Abstract
The utilization of novel compounds as cancer treatments offers enormous potential in this field. The advantages of nanomedicine-based therapy include efficient cellular uptake and selective cell targeting. In this study, we employ selenium nanoparticles’ green-synthesized by apigenin (SeNPs-apigenin) to treat breast cancer. We used various assays to show that SeNPs-apigenin can reduce MCF-7 cell viability and trigger apoptosis in vitro. Flow cytometry and PCR methods were used to detect apoptosis, while cell migration and invasion methods were used to quantify the possible effect of SeNPs-apigenin therapy on cell migration and invasion. According to cytotoxicity testing, the SeNPs-apigenin treatment can successfully limit MCF-7 cell proliferation and viability in a concentration-dependent manner. Flow cytometric and PCR analyses revealed that SeNPs-apigenin treatment induced apoptosis in MCF-7 cells, demonstrating that SeNPs-apigenin treatment could directly target Bcl-2, Bax, and caspase-3 and result in the discharge of cytochrome C from mitochondria into the cytosol, accompanied by the initiation of cell death, leading to permanent DNA damage and killing of MCF-7 cells. Furthermore, treatment with SeNPs-apigenin increased reactive oxygen species production and oxidative stress in MCF-7 cells. Our findings indicate that SeNPs-apigenin has cytotoxic potential in the treatment of breast cancer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cancer is a disease that originates from different biological changes that cause uncontrolled cell division (Bray et al. 2018). Breast cancer caused 0.62 million deaths and 2.08 million new cases in 2018, according to data from the International Agency for Research on Cancer (IARC) and Globocan (approximately 11.6% of all types of cancer recorded). By 2040, if current trends continue, the mortality troll will have risen to a nerve-wracking high of 6.99 million (Sung et al. 2021). In this context, breast cancer is the most common malignant tumor in females, and its prevalence and death rate have climbed quickly in recent years. Because of the high risks of recurrence and metastasis in breast cancer, the long survival rate of breast cancer patients after surgery or chemotherapy therapies remains dismal. Breast cancer metastasis is the biggest cause of patient death (Li et al. 2017). Triple-negative breast cancer is the most aggressive of all breast cancer types; it is difficult to cure and more likely to spread in diagnosed patients (Razak et al. 2019). As a result, novel natural-source treatment medicines with efficient and safe effects for this type of malignancy are desperately needed.
Apigenin (4′,5,7-trihydroxyflavone) is one of the most prevalent flavonoids in plants and is chemically classified as a flavone. It is isolated from a variety of plants in the Asteraceae family. The Lamiaceae and Fabaceae families, on the other hand, have a sufficient amount of apigenin (Ali et al. 2017; Salehi et al. 2019). Apigenin has a wide range of pharmacological actions, including neuromodulatory, hypoglycemia, cell cycle arrest, apoptosis, antiallergic, anti-inflammatory, antibacterial, antiviral, and antioxidant properties (Khandelwal et al. 2020; Singh et al. 2012). Apigenin is thus used to treat various disorders, including amnesia, Alzheimer’s, depression, insomnia, diabetes, and osteoarthritis (Ali et al. 2017; Salehi et al. 2019). In addition, numerous investigations have demonstrated apigenin’s anticancer properties (Javed et al. 2021; Kaur et al. 2008). Apigenin exerts anticancer activity in cancerous cells and transgenic animals via a variety of mechanisms, including inhibition of the GSK-3/NF-кB signaling cascade in pancreatic cancer cell lines (Johnson and de Mejia 2013), targeting both ER alpha-dependent and ERalpha-independent pathways on estrogen-responsive, anti-estrogen sensitive MCF-7 breast cancer cells (Long et al. 2008), and regulation of a p53-Ba (Shukla et al. 2014).
Selenium (Se) has been recognized as an essential micronutrient involved in a variety of biological processes. Se is required for the proper processing of many critical issues such as neuronal, muscular, and reproductive tissues and their role in the immune system due to its integration into the structure of selenoproteins (Al-Brakati et al. 2021; Kursvietiene et al. 2020). Several studies have found a link between Se deficiency and an increased risk of cancer. However, Se’s anticancer properties remain controversial. Se supplementation, in addition to standard chemotherapy therapies, has been found to boost the efficacy of anticancer drugs, reduce side effects, and improve patients’ overall health (Yakubov et al. 2014). The ability of Se to neutralize heavy metal toxicity, maintain DNA stability, enhance DNA repair enzymes, regulate inflammatory and immune reactions, initiate cell cycle arrest and cell death, suppress local invasion and migration of cancer cells, prevent angiogenesis, control cell proliferation, and stimulate phase II carcinogen-detoxifying system are some of the possible mechanisms of Se’s anticancer effect (Chen et al. 2013; Yuan et al. 2020). As a result, Se has a strong anticancer effect in various malignancies, including lung, breast, prostate, melanoma, and glioma (Chen et al. 2008; Liao et al. 2020; Luo et al. 2012). Because of their unique biological properties, low toxicity, and great biocompatibility, elemental Se nanoparticles (SeNPs) have recently been widely exploited in nanotechnology (Al-Brakati et al. 2021).
Considering the anticancer actions of apigenin and Se, the purpose of this study was to evaluate the anticancer effect of biosynthesized SeNPs-apigenin, based on the hypothesis that bio- and nano-counterparts present in the form of a nanohybrid system could lead to promising synergistic anticancer effects.
Material and methods
Cell line and culture condition
ATCC provided the MCF-7 cell line. MCF-7 cells were cultivated at 37 °C in a humidified environment of 5% CO2 in Dulbecco’s Modified Eagle’s Medium (DMEM) (Gibco Life Technologies) supplemented with 10% fetal bovine serum and 100 g/mL penicillin/streptomycin.
Chemicals and reagents
Sigma-Aldrich provided the apigenin and sodium selenite (USA). Unless otherwise noted, the reagents used were of the highest analytical grade. As a solvent for selenium, deionized distilled water (ddH2O) was utilized, whereas dimethyl sulfoxide (DMSO; Sigma-Aldrich, USA) was used to prepare the primary stock solution apigenin and SeNPs-apigenin at a concentration of 1 mg/mL and held at − 20 °C until use.
SeNPs biosynthesis
Two ml of apigenin (0.1 mM/ml) and 10 ml of Na2SeO3 (0.1 mM/ml) were swirled together for 24 h at room temperature. The average size of the SeNPs-apigenin was determined using a Zetasizer (Nano series, ZEN 3600, Malvern, UK).
MTT assay
Following the previous publication of Othman et al. (2021a), the MTT (dimethylthiazolyltetrazolium bromide) colorimetric test was used to determine the cytotoxicity of SeNPs-apigenin. In brief, 80% confluent MCF-7 cells (1 × 104 cells/ml) planted in 96-well plates were individually treated with different concentrations of SeNPs-apigenin for 24 h at 37 °C in a humidified atmosphere of 5% CO2. The cells were then washed with phosphate buffer saline (PBS) and treated with 20 µL of MTT (5 mg/ml) at 37 °C. After 30 min of treatment, the produced formazan crystals were dissolved in 200 µL of DMSO and re-incubated at 37 °C for another 30 min. The intensity of the dark blue color was measured using a microplate reader at 570 nm.
Apoptosis marker determination
According to the manufacturer’s protocols, ELISA kits (Abcam, Cambridge, UK) were used to determine apoptosis markers (Bcl-2, Bax, and cleaved caspase-3). In brief, 2 × 106 MCF-7 cells were individually pretreated with SeNPs-apigenin and DOX at IC30 concentrations and incubated for 24 h, whereas control cells were incubated with the vehicle. The cells were collected by centrifugation at 1800 × g for 5 min to remove the medium, and they were washed twice with PBS. The pellets were lysed in 50 µL of cold lysis buffer. The resultant cell lysate was centrifuged at 12,000 × g for 1 min at 4 °C, and the supernatant was collected. The Bradford method was used to determine the protein levels in each cell lysate. If the protein levels in the sample exceeded 4 g/L, the cell extraction buffer PTR was employed to dilute it. Finally, the developed color of the samples was measured in a microplate reader at 405 nm (Biotech, Inc., USA).
Transwell migration and invasion assay
Corning Transwell 8.0-m pore membranes were used to assess cell migration and invasion (Corning, USA). In a nutshell, 1 × 105 MCF-7 cells were put into the upper chamber of each Transwell. Before adding MCF-7 cells, the upper chamber of the Transwell was coated with 100 μL of 1:8 DMEM-diluted Matrigel (BD, USA). Lower chambers of Transwell were filled with the fresh medium as a chemoattractant, with or without SeNPs-apigenin or DOX. After 48 h of incubation at 37 °C, the cells on the upper surface of the membrane were removed with a cotton swab, while those that had migrated or penetrated to the lower membrane surface were fixed with 4% paraformaldehyde and stained with 0.1% crystal violet solution. A light microscope with a magnification of 200 × was used to photograph and count the number of MCF-7 cells that migrated and infiltrated through the filter.
Molecular assay for the expression level of caspase-3 (Casp-3), Bax, and Bcl-2
According to the manufacturer’s instruction, total RNA was extracted from MCF-7 cells using the TRIzol reagent (Life Technologies, USA), and cDNA was produced using RevertAid™ H Minus Reverse Transcriptase (Fermentas, Thermo Fisher Scientific, USA). SYBR Green Supermix (Biorad, USA) performed RT-PCR operations on a ViiATM 7 System (Thermo Fisher Scientific, USA). GAPDH was chosen as the housekeeping gene for all data standardization.
The sequences of the qPCR primers are as follows:
-
Bax F: 5′-AGGATGCGTCCACCAAGAAG-3′
-
R: 5′-CTTGGATCCAGACAAGCAGC-3′
-
Bcl-2 F: 5′-AGCATGCGACCTCTGTTTGA-3′
-
R: 5′-GCCACACGTTTCTTGGCAAT-3′
-
Cas-3 F: 5′-GGTATTGAGACAGACAGTGG-3′
-
R: 5′-CATGGGATCTGTTTCTTTGC-3′
-
β-actin F: 5′-CCACCATGTACCCAGGCATT-3′
-
R: 5′-AGGGTGTAAAACGCAGCTCA-3′
Flow cytometry
MCF-7 cells (2 × 105 cell/ml) cultured in T25 cell culture flasks were treated for 24 h with SeNPs-apigenin or DOX. MCF-7 cells were collected and resuspended in the binding buffer before being treated for 10 min in the dark at 30 °C with annexin V-FITC (Cat. No.: ab14085, Abcam, UK). The cells were then spun down at 4,000 × g at 4 °C, resuspended in binding buffer, and finally, propidium iodide (PI) (Cat. No: ab14083, Abcam, UK) was added, and the stained cells were analyzed by flow cytometry (BD FACSCalibur flow cytometer, USA).
Determination of oxidative stress markers
MCF-7 cells (2 × 105 cell/ml) cultured in T25 cell culture flasks were treated for 24 h with SeNPs-apigenin or DOX. After harvesting, cells were lysed in a lysis buffer, and the lysate was spun down by centrifugation at 12,000 × g at 4 °C for 1 min at 4 °C, and the supernatant was collected. The Bradford method was used to determine the protein levels in each cell lysate. The resultant cell lysate was utilized to detect ROS levels using the green fluorescence strain 2,7-dichlorofluorescein diacetate (DCFH-DA) described by Othman et al. (2021a). Ohkawa et al. (1979) and Green et al. (1982) described colorimetric techniques for determining lipid peroxidation (LPO) and nitric oxide (NO), respectively. Antioxidant indicators such as glutathione (GSH), superoxide dismutase (SOD), and glutathione peroxidase (GPx) were measured using the known procedures of Ellman (1959), Nishikimi et al. (1972), and Paglia and Valentine (1967), respectively.
Statistical analysis
The mean and standard deviation are used to express all data (SD). One-way ANOVA and post hoc Duncan’s test were used to compare more than two groups. In the analysis, the statistical tool SPSS (20.0) was used. If P values were less than 0.05, significant differences were considered.
Results
SeNP characterization
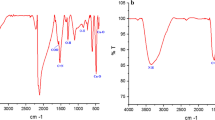
SeNPs-apigenin had a mean zeta potential of − 26.2 mV and a mean diameter of 124.3 nm (Fig. 1a and b). Figure 1c demonstrates the outcome of FT-IR study of the synthesized SeNPs-apigenin. O–H group is represented by a broad peak at 3338.81 cm−1. C–H stretch alkynes have an absorption peak of 2110.81 cm−1. The C–O asymmetric stretch carbon compounds are responsible for the band at 1636.25 cm−1. The amines’ C–N stretching is responsible for the absorption peak at 1438.17 cm−1. The C–O–C asymmetric stretch carbon compounds are responsible for the bands at 1011.07 and 950.71 cm−1. In alkyl halides, C–X expanding produces bands at 451.00 cm−1. These observations indicate the existence of many functional groups that could be accountable for SeNPs-apigenin reduction and stabilization.
Furthermore, the XRD pattern result showed a broader pattern without any definite Braggs peaks. The obtained results indicate that SeNPs-apigenin is more amorphous, not crystalline (Fig. 1d). TEM image of SeNPs-apigenin revealed spherical particles within the diameter < 200 nm. These particles were well distributed with no aggregation (Fig. 1e).
Cytotoxic effect and growth inhibition of SeNPs-apigenin
In the present investigation, the anticancer effect of SeNPs-apigenin was examined against breast cancer cells MCF-7 by using MTT test. As shown in Fig. 2, SeNPs-apigenin markedly inhibited the growth of MCF-7 cancer cells in a dose-dependent manner. The growth inhibition of SeNPs-apigenin against MCF-7 cells was less than 5% at the concentration of 1000 μM/ml, and IC50 was found to be at 51.74 μM/ml. However, the cytotoxic effect of SeNPs-apigenin was lesser than the cytotoxic effect of DOX that has an IC50 value at 0.59 μM/ml against MCF-7 cells.
SeNPs-apigenin treatment leads to enhanced apoptosis in MCF-7 cells
Compared with the control cells, the early and late apoptosis percent in MCF-7 cells was significantly increased after treatment with SeNPs-apigenin and DOX (Fig. 3). Furthermore, an evaluation of the apoptotic response is presented in Fig. 4. The anti-apoptotic gene and protein expression (Bcl-2) and Bcl2/Bax ratio showed significant reductions in cells treated with SeNPs-apigenin and DOX compared to the untreated control cells. The mRNA and protein expressions of the pro-apoptotic markers Bax and caspase-3 showed significant elevations in the groups treated with SeNPs-apigenin and DOX compared to the untreated control cells. The obtained results lead to the conclusion that SeNPs-apigenin treatment can cause apoptosis enhancement.
Induction of cell apoptosis DOX and SeNPs-apigenin on MCF-7 cells. A Annexin V-PI co-staining assay to evaluate apoptosis in MCF-7 cells. B Percent of apoptotic cells. Values represent the mean of three experiments. Ψ, significant with respect to the control (P ˂ 0.05). Δ, significant with respect to the DOX (P ˂ 0.05)
Inhibition of cancer cell migration and invasion by SeNPs-apigenin
MCF-7 cells are characterized by their ability to invade the surrounding tissues. Moreover, they show a good cloning ability. They can break through the basement membrane from carcinoma in situ to form metastatic foci and worsen the disease through the invasion-metastasis cascade reaction. In the present study, we investigated the ability of SeNPs-apigenin to inhibit the growth of tumor cells by inhibiting the migration and invasion of tumor cells. The invasion experiment showed that the effect of the SeNPs-apigenin has no or little inhibiting the invasion and migration of MCF-7 cells (Fig. 5).
Oxidative stress biomarkers
Since ROS generation and antioxidant collapse are basic pathways that enhance apoptotic in cancer cells, ROS, NO, and MDA levels were estimated as oxidants markers, while antioxidant markers were assessed by determining the GSH level and SOD and GPx activities. Noticeable rises were noticed in ROS, NO, and MDA paralleled with significant exhaustion in GSH level and antioxidant enzymes in DOX-treated cells compared with untreated cells. MCF-7 cells treated with SeNPs-apigenin showed notable increases in measured oxidant markers and a decline in antioxidant markers compared to control cells. Compared to DOX-treated cells, MCF-7 cells treated SeNPs-apigenin displayed higher GSH and GPx than DOX-treated cells (Fig. 6).
Discussion
Cancer has a high death rate worldwide; consequently, innovative techniques to control this disease should be researched. Resistance to routinely used chemotherapy lowers apoptotic cancer cells, posing a challenge to cancer treatment success (Geretto et al. 2017). DOX (antineoplastic chemotherapy) causes cancer cells to die by intercalation into DNA and disruption of topoisomerase-II-mediated DNA repair and (ii) the formation of free radicals and their damage to cellular membranes, DNA, and proteins, followed by apoptosis. However, because DOX is mainly unspecific, its administration frequently results in long-term tissue harm, including neurotoxicity, nephrotoxicity, hepatotoxicity, and cardiotoxicity. Drug resistance affects more than 90% of patients (Ansari et al. 2017). That is why finding the botanical natural derived drug with an anticancer effect with minimum side effects would be beneficial.
In MCF-7 cells, the cytotoxic activities of DOX and SeNPs-apigenin were examined, and the IC50 values were 0.59 and 51.74 µM, respectively. MCF-7 cell lines treated with SeNPs-apigenin showed significant cytotoxicity. Previously, apigenin at doses of 60, 40, 25, and 20 µM was shown to rescue the viability, self-renewal capacity, proliferation, and invasion of cultured HL60, HSC-3, Hela, LNCaP, PC-3, DU145, SW480, MDA-MD-231, H1299, and H460 cells, indicating that apigenin at 40 µM almost completely prevented apoptosis (Lee et al. 2016; Seo et al. 2015; Xu et al. 2016; Yan et al. 2017). Azab et al. (2015) discovered that Se and/or apigenin had better antiproliferation efficiency in Ehrlich-bearing mice. According to previous research, green synthesis of SeNPs with phytochemicals as reducing and capping agents was positively connected with improved anticancer activity (Ezhuthupurakkal et al. 2018; Othman et al. 2021b; Rajeshkumar et al. 2018; Umar et al. 2019).
Induction of oxidative stress by metal nanoparticles is a characteristic of cancer cell damage induction (Song et al. 2014). High amounts of ROS may be linked to oxidative damage and may alter mitochondrial membrane malfunction, resulting in the release of mitochondrial factors, the activation of caspase cascades, and apoptosis (Buranrat et al. 2020). As a result, detecting intracellular ROS production is a crucial signal for early cellular responses to NPs and the first stage in cellular toxicity cascade events (Lee et al. 2011). Normally, mitochondria emit moderate quantities of ROS as signaling molecules for cell upkeep. In cases of excessive intracellular ROS generation by NPs, however, cells lost their normal functions, resulting in cell death. Compared to other metal NPs, SeNPs can generate more ROS, resulting in oxidative damage (Song et al. 2014). When MCF-7 cells were treated to SeNPs-apigenin, there was a considerable increase in ROS compared to the control. These findings are consistent with earlier research (Horinaka et al. 2006; Wang and Zhao 2017). Apigenin caused significant ROS generation in human colon carcinoma HCT-116 cells, demonstrating its powerful anticancer potential, supporting the findings of Wnag and Zhao (2017). Furthermore, it’s also been proven that stimulating plant extracts causes an overabundance of reactive oxygen species (ROS), which leads to oxidative and apoptotic cancer cell death (Othman et al. 2021b).
In addition to inducing excessive ROS, SeNPs-apigenin significantly reduced intracellular GSH when compared to the control. GSH protects thiol groups in enzymes and reacts with free radicals such as single oxygen and hydroxyl radicals. Because GSH content can reflect a cell’s antioxidant capacity (Li et al. 2021), exhaustion of its content indicates cytotoxicity of designed NPs in MCF-7 cells, which is consistent with Song et al. (2014). Furthermore, there were significant increases in MDA and NO levels, both oxidative indicators, in SeNPs-apigenin-treated cells compared to the control. MDA and NO levels in MCF-7 cells are significantly raised after treatment with SeNPs, similar to the findings of Yang et al. (2021). Increased MDA suggested cell death mediated by lipid peroxidation of the cell membrane. Surprisingly, the combination of apigenin and SeNPs increased MDA and NO levels in cancer cells, making this therapeutic combination a standout recipe for combating breast carcinogenesis by developing oxidative stress in cancer cells.
Previous research has shown that ROS can interact with the cell’s nucleophilic center and covalently attach to nucleic acid, RNA, and proteins, causing DNA damage (Othman et al. 2021b). Furthermore, ROS regulates the translocation, phosphorylation, and cleavage of pro-apoptosis Bcl-2 members, resulting in the triggering of apoptosis (Wang et al. 2020). Previously, SeNPs were shown to cause ROS production, DNA damage, and apoptosis in C6 (rat glioma cells), A375 (human malignant melanoma cells), (PC-3) prostate cancer cell line, and Caco-2 cells (Chan et al. 2017; Khurana et al. 2019; Sonkusre and Cameotra 2017). Following treatment with apigenin or SeNPs-apigenin, we found a significant increase in pro-apoptotic markers (caspase-3 and Bax) and a decrease in anti-apoptotic markers (Bcl-2). The ratio of pro-apoptotic to anti-apoptotic proteins determines cell fate (Othman et al. 2021b). Pan et al. (2013) discovered that apigenin decreased Bcl-2 expression while increasing caspase-3 expression in a human lung cancer cell line, NCI-H460. Similar findings were observed in apigenin-treated human cervical cancer cells, Hela (Kayacan et al. 2021). These findings are in line with prior research that found plant extracts or natural products can boost enzyme activity and induce caspase-dependent apoptosis in human breast cancer cells and other cell types (Panicker et al. 2020).
Metastasis is a significant aspect of cancer, and the leading cause of mortality is around 90% of cancer patients. Cancer cells’ migratory and invasion functions are critical throughout this process, which leads to major repercussions such as tumor metastases and poor prognosis (Tian et al. 2020). As a result, in addition to investigating the effects of SeNPs on cell proliferation, this study also investigated the effect of combining MCF-7 cells’ migration and invasion. SeNPs therapy, as expected, can limit cell migration and invasion to some extent. The findings are consistent with Fang et al. (2017), who discovered that SeNPs mixed with baicalin suppressed liver cancer cells, HepG2215, migration, and invasion.
Nonetheless, our research has limitations. No in vivo data, including animal and human tests, are available to support this study. As a result, additional research should be conducted to corroborate the findings of the in vivo experiments.
Conclusion
This study found that SeNPs-apigenin had a positive anticancer effect on MCF-7 cells. The findings will give a basis for nanomedicine alone or as a supplementary medication during the chemotherapy stage of breast cancer treatment.
Data availability
All relevant data are within the paper.
References
Seo HS et al. (2015) Induction of caspase-dependent extrinsic apoptosis by apigenin through inhibition of signal transducer and activator of transcription 3 (STAT3) signalling in HER2-overexpressing BT-474 breast cancer cells. Biosci Rep 35. https://doi.org/10.1042/BSR20150165
Al-Brakati A et al (2021) Using green biosynthesized lycopene-coated selenium nanoparticles to rescue renal damage in glycerol-induced acute kidney injury in rats. Int J Nanomedicine 16:4335–4349. https://doi.org/10.2147/IJN.S306186
Ali F, Naz F, Jyoti S, Siddique YH (2017) Health functionality of apigenin: a review. Int J Food Prop 20:1197-1238.https://doi.org/10.1080/10942912.2016.1207188
Ansari L, Shiehzadeh F, Taherzadeh Z, Nikoofal-Sahlabadi S, Momtazi-borojeni AA, Sahebkar A, Eslami S (2017) The most prevalent side effects of pegylated liposomal doxorubicin monotherapy in women with metastatic breast cancer: a systematic review of clinical trials. Cancer Gene Ther 24:189–193. https://doi.org/10.1038/cgt.2017.9
Azab KS, Hanafy SM, Fateh NME, Badr N (2015) Antiangiogenic effects of the apigenin and/or selenium on Ehrlish bearing mice Res J Appl. Biotechnol 1:21–43. https://doi.org/10.21608/rjab.2015.53282
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin 68:394–424
Buranrat B, Boontha S, Temkitthawon P, Chomchalao P (2020) Anticancer activities of Careya arborea Roxb on MCF-7 human breast cancer cells. Biologia 75:2359–2366. https://doi.org/10.2478/s11756-020-00535-6
Chan L et al (2017) Cancer-targeted selenium nanoparticles sensitize cancer cells to continuous γ radiation to achieve synergetic chemo-radiotherapy chemistry – an. Asian J 12:3053–3060. https://doi.org/10.1002/asia.201701227
Chen T, Wong Y-S, Zheng W, Bai Y, Huang L (2008) Selenium nanoparticles fabricated in Undaria pinnatifida polysaccharide solutions induce mitochondria-mediated apoptosis in A375 human melanoma cells. Colloids Surf B: Biointerf 67:26–31. https://doi.org/10.1016/j.colsurfb.2008.07.010
Chen YC, Prabhu KS, Mastro AM (2013) Is selenium a potential treatment for cancer metastasis? Nutrients 5:1149–1168. https://doi.org/10.3390/nu5041149
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Ezhuthupurakkal PB et al (2018) Anticancer potential of ZnO nanoparticle-ferulic acid conjugate on Huh-7 and HepG2 cells and diethyl nitrosamine induced hepatocellular cancer on Wistar albino rat Nanomedicine: Nanotechnology. Biol Med 14:415–428
Fang X, Wu X, Li C, Zhou B, Chen X, Chen T, Yang F (2017) Targeting selenium nanoparticles combined with baicalin to treat HBV-infected liver cancer. RSC Adv 7:8178–8185. https://doi.org/10.1039/c6ra28229f
Geretto M, Pulliero A, Rosano C, Zhabayeva D, Bersimbaev R, Izzotti AJAjocr (2017) Resistance to cancer chemotherapeutic drugs is determined by pivotal microRNA regulators. 7:1350
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem 126:131–138
Horinaka M, Yoshida T, Shiraishi T, Nakata S, Wakada M, Sakai T (2006) The dietary flavonoid apigenin sensitizes malignant tumor cells to tumor necrosis factor-related apoptosis-inducing ligand. Mol Cancer Ther 5:945–951. https://doi.org/10.1158/1535-7163.MCT-05-0431
Javed Z et al (2021) Apigenin role as cell-signaling pathways modulator: implications in cancer prevention and treatment. Cancer Cell Int 21:189. https://doi.org/10.1186/s12935-021-01888-x
Johnson JL, de Mejia EG (2013) Flavonoid apigenin modified gene expression associated with inflammation and cancer and induced apoptosis in human pancreatic cancer cells through inhibition of GSK-3β/NF-κB signaling cascade. Mol Nutr Food Res 57:2112–2127. https://doi.org/10.1002/mnfr.201300307
Kaur P, Shukla S, Gupta S (2008) Plant flavonoid apigenin inactivates Akt to trigger apoptosis in human prostate cancer: an in vitro and in vivo study. Carcinogenesis 29:2210–2217. https://doi.org/10.1093/carcin/bgn201
Kayacan S, Yilancioglu K, Akdemir AS, Kaya-Dagistanli F, Melikoglu G, Ozturk M (2021) Synergistic effect of apigenin and curcumin on apoptosis, paraptosis and autophagy-related cell death in HeLa cells. Anticancer Res 41:1271. https://doi.org/10.21873/anticanres.14884
Khandelwal N et al (2020) Antiviral activity of apigenin against buffalopox: novel mechanistic insights and drug-resistance considerations. Antiviral Res 181:104870. https://doi.org/10.1016/j.antiviral.2020.104870
Khurana A, Tekula S, Saifi MA, Venkatesh P, Godugu C (2019) Therapeutic applications of selenium nanoparticles. Biomed Pharmacother 111:802–812. https://doi.org/10.1016/j.biopha.2018.12.146
Kursvietiene L, Mongirdiene A, Bernatoniene J, Sulinskiene J, Staneviciene I (2020) Selenium anticancer properties and impact on cellular redox status antioxidants (Basel) 9. https://doi.org/10.3390/antiox9010080
Lee K, Lee H, Lee KW, Park TG (2011) Optical imaging of intracellular reactive oxygen species for the assessment of the cytotoxicity of nanoparticles. Biomaterials 32:2556–2565
Lee YM et al (2016) Inhibition of glutamine utilization sensitizes lung cancer cells to apigenin-induced apoptosis resulting from metabolic and oxidative stress. Int J Oncol 48:399–408. https://doi.org/10.3892/ijo.2015.3243
Li J et al (2017) AKR1B10 promotes breast cancer cell migration and invasion via activation of ERK signaling. Oncotarget 8:33694–33703. https://doi.org/10.18632/oncotarget.16624
Li Z et al. (2021) Neuroprotective effects of protocatechuic acid on sodium arsenate induced toxicity in mice: role of oxidative stress, inflammation, and apoptosis. Chemico-Biol Interact 109392
Liao G, Tang J, Wang D, Zuo H, Zhang Q, Liu Y, Xiong H (2020) Selenium nanoparticles (SeNPs) have potent antitumor activity against prostate cancer cells through the upregulation of miR-16. World J Surg Oncol 18
Long X, Fan M, Bigsby RM, Nephew KP (2008) Apigenin inhibits antiestrogen-resistant breast cancer cell growth through estrogen receptor-alpha-dependent and estrogen receptor-alpha-independent mechanisms. Mol Cancer Ther 7:2096–2108. https://doi.org/10.1158/1535-7163.MCT-07-2350
Luo H, Wang F, Bai Y, Chen T, Zheng W (2012) Selenium nanoparticles inhibit the growth of HeLa and MDA-MB-231 cells through induction of S phase arrest. Colloids Surf B Biointerfaces 94:304–308. https://doi.org/10.1016/j.colsurfb.2012.02.006
Nishikimi M, Appaji N, Yagi K (1972) The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun 46:849–854
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Othman MS, Al-Bagawi AH, Obeidat ST, Fareid MA, Habotta OA, Moneim AEA (2021) Antitumor activity of zinc nanoparticles synthesized with berberine on human epithelial colorectal adenocarcinoma (Caco-2) cells through acting on Cox-2/NF-kB and p53 pathways. Anticancer Agents Med Chem. https://doi.org/10.2174/1871520621666211004115839
Othman MS, Obeidat ST, Al-Bagawi AH, Fareid MA, Fehaid A, Abdel Moneim AE (2021b) Green-synthetized selenium nanoparticles using berberine as a promising anticancer agent J. Integr Med. https://doi.org/10.1016/j.joim.2021.11.002
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–169
Pan X, Yang Z, Zhou S, Zhang H, Zang L (2013) Effect of apigenin on proliferation and apoptosis of human lung cancer NCI-H460 cells. Nan Fang Yi Ke Da Xue Xue Bao 33:1137–1140
Panicker NG et al (2020) Organic Extracts from Cleome Droserifolia Exhibit Effective Caspase-Dependent Anticancer Activity. BMC Complement Med Ther 20:74. https://doi.org/10.1186/s12906-020-2858-0
Rajeshkumar S, Kumar SV, Ramaiah A, Agarwal H, Lakshmi T, Roopan SM (2018) Biosynthesis of zinc oxide nanoparticles using Mangifera indica leaves and evaluation of their antioxidant and cytotoxic properties in lung cancer (A549) cells. Enzym Microb Technol 117:91–95
Razak NA et al (2019) Cytotoxicity of eupatorin in MCF-7 and MDA-MB-231 human breast cancer cells via cell cycle arrest, anti-angiogenesis and induction of apoptosis. Sci Rep 9:1514. https://doi.org/10.1038/s41598-018-37796-w
Salehi B et al (2019) The therapeutic potential of apigenin. Int J Mol Sci 20. https://doi.org/10.3390/ijms20061305
Shukla S, Bhaskaran N, Babcook MA, Fu P, Maclennan GT, Gupta S (2014) Apigenin inhibits prostate cancer progression in TRAMP mice via targeting PI3K/Akt/FoxO pathway. Carcinogenesis 35:452–460. https://doi.org/10.1093/carcin/bgt316
Singh P, Mishra SK, Noel S, Sharma S, Rath SK (2012) Acute Exposure of Apigenin Induces Hepatotoxicity in Swiss Mice. PLOS ONE 7:e31964. https://doi.org/10.1371/journal.pone.0031964
Song Y, Guan R, Lyu F, Kang T, Wu Y, Chen X (2014) In vitro cytotoxicity of silver nanoparticles and zinc oxide nanoparticles to human epithelial colorectal adenocarcinoma (Caco-2) cells. Mutat Res/Fund Mol Mech Mutagen 769:113–118
Sonkusre P, Cameotra SS (2017) Biogenic selenium nanoparticles induce ROS-mediated necroptosis in PC-3 cancer cells through TNF activation. J Nanobiotechnol 15:43. https://doi.org/10.1186/s12951-017-0276-3
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin 71:209–249 doi:https://doi.org/10.3322/caac.21660
Tian J, Wei X, Zhang W, Xu A (2020) Effects of selenium nanoparticles combined with radiotherapy on lung cancer cells. Front Bioeng Biotechnol 8. https://doi.org/10.3389/fbioe.2020.598997
Umar H, Kavaz D, Rizaner N (2019) Biosynthesis of zinc oxide nanoparticles using Albizia lebbeck stem bark, and evaluation of its antimicrobial, antioxidant, and cytotoxic activities on human breast cancer cell lines. Int J Nanomed 14:87
Wang B, Zhao X-H (2017) Apigenin induces both intrinsic and extrinsic pathways of apoptosis in human colon carcinoma HCT-116 cells. Oncol Rep 37:1132–1140. https://doi.org/10.3892/or.2016.5303
Wang LS et al (2020) ZnO Nanoparticles induced caspase-dependent apoptosis in gingival squamous cell carcinoma through mitochondrial dysfunction and p70S6K signaling pathway. Int J Mol Sci 21. https://doi.org/10.3390/ijms21051612
Xu M, Wang S, Song YU, Yao J, Huang K, Zhu X (2016) Apigenin suppresses colorectal cancer cell proliferation, migration and invasion via inhibition of the Wnt/beta-catenin signaling pathway. Oncol Lett 11:3075–3080. https://doi.org/10.3892/ol.2016.4331
Yakubov E, Buchfelder M, Eyüpoglu IY, Savaskan NE (2014) Selenium action in neuro-oncology. Biol Trace Elem Res 161:246–254. https://doi.org/10.1007/s12011-014-0111-8
Yan X, Qi M, Li P, Zhan Y, Shao H (2017) Apigenin in cancer therapy: anti-cancer effects and mechanisms of action. Cell Biosci 7:50. https://doi.org/10.1186/s13578-017-0179-x
Yang Y, Zhang Z, Chen Q, You Y, Li X, Chen T (2021) Functionalized selenium nanoparticles synergizes with metformin to treat breast cancer cells through regulation of selenoproteins. Front Bioeng Biotechnol 9:758482. https://doi.org/10.3389/fbioe.2021.758482
Yuan X et al (2020) Selenium nanoparticles pre-treatment reverse behavioral, oxidative damage, neuronal loss and neurochemical alterations in pentylenetetrazole-induced epileptic seizures in mice. Int J Nanomed 15:6339–6353. https://doi.org/10.2147/IJN.S259134
Funding
This research was funded by the Deanship of Scientific Research at Princess Nourah Bint Abdulrahman University (Saudi Arabia), through the Research Funding Program (Grant No# FRP-1441–2).
Author information
Authors and Affiliations
Contributions
A. M. Al-Otaibi, R. S. Almeer, and A. E. Abdel Moneim designed the project. A. M. Al-Otaibi, A. S. Al-Gebaly, R. S. Almeer, G. Albasher, and W. S. Al-Qahtani performed the experiments. A. E. Abdel Moneim analyzed the data, interpreted the data, and drafted and edited the manuscript. A. M. Al-Otaibi, A. S. Al-Gebaly, R. S. Almeer, G. Albasher, and W. S. Al-Qahtani supplied the chemicals and reagents. All authors read and approved the final draft.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible editor: Lotfi Aleya
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Al-Otaibi, A.M., Al-Gebaly, A.S., Almeer, R. et al. Potential of green-synthesized selenium nanoparticles using apigenin in human breast cancer MCF-7 cells. Environ Sci Pollut Res 29, 47539–47548 (2022). https://doi.org/10.1007/s11356-022-19166-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-19166-2