Abstract
Deposits remained after coal combustion are a well-known occurrence in the world; unfortunately, only a small percentage of such deposits are adequately regulated and, consequently, pose a serious threat to the local environment. Attenuation of negative consequences presupposes knowledge of a number of features, both of the deposit and the local environment as well the interaction with local biota. In this study, unregulated waste generated from decades of coal mining and combustion of superhigh-organic-sulfur Raša coal, enriched in Se-U–Mo-V and located in a vulnerable karst region, was investigated. To assess the impact of landfill on the environment, in addition to its general geochemical and mineralogical features, the human health risk was assessed and the leaching of elements from the landfill, local soil, and the coal itself was investigated. For the latter, three extraction procedures, ASTM, EP, and TCLP (pH 4.93 and 2.9), were employed, mimicking different environmental conditions, including the sporadic occurrence of acid rains in the region. The soil around the landfill displayed enrichment in the majority of elements compared to expected values, with exception of Se, Mo, U, V, Sr, and Cu found at the highest levels in landfill samples. Mobility of elements was found to be controlled by both pH and mineralogy (carbonates and sulfates), whereby the overall highest relative mobility was observed in landfill samples for elements prevalently bound to sulfate phases. Calculated Hazard Quotient describes this landfill as a risk to the environment and human health through different pathways.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coal, as a combustible material, is one of the world's most important energy sources and contributes to the production of almost 40% of the world's electricity (WEC 2016). In addition to the energy produced, the by-products of the combustion process, called coal combustion products (CCP), include the non-combustible part of coal in the form of fly ash, ash, boiler slag, and flue-gas extraction residues (Thorneloe et al. 2010). While fly ash is a very fine, powdery material collected by filters and mainly composed of silica, the bottom ash is a coarse-grained product that is too heavy to float and is deposited at the bottom of the boiler. Boiler slag is a molten form of coal ash, and the flue-gas desulfurization material is a remnant of the sulfur dioxide reduction process mainly composed of calcium sulfite and sulfate (Álvarez-Ayuso et al. 2006).
Due to the high temperatures in coal combustion processes (≥ 1000 °C), the organic and inorganic constituents of coal are transformed, making the composition of coal combustion products different from the initial composition of coal (Vejahati et al. 2010). While elements associated with organic coal fractions typically evaporate or are adsorbed on fine particles, elements related to minerals generally remain in the ash, causing CCPs to be enriched in trace elements (Vejahati et al. 2010; Miller 2011; Speight 2012).
If not properly disposed of, coal combustion products can become a major environmental concern (Baba and Kaya 2004; Equeenuddin 2015; Kalembkiewicz and Sitarz-Palczak 2015; Medunić et al. 2016b, 2018b; Fiket et al. 2019). Namely, under different environmental conditions, waste particles could be transported from these repositories by wind action or rainfall erosion and consequently pollute the soil around the disposal site, reach the water bodies in the region, or even enter the local groundwater.
Nowadays, various extraction methods are used to estimate the release of inorganic constituents of potential concern for a wide range of solid materials (Yue and Zhao 2008; Wang et al. 2009; Izquierdo and Querol 2012; Miguel et al. 2012; Equeenuddin 2015; Tiwari et al. 2015; Zhang et al. 2019). They are designed to consider the effect of key environmental conditions and waste properties on leaching either for screening purposes as well as for specific scenarios of its use or disposal. Over time, more than two dozen leaching test methods have been developed to simulate the leaching process (e.g., Miguel et al. 2012; Tiwari et al. 2015; Zhang et al. 2019) and no consensus has been reached on which method gives the most accurate estimate of toxic substance leaching.
Nonetheless, executed under specified laboratory conditions, leaching tests can be used to approximate the amount of constituents that can be released from a solid material into the environment, under certain conditions. The most commonly used methods are The Shake Extraction of Solid Waste with Water (ASTM D-3987-85) and EP tests developed by Environmental Protection Agency (EPA). While the ASTM test is intended to simulate conditions where the solid waste is the dominant factor in determining the pH of the leachate; the EP test simulates leaching under weak acid conditions (pH 5.0 ± 0.2). In case of the latter, the waste is considered hazardous if the extract contains any of the listed contaminants at a concentration greater than the specified value (US EPA, 1980; Table S1). The Toxicity Characteristic Leaching Procedure (TCLP or Method 1311; US EPA, 1993) represents an expanded version of the EP as it covers a broader range of waste types and can be used for more organic and inorganic compounds. It is usually conducted using buffered acetic acid solutions, at pH = 4.93 or pH = 2.88, and its main drawback is that it can overestimate or underestimate leaching potential under other extreme conditions (e.g., alkaline waste).
Raša coal belongs to a class of superhigh-organic-sulfur coal (SHOS), as it contains exceptionally high levels of sulfur (up to 11–14%) present mainly in organic form (Sinninghe Damsté et al. 1999; Medunić et al. 2016a). Decades of its use in the nearby thermal power plant and other local factories left an imprint on the environment, reflected in elevated concentrations of various metal(loid)s as well as PAHs in different environmental compartments of this area (Medunić et al. 2016b). Furthermore, in the nearby Raša Bay area, levels of Hg, Cd, V, Se, Pb, Cr, Zn, Cu, and U in the local soil belong to the category of extremely high levels of soil pollution. In addition to their elevated levels in soil, S, Se, V, and U were also found increased in surface water and home-grown lettuce (Medunić et al. 2018a,b), Mo, U, V, and Sr displayed high levels in the majority of water samples as well as in analyzed vegetables, soil, and aquatic sediments, while Cu, Zn, Pb, and V were found slightly increased in liver samples of birds (Medunić et al. 2018c).
Additionally, the soil around Štrmac was reported to contain anomalously high Cu, Zn, and Pb levels, while low Se levels were prescribed to leaching (Medunić et al. 2018a). There are numerous other landfills in the region as a remnant of the centuries-old tradition of coal mining and its application in the local thermal power plant and factories. Chemical and toxicological evaluation of aqueous extracts of soil collected near a coal-fired Plomin power plant, demonstrated that Plomin soil extracts induced phytotoxic effects (Radić et al. 2018). The same was also recently reported for soil from the Štrmac area (Fiket et al. 2019).
Following the above, the presented study aims to i) evaluate the leaching potential of metal(loid)s by natural weathering processes from an ash and slag landfill remained after SHOS Raša coal combustion; ii) explain the mechanisms of metal(loid)s release from these types of waste deposits and their mobility under natural conditions and iii) to assess the risk to the environment and human health.
Materials and methods
Study area
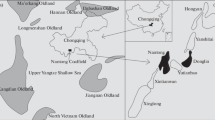
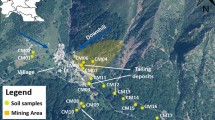
In Istria, eastern Croatia (Fig. 1a), there are a number of landfills consisting of coal ash, slag, and other mining residues. They are the remnants of a four-century-long tradition of coal mining in the region and its application in the local industrial sector. Due to the lack of clean coal technology, the excavation and burning of coal created spoil heaps and illegal landfills that still exist in the villages located in the karst area.
This study included one such landfill located in the village of Štrmac, in the south-eastern part of the Istrian peninsula, the westernmost part of Croatia (Fig. 1b). The geological setting of the wider study area consists of sedimentary Mesozoic (mostly Cretaceous) and Paleogene (Paleocene, Eocene) rocks (Vlahović et al. 2005) with Raša coal seams (Medunić et al. 2016a). Štrmac lays on Eocene limestones with the thrust fault going across the village (Šikić et al. 1969). Soils that predominate in this area are red and brown soils of Chromic Cambisols class (Miko et al. 2003).
Details on Raša coal geochemistry and mineralogy, and influence of its combustion products on local soil, biota and water systems are beyond the scope of this paper, and can be found elsewhere (Bauman and Horvat 1981; Valković et al. 1984; White et al. 1990; Sinninghe Damsté et al. 1999; Medunić et al. 2016a,b; Peco 2018; Fiket et al. 2018).
Sampling and sample preparation
The samples were collected in the Štrmac area (Fig. 1a and b) and include landfill samples (L1, L2, and L3; Fig. 1c), soil sample taken at the foot of the landfill and Raša coal sample taken from the old mine. At all locations, samples (ca. 1 kg) were collected with a sampling shovel and stored in a plastic bag. All samples were air dried, sieved through a 2-mm sieve to remove the gravel fraction, and stored until further analysis.
For total element analysis, sub-samples (0.05 g), previously homogenized in an agate mill, were subjected to a total digestion in the microwave oven (Multiwave 3000, Anton Paar, Graz, Austria) in a two-step procedure. The latter consisted of digestion with a mixture of 4 mL nitric acid (HNO3, 65%, pro analysis, Kemika, Zagreb, Croatia)—1 mL hydrochloric acid (HCl)—1 mL hydrofluoric acid (HF, 48%, pro analysi, Kemika, Zagreb, Croatia) followed by addition of 6 mL of boric acid (H3BO3, Fluka, Steinheim, Switzerland). Prior to analysis, digests were tenfold diluted, acidified with 2% (v/v) HNO3 (65%, supra pur, Fluka, Steinheim, Switzerland) and In (1 µgL−1) as internal standard was added.
Extraction procedure
For extraction, sieved samples (< 2 mm) were mixed with a specified extraction medium. Three extracting methods were tested, ASTM (Method D-3987, ASTM 1995), EP (Method 1310B, US EPA 2005) and TCLP (at pH 4.9 and 2.9; Method 1311, US EPA 1993). The conditions of all extraction procedures are represented in Table 1.
For ASTM procedure, the extraction medium was Milli-Q (MQ) water with a liquid-to-solid ratio 4:1 (40 mL MQ, 10 g sample; Table 1). For EPA procedure, an appropriate quantity of MQ water was added to the sample and pH was adjusted to 5.0 by adding 0.5 N acetic acid. As described in Method 1310B, 0.5 N acetic acid was prepared by diluting concentrated glacial acetic acid (CH3CH2OOH, 17.5 N) by adding 28.5 mL glacial acetic acid to 500 mL of MQ water and further diluting to 1 L. For TCLP procedure, the extraction medium was prepared according to Method 1311 (at pH 4.9 and pH 2.9): a) addition of 2.85 mL glacial acetic acid (CH3CH2OOH) to 250 mL MQ water, followed by the addition of 3.2 mL of 10 N NaOH and diluting to a volume of 500 mL gives a solution with a pH of 4.93 + 0.05; b) diluting 2.85 mL of glacial CH3CH2OOH with MQ water to a volume of 500 mL gives a solution with a pH of 2.88 + 0.05. In both cases, the liquid-to-solid ratio for extraction procedure was 20:1 (40 mL puffer, 2 g sample; Table 1).
Prepared samples were agitated with a horizontal shaker for a specified period, 48 h (ASTM), 24 h (EPA), and 18 h (TCLP). After extraction time finished, the leachates (20 mL) were separated from the solids by filtration through 0.45 µm filters and acidified with 1% (v/v) HNO3 (supra pur, Fluka, Steinheim, Switzerland).
Prior to analysis samples were 100-fold diluted, acidified with 2% (v/v) HNO3 (65%, supra pur, Fluka, Steinheim, Switzerland) and In (1 µgL−1) as internal standard was added.
Multielement analysis
Multielemental analysis of prepared digests and extracts was performed by High Resolution Inductively Coupled Plasma Mass Spectrometry (HR-ICP-MS) using an Element 2 instrument (Thermo, Bremen, Germany). Typical instrument conditions and measurement parameters used throughout the work are reported earlier (Fiket et al. 2017). All samples were analyzed for total concentration of 29 elements (Al, As, Ba, Be, Bi, Cd, Co, Cr, Cs, Cu, Fe, Li, Mn, Mo, Ni, P, Pb, Rb, S, Sb, Sc, Se, Sn, Sr, Ti, Tl, U, V, and Zn).
Quality control of analytical procedure was performed by simultaneous analysis of the blank and certified reference material for soil (NCS DC 77,302, China National Analysis Center for Iron and Steel, Beijing, China). Good agreement between the analyzed and certified concentrations within their analytical uncertainties for all elements was obtained (~ 10%).
Minerological composition by XRD
The mineralogical composition of landfill sample L1 was analyzed using X-ray powder diffraction (XRD, “PANalytical"), equipped with a Ni-filtered Cu Kα radiation source and a scintillation detector. The XRD pattern of sample was recorded over a 2θ interval of 5°- 90°, with a step size of 0.03° on a PANalytical X-ray diffractometer (X’Pert PRO) using Ni-filtered Cu Kα radiation at CSIR-NML. The qualitative mineralogical analysis of all acquired patterns was performed on High Score plus along with manual analysis procedure on the basis of JCPDS database.
The electron-probe micro-analysis (EPMA) was carried out at the EPMA lab of CSIR-NML, Jamshedpur on JXA 8230 (JEOL MAKE) electron microprobe. The operating conditions for analysis were 15 kv of high voltage and 10 nA beam current with beam diameter of 1 micron or less.
Risk assessment
Human health risk assessment was carried out using a model developed by US EPA (USEPA 1996, 2011) and implemented in Octave 5.1.0. Elemental Dose (Di) was estimated for adults and children for three different routes of exposure to potentially hazardous substance (oral exposure – Doral; dermal exposure – Ddermal, inhalation – Dinhal). The non-carcinogenic effects of the elements were estimated by calculating the Hazard Quotient (HQ) and the Hazard Index (HI) using the equations:
The Di is the Elemental Dose and is calculated for each element, while Rfi is the Reference Dose, i.e., maximum acceptable daily dose of element published by US EPA. If HQ is > 1, adverse health effects are possible. The HI is the sum of all three exposure pathways.
Result
Mineral composition
The result of XRD analysis shows a very simple mineralogical pattern in Fig. 2. Mainly two phases are identified in landfill sample L1. The dominant mineral phases present in the sample are calcite and gypsum (Table 2), which is in accordance with data reported by Valković et al. (1984) on fly ash fraction. The variations in mineralogy and texture of the sample examined using EPMA imaging and EDS analysis are presented in Figs. 3a (for gypsum) and 3b (for calcite). Iron oxide also observed rarely.
Major and trace element geochemistry
Total concentrations of major and trace elements in analyzed samples are shown in Table 3, with major elements expressed as oxides.
The shares of major oxides (Al2O3, Fe2O3, MnO, TiO2, P2O5 and SO3) ranged over four orders of magnitude with the highest values observed in soil sample. The only exception was S, showing highest level in coal. In soil and landfill samples, Al was present at highest concentrations, followed by Fe and Ti, while Mn and P were present at concentrations < 1%. Among landfill samples, overall highest levels of oxides were recorded in sample L3. In coal sample, in addition to S as the most abundant, the other major oxides followed the similar decreasing order as in soil and landfill samples.
Concentrations of trace elements (As, Ba, Be, Bi, Cd, Co, Cr, Cs, Li, Ni, Pb, Rb, Sb, Sc, Sn, Tl, and Zn) ranged from below the detection limits (Bi in L3) to 1.1 g/kg (Sr in L1). Generally, the highest concentrations of most elements were observed in soil, with levels of Bi, Co, Cs, Pb, and Rb up to 7–30 times higher than in other samples. On the other hand, Cu, Mo, Se, Sr, U, and V had the highest concentrations in one of the three samples from the landfill. For majority of elements, the lowest concentration were observed in the coal sample, with exception of Bi, Cd, Pb, Se, Tl, Rb, and Zn having the lowest levels in one of the landfill samples and Sr having the lowest concentration in the soil sample.
Extract concentrations
Results are shown in Table S1 (Supplementary material) as extract concentrations (mg L−1) and in Table 4 as Relative Mass Leached (RML, in %).
Extract concentrations ranged from below the detection limits (< LOD) to 507 mg L−1 in ASTM, from < LOD to 293 mg L−1 in EP, from < LOD to 427 mg L−1 in TCLP (pH = 4.93) and from < LOD to 272 mg L−1 in TCLP2 (pH = 2.9); whereby all maximum concentrations are related to sulfur. The extract concentrations of Be, Bi, Sb, Sc, and Tl were below the limits of detection in majority of samples and are omitted from the discussion.
The highest concentrations were observed for S and Sr in landfill and coal samples, and for Mn and S in soil samples. In general, the highest extract concentrations for most elements were observed in landfill samples, while the lowest element concentrations were observed in coal sample. Regarding methods, ASTM resulted in similar to lower extract concentrations compared to those obtained by EPA method. Element concentrations obtained by TCLP and TCLP2 method were higher, up to two orders of magnitude in case of the latter, with exception of S and Mo which had the highest extract concentrations in ASTM method.
Discussion
Element levels in samples
In landfill samples, an increase with depth was observed for all oxides and most trace elements, probably due to direct exposure of surface layer to rainfall. The overall highest variability in element distribution was observed for Se (5.39–35.9 mg/kg) with highest value in the L2 (Table 3), probably reflecting the combined action of leaching and secondary adsorption. Compared to the previous study of this landfill (Medunić et al. 2018a), which encompassed a much smaller set of elements (Cd, Cr, Cu, Pb, Se, Sr, U, V, and Zn), the measured concentrations are only to some extent comparable. While for Cd, Cu, Pb, V, and Zn the measured concentrations fall within the range of those previously published, U was found only slightly higher, and concentrations of Sr, Cr and Se were, respectively, 1.7, 4 and tenfold higher than the highest previously reported. Compared to data reported for the geochemically similar ash landfill in eastern Croatia (Oreščanin et al. 2005), U was also only slightly elevated, Se and Sr exhibited, respectively, threefold and twofold, higher levels, while only Pb and Zn were found at lower levels. Compared to solid wastes from thermal power plants of western Turkey (Baba and Kaya 2004), coal combustion bottom ash from Poland (Kierczak and Chudy 2014) and alkaline coal combustion by-products from China (Zhang et al. 2019), obtained data are lower to comparable for majority of elements. Also, all elements were found below the coal ash Clarke values (Ketris and Yudovich 2009), again with exception of Se and Sr which showed elevated values.
In soil, fifteen elements (Al, Ba, Co, Cr, Cu, Fe, Ni, Pb, Sc, Sr, Ti, V, and Zn) out of seventeen listed in Geochemical atlas of Croatia (Halamić and Miko 2009) had higher concentrations than the median background values for the region, while only As and Cd were found at slightly lower levels. Nonetheless, distribution of major and several trace elements (Cd, Cr, U, and V) was found in agreement with previous studies of this area (Fiket et al. 2018; Medunić et al. 2018a). Only Se was found at higher concentrations (up to ten times), while concentrations of Pb, Zn, Sr, and Cu were more than two times lower.
The content of major elements and majority of trace elements in coal sample is in the agreement with previously reported values (Jakšić et al. 1993; Peco 2018). However, some trace elements (As, Cr, Mo, Ni, Pb, Sr, U, and V) were more than two times lower than those previously reported. Also, all trace elements were lower or comparable to the coal Clarke values (Ketris and Yudovich 2009), with exception of Mo and Se. The latter is in accordance with Se-U–Mo-V enrichment characteristic for Raša coal (Medunić et al. 2016a).
Impact of Štrmac landfill on the environment
Extraction procedures resulted in different extract concentrations of analyzed elements (ASTM < EPA < TCLP < TCLP2), which indicates that pH is an important parameter affecting the leaching rate of studied elements. It also raises a question which procedure is the most effective in predicting subsurface contamination due to leaching.
Selection of a method that can closely simulate the real-life scenario depends on different factors—physical properties of wastes, the composition of the source, age of waste disposal, and the climatic conditions of the disposal area (Tiwari et al. 2015). Most authors agree that ASTM is the best method in representing natural weathering, since the waste is the dominant factor in determining pH of the extract (Baba and Kaya 2004). EPA and TCLP method are considered too aggressive for majority of wastes, which often results in misinterpreting the results and wrongly classifying waste (Izquierdo and Querol 2012; Miguel et al. 2012; Tiwari et al. 2015). In addition, both of these methods were shown to mask leaching of oxyanionic species, like As and Se which are usually more mobile in neutral to alkaline conditions (Thorneloe et al. 2010; Tiwari et al. 2015). And although TCLP may not always be the optimal choice when assessing natural weathering conditions, its application provides insight into the mobility of elements in acidic conditions, especially in parts of the world affected, even occasionally, by acid rain (Kalembkiewicz and Sitarz-Palczakm 2015).
Compared to EPA toxicity levels (in mg L−1: As 5.0; Ba 100; Cd 1.0; Cr 5.0; Pb 5.0; Se 1.0), the landfill extract concentrations, define Štrmac landfill as non-hazardous. However, extract concentrations of several elements (Al, S, Se, and V) were above limit values listed in Croatian regulation of wastewater treatment (Ministry of Agriculture, Croatian Government 2010; Table S1). Not only are the S, Se, and V naturally enriched in SHOS Raša coal (Medunić et al. 2016a,b), but previous studies in the region reported their increased levels in water, vegetables, soil, and aquatic sediment (Medunić et al. 2018a,b,c), suggesting that leaching from the Štrmac landfill presents one of the possible sources of pollution.
Additionally, all these elements were above prescribed limits only in TCLP2 extracts suggesting that their release from the landfill could pose serious threat to local soil and groundwater in case of acid rain. The above scenario is quite realistic given that for the first half of 2019 pH of rainfall measured in the region (Rijeka, Croatia) ranged from 4.8–7.2, while in 1996 pH as low as 3.0 was reported (e.g., DHMZ 1996, 2020). In soils, only Mn was found above prescribed limits in EP and TCPL schemes.
Mobility of elements
Leaching pattern of elements
Under different pH conditions, elements can display one of the following leaching patterns: (i) cationic, displaying highest mobility in acidic pH, which decreases as pH increases; (ii) oxyanionic, displaying slight increase at acidic pH and more prominent increase at alkaline pH; and (iii) amphoteric, having high mobility at both alkaline and acidic pH values (Izquierdo and Querol 2012; Komonweeraket et al. 2015; Zhang et al. 2019).
In our study, the Al, Ba, Cd, Co, Cr, Cu, Fe, Li, Mn, Ni, Pb, Sr, Ti, U, and V displayed an increase in all extract concentrations with a decrease in pH, implying their cationic character (e.g., Cu in Fig. 4a). As and Se displayed such behavior only in landfill samples, whereas in soil and coal their concentrations were similar, regardless of the pH (e.g., As in Fig. 4b).
As expected, S displayed oxyanionic pattern with highest extract concentrations in ASTM (Fig. 4c). Similarly, P displayed highest extract concentrations in EP.
Oxyanion Mo, on the other hand, showed amphoteric pattern (Fig. 4d). Contrary to other publications (Zhang et al. 2019, Izquierdo and Querol 2012), which reported that other oxyanion species (e.g., As, Se) usually have similar leaching pattern as Mo, As and Se in landfill extracts had, on average, the lowest concentrations in ASTM. Due to its mineral composition and the prevalence of calcite (Fig. 2, Table 2), conditions within the landfill are predominately alkaline. And although alkaline conditions enhance desorption of oxyanions, they also decrease dissolved As and Se through incorporation into secondary calcium precipitates, like ettringite (Wang et al. 2009; Izquierdo and Querol 2012; Schwartz et al. 2018). Sulfur concentration hasn’t decreased in ASTM conditions, suggesting that such precipitates may not have formed, but that does not exclude forming of other secondary calcium precipitates. Uptake of Mo by secondary precipitates is usually lower due to its larger size (Kumarathasan et al. 1989), which is in accordance with the high mobility of Mo in ASTM, unlike other oxyanions. Uptake of Se by Ca-precipitates is further supported by higher total values of Se in landfill samples L1 and L2 compared to L3 (Table 3).
Contrary to other elements, the Cs, Pb, Rb, Sn, and Zn showed similar concentrations at all tested pH. However, according to Yue and Zhao (2008), the behavior of elements during leaching is not only controlled by the pH, but also by associated mineral assemblage, as discussed below.
Relative mobility of elements
Compared to the literature (Baba and Kaya 2004; Equeenuddin 2015; Kierczak and Chudy 2014; Zhang et al. 2019; with RML from 0.1% to > 10.0%), all elements had noticeably lower RML values (0.00001% to 1.94%). High content of carbonates and low pyritic value (White et al. 1990; Peco 2018) of samples are probably responsible for the low RML. Namely, under alkaline conditions, Ca-precipitates are known to occur, causing the precipitation of trace elements (Jones 1995). Absence of pyrite, a promotor of acidic discharge (Equeenuddin 2015), further promotes the stated conditions.
Nonetheless, for purpose of discussion the elements were grouped as follows: elements with RML 0.5% or higher were referred to as high mobility class, elements with RML between 0.5% and 0.05% as moderate mobility class and elements with RML below 0.05% as low mobility class.
Element mobility related to matrice properties
High mobility in all sample types was observed for Mn, Sr, and Cd (Table 4), suggesting their association with carbonates and sulfates. Substitution of Mn2+ and Sr2+ for Ca2+ in calcite was reported for many coals (Swaine 1990), while Sr can also be associated with gypsum (Spivak-Birndorf et al. 2012). Cadmium is usually associated with sulfides and considered immobile in neutral to alkaline conditions (Izquierdo and Querol 2012; Zhang et al. 2019). However, due to its moderate to high mobility in studied samples, it is likely that Cd is associated with sulfates or soluble salts like CdSO4 and ZnSO4 (Querol et al. 1996). Although they are not present as major phases, we cannot rule out their presence as minor minerals.
Similarly to Sr, Ba forms sparingly soluble compounds with carbonates (Fruchter et al. 1990), further corroborating its moderate to high mobility in samples.
High mobility of S in landfill and soil samples is probably due to high amounts of soluble sulfates (gypsum, Table 2), while low mobility of S in coal sample reflects its prevalence in less-mobile organic form (Markuszewski et al. 1981).
Alkali metals Cs, Li, and Rb showed the same leaching pattern, moderate to high leaching in landfill samples and low mobility in soil and coal. Reported that the peak of Rb was present in non-magnetic fraction of fly ash where CaCO3 and CaSO4 were main constituents, suggesting that these elements are also associated with sulfates in the landfill samples. Although Raša coal is characterized by a high sulfur content with calcite and dolomite as the main mineral phases, White et al. (1990) reported that from 10.77 wt.% of the total sulfur (as-received basis), 10.45% is organic sulfur, 0.3–0.63% is pyrite, and only 0.02% is sulfate. Low mobility of Rb, Cs, and Li in coal samples, thus, may be due to these elements being bound to small pyritic fraction, clay minerals or organic fraction (Izquierdo and Querol 2012; Zhao et al. 2017). In soil samples, those elements are probably absorbed on potassium alumino-silicates (e.g., ilite) due to common substitution of those elements for K ion in mentioned minerals.
The Mo, Se, and U also showed high mobility in landfill samples. Mobility of Mo is mostly affected by pH mentioned earlier. As volatile element, Mo readily condenses on the surface of fly ash particles during coal combustion and is consequently prone to leaching. Additionally, high mobility of Mo and U could be linked to their association with sulfates, oxides, and salts (Querol et al. 1996).
In the coal, P and Co demonstrated highest mobility. Phosphorus may be bound to organic matter in coal but also to inorganic phases (e.g., apatite and aluminophosphate), whereby, depending on the pH and metal supply during coalification, typically one mineral group dominates (Ward et al. 2005). Apatite is more likely to form in natural to alkaline conditions, meaning that apatite is more likely to appear in Raša coal due to alkaline conditions prevailing during coalification of Raša coal (Medunić et al. 2016a, b). However, mobility of P was the highest in ASTM procedure, which is contrary to solubility of apatite which has lower solubility as pH increases (Smith et al. 1977). Lack of literature data about mobility of phosphorus from coal precludes further discussion. Cobalt, on the other hand, is usually associated with pyrite, calcite, and dolomite. Considering low pyrite value of Raša coal, high mobility of Co in coal could be attributed to solubility of carbonates in acidic conditions.
The low mobility of other elements in the coal sample is probably due to its complex structure and the fact that many elements in the matrix are associated with organic matter, as well as the presence of soluble iron sulfates (rozenite and siderotil; Peco, 2018). Namely, the dissolution of these phases releases iron which is then precipitated as Fe-oxyhydroxide, readily binding other present trace elements (Herbert 1996; Hudson-Edwards et al. 1999; Kairies et al. 2005).
Although total values of elements were highest in soil samples, mobility of elements was lower in soil than in landfill samples. This is probably due to slow dissolution rate of silicates in acid conditions and association of elements with clay minerals, which adsorb trace elements, especially in alkaline conditions.
Most elements showed highest mobility in landfill samples, probably due to transformation of minerals during coal combustion (Unver and Terzi 2018), as well as higher content of water-soluble sulfates, unlike in soil where main minerals are clay minerals (Peco 2018).
Health assessment
Of all the elements, only for As HQ was > 1 for all three routes of exposure (oral, dermal, and inhalation). For other elements, HQ < 1 for inhalation and dermal exposure, while for oral exposure it was higher for Co and V for the adult population, and for Co, Li, Mn, and V for children. In addition, the HI was > 1 for As, indicating that it would be desirable to limit any contact or prolonged stay at the studied landfill. Although As, Co, and Li were not above the prescribed limit in leachate, the calculated HQs nevertheless indicate a possible threat to human health, which further emphasizes the importance of considering different routes of exposure. All the more so as elements such as Mn and V in leachate have been recorded above the prescribed limit, confirming that the soil from this site poses a risk to the environment and human health through various forms of exposure.
Conclusion
In the studied landfill remained after deposition of SHOS coal combustion products, elements with highest leaching potential are those associated with sulfates and to a lesser extent with carbonates. Overall low RML values of all elements could, thus, be prescribed primarily to the alkalinity of this type of matrix which attenuates the release of elements associated with silicates (the majority of analyzed elements) and enhances the release of oxyanionic species (As, Cr, Sb, Se, and V), while simultaneously causes the uptake of released elements by Ca-precipitates. Nonetheless, both pH and mineralogy affect the leaching pattern. This fact not only highlights the complex interaction between mineral matter solubility, pH, and the leaching of potentially hazardous elements but also emphasizes the necessity to consider also the mineralogy of inorganic deposits in the assessment of their possible impact on the environment.
Results also showed the non-cancerogenic risk for As (oral, inhalation, and dermal exposure) and also for oral exposure for Co and V (adults), and Co, Li, Mn and V (children), emphasizing also the necessity to consider different pathways of exposure of this type of waste deposits.
Data availability
All relevant data are contained herein.
References
Álvarez-Ayuso E, Querol X, Tomás A (2006) Environmental impact of a coal combustion-desulphurisation plant: Abatement capacity of desulphurisation process and environmental characterisation of combustion by-products. Chemosphere 65(11):2009–2017. https://doi.org/10.1016/j.chemosphere.2006.06.070
American Society for Testing and Materials (ASTM) (1995) D-3987. Standard Test Method for Shake Extraction of Solid Waste with Water, Annual Book of ASTM Standards 11(04):14–17
Baba A, Kaya A (2004) Leaching characteristics of solid wastes from thermal power plants of western Turkey and comparison of toxicity methodologies. J Environ Manage 73(3):199–207. https://doi.org/10.1016/j.jenvman.2004.06.005
Bauman A, Horvat Đ (1981) The impact of natural radioactivity from a coal-fired power plant. Sci Total Environ 17(1):75–81. https://doi.org/10.1016/0048-9697(81)90109-1
DHMZ, State Hydrometeorological Institute (1996): Meteorological and hydrological bulletin 1996 (in Croatian).
DHMZ, State Hydrometeorological Institute (2020): Meteorological and hydrological bulletin 2020 (in Croatian).
Equeenuddin SM (2015) Leaching of trace elements from Indian Coal. Journal Geological Society of India 86:102–106. https://doi.org/10.1007/s12594-015-0285-5
Fiket Ž, Medunić G, Furdek Turk M, Kniewald G (2018) Rare earth elements in superhigh-organic-sulfur Raša coal ash (Croatia). Int J Coal Geol 194:1–10. https://doi.org/10.1016/j.coal.2018.05.002
Fiket Ž, Medunić G, Vidaković-Cifrek Ž, Jezidžić P, Cvjetko P (2019) Effect of coal mining activities and related industry on composition, cytotoxicity and genotoxicity of surrounding soils. Environ Sci Pollut Res 27:6613–6627. https://doi.org/10.1007/s11356-019-07396-w
Fiket Ž, Mikac N, Kniewald G (2017) Mass Fractions of Forty-Six Major and Trace Elements, Including Rare Earth Elements, in Sediment and Soil Reference Materials Used in Environmental Studies. Geostand Geoanal Res 41:123–135. https://doi.org/10.1111/ggr.12129
Fruchter JS, Rai D, Zachara JM (1990) Identification of solubility-controlling solid phases in a large fly ash field lysimeter. Environ Sci Technol 24(8):1173–1179. https://doi.org/10.1021/es00078a004
Halamić, J. Miko, S. (eds) (2009): Geochemical Atlas of the Republic of Croatia. Croatian Geological Survey, 87, Zagreb.
Herbert RB Jr (1996) Metal retention by iron oxide precipitation from acidic ground water in Dalarna, Sweden. Appl Geochem 11:229–235. https://doi.org/10.1016/0883-2927(95)00070-4
Hudson-Edwards KA, Schell C, Macklin MG (1999) Mineralogy and geochemistry of alluvium contaminated by metal mining in the Rio Tinto area, southwest Spain. Appl Geochem 14:1015–1030. https://doi.org/10.1016/S0883-2927(99)00008-6
Izquierdo M, Querol X (2012) Leaching behaviour of elements from coal combustion fly ash: an overview. Int J Coal Geol 94:54–66. https://doi.org/10.1016/j.coal.2011.10.006
Jakšić M, Bogdanović I, Cereda E, Fazinić S, Valković V (1993) Quantitative PIXE analysis of single fly ash particles by a proton microbeam. Nucl Instrum Methods Phys Res, Sect B 77:505–508. https://doi.org/10.1016/0168-583X(93)95587-U
Jones DR (1995) The leaching of major and trace elements from coal ash. In: Swaine DJ, Goodarzi F (eds) Environmental Aspects of Trace Elements in Coal. Kluwer Academic Publishers, Netherlands, pp 221–262
Kairies CL, Capo RC, Watzlaf GR (2005) Chemical and physical properties of iron hydroxide precipitates associated with passively treated coal mine drainage in the Bituminous Region of Pennsylvania and Maryland. Appl Geochem 20(8):1445–1460. https://doi.org/10.1016/j.apgeochem.2005.04.009
Kalembkiewicz J, Sitarz-Palczak E (2015) Efficiency of leaching tests in the context of the influence of the fly ash on the environment. Journal of Ecological Engineering 16:67–80. https://doi.org/10.2478/environ-2019-0012
Ketris MP, Yudovich YE (2009) Estimations of Clarkes for carbonaceous biolithes: world averages for trace element contents in black shales and coals. Int J Coal Geol 78(2):135–148. https://doi.org/10.1016/j.coal.2009.01.002
Kierczak J, Chudy K (2014) Mineralogical, chemical, and leaching characteristics of coal combustion bottom ash from a power plant located in northern Poland. Pol J Environ Stud 23(5):1627–1636
Komonweeraket K, Cetin B, Benson CH, Aydilek AH, Edil TB (2015) Leaching characteristics of toxic constituents from coal fly ash mixed soils under the influence of pH. Waste Manage 38:174–218. https://doi.org/10.1016/j.wasman.2014.11.018
Kumarathasan P, McCarthy GJ, Hassett DJ, Pflughoeft-Hassett DF (1989) Oxyanion substituted ettringites: synthesis and characterization, and their potential role in immobilization of As, B, Cr, Se, and V. MRS Proc 178:83–104. https://doi.org/10.1557/PROC-178-83
Markuszewski R, Miller LJ, Straszheim WE (1981) Evalution of the removal of organic sulfur from coal. Coal Chemistry 23:401–414
Medunić G, Ahel M, Božičević Mihalić I, Gaurina Srček V, Kopjar N, Fiket Ž, Bituh T, Mikac I (2016a) Toxic airborne S, PAH, and trace element legacy of the superhigh-organic- sulfur Raša coal combustion: cytotoxicity and genotoxicity assessment of soil and ash. Sci Total Environ 566–567:306–319. https://doi.org/10.1016/j.scitotenv.2016.05.096
Medunić G, Kuharić Ž, Fiket Ž, Bajramović M, Singh AL, Krivohlavek A, Kniewald G, Dujmović L (2018a) Selenium and other potentially toxic elements in vegetables and tissues of three non-migratory birds exposed to soil, water, and aquatic sediment contaminated with seleniferous Raša coal. Rudarsko-Geološko-Naftni Zbornik 33(3):53–62. https://doi.org/10.17794/rgn.2018.3.6
Medunić G, Kuharić Ž, Krivohlavek A, Đuroković M, Dropučić K, Rađenović A, Lužar Oberiter B, Krizmanić A, Bajramović M (2018b) Selenium, Sulphur, Trace Metal, and BTEX Levels in Soil, Water, and Lettuce from the Croatian Raša Bay Contaminated by Superhigh-Organic-Sulphur Coal. Geosciences 8:408–426. https://doi.org/10.3390/geosciences8110408
Medunić, G., Kuharić, Ž., Krivohlavek, A., Fiket, Ž., Rađenović, A., Gödel, K., Kampić, Š., Kniewald, G. (2018b) Geochemistry of Croatian superhigh-organic- sulphur Raša coal, imported low-S coal, and bottom ash: their Se and trace metal fingerprints in seawater, clover, foliage, and mushroom specimens. International Journal of Oil Gas and Coal Technology, 18, 1/2; 3–24.
Medunić G, Rađenović A, Bajramović M, Švec M, Tomac M (2016b) Once grand, now forgotten: what do we know about the superhigh-organic-sulfur Raša coal? Mining- Geological-Petroleum Engineering Bulletin 31(1):27–45. https://doi.org/10.17794/rgn.2016.3.3
Miguel RE, Ippolito JA, Porta AA, Banda Noriega RB, Dungan RS (2012) Use of standardized procedures to evaluate metal leaching from waste foundry sands. J Environ Qual 42(2):615–620. https://doi.org/10.2134/jeq2012.0356
Miko S, Durn G, Adamcová R, Čović., M., Dubiková, M., Skalský, R., Kapelj, S., Ottner, F. (2003) Heavy metal distribution in karst soils from Croatia and Slovakia. Environ Geol 45:262–272. https://doi.org/10.1007/s00254-003-0878-y
Miller, B. (2011): Clean coal engineering technology. Butterworth Heinemann, Burlington, 696 pp.
Ministry of Agriculture, Croatian Government (2010): Pravilnik o graničnim vrijednostima emisija otpadnih voda. Zagreb, Croatia: Official Gazette 80/13, 43/14, 27/15, 3/16, (in Croatian).
Oreščanin V, Barišić D, Mikelić L, Lovrenčić I, Mačefat MR, Pavlović G, Lulić S (2005) The influence of fly and bottom ash deposition on the quality of Kaštela Bay sediments. Environ Geol 49(1):53–64. https://doi.org/10.1007/s00254-005-0058-3
Peco, J. (2018): Potential mobility of toxic elements from Plomin soil contaminated with superhigh sulphur Raša coal and ash. Master Thesis. Faculty of Science, University of Zagreb, 68 pp. (In Croatian)
Querol X, Cabrera L, Pickel W, López-Soler A, Hagemann HW, Fernádez-Turiel JL (1996) Geological controls on the coal quality of the Mequinenza subbituminous coal deposit, northeast Spain. Int J Coal Geol 29:67–91. https://doi.org/10.1016/0166-5162(95)00009-7
Radić S, Medunić G, Kuharić Ž, Roje V, Maldini K, Vujčić V, Krivohlavek A (2018) The effect of hazardous pollutants from coal combustion activity: Phytotoxicity assessment of aqueous soil extracts. Chemosphere 199:191–200. https://doi.org/10.1016/j.chemosphere.2018.02.008
Schwartz GE, Hower JC, Phillips AL, Rivera N, Vengosh A, Hsu-Kim H (2018) Ranking Coal Ash Material for their potential to leach arsenic and selenium: Relative importance of ash chemistry and site biogeochemistry. Environ Eng Sci 35(7):728–738. https://doi.org/10.1089/ees.2017.0347
Sinninghe Damsté JS, White CM, Green JB, de Leeuw JW (1999) Organosulfur compounds in sulfur-rich Raša coal. Energy Fuels 13(3):728–738. https://doi.org/10.1021/ef980236c
Smith EA, Mayfield CI, Wong PTS (1977) Physical and chemical characterization of selected natural apatites in synthetic and natural aqueous solutions. Water, Air, Soil Pollution 8(4):401–415. https://doi.org/10.1007/BF00228655
Speight, J.G. (2012): The Chemistry and Technology of Coal. 3rd Edition. Engineering and Technology. Boca Raton. doi:https://doi.org/10.1201/b12497
Spivak-Birndorf LJ, Stewart BW, Capo RC, Chapman EC, Schroeder KT, Brubaker TM (2012) Strontium Isotope Study of Coal Utilization By-Products Interacting with Environmental Waters. Journal of Environment Quality 41(1):144–154. https://doi.org/10.2134/jeq2011.0222
Swaine DJ (1990) Trace Elements in Coal. Butterworth-Heinemann, London, p 294
Šikić, D., Polšak, A., Magaš, N. (1969): The basic geological map of SFRJ 1:100 000, sheet Labin (L33–101). Institute for geological research Zagreb, Federal geological institute Beograd.
Thorneloe SA, Kosson DS, Sanchez F, Garrabrants AC, Helms G (2010) Evaluating the fate of metals in air pollution control residues from coal fired power plants. Environ Sci Technol 44:7351–7356. https://doi.org/10.1021/es1016558
Tiwari MK, Bajpai S, Dewangan UK, Tamrakar RK (2015) Suitability of leaching test methods for fly ash and slag: A review. Journal of Radiation Research and Applied Sciences 8(4):523–537. https://doi.org/10.1016/j.jrras.2015.06.003
United States Environmental Protection Agency (1980) Extraction Procedure Toxictiy Characteristic. Fed Reg 45(98):33063–33285
United States Environmental Protection Agency (1993): Toxicity characteristic leaching procedure, Method 1311, Revision 1, July 1992, Final Update I to the Third Edition of Test Methods for Evaluating Solid Waste, Physical/Chemical Methods, EPA publication SW-846.
United States Environmental Protection Agency (1996): US Environmental Protection Agency, Soil screening guidance: User`s guide, 4–23. Washington, DC 20460: Office of Solid Waste and Emergency Response, Publication 9355.
United States Environmental Protection Agency (2005): Extraction Procedure (EP) Toxicity Test Method and Structural Integrity Test, Method 1310B, Revision 1, November 2004, Final Update III to the Third Edition of Test Methods for Evaluating Solid Waste, Physical/Chemical Methods, EPA publication SW-846.
United States Environmental Protection Agency (2011): US Environmental Protection Agency, Exposure Factors Handbook: 2011 Edition. EPA/600/R-09/052F. Washington, DC 20460: National Center for Environmental Assessment, Office of Research and Development.
Unver K, Terzi M (2018) Distribution of trace elements in coal and coal fly ash and their recovery with mineral processing practices: A review. Journal of Mining and Environment 9(3):641–655. https://doi.org/10.22044/JME.2018.6855.1518
Valković V, Makjanić J, Jaksić M, Popović S, Bos AJJ, Vis RD, Wiederspahn K, Verheul H (1984) Analysis of fly ash by X-ray emission spectroscopy and proton microbeam analysis. Fuel 63:1357–1362. https://doi.org/10.1016/0016-2361(84)90337-5
Vejahati F, Xu Z, Gupta R (2010) Trace elements in coal: Associations with coal and minerals and their behavior during coal utilization – A review. Fuel 89:904–911. https://doi.org/10.1016/j.fuel.2009.06.013
Vlahović I, Tišljar J, Velić I, Matičec D (2005) Evolution of the Adriatic Carbonate Platform: Palaeogeography, main events and depositional dynamics. Palaeogeogr Palaeoclimatol Palaeoecol 220:333–360. https://doi.org/10.1016/j.palaeo.2005.01.011
Wang T, Wang J, Tang Y, Shi H, Ladwig K (2009) Leaching characteristics of arsenic and selenium from coal fly ash: Role of calcium. Energy Fuels 23(6):2959–2966. https://doi.org/10.1021/ef900044w
Ward, C.R., Li, Z. French, D. (2005): Geological sources of metals in coal and coal products, in Metal Contaminants in New Zealand, T.A. Moore, A. Black, J.A. Centeno, J.S. Harding, D.A. Trumm (Eds.), resolutionz press, Christchurch, NZ, p. 49–79.
WEC, 2016. World Energy Council. World Energy Resources – Coal 2016. https://www.worldenergy.org/wp-content/uploads/2017/03/WEResources_Coal_2016.pdf
White, C. M., Douglas, L. J., Anderson, R. R., Schmidt, C. E., Grax, R., J. (1990): Organosulfur constituents in Rasa coal. In: Geochemistry of sulfur in fossil fuels, W.L. Orr and C.M. White, (ed.), ACS Symposium Series; American Chemical Society, Washington, DC, 261–286. doi: https://doi.org/10.1021/bk-1990-0429.fw001
Yue M, Zhao F (2008) Leaching experiments to study the release of trace elements from mineral separates from Chinese coals. Int J Coal Geol 73(1):43–51. https://doi.org/10.1016/j.coal.2007.07.002
Zhang S, Dai S, Finkelman RB, Graham IT, French D, Hower JC, Li X (2019) Leaching characteristics of alkaline coal combustion by-products: A case study from a coal-fired power plant, Hebei Province, China. Fuel 255:0016–2361. https://doi.org/10.1016/j.fuel.2019.115710
Zhao C, Liu B, Ma J, Liu S, Blokhin MG (2017) Occurrence of rubidium and cesium in Iqe coal, Qinghai-Tibet Plateau: Evidence from sequential chemical extraction experiment. Energy Explor Exploit 35(3):376–387. https://doi.org/10.1177/0144598717690088
Funding
This study has been supported in part by Croatian Science Foundation under the project FORtIS (IP-2019-04-9354).
Author information
Authors and Affiliations
Contributions
Ž Fiket and G Medunić conceived the study design. G Medunić and M Petrović contributed to sampling. M Petrović contributed to sample preparation for ICP-MS analysis, including digestion and extraction. S Chakravarty carried out the XRD and EPMA analysis. M Petrović, Ž Fiket, G Medunić, and S Chakravarty performed data analysis and interpretation. M Petrović and Ž Fiket wrote the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Petrović, M., Fiket, Ž., Medunić, G. et al. Mobility of metals and metalloids from SHOS coal ash and slag deposit: mineralogical and geochemical constraints. Environ Sci Pollut Res 29, 46916–46928 (2022). https://doi.org/10.1007/s11356-022-19074-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-19074-5