylene oxide-polybutylene terephthalate (PEO-PBT)-based co-polymeric composite membrane for CO2 gas separation in various gas mixtures (N2, CH4, and H2). The prepared membrane exhibits outstanding gas separation performance and stability. Gu and Lodge (2011), synthesized the triblock co-polymer ion gel composite membrane composed of poly (styrene-b-oxy-ionic liquid-amide-b-styrene)/PVDF for CO2 gas separation from N2 and CH4 gases. The membrane parades higher gas separation efficiency and more stability in a given environment. Ji and co-worker (Ji et al. 2009, 2010) prepared a new kind of PDMAEMA-PEGMEMA/PSf co-polymeric composite membranes for CO2/N2 separation via interfacial polymerization process; the membrane exhibits better gas separation efficiency along with good CO2 plasticization resistance and physical aging. Another work by Karunakaran et al. (2017) introduced a PAN-r-PEGMA/PAN co-polymeric composite membrane for CO2, N2, CH4, and H2 separation; the membrane shows remarkable stability.
Based on the various studies, it can be concluded that the numerous co-polymers composed of composite membrane show higher gas separation efficiency and thus occupied the top position in Robeson upper bound 2008 compared to glassy and rubbery polymers (Ji et al. 2010; Yave et al. 2010a). But still they have some stability issues that need to be addressed, namely physical aging and the CO2 plasticization phenomenon. Cross-linking the polymer network (Sridhar et al. 2007c) or changing the functionality of co-polymer segments with different functional groups (metals, amino, imido, fluro, etc.) addresses the aforementioned issues. Wang et al. (2006) prepared a CO2-philic co-polymeric composite membrane composed of poly(N-viny-γ-sodium aminobutyrate-co-sodium acrylate) (PVSA-SA)/PSf. The membrane displays outstanding performance with good stability against CO2 plasticization in CO2/CH4 mixture. Shen et al. (2006) developed the metal functionalized poly(2-N,N-dimethyl aminoethyl methacrylate-co-acrylic acid sodium) (PDMAEMA-Na) co-polymer shown there effective CO2/CH4 separation performance and metal substitution increased the stability and gas transportation of CO2 gas molecules with a specific interaction. Similarly, fluorinated poly(ethylene glycol)-block-poly(pentafluoropropyl acrylate) diblock copolymer network for CO2/N2 gas separation was synthesized by another group (Scofield et al. 2016). After fluorination of co-polymer network enhanced the membrane stability against temperature, pressure, and CO2 plasticization. Nguyen et al. (2013) synthesized the alkyl-imidazolium-based RTIL block copolymer (BCP) composite membrane for CO2/N2 gas separation application. They claimed alkyl and imidazolium combination created the well-ordered nano-structural polymer network and the formed nanostructure greater affinity with CO2 gas molecules, which enhanced the CO2 gas transportation through this polymer network.
Moreover, the form ordered nanostructure stable against CO2 plasticization and physical aging problems. Some other studies also reported the improvement in the membrane stability and performance by changing the functionality of the co-polymer segment (Yave et al. 2010a; Zhu et al. 2015; Karunakaran et al. 2017).
Though the physical aging and CO2 plasticization problems of co-polymer were minimized by changing the functionality of the co-polymer segment, still the issues were not toggled fully. Further, the researcher identified another solution to improve the gas separation performance and stability of co-polymer composite membrane by blending of polymers or inoculation of inorganic particles into the copolymer matrix and multilayer formation (Sridhar et al. 2007c; Murali et al. 2010; Li et al. 2013a; Halim et al. 2014; Scofield et al. 2015, 2016; Peng et al. 2017; Sarmadi and Salimi 2020; Taheri et al. 2021). In the above former cases (blending or inoculation), extra functionality arises to the co-polymer matrix which promotes the solubility and diffusivity of CO2 gas molecules and also enhanced the membrane stability; on the other side of latter cases (multilayer formation), it helps to make a defect-free ultra-thin polymer selective layer. The identification of suitable polymer blend, inorganic particles, and gutter or productive layer material are more essential to the effective membrane preparation because the right polymer blends or inorganic particles facilitate the greater gas separation efficiency as well as improve the thermal, mechanical, anti-plasticization, and physical aging characteristic of the copolymer matrix (Ji et al. 2009, 2010; Yave et al. 2010a; Jomekian et al. 2011, 2017a; Kim et al. 2013a, b; Scofield et al. 2015; Karunakaran et al. 2017). The effective blending of polymer (e.g., PDMS, PEG, PEI, PI) into co-polymer matrix considers as fine miscible of polymers on a molecular level and possesses both good mechanical strength and gas separation efficiency (Car et al. 2008; Ji et al. 2009; Yave et al. 2009, 2010a; Kim et al. 2013a, b; Ahmadpour et al. 2014). Similarly, incorporation of filler particles (e.g., metal oxide, zeolites, CMS, CNTs, MOFs, and ZIF) to the copolymer matrix needs good adhesive nature, a defect-free filler–polymer interface which after greater CO2 gas separation efficiency (Murali et al. 2010; Jomekian et al. 2011, 2017b; Li et al. 2013a, b, c; Fu et al. 2014; Chen et al. 2015; Friebe et al. 2016; Dai et al. 2019a). Many studies proved that blending of polymer or addition of filler to the co-polymer matrix enhanced the CO2 gas separation efficiency with good stability. The separation performances of blend and multilayer co-polymeric membranes are also listed in Table 3.
Sridhar et al. (2007a) produced the silver incorporated PEBAX2533/PVDF co-polymeric composite membrane for CO2/CH4 separation. The Ag- incorporation enhanced the gas pair selectivity compare to pure PEBAX2533 and also the gas separation efficiency of membrane feasible for commercial purposes. Murali et al. (2010) prepared composite membranes composed of MWCNTs-PEBAX 1657/PSf, which shows remarkable stability against thermal and CO2 plasticization as well as good gas separation efficiency after incorporation of MWCNTs. Moreover, the authors claimed that the gas separation efficiency of modified co-polymer membrane proved their suitability in various gas separation applications, such as CO2/N2 separation in the power plant industry, H2/NH3 for purge gas recycling, O2 from the air in medical application, and CO2 from water gas shifting for H2 purification. Similarly, Li et al. (2013b) prepared the ZIF-7 nanoparticles incorporated into PEBAX1657/PAN composite membrane which exhibit good separation efficiency, as well as better mechanical stability in CO2/CH4 gas mixture combination. Another work by Fu et al. (2014) introduced the PEG-based cross-linked soft nanoparticles incorporated into the PEBAX2533/PAN composite membranes for CO2/N2 separation. The membrane exhibited the highest CO2 permeability with reasonable selectivity and the performance of the membrane denoted membrane suitable for up-scaling in post-combustion carbon capture, such as power plant CO2 capture. Further, research continued with the blending of polymer. Halim et al. (2014) prepared a co-polymeric composite membrane composed of PEGand PEG-b-PDMS polymer blend PEBAX2533/PAN showing remarkable CO2 flux after blending of polymer, and the membrane shows long time stability in CO2/N2 separation. Similarly, Car et al. (2008) prepared a PEG200 blended PEBAX1657/PAN co-polymeric composite membrane for CO2/N2/H2 separation; the membrane exhibited good gas separation efficiency in both CO2/N2 and CO2/H2 gas mixture up to 20 bar. Yave et al. (2010a) introduced the different types of PEGpolymer blended PEO-PBT/PAN co-polymeric composite membrane which show outstanding CO2 separation performance over N2 and H2. The gas separation performances of this new kind of polymer combination boost up the co-polymeric composite membranes for commercial purposes. Similarly, some other studies (Anson et al. 2004; Kim et al. 2006; Yave et al. 2009; Chen et al. 2015; Shen et al. 2016; Park et al. 2019; Sanchez-Laınez et al. 2019; Jomekian et al. 2020; Scofield et al. 2015, 2016) proved that the blending or incorporation of inorganic particles, such as activated carbon (CA), MOF, ILs, PEG, PEG-b-PPFPA, and ZIF, into co-polymer network, improves their gas separation performance and stability. The overall literature studies denoted that the blending or incorporation polymer or inorganic particle into co-polymer network offers essential membrane stability without compromising the gas separation ability, thus literally membranes are gone for behind the Robeson upper boundary 2008.
The aforementioned different strategies improve the gas separation performance and long-term stability of membrane (anti-CO2 plasticization and physical aging), but the improvements in CO2 gas separation performance are not adequate to replace the currently existing amine absorption process (Yang et al. 2008; Basu et al. 2010b; Widjojo et al. 2012). Further, researchers realize that the polymeric composite membrane with solution-diffusion mechanism is still not much approachable for effective industrial CO2 gas separation process in later research. Hence further research focused on an alternative solution to overcome the persisting problems than the researchers’ move to facilitate transport phenomenon in CO2 gas separation.
Facilitated transport polymeric composite membranes (FTPCMs)
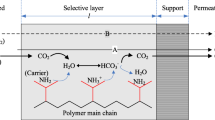
The idea of facilitated transport is originated from the biological process since the molecular transport from the bloodstream into cells is accomplished by the facilitated transport phenomenon. Thereby researchers applied the concept of facilitated transport phenomenon in polymeric composite membranes for various gas separation processes (CO2/N2, CO2/CH4, H2/CO2, O2/N2, etc.) (Yamaguchij et al. 1995; Kim et al. 2004; Dong et al. 2006; Sandru et al. 2010; Yu et al. 2010; Yao et al. 2012; Chen et al. 2016; Prasad and Mandal 2017; Saeed et al. 2021). In the gas separation process, FTPCMs exhibit fairly higher CO2 permeability and selectivity than the solution-diffusion polymeric composite membrane. Because the polymer selective layer contains the CO2 reactive carrier molecules in their backbone trigger the CO2 gas transport by both facilitation transport and solution-diffusion manner. While the non-interacting gases (N2, H2, and CH4) are transported only by the solution-diffusion, which is facile to achieve high CO2/light gas selectivity without sacrificing permeability (Scholes et al. 2008; Rafiq et al. 2016; Maheswari and Palanivelu 2017). In the following functional groups, -NH2, PO43−, F−, CO32−, and COO−, some metals (Ag, Zn, Fe, etc.) are employed as CO2 reactive carriers in many pieces of literature (Matsuyama et al. 1999a; Kim et al. 2004; Wang et al. 2007; Bara et al. 2010; Mao et al. 2011; Yao et al. 2012; Jie et al. 2015; Tomé et al. 2015; Shankar and Kandasamy 2019). Amongst the functional groups, amine (-NH2) is considered as most effective CO2 reactive carrier in FTPCMs for gas separation process (Yamaguchij et al. 1995; Kim et al. 2004; Dong et al. 2006; Yu et al. 2010; Yuan et al. 2011; Shen et al. 2015; Prasad and Mandal 2017). Due to its fast CO2 reaction rates, high absorption capacity, and ease of regeneration, the CO2 gas transportation of amine CO2 carrier presented polymers is accomplished by a reversible reaction manner. From the literature, it is observed that possible reversible reactions between CO2 and amine carrier molecules present in the polymer selective layer are given below (Eqs. 1, 2, 3, 4, and 5).
Primary amine:
Secondary amine:
Tertiary amines:
The reversible reaction between CO2 vs primary (Eqs. 1 and 2) and secondary amines (Eqs. 3 and 4) functionality polymer selective layers is happening by carbamate intermediate ion formation in dry state and bicarbonate intermediate formation in a wet state. Whereas in the case of reversible reaction between CO2 vs tertiary amines (Eqs. 5), functionality polymer selective layers are deferent from primary and secondary amines, the formation of carbamate intermediate ion is not possible in a dry state, an alternative bicarbonate formation is happening at wet state, and so tertiary amines in the CO2 reversible reaction are only possible at the presence of water content. Therefore, the performance of ternary amine composed polymeric composite membranes is much more dependent on the environment humidity. Further information about the facilitated transport membrane is found in the reviews of Rafiq et al. (2016) and Maheswari and Palanivelu (2017).
Normally, polymers used in FTPCMs are categorized into two types: fixed-site polymers and hydrogel polymers. Based on the polymer used, the FTPCMs are classified as fixed-site polymeric composite membrane and hydrogel polymer composite membrane. The recent research developments in the area of the fixed site and hydrogel polymeric composite membranes for CO2 gas separation are discussed in a further session.
Fixed site polymeric composite membranes for CO2 gas separation (FSPCMs)
Over the decades, more than thousands of papers have been published in the field of a fixed-site polymeric composite membrane in gas separation applications. Various FTPCMs and their gas separation performances are furnished in Table 4. Through the literature polyvinyl amine (PVAm), polyallylamine (PAAm), polyethyleneimine (PEI), chitosan, PAMAM, and amine-functionalized co-polymers are the most used fixed-site polymers in CO2 gas separation application (Matsuyama et al. 1999b; Kim et al. 2004; Yu et al. 2010; Duan et al. 2012; Zhao and Ho 2012; Li et al. 2015b; Shen et al. 2015). Certain literature described these fixed-site polymers cannot sustain without support material and they are stable in composite form because the unsupported polymers are sticky and less permeable; moreover, they are not able to stand in self-standing form during the operation conditions (Matsuyama et al. 1999b; Kim et al. 2004; Duan et al. 2012; Zhao and Ho 2012). For example, Kim et al. (2004) reported that the FT polymer of PVAm is a topmost CO2 carrier polymer; however, its nature is like a high viscosity fluid to sticky the self-standing form; hence, they are not capable to handle without support materials. On the other hand, a lot of FTPCMs drop their better gas separation ability in a short time period, due to some interior characteristics (crystallinity, free volume, CO2 and carrier reaction rate, etc.) of the polymer matrix. Some studies proved that this may be overcome by using different support materials (Kim et al. 2004; Sandru et al. 2010), cross-linking, and copolymerization with various CO2 selective molecules (Zhang et al. 2002; Kim et al. 2004; Dong et al. 2006; Yi et al. 2006; Li et al. 2015b; He et al. 2016), different treatment process (pH control (Kim and Lee 2013), preparation method (Yu et al. 2010)), and operation mode (sweep or vacuum mode) (Sandru et al. 2010). For example, Kim et al. (2004) examined the PVAm composite membrane for CO2/CH4 separation with the different cross-linking agents and various support materials. Among the various cross-linker and support materials used, ammonium fluoride (NH4F) and PSf support show remarkable higher performance, due to the higher electronegativity of the fluoride ion anticipated more CO2 facilitated transport through the PVAm membrane; moreover, the film formation of NH4F/PVAm polymer composition is better on PSf support surface. Similarly, Dong et al. (2006) and Yi et al. (2006) studied the GA cross-linked PVAm/PSf hollow fiber membrane for CO2/CH4 separation. The cross-linked membrane shows well separation efficiency along with good stability (6 days stable performance) at lower pressure range (96 cmHg). In another work, Kim et al. (2013a, b) demonstrated the CO2/N2 separation efficiency of PVAm/PSf composite membranes with three different pH of PVAm casting solution. From the experimental results, they observed that pH of casting solution directly influence the membrane characteristic (degree of free amine groups and viscosity of casting solution) and CO2 gas separation efficiency. The higher pH of casting solution increase the degree of free amine groups, which facilitated more CO2 transport through the membrane; likewise, the lower pH of the casting solution increase the viscosity of casting solution, thus reducing the gas transportation. Yu et al. (2010) prepared a novel amine contains fixed-site membranes via an interfacial polymerization on porous polysulfone support (DNMDAm-TMC/PS) for CO2/N2 and CO2/CH4 mixture separation. The membrane formed ultrathin (l = 300 nm) defect selective layer shows remarkable higher CO2 permeability and selectivity over CH4 and N2, and the study revealed the performance of membrane depending on concentration of TMC in IP process.
Li et al. (2015b) synthesized a new kind of co-polymer as a selective layer for the separation of CO2 from N2, CH4, and H2 mixture. The membrane material contains three types of CO2-reactive molecules (primary amino groups, quaternary ammonium groups, and carbonate groups) that make more CO2 facilitation to the membranes. Further, the study exposed membrane has long-term stability and resistance to impurity gases. Similarly, other studies (multilayer composite membranes formation (Li et al. 2015a), sterically hindrance (Zhao and Ho 2012), and different support usages (Kim et al. 2004; Sandru et al. 2010), implied the stability of facilitated transport composite membranes. Later researchers found the aforementioned process addresses the stability issues of facilitated transport polymeric composite membrane, but the gas separation efficiency is not adequate to beat the existing trade-off relationship between permeability and selectivity. This was over-come by immobilization of mobile carriers (small organic and inorganic molecules that possess CO2 facilitation transport nature, e.g., amines, amino alcohols, salts of amino acids, dendrimers, enzymes, metal carbonates, and hydroxide) into polymers of fixed-site carrier composite membranes.
A moment ago, many authors (Yamaguchij et al. 1995; Yi et al. 2006; Duan et al. 2008; El-azzami and Grulke 2009; Yuan et al. 2011; Zhao et al. 2012; Qiao et al. 2013; Jie et al. 2015; Chen et al. 2016; Yu et al. 2016; Prasad and Mandal 2017) demonstrated that the addition of mobile carrier into the polymer matrix enhanced the membrane permeability and selectivity, because the addition of mobile carrier increased the number of CO2 reactive site and also easily move CO2-carrier complex through the membrane, which resulting higher gas transport and higher gas separation efficiency. Massive mobile carrier incorporated facilitated transport polymeric composite membranes drop their gas separation performance earlier during continuous operation, due to the carrier loss and degradation of the mobile carrier at given operation condition (El-azzami and Grulke 2009)(Yuan et al. 2011). From an applications’ point of view, selection of suitable membranes are based on separation ability along with stability and lifetime of membrane. Therefore, the researcher step forwarded to the grownup the improvement of stable and longer lifetime of mobile carrier containing FTPCMs for CO2 gas separation application. Then they are introduced hyperbranched or sterically hindered molecules to attain the maximum carrier stability in a polymer matrix (Yu et al. 2011; Zhao and Ho 2012; Duan et al. 2014; He et al. 2015). Alternatively, the researchers (Yi et al. 2006; Cai et al. 2008; Deng et al. 2009; Zhao et al. 2012, 2013, 2015b; Shen et al. 2013, 2015; Casado-Coterillo et al. 2015; Li et al. 2015a; Wang et al. 2015; Prasad and Mandal 2017; Bharali et al. 2020) identified the blending of another polymer or inorganic particles into the facilitated polymer matrix enhanced both separation efficiency and membrane stability.
Yi et al. (2006) prepared a PEG blended PVAm/PES facilitated transport composite membranes for CO2/CH4 separation. The author observed the addition of PEG decreases the crystallinity of the PVAm matrix, thus increasing mechanical stability and gas separation ability. Similarly, Shen et al. (2013) investigated the CO2/N2 separation ability of PEI blended carboxymethyl chitosan/PSf membrane. The author indicated that this membrane shows remarkable higher separation performance along with good stability than the other facilitated transport membranes. Another work by Zhao et al. (2012, 2013) demonstrated the incorporation of polyaniline nanoparticles (PANI nono particles) into the PVAm matrix, and the membrane displays higher CO2 permeance (up to 1200 GPU) and higher CO2/N2 selectivity (up to 120). Moreover, the performance deterioration was not observed up to 300-h operation; hence, the study denoted membrane has on long time stability and suitable for effective CO2 separation. Similarly, some other research works also performed with different polymer and inorganic materials incorporated fixed-site facilitated polymeric composite membrane for CO2 gas separation applications in various gas mixtures, and they are shown well throughput in given operation conditions (Gao et al. 2018; Han et al. 2018; Wang et al. 2019, 2020; Xu et al. 2019; Janakiram et al. 2020a; Zhang et al. 2021). Most recently, some studies demonstrated the facilitated polymeric composite membranes in a field trial in the form of high membrane surface area comprised hollow fiber and spiral wound modules (Salim et al. 2018; Dai et al. 2019c; Han et al. 2019a; Janakiram et al. 2020b). The authors observed the outstanding gas separation performance with long-term stability in-field operation; thereby, they suggested FTPCMs are a good candidate for industrial CO2 gas separation operation.
Hydrogel polymeric composite membrane (HPCMs) in CO2 capture
On the other side, researchers demonstrated that hydrogel polymeric composite membranes are another class of facilitated polymeric composite membranes used in CO2 gas separation (Bai et al. 1988; Ito et al. 1997; Bae et al. 1998; Matsuyama et al. 1999b; Zhang et al. 2002; Adams et al. 2011; Mao et al. 2011; Liu et al. 2014; Saedi et al. 2016; Jahan et al. 2018; Borgohain and Mandal 2019; Lee and Kang 2020). In HPCMs, the polymer selective layer is made of hydrogel polymeric materials. Normally, hydrogel polymeric materials are defined as cross-linked hydrophilic polymer network (cross-linked polyvinyl alcohol (Moulay 2015), polyvinyl acetate (PVAc) (Semsarzadeh and Ghalei 2012), polyvinyl pyrrolidone (PVP) (Wenthold et al. 1987), chitosan (Ito et al. 1997), and cellulose (Mao et al. 2011)), which is swelling significantly in presence of solvent (water as a common solvent) but not dissolving to it (Scholes et al. 2008). The various works on hydrogel polymeric composite membranes are provided in Table 5. Generally, in hydrogel polymeric composite membranes, gas separation ability is poor in dry condition, but after being swollen by water, the performance of the membranes is remarkably high which indicated water molecules one of the key crucial in hydrogel membranes for gas separation process (Ito et al. 1997; Bae et al. 1998; Deng and Hägg 2010; Mao et al. 2011; Mondal and Mandal 2014a; Saedi et al. 2016). For example, Ito et al. (1997) reported that the CO2 permeability of the chitosan membrane is very lower (0.4 barrer) in a dry state, but after being swollen by water, the CO2 permeability increased subsequently (up to 200 barrer at 20 °C). Here they observed the swollen membranes contains bounded or sorbed water molecules, which disturbed the chain packing of polymer matrix as a resultant broaden the free-volume (gas transport channels) of CS polymer network and facilitate more gas transport through the CS matrix. From that, they are concluded that water molecules play an important role on CO2 gas transport through hydrogel polymeric composite membranes. Further studies explore the water molecules take part in this process are classified in two types: free water molecules and bound water molecules. The CO2 gas transportation in free water molecule is assisted by solution-diffusion whereas in the bound water molecule it is mainly by facilitated transport. Liu et al. (2008) discover the gas transport of hydrogel polymeric composite membranes anticipated by dual ways; one is channel transport, and the other is water passage way. Increase in the CO2 gas separation performance could be achieved by constructing more number of channels to the polymer matrix as well as increase in the amount of water content during the process in HPCMs.
Though the higher CO2 permeability is obtained in hydrogel polymeric composite membrane with high water content, on the other side problem facing by falling down the gas pair selectivity and membrane stability by excess water content, this can be overcome by controlling the degree of cross-linking to the polymer matrix with effective techniques (Philipp and Hsu 1979). Nevertheless, the higher degree of cross-linking makes membranes brittle and hard to handle, which may also aid poor performance (CO2 permeability) of hydrogel polymeric composite membrane in CO2 gas separation (Huang et al. 2008; Deng et al. 2009; Uma Maheswari and Palanivelu 2017). Further, an improvement in the gas separation performance of the hydrogel membrane is observed by the addition of a CO2 facilitating carrier (amine, amino acids, liquid enzymes, etc.) to the hydrogel polymeric network.
The gas permeation results of some CO2 facilitating carrier incorporated HPCMs are listed in Table 5. In recent times, various authors (Francisco et al. 2007; El-azzami and Grulke 2008; Huang et al. 2008; Yao et al. 2012; Saeed and Deng 2015; Yu et al. 2016; Uma Maheswari and Palanivelu 2017; Dai et al. 2019b; Klemm et al. 2020; Lee and Kang 2020) demonstrated that the addition of CO2 facilitated carriers (liquid enzymes, both fixed and mobile carriers) into hydrogel polymer network enhanced the CO2 permeability and selectivity. For this, enhanced gas separation efficiency denoted HPCMs as a good candidate for CO2 gas separation application, but still, there are some limitations alleviated in terms of stability issues (instability of CO2 facilitating carriers into hydrogel polymer matrix) not able to go for field application. Further researches address the existing carrier instability problems via the addition of some inherent properties (low volatile, high polarity and ionic conductivity, thermal stability, steric hindrance, etc.) which contain ILs (Casado-coterillo et al. 2014) (Kamio et al. 2020b)(Klemm et al. 2020) and hyperbranched dendrimer networks (Kai et al. 2008)(Duan et al. 2012) (Kunalan et al. 2021) as CO2 facilitating carrier into the hydrogel polymer matrix. The aforementioned solution has not fully resolved the instability problems of CO2 facilitated carriers. Further, researchers (Wang et al. 2007; Kouketsu et al. 2007; Duan et al. 2012, 2014; Deng and Hägg 2014; Ansaloni et al. 2015; Shen et al. 2015; Casado-Coterillo et al. 2015; Saedi et al. 2016; Jahan et al. 2018; Borgohain and Mandal 2019; Torstensen et al. 2019; Lilleby Helberg et al. 2020) established that another aspect by blending of polymers or inorganic particle into HPCMs enhanced the gas separation performance without compromising the membrane stability.
Shen et al. (2015) prepared a PEI hyperbranched GO nanofiller incorporated PVAm-CS/PSf composite membranes for CO2/N2 separation. The HBPEI-GO-PVAm-CS/PSf membrane shows double the performance than PVAm-CS/PSf membrane, which may happen by GO addition providing more CO2 channel to the PVAm-CS/PSf composite membrane. Moreover, the stability study revolved the membrane possesses in long time stability against given operating conditions. Similarly, Ansaloni et al. (2015) demonstrated that the amine-functionalized MWCNT incorporated into cross-linked polyvinylalcohol-polysiloxane/amine blend/PSf composite membrane for CO2 separation from H2/N2/CH4 mixture. The authors observed that the gas separation performance of the MMM-composite membrane was incredibly higher after the addition of MWCNTs. Moreover, the addition of amine-functionalized MWCNTs enhanced the polymer filler comfortability and mechanical strength remarkably. The highest CO2 permeability and selectivity along with greater mechanical stability offers this MMMs-composite membrane a good candidate for industrial CO2 gas separation application. One more work by Deng and Hägg (2014) developed a CNT-PVAm-PVA/PSf composite membrane for CO2/CH4 separation. The membrane displays long durability with good CO2/CH4 separation ability. Some other studies (Bai and Ho 2011; Duan et al. 2013; He et al. 2014; Zhao et al. 2014; Casado-Coterillo et al. 2015; Saedi et al. 2016; Jahan et al. 2018; Barooah and Mandal 2019; Borgohain and Mandal 2020) also demonstrated the incorporation of filler particle to the facilitated hydrogel polymeric composite membrane for CO2 gas separation from the different gas mixture, and the membranes show remarkably high gas separation performance and stability.
Since the great potential for the development of facilitating transport polymeric composite membranes of fixed-site polymeric composite membranes and hydrogel polymeric composite membranes in CO2 gas separation application, they have some limitations to the application point of view. Due to the higher CO2 partial pressure (above 5 bar), these composite membranes drop their gas separation performance (both permeability and selectivity). On the other side, low CO2 concentration, low CO2 partial pressure, and desirable temperature (up to 100 °C), the FPCMs, and HPCMs show extraordinary gas separation efficiency and membrane stability. In this point of view, the flue gas (10–15%) CO2 capture applications facilitated transport polymeric composite membranes more favorable than conventional solution-diffusion polymeric composite membrane because the pressure and temperature range of flue gas is less than 2 bar and 100 °C. At the same time, the functionalized inorganic filler incorporated facilitated transport composite membranes sustained at higher pressure (up to 30 bar) and higher temperature (150 °C), so the hybrid facilitated transport polymeric composite membranes (HFTPCMs) are suitable for fuel gas separation (biogas, natural gas, syngas).
The merits and demerits of the polymeric composite membranes in CO2 gas separation
The overall investigation on the polymeric composite membrane in CO2 gas separation from various gas mixtures revealed that each type of polymeric composite membrane exhibited different CO2 gas separation efficiency. Table 6 provides information about various polymeric composite membranes’ merits, demerits, and possible ways to overcome the problems.
Whereas in solution-diffusion polymeric composite membranes, the gas separation performance of rubbery polymer materials suggested that the rubbery polymer material is the most suitable for gutter and productive layer formation. On the other side, composite membranes composed with glassy or co-polymers material offer a better gas separation performance by blending with other polymers or inorganic materials (MMMs), but the gas separation performance has not yet reached up to the level of real field application. Further, the composite membrane composed with facilitated transport polymers exhibited the highest CO2 permeability and CO2/light gas selectivity than polymeric composite membranes relating to solution-diffusion. Even though the higher gas separation performance of FPCMs are not yet commercialized due to the gap between lab results and results obtained from the industrial condition though their gas separation performance is favourable. Therefore, the developing facilitated polymeric composite membranes exhibiting a larger CO2 absorption capacity along with a long lifetime at given industrial operation conditions are more preferred for effective implementation in-field application and compete for the current existing CO2 capturing system (amine absorption process).
Critical outlook and the new approaches to improve the existing membrane system
In CO2 gas separation amine-based absorption process well-established technology in over the long period. Nowadays, membrane technology also competes with the current mature technology of the amine absorption process intensively in terms of energy and cost. A review of the overall literature results points out that each type of polymeric composite membrane has some interstice trade-offs like selectivity-permeability, synthesis route, processability, and membrane stability. However, the efficiency of polymeric composite in CO2 captures somewhat greater effort in the current scenario. The well efforts on the polymeric composite membranes in CO2 gas separation yield a good positive result on lab scale, but still, there is a large gap between labs to industrial scale. If the polymeric composite membranes have to compete with the current existing CO2 amine absorption capturing technology, the membrane systems need to improve in the following aspects.
-
✓ Defect-free membrane formation through guttering or productive layer formation and using advanced membrane fabrication system (lamination, plasma polymerization, layer-by-layer deposition, etc.)
-
✓ Hybrid membrane formation is done by the controlled atmosphere, with an appropriate pre-treatment process. Further, the membrane system was adopted with some pre-treatment systems (i.e., gas compression, particulate matter removal, and desulfonation).
-
✓ In most of the studies, the efficiency of polymeric composite membranes was determined only with simple gases streams (single and binary gas mixture), and further research needs to evaluate the effect of traces impurities (NOx and SOx) on gas separation performance.
-
✓ The viability of polymeric composite membranes in real field harsh environmental conditions (e.g., feed gas composition, higher pressure, and temperature) has not been performed systematically, and also the long-term stability of membranes in those conditions has not yet been reported and so the study toward on membrane stability in harsh environmental condition to be addressed.
-
✓ Since in industrial-scale enormous volume of the gaseous stream needs to be processed, at this stage a large membrane area is required for this process, using hollow fiber and spiral wound module satisfied the purpose. But, effective studies have yet not been performed in hollow fiber and spiral wound membrane modules.
If the aforementioned points are addressed systematically, the membrane technology will outperform the current conventional process of the amine absorption process.
Conclusion
Through the literature studies with polymeric composite membrane for CO2 gas separation, the polymeric composite membrane with facilitated transport shown their good throughput in CO2 capture process than solution-diffusion membranes. In the case of SDPCMs, the combination of polymer matrix and inorganic filler (mixed matrix) partially fulfill the required CO2 gas separation ability along with membrane stability, but the gas separation performance is not at the level of facilitated transport polymeric composite membranes. On the other side, FTPCMs well-adapted power plant industrial flue gas CO2 capture, due to suitability of operation environmental (CO2 concentration, partial pressure, and temperature). Further, it is hoped that the polymeric composite membrane better choose to real field CO2 capture in the future. The review discussed all those various kinds of approaches and some of the requirements (defect-free layer formation, impurity present, harsh condition, and module type) that are more important for implementation than only success with the membranes for gas separation process in real field application.
Availability of data and materials
Not applicable.
References
Achalpurkar MP, Kharul UK, Lohokare HR, Karadkar PB (2007) Gas permeation in amine functionalized silicon rubber membranes. Sep Purif Technol 57:304–313. https://doi.org/10.1016/j.seppur.2007.05.002
Adams RT, Lee JS, Bae TH et al (2011) CO2-CH4 permeation in high zeolite 4A loading mixed matrix membranes. J Membr Sci 367:197–203. https://doi.org/10.1016/j.memsci.2010.10.059
Adewole JK and Sultan AS (2019) Functional polymers, polymers and polymeric composites: A reference series. Springer Nature, Switzerland, pp. 941–971
Ahmad AL, Jawad ZA, Low SC, Zein SHS (2014) A cellulose acetate / multi-walled carbon nanotube mixed matrix membrane for CO2/N2 separation. J Membr Sci 451:55–66. https://doi.org/10.1016/j.memsci.2013.09.043
Ahmadpour E, Shamsabadi AA, Behbahani RM et al (2014) Study of CO2 separation with PVC/Pebax composite membrane. J Nat Gas Sci Eng 21:518–523. https://doi.org/10.1016/j.jngse.2014.09.021
Alavi SA, Kargari A, Sanaeepur H, Karimi M (2017) Preparation and characterization of PDMS/zeolite 4A/PAN mixed matrix thin film composite membrane for CO2/N2 and CO2/CH4 separations. Res Chem Intermed 43:2959–2984. https://doi.org/10.1007/s11164-016-2806-2
Aliyev EM, Khan MM, Nabiyev AM et al (2018) Covalently modified graphene oxide and polymer of intrinsic microporosity (PIM-1) in mixed matrix thin-film composite membranes. Nanoscale Res Lett 13:359–371. https://doi.org/10.1186/s11671-018-2771-3
Alqaheem Y, Alomair A, Vinoba M, Pérez A (2017) Polymeric gas-separation membranes for petroleum refining. Int J Polym Sci 2017:1–19. https://doi.org/10.1155/2017/4250927
Amooghin AE, Sharifzadeh MMM, Pedram MZ (2017) Rigorous modeling of gas permeation behavior in facilitated transport membranes (FTMs); evaluation of carrier saturation effects and double-reaction mechanism. Greenh Gases Sci Technol 8:429–443. https://doi.org/10.1002/ghg.1750
Ansaloni L, Zhao Y, Jung BT et al (2015) Facilitated transport membranes containing amino-functionalized multi-walled carbon nanotubes for high-pressure CO2 separations. J Membr Sci 490:18–28. https://doi.org/10.1016/j.memsci.2015.03.097
Anson M, Marchese J, Garis E et al (2004) ABS copolymer-activated carbon mixed matrix membranes for CO2/CH4 separation. J Membr Sci 243:19–28. https://doi.org/10.1016/j.memsci.2004.05.008
Awad A, Aljundi IH (2018) Layer-by-layer assembly of carbide derived carbon-polyamide membrane for CO2 separation from natural gas. Energy 157:188–199. https://doi.org/10.1016/j.energy.2018.05.136
Bae SY, Lee KH, Yi SC et al (1998) CO2, N2 gas sorption and permeation behavior of chitosan membrane. Korean J Chem Eng 15:223–226
Bai H, Ho WSW (2011) Carbon dioxide-selective membranes for fhigh-pressure synthesis gas purification. Ind Eng Chem Res 50:12152–12161. https://doi.org/10.1021/ie2007592
Bai R, Huang M, Jiang Y (1988) Selective permeabilities of chitosan-acetic acid complex membrane and chitosan-polymer complex membranes for oxygen and carbon dioxide. Polym Bull 20:83–88
Baker RW, Low BT (2014) Gas separation membrane materials: a perspective. Macromolecules 47:6999–7013. https://doi.org/10.1021/ma501488s
Bara JE, Camper DE, Gin DL, Noble RD (2010) Room-temperature ionic liquids and composite materials : platform technologies for CO2 capture. Acc Chem Res 43:152–159. https://doi.org/10.1021/ar9001747
Barooah M, Mandal B (2019) Synthesis, characterization and CO2 separation performance of novel PVA/PG/ZIF-8 mixed matrix membrane. J Membr Sci 572:198–209. https://doi.org/10.1016/j.memsci.2018.11.001
Basu S, Cano-odena A, Vankelecom IFJ (2010a) Asymmetric Matrimid ® /[Cu3(BTC)2] mixed-matrix membranes for gas separations. J Membr Sci 362:478–487. https://doi.org/10.1016/j.memsci.2010.07.005
Basu S, Khan AL, Cano-Odena A et al (2010b) Membrane-based technologies for biogas separations. Chem Soc Rev 39:750–768. https://doi.org/10.1039/b817050a
Bei P, Liu H, Zhang Y et al (2021) Preparation and characterization of polyimide membranes modified by a task-specific ionic liquid based on Schiff base for CO2/N2 separation. Environ Sci Pollut Res 28:738–753. https://doi.org/10.1007/s11356-020-10533-5
Berchtold KA, Singh RP, Young JS, Dudeck KW (2012) Polybenzimidazole composite membranes for high temperature synthesis gas separations. J Membr Sci 415–416:265–270. https://doi.org/10.1016/j.memsci.2012.05.005
Bharali P, Borthakur S, Hazarika S (2020) Selective permeation of CO2 through amine bearing facilitated transport membranes. J Membr Sci Technol 10:203–210. https://doi.org/10.35248/2155-9589.2020.10.203
Blinova NV, Stejskal J, Fréchet JMJ, Svec F (2012) Effect of reaction conditions on film morphology of polyaniline composite membranes for gas separation. J Polym Sci A Polym Chem 50:3077–3085. https://doi.org/10.1002/pola.26093
Blinova NV, Svec F (2012) Functionalized polyaniline-based composite membranes with vastly improved performance for separation of carbon dioxide from methane. J Membr Sci 423–424:514–521. https://doi.org/10.1016/j.memsci.2012.09.003
Blume I and Pinnau I (1990) Composite membrane, method of preparation and use. Google Patents
Borgohain R, Mandal B (2019) PH responsive carboxymethyl chitosan/poly(amidoamine) molecular gate membrane for CO2/N2 separation. ACS Appl Mater Interfaces 11:42616–42628. https://doi.org/10.1021/acsami.9b15044
Borgohain R, Mandal B (2020) Thermally stable and moisture responsive carboxymethyl chitosan/dendrimer/hydrotalcite membrane for CO2 separation. J Membr Sci 608:118214. https://doi.org/10.1016/j.memsci.2020.118214
Brinkmann T, Lillepärg J, Notzke H et al (2017) Development of CO2 selective poly(ethylene oxide)-based membranes: from laboratory to pilot plant scale. Engineering 3:485–493. https://doi.org/10.1016/J.ENG.2017.04.004
Brunetti A, Cersosimo M, Kim JS et al (2017) Thermally rearranged mixed matrix membranes for CO2 separation: an aging study. Int J Greenh Gas Control 61:16–26. https://doi.org/10.1016/j.ijggc.2017.03.024
Brunetti A, Scura F, Barbieri G, Drioli E (2010) Membrane technologies for CO2 separation. J Membr Sci 359:115–125. https://doi.org/10.1016/j.memsci.2009.11.040
Bum H, Hoon S, Ho C et al (2010) Thermally rearranged (TR) polymer membranes for CO2 separation. J Membr Sci 359:11–24. https://doi.org/10.1016/j.memsci.2009.09.037
Cai Y, Wang Z, Yi C et al (2008) Gas transport property of polyallylamine-poly(vinyl alcohol)/polysulfone composite membranes. J Membr Sci 310:184–196. https://doi.org/10.1016/j.memsci.2007.10.052
Car A, Stropnik C, Yave W, Peinemann K (2008) Pebax®/polyethylene glycol blend thin film composite membranes for CO2 separation : performance with mixed gases. Sep Purif Technol 62:110–117. https://doi.org/10.1016/j.seppur.2008.01.001
Casado-Coterillo C, Fernández-Barquín A, Zornoza B et al (2015) Synthesis and characterisation of MOF/ionic liquid/chitosan mixed matrix membranes for CO2/N2 separation. RSC Adv 5:102350–102361. https://doi.org/10.1039/C5RA19331A
Casado-coterillo C, López-guerrero MM, Irabien Á (2014) Synthesis and characterisation of ETS-10/acetate-based ionic liquid/chitosan mixed matrix membranes for CO2/N2 Permeation. Membranes (Basel) 4:287–301. https://doi.org/10.3390/membranes4020287
Chen HZ, Thong Z, Li P, Chung T (2014) High performance composite hollow fiber membranes for CO2/H2 and CO2/N2 separation. Int J Hydrogen Energy 39:5043–5053. https://doi.org/10.1016/j.ijhydene.2014.01.047
Chen Y, Wang B, Zhao L et al (2015) New Pebax/zeolite Y composite membranes for CO2 capture from flue gas. J Membr Sci 495:415–423. https://doi.org/10.1016/j.memsci.2015.08.045
Chen Y, Zhao L, Wang B et al (2016) Amine-containing polymer/zeolite Y composite membranes for CO2/N2 separation Solution-diffusion. J Membr Sci 497:21–28. https://doi.org/10.1016/j.memsci.2015.09.036
Chern Y, Wu B (1997) Preparation of composite membranes with polyimides and poly(amide-imide) skin via interfacial condensation for air separation. J Appl Polym Sci 63:693–701. https://doi.org/10.1002/(SICI)1097-4628(19970207)63:6%3c693::AID-APP2%3e3.0.CO;2-I
Choi S, Tasselli F, Jansen JC et al (2010) Effect of the preparation conditions on the formation of asymmetric poly (vinylidene fluoride) hollow fibre membranes with a dense skin. Eur Polym J 46:1713–1725. https://doi.org/10.1016/j.eurpolymj.2010.06.001
Chung T, Shieh J, Lau WWY et al (1999) Fabrication of multi-layer composite hollow fiber membranes for gas separation. J Membr Sci 152(152):211–225. S0376-7388(98)00225-7
Cong H, Radosz M, Towler BF, Shen Y (2007) Polymer-inorganic nanocomposite membranes for gas separation. Sep Purif Technol 55:281–291. https://doi.org/10.1016/j.seppur.2006.12.017
Dai Z, Ansaloni L, Deng L (2016) Recent advances in multi-layer composite polymeric membranes for CO2 separation : a review. Green Energy Environ 1:102–128. https://doi.org/10.1016/j.gee.2016.08.001
Dai Z, Bai L, Hval KN et al (2019a) Pebax ® / TSIL blend thin film composite membranes for CO2 separation. Sci China Chem 59:538–546. https://doi.org/10.1007/s11426-016-5574-3
Dai Z, Deng J, Ansaloni L et al (2019b) Thin-film-composite hollow fiber membranes containing amino acid salts as mobile carriers for CO2 separation. J Membr Sci 578:61–68. https://doi.org/10.1016/j.memsci.2019.02.023
Dai Z, Fabio S, Giuseppe Marino N et al (2019c) Field test of a pre-pilot scale hollow fiber facilitated transport membrane for CO2 capture. Int J Greenh Gas Control 86:191–200. https://doi.org/10.1016/j.ijggc.2019.04.027
Deng L, Hägg M (2010) Swelling behavior and gas permeation performance of PVAm/PVA blend FSC membrane. J Membr Sci 363:295–301. https://doi.org/10.1016/j.memsci.2010.07.043
Deng L, Hägg M (2014) Carbon nanotube reinforced PVAm/PVA blend FSC nanocomposite membrane for CO2/CH4 separation. Int J Greenh Gas Control 26:127–134. https://doi.org/10.1016/j.ijggc.2014.04.018
Deng L, Kim T, Hägg M (2009) Facilitated transport of CO2 in novel PVAm/PVA blend membrane. J Membr Sci 340:154–163. https://doi.org/10.1016/j.memsci.2009.05.019
Dilshad MR, Islam A, Sabir A et al (2017) Fabrication and performance characterization of novel zinc oxide filled cross-linked PVA/PEG 600 blended membranes for CO2/N2 separation. J Ind Eng Chem 55:65–73. https://doi.org/10.1016/j.jiec.2017.06.029
Dong C, Wang Z, Yi C, Wang S (2006) Preparation of polyvinylamine / polysulfone composite hollow-fiber membranes and their CO2/CH4 separation performance. J Appl Polym Sci 101:1885–1891. https://doi.org/10.1002/app.23342
Du N, Park HB, Dal-cin M, Guiver MD (2012) Advances in high permeability polymeric membrane materials for CO2 separations. Energy Environ Sci 5:7306–7322. https://doi.org/10.1039/C1EE02668B
Du R, Chakma A, Feng X (2007) Interfacially formed poly (N, N-dimethylaminoethyl methacrylate)/ polysulfone composite membranes for CO2/N2 separation. J Membr Sci 290:19–28. https://doi.org/10.1016/j.memsci.2006.12.010
Du R, Feng X, Chakma A (2006) Poly(N, N-dimethylaminoethyl methacrylate)/polysulfone composite membranes for gas separations. J Membr Sci 279:76–85. https://doi.org/10.1016/j.memsci.2005.11.048
Duan S, Chowdhury FA, Kai T et al (2008) PAMAM dendrimer composite membrane for CO2 separation : addition of hyaluronic acid in gutter layer and application of novel hydroxyl PAMAM dendrimer. Desalination 234:278–285. https://doi.org/10.1016/j.desal.2007.09.095
Duan S, Kai T, Saito T et al (2014) Effect of cross-linking on the mechanical and thermal properties of poly(amidoamine) dendrimer/poly(vinyl alcohol) hybrid membranes for CO2 separation. Membranes (Basel) 4:200–209. https://doi.org/10.3390/membranes4020200
Duan S, Taniguchi I, Kai T, Kazama S (2012) Poly(amidoamine)dendrimer/poly(vinyl alcohol) hybrid membranes for CO2 capture. J Membr Sci 423–424:107–112. https://doi.org/10.1016/j.memsci.2012.07.037
Duan S, Taniguchi I, Kai T, Kazama S (2013) Development of poly(amidoamine)dendrimer/polyvinyl alcohol hybrid membranes for CO2 capture at elevated pressures. Energy Procedia 37:924–931. https://doi.org/10.1016/j.egypro.2013.05.187
Duval J-M, Folkers B, Mulder MHV et al (1993) Adsorbent filled membranes for gas separation. Part 1: Improvement of the gas separation properties of polymeric membranes by incorporation of microporous adsorbents. J Membr Sci 80:189–198
El-azzami LA, Grulke EA (2009) Carbon dioxide separation from hydrogen and nitrogen Facilitated transport in arginine salt-chitosan membranes. J Membr Sci 328:15–22. https://doi.org/10.1016/j.memsci.2008.08.038
El-azzami LA, Grulke EA (2008) Carbon dioxide separation from hydrogen and nitrogen by fixed facilitated transport in swollen chitosan membranes. J Membr Sci 323:225–234. https://doi.org/10.1016/j.memsci.2008.05.019
Escorihuela S, Tena A, Id SS et al (2018) Gas separation properties of polyimide thin films on ceramic supports for high temperature applications. Membranes (Basel) 8:16–36. https://doi.org/10.3390/membranes8010016
Fang M, Okamoto Y, Koike Y et al (2016) Gas separation membranes prepared with copolymers of perfluoro(2-methylene-4,5-dimethyl-1,3-dioxlane) and chlorotrifluoroethylene. J Fluor Chem 188:18–22. https://doi.org/10.1016/j.jfluchem.2016.05.013
Fauzan NAB, Mukhtar H, Nasir R et al (2020) Composite amine mixed matrix membranes for high- pressure CO2-CH4 separation : synthesis, characterization and performance evaluation. R Soc Open Sci 7:200795. https://doi.org/10.6084/m9.figshare.c.5111458
Fernández-barquín A, Casado-coterillo C, Palomino M et al (2016) Permselectivity improvement in membranes for CO2/N2 separation. Sep Purif Technol 157:102–111. https://doi.org/10.1016/j.seppur.2015.11.032
Firpo G, Angeli E, Repetto L, Valbusa U (2015) Permeability thickness dependence of polydimethylsiloxane (PDMS) membranes. J Membr Sci 481:1–8. https://doi.org/10.1016/j.memsci.2014.12.043
Francisco GJ, Chakma A, Feng X (2007) Membranes comprising of alkanolamines incorporated into poly (vinyl alcohol) matrix for CO2/N2 separation. J Membr Sci 303:54–63. https://doi.org/10.1016/j.memsci.2007.06.065
Francisco GJ, Chakma A, Feng X (2010) Separation of carbon dioxide from nitrogen using diethanolamine-impregnated poly (vinyl alcohol) membranes. Sep Purif Technol 71:205–213. https://doi.org/10.1016/j.seppur.2009.11.023
Freeman BD (1999) Basis of permeability/selectivity tradeoff relations in polymeric gas separation membranes. Macromolecules 32:375–380. https://doi.org/10.1021/ma9814548
Friebe S, Mundstock A, Unruh D et al (2016) NH 2 -MIL-125 as membrane for carbon dioxide sequestration : thin supported MOF layers contra mixed-matrix-membranes. J Membr Sci 516:185–193. https://doi.org/10.1016/j.memsci.2016.06.015
Fritsch D, Peinemann K-V, Behling R-D (1993) Silicone/non-silicone grafted blend composite membranes for air/vapor separation. Desalination 90:235–247. 0011-9164/93/$06.00
Fu J, Das S, Xing G et al (2016) Fabrication of COF-MOF composite membranes and their highly selective separation of H2/CO2. J Am Chem Soc 138:7673–7680. https://doi.org/10.1021/jacs.6b03348
Fu Q, Wong EHH, Kim J et al (2014) The effect of soft nanoparticle morphologies on thin film composite membrane performance. J Mater Chem A 2:17751–17756. https://doi.org/10.1039/C4TA02859G
Gao Y, Qiao Z, Zhao S et al (2018) In situ synthesis of polymer grafted ZIFs and application in mixed matrix membrane for CO2 separation. J Mater Chem A 6:3151–3161. https://doi.org/10.1039/c7ta10322k
Gu Y, Lodge TP (2011) Synthesis and gas separation performance of triblock copolymer ion gels with a polymerized ionic liquid mid-block. Macromolecules 44:1732–1736. https://doi.org/10.1021/ma2001838
Halim A, Fu Q, Yong Q et al (2014) Soft polymeric nanoparticles additives for next generation gas separation membranes. J Mater Chem A 2:4999–5009. https://doi.org/10.1039/C3TA14170E
Hamad FA, Chowdhury G, Matsuura T (2001) Sulfonated polyphenylene oxide – polyethersulfone thin-film composite membranes : effect of counterions on the gas transport properties. J Membr Sci 191:71–83. S0376-7388(01)00451-3
Han Y, Wu D, Ho WSW (2018) Nanotube-reinforced facilitated transport membrane for CO2/N2 separation with vacuum operation. J Membr Sci 567:261–271. https://doi.org/10.1016/j.memsci.2018.08.061
Han Y, Salim W, Chen KK et al (2019a) Field trial of spiral-wound facilitated transport membrane module for CO2 capture from flue gas. J Membr Sci 575:242–251. https://doi.org/10.1016/j.memsci.2019.01.024
Han Y, Wu D, Ho WSW (2019b) Simultaneous effects of temperature and vacuum and feed pressures on facilitated transport membrane for CO2/N2 separation. J Membr Sci 573:476–484. https://doi.org/10.1016/j.memsci.2018.12.028
Harra KEO, Kammakakam I, Devriese EM et al (2019) Synthesis and performance of 6FDA-based polyimide-ionenes and composites with ionic liquids as gas separation membranes. Membranes (Basel) 9:79–96
He W, Wang Z, Li W et al (2015) Cyclic tertiary amino group containing fixed carrier membranes for CO2 separation. J Membr Sci 476:171–181. https://doi.org/10.1016/j.memsci.2014.11.039
He X, Hagg M-B, Kim T-J (2014) Hybrid FSC membrane for CO2 removal from natural gas : experimental, process simulation, and economic feasibility analysis. AIChE J 60:4174–4184. https://doi.org/10.1002/aic.14600
He Y, Wang Z, Dong S et al (2016) Polymeric composite membrane fabricated by 2-aminoterephthalic acid chemically cross-linked polyvinylamine for CO2 separation under high temperature. J Membr Sci 518:60–71. https://doi.org/10.1016/j.memsci.2016.06.039
Hellums MW, Koros WJ, Husk GR, Paul DR (1989) Fluorinated polycarbonates for gas separtion applications. J Membr Sci 46:93–112
Hernández-Martínez H, Ruiz-Treviño FA, Ortiz-Espinoza J et al (2018) Simultaneous thermal cross-linking and decomposition of side groups to mitigate physical aging in poly(oxyindole biphenylylene) gas separation membranes. Ind Eng Chem Res 57:4640–4650. https://doi.org/10.1021/acs.iecr.8b00529
Hillock AMW, Koros WJ (2007) Cross-linkable polyimide membrane for natural gas purification and carbon dioxide plasticization reduction. Macromolecules 40:583–587. https://doi.org/10.1021/ma062180o
Hong T, Chatterjee S, Mahurin SM et al (2017) Impact of tuning CO2-philicity in polydimethylsiloxane-based membranes for carbon dioxide separation. J Membr Sci 530:213–219
Hosseinia SS, Penga N, Chung TS (2010) Gas separation membranes developed through integration of polymer blending and dual-layer hollow fiber spinning process for hydrogen and natural gas enrichments. J Memb Sci 349:156–166. https://doi.org/10.1016/j.memsci.2009.11.043
Huang J, Zou J, Ho WSW (2008) Carbon dioxide capture using a CO2-selective facilitated transport membrane. Ind Eng Chem Res 47:1261–1267. https://doi.org/10.1021/ie070794r
Hussain M, König A (2012) Mixed-matrix membrane for gas separation : polydimethylsiloxane filled with zeolite. Chem Eng Technol 35:561–569. https://doi.org/10.1002/ceat.201100419
IPCC sixth assessment report: climate change (2021) (AR6) from IPCC website. https://www.ipcc.ch/2021/06/29/wg1-ar6. Accessed 29 June 2021
Iqbal M, Man Z, Mukhtar H, Dutta BK (2008) Solvent effect on morphology and CO2/CH4 separation performance of asymmetric polycarbonate membranes. J Membr Sci 318:167–175. https://doi.org/10.1016/j.memsci.2008.02.040
Ito A, Sato M, Anma T (1997) Permeability of CO2 through chitosan membrane swollen by water vapor in feed gas. Die Angew Makromol Chem 248:85–94
Jahan Z, Niazi MBK, Hägg MB, Gregersen ØW (2018) Cellulose nanocrystal/PVA nanocomposite membranes for CO2/CH4 separation at high pressure. J Membr Sci 554:275–281. https://doi.org/10.1016/j.memsci.2018.02.061
Janakiram S, Martín Espejo JL, Høisæter KK et al (2020a) Three-phase hybrid facilitated transport hollow fiber membranes for enhanced CO2 separation. Appl Mater Today 21:100801–100811. https://doi.org/10.1016/j.apmt.2020.100801
Janakiram S, Santinelli F, Costi R et al (2020b) Field trial of hollow fiber modules of hybrid facilitated transport membranes for flue gas CO2 capture in cement industry. Chem Eng J 413:127405. https://doi.org/10.1016/j.cej.2020.127405
Jansen JC, Tasselli F, Tocci E, Drioli E (2006) High-flux composite perfluorinated gas separation membranes of Hyflon® AD on a hollow fibre ultrafiltration membrane support. Desalination 192:207–213. https://doi.org/10.1016/j.desal.2005.04.134
Ji P, Cao Y, Jie X et al (2010) Impacts of coating condition on composite membrane performance for CO2 separation. Sep Purif Technol 71:160–167. https://doi.org/10.1016/j.seppur.2009.11.015
Ji P, Cao Y, Zhao H et al (2009) Preparation of hollow fiber poly (N, N-imethylaminoethyl methacrylate)-poly (ethylene glycol methyl ether methyl acrylate)/polysulfone composite membranes for CO2/N2 separation. J Membr Sci 342:190–197. https://doi.org/10.1016/j.memsci.2009.06.038
Jia M, Peinemann K, Behling R (1991) Molecular sieving effect of the zeolite-filled silicone rubber membranes in gas permeation. J Membr Sci 57:289–296
Jiang L, Chung T, Fei D et al (2004) Fabrication of Matrimid/polyethersulfone dual-layer hollow fiber membranes for gas separation. J Membr Sci 240:91–103. https://doi.org/10.1016/j.memsci.2004.04.015
Jiao C, Li Z, Li X et al (2021) Improved CO2/N2 separation performance of Pebax composite membrane containing polyethyleneimine functionalized ZIF-8. Sep Purif Technol 259:118190. https://doi.org/10.1016/j.seppur.2020.118190
Jie X, Chau J, Obuskovic G, Sirkar KK (2015) Microporous ceramic tubule based and dendrimer-facilitated immobilized ionic liquid membrane for CO2 separation. Ind Eng Chem Res 54:10401–10418. https://doi.org/10.1021/acs.iecr.5b01214
Jomekian A, Ali S, Mansoori A et al (2011) Gas transport behavior of DMDCS modified MCM-48 / polysulfone mixed matrix membrane coated by PDMS. Korean J Chem Eng 28:2069–2075. https://doi.org/10.1007/s11814-011-0075-8
Jomekian A, Bazooyar B, Behbahani RM (2020) Experimental and modeling study of CO2 separation by modified Pebax 1657 TFC membranes. Korean J Chem Eng 37:2020–2040. https://doi.org/10.1007/s11814-020-0598-y
Jomekian A, Bazooyar B, Behbahani RM, Mohammadi T, Kargari A (2017a) Ionic liquid-modified Pebax® 1657 membrane filled by ZIF-8 particles for separation of CO2 from CH4, N2 and H2. J Membr Sci 524:652–662. https://doi.org/10.1016/j.memsci.2016.11.065
Jomekian A, Behbahani RM, Mohammadi T, Kargari A (2017b) High speed spin coating in fabrication of Pebax 1657 based mixed matrix membrane filled with ultra-porous ZIF-8 particles for CO2/CH4 separation. Korean J Chem Eng 34:440–453. https://doi.org/10.1007/s11814-016-0269-1
Kai T, Kouketsu T, Duan S et al (2008) Development of commercial-sized dendrimer composite membrane modules for CO2 removal from flue gas. Sep Purif Technol 63:524–530. https://doi.org/10.1016/j.seppur.2008.06.012
Kamio E, Kasahara S, Moghadam F, Matsuyama H (2020a) Development of facilitated transport membranes composed of a dense gel layer containing CO2 carrier formed on porous cylindrical support membranes. Chem Eng Res Des 153:284–293. https://doi.org/10.1016/j.cherd.2019.10.046
Kamio E, Tanaka M, Shirono Y et al (2020b) Hollow fiber-type facilitated transport membrane composed of a polymerized ionic liquid-based gel layer with amino acidate as the CO2 carrier. Ind Eng Chem Res 59:2083–2092. https://doi.org/10.1021/acs.iecr.9b05253
Kanehashi S, Chen GQ, Scholes CA et al (2015) Enhancing gas permeability in mixed matrix membranes through tuning the nanoparticle properties. J Membr Sci 482:49–55. https://doi.org/10.1016/j.memsci.2015.01.046
Kapantaidakis GC, Koops GH, Wessling M (2003) CO 2 Plasticization of polyethersulfone / polyimide gas-separation membranes. AIChE J 49:1702–1711
Kapoor R, Ghosh P, Kumar M, Vijay VK (2019) Evaluation of biogas upgrading technologies and future perspectives : a review. Environ Sci Pollut Res 26:11631–11661. https://doi.org/10.1007/s11356-019-04767-1
Karunakaran M, Kumar M, Shevate R et al (2017) CO2-philic thin film composite membranes : synthesis and characterization of PAN-r-PEGMA copolymer. Polymers (Basel) 9:219–233. https://doi.org/10.3390/polym9070219
Khalilinejad I, Sanaeepur H, Kargari A (2015) Preparation of poly ( ether-6-block amide )/ pvc thin film composite membrane for CO2 separation : effect of top layer thickness and operating parameters. J Membr Sci Res 1:124–129
Khan MM, Filiz V, Bengtson G et al (2012) Functionalized carbon nanotubes mixed matrix membranes of polymers of intrinsic microporosity for gas separation. Nanoscale Res Lett 7:504–515
Kim S, Lee YM (2013) High performance polymer membranes for CO2 separation. Curr Opin Chem Eng 2:238–244. https://doi.org/10.1016/j.coche.2013.03.006
Kim S, Lee YM (2014) Thermally rearranged (TR) polymer membranes with nanoengineered cavities tuned for CO2 separation. J Nanopart Res 14:265–275. https://doi.org/10.1007/978-3-319-05041-6
Kim J, Choi J, Kang YS, Won J (2016a) Matrix effect of mixed-matrix membrane containing CO2-selective MOFs. J Appl Polym Sci 132:42853–42860. https://doi.org/10.1002/app.42853
Kim J, Fu Q, Scofield JMP et al (2016b) Ultra-thin film composite mixed matrix membranes incorporating bio-inspired Iron(III)-dopamine nanoparticles for CO2 separation. Nanoscale 8:8312–8323. https://doi.org/10.1039/C5NR08840B
Kim J, Fu Q, Xie K et al (2016c) CO2 separation using surface-functionalized SiO2 nanoparticles incorporated ultra-thin film composite mixed matrix membranes for post- combustion carbon capture. J Membr Sci 515:54–62. https://doi.org/10.1016/j.memsci.2016.05.029
Kim K, Ingole PG, Kim J, Lee H (2013a) Separation performance of PEBAX/PEI hollow fiber composite membrane for SO2/CO2/N2 mixed gas. Chem Eng J 233:242–250. https://doi.org/10.1016/j.cej.2013.08.030
Kim T, Vralstad H, Sandru M, May-britt H (2013b) Separation performance of PVAm composite membrane for CO2 capture at various pH levels. J Membr Sci 428:218–224. https://doi.org/10.1016/j.memsci.2012.10.009
Kim K, Park S, So W et al (2003) CO2 separation performances of composite membranes of 6FDA-based polyimides with a polar group. J Membr Sci 211:41–49. S0376-7388(02)00316-2
Kim S, Hou J, Wang Y et al (2018) Highly permeable thermally rearranged polymer composite membranes with a graphene oxide scaffold for gas separation. J Mater Chem A 6:7668–7674. https://doi.org/10.1039/c8ta02256a
Kim S, Pechar TW, Marand E (2006) Poly (imide siloxane) and carbon nanotube mixed matrix membranes for gas separation. Desalination 192:330–339. https://doi.org/10.1016/j.desal.2005.03.098
Kim T-J, Li B, Hagg M-B (2004) Novel fixed-site-carrier polyvinylamine membrane for carbon dioxide capture. J Polym Sci B Polym Phys 42:4326–4336. https://doi.org/10.1002/polb.20282
Kimmerlea K, Hofmannb T, Strathmannb H (1991) Analysis of gas permeation through composite membranes. J Membr Sci 61:1–17. 0376-7388/91/$03.5
Kinoshita Y, Wakimoto K, Gibbons AH et al (2017) Enhanced PIM-1 membrane gas separation selectivity through efficient dispersion of functionalized POSS fillers. J Membr Sci 539:178–186. https://doi.org/10.1016/j.memsci.2017.05.072
Klemm A, Lee YY, Mao H, Gurkan B (2020) Facilitated transport membranes with ionic liquids for CO2 separations. Front Chem 8:1–8. https://doi.org/10.3389/fchem.2020.00637
Koschine T, Ratzke K, Faupel F et al (2015) Correlation of gas permeation and free volume in new and used high free volume thin film composite membranes. J Polym Sci B Polym Phys 53:213–217. https://doi.org/10.1002/polb.23616
Kouketsu T, Duan S, Kai T et al (2007) PAMAM dendrimer composite membrane for CO2 separation : formation of a chitosan gutter layer. J Membr Sci 287:51–59. https://doi.org/10.1016/j.memsci.2006.10.014
Kunalan S, Dey K, Roy PK et al (2021) Efficient facilitated transport PETIM dendrimer-PVA-PEG/PTFE composite flat-bed membranes for selective removal of CO2. J Membr Sci 622:119007
Kusakabe K, Ichiki K, Hayashi J et al (1996) Preparation and characterization of silica-polyimide composite membranes coated on porous tubes for CO2 separation. J Membr Sci 115:65–75. 0376-7388(95)00290-1
Lasseuguette E, Ferrari MC, Brandani S (2014) Humidity impact on the gas permeability of PIM-1 membrane for post-combustion application. Energy Procedia 63:194–201. https://doi.org/10.1016/j.egypro.2014.11.020
Lee WG, Kang SW (2019) Highly selective poly(ethylene oxide)/ionic liquid electrolyte membranes containing CrO3 for CO2/N2 separation. Chem Eng J 356:312–317. https://doi.org/10.1016/j.cej.2018.09.049
Lee HJ, Kang SW (2020) CO2 separation with polymer/aniline composite membranes. Polymers (Basel) 12:1–10. https://doi.org/10.3390/POLYM12061363
Lee J, Kim JS, Moon S, Young et al (2020) Dimensionally-controlled densification in crosslinked thermally rearranged (XTR) hollow fiber membranes for CO2 capture. J Membr Sci 595:117535–117543. https://doi.org/10.1016/j.memsci.2019.117535
Li P, Chen HZ, Chung T (2013a) The effects of substrate characteristics and pre-wetting agents on PAN-PDMS composite hollow fiber membranes for CO2/N2 and O2/N2 separation. J Membr Sci 434:18–25. https://doi.org/10.1016/j.memsci.2013.01.042
Li S, Wang Z, Zhang C et al (2013b) Interfacially polymerized thin film composite membranes containing ethylene oxide groups for CO2 separation. J Membr Sci 436:121–131. https://doi.org/10.1016/j.memsci.2013.02.038
Li T, Pan Y, Peinemann K, Lai Z (2013c) Carbon dioxide selective mixed matrix composite membrane containing ZIF-7 nano-fillers. J Membr Sci 425–426:235–242. https://doi.org/10.1016/j.memsci.2012.09.006
Li Y, Wang S, Wu H et al (2014) High-performance composite membrane with enriched CO2-philic groups and improved adhesion at the interface. ACS Appl Mater Interfaces 6:6654–6663. https://doi.org/10.1021/am500356g
Li P, Wang Z, Li W et al (2015a) High-performance multilayer composite membranes with mussel- inspired polydopamine as a versatile molecular bridge for CO2 separation. ACS Appl Mater Interfaces 7:15481–15493. https://doi.org/10.1021/acsami.5b03786
Li P, Wang Z, Liu Y et al (2015b) A synergistic strategy via the combination of multiple functional groups into membranes towards superior CO2 separation performances. J Membr Sci 476:243–255. https://doi.org/10.1016/j.memsci.2014.11.050
Lilleby Helberg RM, Dai Z, Ansaloni L, Deng L (2020) PVA/PVP blend polymer matrix for hosting carriers in facilitated transport membranes: synergistic enhancement of CO2 separation performance. Green Energy Environ 5:59–68. https://doi.org/10.1016/j.gee.2019.10.001
Lillepärg J, Georgopanos P, Shishatskiy S (2014) Stability of blended polymeric materials for CO2 separation. J Membr Sci 467:269–278. https://doi.org/10.1016/j.memsci.2014.05.039
Lin R, Ge L, Liu S et al (2015) Mixed-matrix membranes with metal − organic framework-decorated CNT fillers for efficient CO2 separation. ACS Appl Mater Interfaces 7:14750–14757. https://doi.org/10.1021/acsami.5b02680
Lindemann P, Tsotsalas M, Shishatskiy S et al (2014) Preparation of freestanding conjugated microporous polymer nanomembranes for gas separation. Chem Mater 26:7189–7193. https://doi.org/10.1021/cm503924h
Liu L, Chakma A, Feng X (2004) Preparation of hollow fiber poly (ether block amide )/ polysulfone composite membranes for separation of carbon dioxide from nitrogen. Chem Eng J 105:43–51. https://doi.org/10.1016/j.cej.2004.08.005
Liu L, Chakma A, Feng X (2005) CO2/N2 separation by poly (ether block amide) thin film hollow fiber composite membranes. Ind Eng Chem Res 44:6874–6882
Liu L, Chakma A, Feng X (2008) Gas permeation through water-swollen hydrogel membranes. J Membr Sci 310:66–75. https://doi.org/10.1016/j.memsci.2007.10.032
Liu Y, Chung T, Wang R et al (2003) Chemical cross-linking modification of polyimide/poly(ether sulfone) dual-layer hollow-fiber membranes for gas separation. Ind Eng Chem Res 42:1190–1195. https://doi.org/10.1021/ie020750c
Liu Y, Yu S, Wu H et al (2014) High permeability hydrogel membranes of chitosan/poly ether-block-amide blends for CO2 separation. J Membr Sci 469:198–208. https://doi.org/10.1016/j.memsci.2014.06.050
Lu HT, Kanehashi S, Scholes CA, Kentish SE (2016) The potential for use of cellulose triacetate membranes in post combustion capture. Int J Greenh Gas Control 55:97–104. https://doi.org/10.1016/j.ijggc.2016.11.002
Lundy KA, Cabasso I (1989) Analysis and construction of multilayer composite membranes for the separation of gas mixtures. Ind Eng Chem Res 28:742–756. 0888-5885/89/2628-0742$01.50/0 0
Ma C, Koros WJ (2013) Ester-cross-linkable composite hollow fiber membranes for CO2 removal from natural gas. Ind Eng Chem Res 52:10495–10505. https://doi.org/10.1021/ie303531r
Madaeni SS, Badieh MMS, Vatanpour V (2013) Effect of coating method on gas separation by PDMS / PES membrane. Polym Eng Sci 53:1878–1885. https://doi.org/10.1002/pen.23456
Maheswari AU, Palanivelu K (2017) Carbon dioxide capture by facilitated transport membranes : a review. Int J Glob Warm 12:1–49. https://doi.org/10.1504/IJGW.2017.084013
Mannan HA, Mukhtar H, Murugesan T et al (2013) Recent applications of polymer blends in gas separation membranes. Chem Eng Technol 36:1–10. https://doi.org/10.1002/ceat.201300342
Mannan HA, Mukhtar H, Shaharun MS et al (2016) Polysulfone/poly(ether sulfone) blended membranes for CO2 separation. J Appl Polym Sci 133:42946. https://doi.org/10.1002/app.42946
Mao Z, Jie X, Cao Y et al (2011) Preparation of dual-layer cellulose/polysulfone hollow fiber membrane and its performance for isopropanol dehydration and CO2 separation. Sep Purif Technol 77:179–184. https://doi.org/10.1016/j.seppur.2010.11.031
Marcq J, Nguyen QT, Langevin D, Brule B (2005) ABatement of CO2 emissions by means of membranes-characterization of industrial Pebax ™ films. Environ Prot Eng 31:13–22
Marek M Jr, Brynda E, Houska M et al (1996) Ultra-thin polyimide film as a gas-separation layer for composite membranes. Polymer (Guildf) 37:2577–2579
Matsuyama H, Hirai K, Teramoto M (1994) Selective permeation of carbon dioxide through plasma polymerized membrane from diisopropylamine. J Membr Sci 92:257–265. 0376-7388(94)00069-B
Matsuyama H, Matsui K, Kitamura Y et al (1999a) Effects of membrane thickness and membrane preparation condition on facilitated transport of CO2 through ionomer membrane. Sep Purif Technol 17:235–241. S1383-5866 (99) 00047-7
Matsuyama H, Terada A, Nakagawara T et al (1999b) Facilitated transport of CO2 through polyethylenimine/poly(vinyl alcohol) blend membrane. J Membr Sci 163:221–227
Matsuyama H, Teramoto M, Sakakura H (1996) Selective permeation of CO2 through poly(2-(N, N-dimethyl) aminoethyl methacrylate) membrane prepared by plasma-graft polymerization technique. J Membr Sci 114:193–200
Mei X, Yang S, Lu P et al (2020) Improving the selectivity of ZIF-8/polysulfone-mixed matrix membranes by polydopamine modification for H2/CO2 separation. Front Chem 8:528. https://doi.org/10.3389/fchem.2020.00528
Merkel TC, Bondar VI, Nagai K et al (2000) Gas sorption, diffusion, and permeation in poly(dimethylsiloxane). J Polym Sci B Polym Phys 38:415–434
Merkel TC, Lin H, Wei X, Baker R (2010) Power plant post-combustion carbon dioxide capture : An opportunity for membranes. J Memb Sci 359:126–139. https://doi.org/10.1016/j.memsci.2009.10.041
Mohamed MH, Gipple MJ, David P et al (2020) Dual-layer MOF composite membranes with tuned interface interaction for postcombustion carbon dioxide separation dual-layer MOG composite membranes with tuned interface interaction for postcombustion carbon dioxide separation. Cell Rep Phys Sci 1:100059. https://doi.org/10.1016/j.xcrp.2020.100059
Molavi H, Shojaei A (2019) Mixed-matrix composite membranes based on UiO-66-derived MOFs for CO2 separation. ACS Appl Mater Interfaces 11:9448–9461. https://doi.org/10.1021/acsami.8b20869
Mondal A, Mandal B (2013) Synthesis and characterization of crosslinked poly(vinyl alcohol)/poly composite membrane for CO2/N2 separation. J Membr Sci 446:383–394. https://doi.org/10.1016/j.memsci.2013.06.052
Mondal A, Mandal B (2014a) CO2 separation using thermally stable crosslinked poly(vinyl alcohol) membrane blended with polyvinylpyrrolidone/polyethyleneimine/ tetraethylenepentamine. J Membr Sci 460:126–138. https://doi.org/10.1016/j.memsci.2014.02.040
Mondal A, Mandal B (2014b) Novel CO2-selective cross-linked poly (vinyl alcohol)/ polyvinylpyrrolidone blend membrane containing amine carrier for CO2-N2 separation : synthesis, characterization and gas permeation study. Ind Eng Chem Res 53:19736–19746. https://doi.org/10.1021/ie500597p
Moulay S (2015) Review : Poly(vinyl alcohol) functionalizations and applications. Polym Plast Technol Eng 54:1289–1319. https://doi.org/10.1080/03602559.2015.1021487
Mozafari M, Abedini R, Rahimpour A (2018) Zr-MOFs incorporated thin film nanocomposite-Pebax 1657 membranes dip coated on polymethylpentyne layer for efficient separation of CO2/CH4. J Mater Chem A 6:12380–12392. https://doi.org/10.1039/C8TA04806A
Murali RS, Sridhar S, Sankarshana T, Ravikumar YVL (2010) Gas permeation behavior of pebax-1657 nanocomposite membrane incorporated with multiwalled carbon nanotubes. Ind Eng Chem Res 49:6530–6538
Nakagawa T, Saito T, Asakawa S, Saito Y (1988) Polyacetylene derivatives as membranes for gas separation. Gas Sep Purif 2:3–8. https://doi.org/10.1016/0950-4214(88)80035-5
Nguyen PT, Wiesenauer EF, Gin DL, Noble RD (2013) Effect of composition and nanostructure on CO2/N2 transport properties of supported alkyl-imidazolium block copolymer membranes. J Membr Sci 430:312–320. https://doi.org/10.1016/j.memsci.2012.12.016
Nikolaeva D, Azcune I, Tanczyk M et al (2018) The performance of affordable and stable cellulose-based poly-ionic membranes in CO2/N2 and CO2/CH4 gas separation. J Membr Sci 564:552–561. https://doi.org/10.1016/j.memsci.2018.07.057
Norahim N, Faungnawakij K, Quitain AT, Klaysom C (2019) Composite membranes of graphene oxide for CO2/CH4 separation. J Chem Technol Biotechnol 94:2783–2791. https://doi.org/10.1002/jctb.5999
Oh S, Zurawsky WP (1996) Gas permeation through poly (dimethylsiloxane)-plasma polymer composite membranes. J Membr Sci 120:89–99. S0376-7388(96)00139-1
Olajire AA (2010) CO2 capture and separation technologies for end-of-pipe applications - a review. Energy 35:2610–2628. https://doi.org/10.1016/j.energy.2010.02.030
Orlov AV, Kiseleva SG, Karpacheva GP et al (2003) Structure and gas separation properties of composite films based on polyaniline. J Appl Polym Sci 89:1379–1384
Orme CJ, Harrup MK, Luther TA et al (2001) Characterization of gas transport in selected rubbery amorphous polyphosphazene membranes. J Membr Sci 186:249–256. S0376-7388(00)00690-6
Pakizeh M, Mansoori SAA, Chenar MP, Namvar-Mahboub M (2013) Modification of PSf membrane nanostructure using different fabrication parameters and investigation of the CO2 separation properties of PDMS-coated PSf composite membranes. Braz J Chem Eng 30:345–354
Park HJ, Bhatti UH, Nam SC et al (2019) Separation and purification technology nafion/TiO2 nanoparticle decorated thin film composite hollow fiber membrane for efficient removal of SO2 gas. Sep Purif Technol 211:377–390. https://doi.org/10.1016/j.seppur.2018.10.010
Park S, Kim K, So W et al (2003) Gas separation properties of 6FDA-based polyimide membranes with a polar group. Macromol Res 11:157–162. 1598-5032/06/157-06©2003
Pedram MZ, Omidkhah M, Amooghin AE (2014) Synthesis and characterization of diethanolamine-impregnated cross-linked polyvinylalcohol/glutaraldehyde membranes for CO2/CH4 separation. J Ind Eng Chem 20:74–82. https://doi.org/10.1016/j.jiec.2013.04.024
Peng D, Wang S, Tian Z et al (2017) Facilitated transport membranes by incorporating graphene nanosheets with high zinc ion loading for enhanced CO2 separation. J Membr Sci 522:351–362. https://doi.org/10.1016/j.memsci.2016.09.040
Peter J, Kosmala B, Bleha M (2009) Synthesis of hyperbranched copolyimides and their application as selective layers in composite membranes. Desalination 245:516–526. https://doi.org/10.1016/j.desal.2009.02.015
Peter J, Peinemann K (2009) Multilayer composite membranes for gas separation based on crosslinked PTMSP gutter layer and partially crosslinked Matrimid ® 5218 selective layer. J Membr Sci 340:62–72. https://doi.org/10.1016/j.memsci.2009.05.009
Petersen J, Peinemann KV (1997) Novel polyamide composite membranes for gas separation prepared by interfacial polycondensation. J Appl Polym Sci 63:1557–1563. https://doi.org/10.1002/(SICI)1097-4628(19970321)63:12%3c1557::AID-APP6%3e3.0.CO;2-N
Philipp HW and Hsu L (1979) NASA Technical Paper. https://apps.dtic.mil/sti/citations/ADA304743. Accessed 3 Sept 2013
Polevaya V, Geiger V, Bondarenko G et al (2019) Chemical modification of poly(1-trimethylsylil-1-propyne) for the creation of highly efficient CO2-selective membrane materials. Materials (Basel) 12:2763–2775. https://doi.org/10.3390/ma12172763
Prasad B, Mandal B (2017) CO2 separation performance by chitosan/tetraethylenepentamine/poly (ether sulfone) composite membrane. J Appl Polym Sci 134:45206–45215. https://doi.org/10.1002/app.45206
Qiao Z, Wang Z, Zhang C et al (2013) PVAm-PIP/PS composite membrane with high performance for CO2/N2 separation. AIChE J 59:215–228. https://doi.org/10.1002/aic.13781
Qin J, Chung T, Cao C, Vora RH (2005) Effect of temperature on intrinsic permeation properties of fabrication of its hollow fiber membranes for CO2/CH4 separation. J Membr Sci 250:95–103. https://doi.org/10.1016/j.memsci.2004.10.021
Rafiq S, Deng L, Hägg M-B (2016) Role of facilitated transport membranes and composite membranes for efficient CO2 capture - a review. ChemBioEng Rev 3:68–85. https://doi.org/10.1002/cben.201500013
Rafiq S, Man Z, Maulud A et al (2012) Separation of CO2 from CH4 using polysulfone/polyimide silica nanocomposite membranes. Sep Purif Technol 90:162–172. https://doi.org/10.1016/j.seppur.2012.02.031
Rahaman MSA, Zhang L, Cheng L-H et al (2012) Capturing carbon dioxide from air using a fixed carrier facilitated transport membrane. RSC Adv 2:9165–9172. https://doi.org/10.1039/c2ra20783d
Ramasubramanian K, Ho WSW (2011) Recent developments on membranes for post-combustion carbon capture. Curr Opin Chem Eng 1:47–54. https://doi.org/10.1016/j.coche.2011.08.002
Reid BD, Ebron VHM, Musselman IH et al (2002) Enhanced gas selectivity in thin film composite membranes of poly (3-(2-acetoxyethyl)thiophene). J Membr Sci 195:181–192. S0376-7388(01)00551-8
Ren X, Ren J, Deng M (2012) Poly (amide-6-b-ethylene oxide) membranes for sour gas separation. Sep Purif Technol 89:1–8. https://doi.org/10.1016/j.seppur.2012.01.004
Rezakazemi M, Amooghin AE, Montazer-rahmati MM et al (2015) State-of-the-art membrane based CO2 separation using mixed matrix membranes (MMMs): An overview on current status and future directions. Prog Polym Sci 39:817–861. https://doi.org/10.1016/j.progpolymsci.2014.01.003
Rhim J, Chowdhury G, Matsuura T (2000) Development of thin-film composite membranes for carbon dioxide and methane separation using sulfonated poly (phenylene oxide). J Appl Polym Sci 76:735–742. https://doi.org/10.1002/(SICI)1097-4628(20000502)76:5%3C735::AID-APP16%3E3.0.CO;2-N
Robeson LM (2008) The upper bound revisited. J Membr Sci 320:390–400. https://doi.org/10.1016/j.memsci.2008.04.030
Sabouni R, Kazemian H, Rohani S (2014) Carbon dioxide capturing technologies : a review focusing on metal organic framework materials (MOFs). Environ Sci Pollut Res 21:5427–5449. https://doi.org/10.1007/s11356-013-2406-2
Sadeghi M, Semsarzadeh MA, Barikani M, Pourafshari Chenar M (2011) Gas separation properties of polyether-based polyurethane-silica nanocomposite membranes. J Membr Sci 376:188–195. https://doi.org/10.1016/j.memsci.2011.04.021
Sadrzadeh M, Amirilargani M, Shahidi K, Mohammadi T (2009) Gas permeation through a synthesized composite PDMS/PES membrane. J Membr Sci 342:236–250. https://doi.org/10.1016/j.memsci.2009.06.047
Saedi S, Madaeni SS, Shamsabadi AA (2014) PDMS coated symmetric PES membrane for natural gas sweetening: effect of preparation and operating parameters on performance. Can J Chem Eng 92:892–904. https://doi.org/10.1002/cjce.21947
Saedi S, Seidi F, Moradi F, Xiang X (2016) Preparation and characterization of an amino-cellulose (AC) derivative for development of thin-film composite membrane for CO2/CH4 separation. Starch 68:1–11. https://doi.org/10.1002/star.201500255
Saeed M, Deng L (2015) CO2 facilitated transport membrane promoted by mimic enzyme. J Membr Sci 494:196–204. https://doi.org/10.1016/j.memsci.2015.07.028
Saeed U, Khan AL, Gilani MA et al (2021) CO2 separation by supported liquid membranes synthesized with natural deep eutectic solvents. Environ Sci Pollut Res 28:33994–34008. https://doi.org/10.1007/s11356-020-10260-x
Sainan L, Gongping L, Wang W et al (2013) Ceramic supported PDMS and PEGDA composite membranes for CO2 separation. Chin J Chem Eng 21:348–356. https://doi.org/10.1016/S1004-9541(13)60478-4
Salim W, Vakharia V, Chen Y et al (2018) Fabrication and field testing of spiral-wound membrane modules for CO2 capture from flue gas. J Membr Sci 556:126–137. https://doi.org/10.1016/j.memsci.2018.04.001
Sánchez-laínez J, Paseta L, Navarro M et al (2018a) Ultrapermeable Thin Film ZIF-8 / Polyamide Membrane for H2/CO2 Separation at High Temperature without Using Sweep Gas. Adv Mater interfaces 1800647:18999647–1899954. https://doi.org/10.1002/admi.201800647
Sanchez-lainez J, Zornoza B, Tellez C, Coronas J (2018b) Asymmetric polybenzimidazole membranes with thin selective skin layer containing ZIF-8 for H2/CO2 separation at pre-combustion capture conditions. J Membr Sci 563:427–434. https://doi.org/10.1016/j.memsci.2018.06.009
Sanchez-Laınez J, Gracia-Guillen I, Zornoza B et al (2019) Thin supported MOF based mixed matrix membranes of Pebax s 1657 for biogas upgrade. New J Chem 43:312–319. https://doi.org/10.1039/c8nj04769c
Sandru M, Haukebø SH, Hägg M (2010) Composite hollow fiber membranes for CO2 capture. J Membr Sci 346:172–186. https://doi.org/10.1016/j.memsci.2009.09.039
Sarmadi R, Salimi M (2020) The assessment of honeycomb structure UiO-66 and amino functionalized UiO-66 metal-organic frameworks to modify the morphology and performance of Pebax ® 1657-based gas separation membranes for CO2 capture applications. Environ Sci Pollut Res 27:40618–40632. https://doi.org/10.1007/s11356-020-09927-2
Sasikumar B, Bisht S, Arthanareeswaran G et al (2021) Performance of polysulfone hollow fiber membranes encompassing ZIF-8, SiO2/ZIF-8, and amine-modified SiO2/ZIF-8 nanofillers for CO2/CH4 and CO2/N2 gas separation. Sep Purif Technol 264:118471. https://doi.org/10.1016/j.seppur.2021.118471
Scholes CA, Chen GQ, Stevens GW, Kentish SE (2010a) Plasticization of ultra-thin polysulfone membranes by carbon dioxide. J Membr Sci 346:208–214. https://doi.org/10.1016/j.memsci.2009.09.036
Scholes CA, Stevens GW, Kentish SE (2010b) The effect of hydrogen sulfide, carbon monoxide and water on the performance of a PDMS membrane in carbon dioxide/nitrogen separation. J Membr Sci 350:189–199. https://doi.org/10.1016/j.memsci.2009.12.027
Scholes CA, Kanehashi S, Stevens GW, Kentish SE (2015) Water permeability and competitive permeation with CO2 and CH4 in perfluorinated polymeric membranes. Sep Purif Technol 147:203–209. https://doi.org/10.1016/j.seppur.2015.04.023
Scholes CA, Kentish SE, Stevens GW (2008) Carbon dioxide separation through polymeric membrane systems for flue gas applications. Recent Patents Chem Eng 1:5266
Scholes CA, Stevens GW, Kentish SE (2012) Membrane gas separation applications in natural gas processing. Fuel 96:15–28. https://doi.org/10.1016/j.fuel.2011.12.074
Scofield JMP, Gurr PA, Kim J et al (2016) Development of novel fluorinated additives for high performance CO2 separation thin-film composite membranes. J Memb Sci 499:191–200. https://doi.org/10.1016/j.memsci.2015.10.035
Scofield JMP, Gurr PA, Kim J et al (2015) High-performance thin film composite membranes with well-defined poly(dimethylsiloxane)-b-poly(ethylene glycol) copolymer additives for CO2 separation. J Polym Sci A Polym Chem 53:1500–1511. https://doi.org/10.1002/pola.27628
Sekizkardes AK, Kusuma VA, Dahe G et al (2016) Separation of carbon dioxide from flue gas by mixed matrix membranes using dual phase microporous polymeric constituents. Chem Commun 52:11768–11771. https://doi.org/10.1039/c6cc04811k
Sekizkardes AK, Kusuma VA, McNally JS et al (2018) Microporous polymeric composite membranes with advanced film properties: pore intercalation yields excellent CO2 separation performance. J Mater Chem A 6:22472–22477. https://doi.org/10.1039/c8ta07424k
Selyanchyn R, Ariyoshi M, Fujikawa S (2018) Thickness effect on CO2/N2 separation in double layer pebax-1657®/PDMS membranes. Membranes (Basel) 8:121–133. https://doi.org/10.3390/membranes8040121
Semsarzadeh MA, Ghalei B (2012) Characterization and gas permeability of polyurethane and polyvinyl acetate blend membranes with polyethylene oxide-polypropylene oxide block copolymer. J Membr Sci 401–402:97–108. https://doi.org/10.1016/j.memsci.2012.01.035
Shankar K, Kandasamy P (2019) Carbon dioxide separation using α-alumina ceramic tube supported cellulose triacetate-tributyl phosphate composite membrane. Greenh Gases Sci Technol 9:287–305. https://doi.org/10.1002/ghg
Shen J, Qiu J, Wu L, Gao C (2006) Facilitated transport of carbon dioxide through poly (2-N, N-dimethyl aminoethyl methacrylate-co-acrylic acid sodium) membrane. Sep Purif Technol 51:345–351. https://doi.org/10.1016/j.seppur.2006.02.015
Shen J, Yu C, Zeng G, Van Der BB (2013) Preparation of a facilitated transport membrane composed of carboxymethyl chitosan and polyethylenimine for CO2/N2 separation. Int J Mol Sci 14:3621–3638. https://doi.org/10.3390/ijms14023621
Shen Y, Wang H, Liu J, Zhang Y (2015) Enhanced performance of a novel polyvinyl amine / chitosan / graphene oxide mixed matrix membrane for CO2 capture. ACS Sustain Chem Eng Interfaces 3:1819–1829. https://doi.org/10.1021/acssuschemeng.5b00409
Shen Y, Wang H, Zhang X, Zhang Y (2016) MoS2 nanosheets functionalized composite mixed matrix membrane for enhanced CO2 capture via surface drop-coating method. ACS Appl Mater Interfaces 8:23371–23378. https://doi.org/10.1021/acsami.6b07153
Shieh J, Chung T (2000) Cellulose nitrate-based multilayer composite membranes for gas separation. J Membr Sci 166:259–269. S0376-7388(99)00270-7
Shieh J, Chung T, Wang R et al (2001) Gas separation performance of poly (4-vinylpyridine)/ polyetherimide composite hollow fibers. J Membr Sci 182:111–123. S0376-7388(00)00560-3
Songolzadeh M, Soleimani M, Ravanchi MT, Songolzadeh R (2014) Carbon dioxide separation from flue gases : a technological review emphasizing reduction in greenhouse gas emissions. Sci World J 2014:1–34. https://doi.org/10.1155/2014/828131
Sridhar S, Aminabhavi TM, Mayor SJ, Ramakrishna M (2007a) Permeation of carbon dioxide and methane gases through novel silver-incorporated thin film composite pebax membranes. Ind Eng Chem Res 46:8144–8151
Sridhar S, Smitha B, Aminabhavi TM (2007b) Separation of carbon dioxide from natural gas mixtures through polymeric membranes - a review. Sep Purif Rev 36:113–174. https://doi.org/10.1080/15422110601165967
Sridhar S, Suryamurali R, Smitha B, Aminabhavi TM (2007c) Development of crosslinked poly (ether-block-amide) membrane for CO2/CH4 separation. Colloids Surf A Physicochem Eng Asp 297:267–274. https://doi.org/10.1016/j.colsurfa.2006.10.054
Strathman H, Bell CM, Kimmerle K (1986) Development of synthetic membranes for gas and vapor separation. Pure Appl Chem 58:1663–1668. https://doi.org/10.1351/pac198658121663
Sun C, Srivastava DJ, Grandinetti PJ, Dutta PK (2016) Microporous and mesoporous materials synthesis of chabazite / polymer composite membrane for CO2/N2 separation. Microporous Mesoporous Mater 230:208–216. https://doi.org/10.1016/j.micromeso.2016.04.042
Sun J, Yi Z, Zhao X et al (2017) CO2 separation membranes with high permeability and CO2/N2 selectivity prepared by electrostatic self-assembly of polyethylenimine on reverse osmosis membranes. RSC Adv 7:14678–14687. https://doi.org/10.1039/C7RA00094D
Suzuki H, Tanaka K, Kita H et al (1998) Preparation of composite hollow fiber membranes of poly(ethylene oxide)-containing polyimide and their CO2/N2 separation properties. J Membr Sci 146:31–37. https://doi.org/10.1016/S0376-7388(98)00081-7
Taheri P, Raisi A, Maleh MS (2021) CO2-selective poly (ether-block-amide)/polyethylene glycol composite blend membrane for CO2 separation from gas mixtures. Environ Sci Pollut Res 1-17.https://doi.org/10.1007/s11356-021-13447-y
Taniguchi I, Wada N, Kinugasa K, Higa M (2017) A strategy to enhance CO2 permeability of well-defined hyper-branched polymers with dense polyoxyethylene comb graft. J Membr Sci 535:239–247. https://doi.org/10.1016/j.memsci.2017.04.046
Teramae T, Kumazawa H (2007) Gas permeability and permselectivity of plasma-treated polypropylene membranes. J Appl Polym Sci 104:3236–3239. https://doi.org/10.1002/app.25951
Thomas DM, Mechery J, Paulose SV (2016) Carbon dioxide capture strategies from flue gas using microalgae : a review. Environ Sci Pollut Res 23:16926–16940. https://doi.org/10.1007/s11356-016-7158-3
Tomé LC, Isik M, Freire CSR et al (2015) Novel pyrrolidinium-based polymeric ionic liquids with cyano counter-anions : High performance membrane materials for post-combustion CO2 separation. J Membr Sci 483:155–165. https://doi.org/10.1016/j.memsci.2015.02.020
Torstensen JØ, Helberg RML, Deng L et al (2019) PVA/nanocellulose nanocomposite membranes for CO2 separation from flue gas. Int J Greenh Gas Control 81:93–102. https://doi.org/10.1016/j.ijggc.2018.10.007
Tseng HH, Lin ZY, Chen SH et al (2019) Reuse of reclaimed tire rubber for gas-separation membranes prepared by hot-pressing. J Clean Prod 237:117739. https://doi.org/10.1016/j.jclepro.2019.117739
Uddin MW, Hagg M-B (2012) Natural gas sweetening-the effect on CO2-CH4 separation after exposing a facilitated transport membrane to hydrogen sulfide and higher hydrocarbons. J Membr Sci 423–424:143–149. https://doi.org/10.1016/j.memsci.2012.08.010
Uma Maheswari A, Palanivelu K (2017) Separation of carbon dioxide and nitrogen gases using novel composite membranes. Can J Chem 95:57–67. https://doi.org/10.1139/cjc-2016-0090
Wang M, Wang Z, Li N et al (2015) Relationship between polymer-filler interfaces in separation layers and gas transport properties of mixed matrix composite membranes. J Membr Sci 495:252–268. https://doi.org/10.1016/j.memsci.2015.08.019
Wang S, Li X, Wu H et al (2016) Advances in high permeability polymer-based membrane materials for CO2 separations. Energy Environ Sci 9:1863–1890. https://doi.org/10.1039/C6EE00811A
Wang Y, Hu T, Li H et al (2014) Enhancing membrane permeability for CO2 capture through blending commodity polymers with selected PEO and PEO-PDMS copolymers and composite hollow fibres. Energy Procedia 63:202–209. https://doi.org/10.1016/j.egypro.2014.11.021
Wang Y, Li L, Zhang X et al (2019) Polyvinylamine/graphene oxide/PANI@CNTs mixed matrix composite membranes with enhanced CO2/N2 separation performance. J Membr Sci 589:117246. https://doi.org/10.1016/j.memsci.2019.117246
Wang Y, Li L, Zhang X, et al (2020) Polyvinylamine/amorphous metakaolin mixed-matrix composite membranes with facilitated transport carriers for highly efficient CO2/N2 separation. J Membr Sci 599.https://doi.org/10.1016/j.memsci.2020.117828
Wang Z, Li M, Cai Y et al (2007) Novel CO2 selectively permeating membranes containing PETEDA dendrimer. J Membr Sci 290:250–258. https://doi.org/10.1016/j.memsci.2006.12.041
Wang Z, Yi C, Zhang Y et al (2006) CO2-facilitated transport through poly(N-vinyl-γ-sodium aminobutyrate-co-sodium acrylate)/polysulfone composite membranes. J Appl Polym Sci 100:275–282. https://doi.org/10.1002/app.23100
Weigelt F, Escorihuela S, Descalzo A et al (2019) Novel polymeric thin-film composite membranes for high-temperature gas separations. Membranes (Basel) 9:51–63. https://doi.org/10.3390/membranes9040051
Wenthold RM, Hall RT, Andrus RG, Brinda PD, Cosentino LC, Reggin RF, Pigott DT (1987) Hollow fiber membranes and method of manufacture. Google patents
Widjojo N, Li Y, Jiang L, and Chung TS (2012) Recent progress and challenges on mixed matrix membranes in both material and configuration aspects for gas separation. In: Buonomenna MJ and Golemme G (eds) Advanced Materials for Membrane Preparation, Bentham Science, United Arab Emirates, pp 64–82
World Energy Outlook (2021) (Released on 18 october 2021). https://www.iea.org/reports/world-energy-outlook-2021. Accessed date 18 Oct 2021
Wu F, Li L, Xu Z et al (2006) Transport study of pure and mixed gases through PDMS membrane. Chem Eng J 117:51–59. https://doi.org/10.1016/j.cej.2005.12.010
Xin Q, Gao Y, Wu X et al (2015) Incorporating one-dimensional aminated titania nanotubes into sulfonated poly (ether ether ketone) membrane to construct CO2-facilitated transport pathways for enhanced CO2 separation. J Membr Sci 488:13–29. https://doi.org/10.1016/j.memsci.2015.02.047
Xing R, Ho WSW (2009) Synthesis and characterization of crosslinked polyvinylalcohol / polyethyleneglycol blend membranes for CO2/CH4 separation. J Taiwan Inst Chem Eng 40:654–662. https://doi.org/10.1016/j.jtice.2009.05.004
Xomeritakis G, Tsai CY, Jiang YB, Brinker CJ (2009) Tubular ceramic-supported sol-gel silica-based membranes for flue gas carbon dioxide capture and sequestration. J Membr Sci 341:30–36. https://doi.org/10.1016/j.memsci.2009.05.024
Xu R, Wang Z, Wang M et al (2019) High nanoparticles loadings mixed matrix membranes via chemical bridging-crosslinking for CO2 separation. J Membr Sci 573:455–464. https://doi.org/10.1016/j.memsci.2018.12.027
Xu X, Coleman MR (1999) Preliminary investigation of gas transport mechanism in a H+ irradiated polyimide-ceramic composite membrane. Nucl Instruments Methods Phys Res B 152:325–334
Yamaguchij T, Boetje LM, Koval C et al (1995) Transport properties of carbon dioxide through amine functionalized carrier membranes. Ind Eng Chem Res 34:4071–4077. 0888-5885/95/2634-40
Yamasaki A (2003) An overview of CO2 mitigation options for global warming-emphasizing CO2 sequestration options. J Chem Eng Japan 36:361–375. https://doi.org/10.1252/JCEJ.36.361
Yampolskii Y (2012) Polymeric gas separation membranes. Macromolecules 45:3298–3311. https://doi.org/10.1021/ma300213b
Yang H, Xu Z, Fan M et al (2008) Progress in carbon dioxide seperation and capture: A review. J Environ Sci 20:14–27
Yao K, Wang Z, Wang J, Wang S (2012) Biomimetic material-poly (N-vinylimidazole)-zinc complex for CO2 separation. Chem Commun 48:1766–1768. https://doi.org/10.1039/c2cc16835a
Yasuda H, Tsai JT (1974) Pore size of microporous polymer membranes. J Appl Polym Sci 18:805–819
Yave W, Car A, Funari SS et al (2010a) CO2-philic polymer membrane with extremely high separation performance. Macromolecules 43:326–333. https://doi.org/10.1021/ma901950u
Yave W, Car A, Wind J, Peinemann K-V (2010b) Nanometric thin film membranes manufactured on square meter scale : ultra-thin films for CO2 capture. Nanotechnology 21:395301–395307. https://doi.org/10.1088/0957-4484/21/39/395301
Yave W, Szymczyk A, Yave N, Roslaniec Z (2010c) Design, synthesis, characterization and optimization of PTT-b-PEO copolymers : A new membrane material for CO2 separation. J Membr Sci 362:407–416. https://doi.org/10.1016/j.memsci.2010.06.060
Yave W, Car A, Peinemann K et al (2009) Gas permeability and free volume in poly (amide-b-ethylene oxide)/polyethylene glycol blend membranes. J Membr Sci 339:177–183. https://doi.org/10.1016/j.memsci.2009.04.049
Yegani R, Hirozawa H, Teramoto M et al (2007) Selective separation of CO2 by using novel facilitated transport membrane at elevated temperatures and pressures. J Membr Sci 291:157–164. https://doi.org/10.1016/j.memsci.2007.01.011
Yeo ZY, Chew TL, Zhu PW et al (2012) Conventional processes and membrane technology for carbon dioxide removal from natural gas : a review. J Nat Gas Chem 21:282–298. https://doi.org/10.1016/S1003-9953(11)60366-6
Yi C, Wang Z, Li M et al (2006) Facilitated transport of CO2 through polyvinylamine / polyethlene glycol blend membranes. Desalination 193:90–96. https://doi.org/10.1016/j.desal.2005.04.139
Yu B, Cong H, Zhao XS, Chen Z (2011) Carbon dioxide capture by dendrimer-modified silica nanoparticles †. Adsorpt Sci Technol 29:781–788. https://doi.org/10.1260/0263-6174.29.8.781
Yu M, Dai Y, Yang K et al (2016) TEA incorporated CS blend composite membrane for high CO2 separation performance. RSC Adv 6:27016–27019. https://doi.org/10.1039/C5RA25029C
Yu X, Wang Z, Wei Z et al (2010) Novel tertiary amino containing thin film composite membranes prepared by interfacial polymerization for CO2 capture. J Membr Sci 362:265–278. https://doi.org/10.1016/j.memsci.2010.06.043
Yuan F, Wang Z, Li S et al (2012) Formation-structure-performance correlation of thin film composite membranes prepared by interfacial polymerization for gas separation. J Membr Sci 421–422:327–341. https://doi.org/10.1016/j.memsci.2012.07.035
Yuan S, Wang Z, Qiao Z et al (2011) Improvement of CO2/N2 separation characteristics of polyvinylamine by modifying with ethylenediamine. J Membr Sci 378:425–437. https://doi.org/10.1016/j.memsci.2011.05.023
Zhang B, Fu J, Zhang Q et al (2019) Study on CO2 facilitated separation of mixed matrix membranes containing surface modified MWCNTs. J Appl Polym Sci 136:1–12. https://doi.org/10.1002/app.47848
Zhang C, Sheng M, Hu Y et al (2021) Efficient facilitated transport polymer membrane for CO2/CH4 separation from oilfield associated gas. Membranes (Basel) 11:118–136. https://doi.org/10.3390/membranes11020118
Zhang Y, Sunarso J, Liu S, Wang R (2013) Current status and development of membranes for CO2/CH4 separation: a review. Int J Greenh Gas Control 12:84–107. https://doi.org/10.1016/j.ijggc.2012.10.009
Zhang Y, Wang Z, Wang SC (2002) Selective permeation of CO2 through new facilitated transport membranes. Desalination 145:385–388
Zhao B, Peng N, Liang C et al (2015a) Hollow fiber membrane dehumidification device for air conditioning system. Membranes (Basel) 5:722–738. https://doi.org/10.3390/membranes5040722
Zhao S, Cao X, Ma Z et al (2015b) Mixed-matrix membranes for CO2/N2 separation comprising a poly (vinylamine) matrix and metal−organic frameworks. Ind Eng Chem Res 54:5139–5148. https://doi.org/10.1021/ie504786x
Zhao H, Cao Y, Ding X et al (2008) Poly (N, N-dimethylaminoethyl methacrylate)-poly (ethylene oxide) copolymer membranes for selective separation of CO2. J Membr Sci 310:365–373. https://doi.org/10.1016/j.memsci.2007.11.014
Zhao J, Wang Z, Wang J, Wang S (2006) Influence of heat-treatment on CO2 separation performance of novel fixed carrier composite membranes prepared by interfacial polymerization. J Membr Sci 283:346–356. https://doi.org/10.1016/j.memsci.2006.07.004
Zhao J, Wang Z, Wang J, Wang S (2012) High-performance membranes comprising polyaniline nanoparticles incorporated into polyvinylamine matrix for CO2/N2 separation. J Membr Sci 403–404:203–215. https://doi.org/10.1016/j.memsci.2012.02.048
Zhao S, Wang Z, Qiao Z et al (2013) Gas separation membrane with CO2-facilitated transport highway constructed from amino carrier containing nanorods and macromolecules. J Mater Chem A 1:246–249. https://doi.org/10.1039/c2ta00247g
Zhao Y, Ho WSW (2013) CO2-selective membranes containing sterically hindered amines for CO2/H2 separation. Ind Eng Chem Res 52:8774–8782. https://doi.org/10.1021/ie301397m
Zhao Y, Ho WSW (2012) Steric hindrance effect on amine demonstrated in solid polymer membranes for CO2 transport. J Membr Sci 415–416:132–138. https://doi.org/10.1016/j.memsci.2012.04.044
Zhao Y, Jung BT, Ansaloni L, Ho WSW (2014) Multiwalled carbon nanotube mixed matrix membranes containing amines for high pressure CO2/H2 separation. J Membr Sci 459:233–243. https://doi.org/10.1016/j.memsci.2014.02.022
Zhou C, Chung T, Wang R et al (2003) The accelerated CO2 plasticization of ultra-thin polyimide films and the effect of surface chemical cross-linking on plasticization and physical aging. J Membr Sci 225:125–134. https://doi.org/10.1016/j.memsci.2003.07.006
Zhu L, Zhou M, Yang S, Shen J (2015) Novel tertiary amino containing blinding composite membranes via raft polymerization and their preliminary CO2 permeation performance. Int J Mol Sci 16:9078–9096. https://doi.org/10.3390/ijms16059078
Zhuang GL, Tseng HH, Wey MY (2018) Facile synthesis of CO2-selective membrane derived from butyl reclaimed rubber (BRR) for efficient CO2 separation. J CO2 Util 25:226–234. https://doi.org/10.1016/j.jcou.2018.04.003
Zou J, Ho WSW (2006) CO2-selective polymeric membranes containing amines in crosslinked poly (vinyl alcohol). J Membr Sci 286:310–321. https://doi.org/10.1016/j.memsci.2006.10.013
Funding
The study is financially supported by the University Grants Commission-Major Research Project (UGC-MRP) (G. No: 43–321/2014 (SR)), New Delhi, India, and Indo-Russian joint project (G. No: 18–58-45003), Department of Science and Technology (DST), New Delhi, India.
Author information
Authors and Affiliations
Contributions
Kunalan Shankar: Literatures collection, interpretation, and article preparation. Palanivelu Kandasamy: Supervision; interpretation, revision, and editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible editor: Angeles Blanco
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kunalan, S., Palanivelu, K. Polymeric composite membranes in carbon dioxide capture process: a review. Environ Sci Pollut Res 29, 38735–38767 (2022). https://doi.org/10.1007/s11356-022-19519-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-19519-x