Abstract
Evidence from previous studies has shown that exposure to cadmium (Cd) is associated with cardiovascular disease, kidney disease, and osteoporosis, but the effects of Cd on liver toxicity in adolescents are unclear. The data of 4411 adolescents who participated in the US The National Health and Nutrition Examination Survey (NHANES) during 1999–2016 was analyzed. Liver function was indicated by the levels of alanine aminotransferase (ALT) and aspartate amino transferase (AST). The associations between the levels of urinary Cd and liver function were evaluated using multivariate logistic regression models adjusted for covariates. The results showed that the odds ratios of ALT and AST in the highest quartiles of urinary Cd were 1.40 (95% confidence interval [CI], 1.07–1.82) and 1.64 (95% CI, 1.10–2.44), respectively, compared with the lowest quartiles, which were similar to using urinary creatinine as the covariate. We also found linear regression of associations of urinary Cd with elevated ALT and AST levels in boys. In addition, one augmented urinary Cd concentration unit (Log10) was associated with a 0.04-mg/dL increase in C-reactive protein and a 0.53-mg/dL decrease in HDL cholesterol in the fully adjusted model. Our results add novel evidence that exposure to Cd might be positively associated with indicators of liver injury, indicating the potential toxic effect of Cd exposure on the adolescent liver. Further confirmatory studies are needed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over the past decades, the prevalence of liver disease in adolescents has increased, placing a great burden on the health care system. It is necessary to better understand the risk factors for liver function to provide information for disease control and prevention in the future (Williams et al. 2015). According to NHANES data study estimates, the incidence of nonalcoholic liver disease (NAFLD) among adolescents increased from 3.9% in 1988 to 10.7% in 2010 (Younossi et al. 2019). NAFLD presents as a range of clinical and pathological liver changes, from elevated circulating alanine aminotransferase (ALT) activity to fatty liver and steatohepatitis, which can progress to more serious manifestations such as cirrhosis and hepatocellular carcinoma (Lazo et al. 2013; Liu et al. 2014; Rinella 2015). Alcoholic liver disease and viral hepatitis, mainly caused by hepatitis B and C, are also common liver diseases among adolescents. Early-stage liver disease is frequently asymptomatic, and there is a need to identify modifiable risk factors that can be targeted for primary prevention (Hadzic et al. 2017). Some of the more commonly observed risk factors for liver injury include genetic factors, unhealthy lifestyles, viral hepatitis infection, hepatic cancer, and metabolic disorders. In addition, accumulating evidence suggests that environmental pollution and chemicals may also contribute to abnormal liver function (Weng et al. 2020; Zhao et al. 2020). Gallo observed that two kinds of serum perfluoroalkyl acid concentrations were positively correlated with serum ALT levels (Gallo et al. 2012). Yu et al. found that exposure to phthalates might be adversely associated with markers of liver injury (Yu et al. 2021). However, to date, there are few studies on the relationship between cadmium (Cd) exposure and liver function in adolescents.
Cd is a widespread industrial and environmental pollutant that mainly comes from various human production activities, such as mining, smelting, fuel burning, waste incineration, and metal recovery (Li et al. 2018; Sall et al. 2020; Wu et al. 2019). Due to extremely high soil–plant transfer rates, the population was exposed to Cd mainly through contaminated food, water, and cigarette smoke (Dennis et al. 2021; Li et al. 2019; Zhao et al. 2016). Because of its stability and permeation, Cd is not rapidly eliminated from the human body, which makes it easy to accumulate in the body and causes harmful health consequences (Wu et al. 2019). In this sense, evidence from epidemiological studies has demonstrated that environmental exposure to Cd, even at low levels, results in the development of cardiovascular disease (Xu et al. 2021b), kidney disease (Hagedoorn et al. 2020), and osteoporosis (Ma et al. 2021). In addition, exposure to Cd inhibits liver detoxification enzymes and leads to liver dysfunction, as the liver plays a crucial role in the detoxification of pollutants, including Cd (Xiong et al. 2020). Studies have shown that Cd is hepatotoxic to mice, zebrafish, and hens (Gao et al. 2018; Wang et al. 2017; Zhu et al. 2020). However, whether human exposure to Cd could induce liver function disorder remains unclear. Given that exposure levels and pharmacokinetics in humans differ from those in animals, epidemiological studies are needed to assess the relationship between cadmium exposure and human liver function.
In this study, we analyzed the association between Cd and liver function in adolescents using NHANES data. Based on these results, urine Cd concentration is considered to be an indicator of long-term exposure to Cd (Li et al. 2021a). We used urinary Cd as an indicator of Cd exposure in vivo. The relationships between urinary Cd and indicators of liver function, including ALT and AST, were analyzed by logistic regression. In addition, a sex-stratified analysis was conducted to explore whether the association between urinary Cd and ALT and AST was different in boys and girls. Furthermore, we investigated whether there were differences in urinary Cd and lipid parameters and CRP and explained the mechanism of elevated urinary Cd and ALT and AST at the epidemiological level. Our study suggests that one of the causes of abnormal liver function may be increased cadmium. The results of our current cross-sectional study can reveal the association between the increase in cadmium and the increase in liver enzymes, which provides a basis for subsequent cohort studies and possible randomized controlled studies.
Methods
Study population
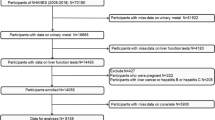
Our study sample included adolescents aged 13 to 19 years old who participated in the 1999–2016 National Health and Nutrition Examination Survey (NHANES). NHANES was a population-based cross-sectional survey conducted by the CDC’s National Center for Health Statistics. The survey contains data on the diet, nutritional status, health, and health behaviors of noninstitutionalized US civilians (Ahluwalia et al. 2016). The NCHS Institutional Review Board approved the NHANES Agreement and obtained a signed informed consent form. We excluded those who did not test for urinary Cd, those who did not test for liver function (ALT), and those who were pregnant. After further excluding participants with missing covariate data listed in Table 1, a total of 3958 adolescents were eligible for urinary Cd analysis.
Detection method of Cd
Urine samples were collected and then subpacked, stored, and transported to multiple laboratories for analysis, which was performed in accordance with the operation manual: https://www.cdc.gov/nchs/data/nhanes/2017-2018/manuals/2017_MEC_Laboratory_Procedures_Manual.pdf. During the NHANES survey period from 1999 to 2000, the detection limit of urinary Cd was 0.01 μg/L and the LOD was 0.042 μg/L from 2001 to 2004. From 2005 to 2010, the LOD was 0.030 μg/L; the LOD was 0.056 μg/L in 2011–2012 and 0.036 μg/L in 2013–2014. For study participants whose urine Cd concentration was less than the detection limit, the results were replaced by a value equal to the square root of the detection limit divided by 2. The file data (CDC official website, https://www.cdc.gov/nchs/data/nhanes/1999-2000/labmethods/lab06_met_lead_and_Cd.pdf, etc.) were used by adjusting the urine creatinine to illustrate urine diluted at present.
Blood biochemical analysis
Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were detected by a Beckman-Coulter DXH800 instrument. According to the NASPGHAN guidelines (Goldner &Lavine 2020), ALT levels ≥ 26 U/L in male adolescents and ≥ 22 U/L in female adolescents were defined as elevated ALT. The AST detection lines were 37 U/L. If the value was greater than or equal to this value, the level increased.
Other covariables
Many covariates were assessed as potential confounders, including age, sex, race/ethnicity, physical activity, alcohol consumption, smoking, and poverty income ratio (PIR). Racial/ethnic categories were “Mexican American,” “Other Hispanic,” “Non-Hispanic white,” “Non-Hispanic Black,” and “Other race.” Physical activity was classified as never, occasionally, and often. We used the income poverty ratio to infer poverty status; if the ratio was < 1, individuals were classified as below the poverty level according to a previous study (Noor et al. 2018). In modeling urinary Cd, urinary creatinine (Cr) concentration (mg/dL) was further considered a confounding factor (Kim et al. 2018). Serum cotinine concentration and BMI were considered potential confounding factors in the sensitivity analysis. Due to active and passive smoking exposures, while the questionnaire only reflects active smoking, serum cotinine was used to reflect the subjects’ smoking exposure. Participants were assessed for exposure to environmental tobacco smoke (ETS) by measuring serum cotinine, a metabolite of nicotine. The unexposed group was defined as having serum cotinine below the detection limit; the low-exposure group was whose serum cotinine level was between the detection limit and 10 ng/mL; and the high-exposure group was whose cotinine level was above 10 ng/mL. BMI was calculated by dividing the measured weight (kg) by the measured height squared (m2). In addition, we selected factors related to liver function, such as physical activity (Ahluwalia et al. 2016), drinking (Le Dare et al. 2019), smoking (Wannamethee &Shaper 2010), family income (Hu et al. 2020), and BMI (Li et al. 2020), as covariates, and they were adjusted in statistical analysis to reduce the impact of these factors on the results.
Statistical analysis
The values are expressed as the mean (± standard deviation) or number (percentage) for continuous variables and categorical variables, respectively. The Mann–Whitney U test was used to test continuous data by group, and the comparison of categorical variables was performed by the chi-square test or Fisher’s exact test. We also investigated the association of urinary Cd with ALT and AST levels by sex. Multivariate logistic regression models were used to calculate odds ratios (ORs) and corresponding 95% confidence intervals (CIs) to determine the liver function parameters associated with urinary Cd according to quartiles, and the lowest quartile was regarded as the reference group. SAS software, version 9.2, was used for all of the statistical analyses. A value of P < 0.05 (two-sided) indicates statistical significance.
Results
Characteristics of the participants
Of all of the adolescents assessed, 52.55% were boys and 47.45% were girls. The sample included 30.17% Mexican Americans, 6.96% other Mexicans, 25.98% non-Hispanic whites, 29.17% non-Hispanic blacks, and 7.7% other races (n = 4411). Table 1 shows the basic characteristics of the study population. Subjects with high total urine Cd levels were more likely to be females and non-Mexican blacks, as were those with low family PIR and vigorous exercise (both trends P < 0.001). The mean ALT concentrations for all subjects were 20.2 ± 16.7, 19.6 ± 12.7, 19.5 ± 14.8, and 19.3 ± 13.3. The mean AST concentrations were 24.0 ± 9.7, 24.2 ± 10.2, 24.3 ± 11.6, and 23.7 ± 9.3.
Relationship between urinary Cd level and ALT and AST
Table 2 shows that ALT and AST levels are affected by age, race, and sex. After adjustment for all covariables, urine Cd concentration was positively correlated with ATL (OR for Q4 v. Q1:1.40, 95% CI 1.07, 1.82, P for trend = 0.002) and AST (OR for Q4 v. Q1:1.64, 95% CI 1.10, 2.44, P for trend = 0.002). For the purpose of rigor, urinary creatinine was used as a covariable, and the results are shown in Supplementary Table S1. The correlations of urinary Cd concentration with ATL (OR for Q4 vs. Q1: 1.60, 95% CI 1.14, 2.23, P = 0.001) and AST (OR for Q3 vs. Q1: 1.60, 95% CI 1.03, 2.51, P for trend = 0.001, OR for Q4 vs. Q1: 1.99, 95% Cl 1.20, 3.32, P for trend = 0.001) were positive, similar to the results with urinary creatinine as the denominator, suggesting that our results are reliable.
Association between urinary Cd levels and ALT AST continuity variables
Since sex has an effect on ALT AST (Bussler et al. 2018), we analyzed Cd and continuous ALT AST for stratification at the same time. The results are shown in Table 3. We found that Cd and ALT and AST were positively correlated in the total population and in boys. In boys, Cd and ALT and AST results were β for 1.45 (95% CI 0.51, 2.40, P = 0.003) and β for 1.06 (95% CI 0.37, 1.75, P = 0.003, respectively). For girls, β was 0.57 (95% CI 0.18, 1.32, P = 0.134) and β was 0.32 (95% CI 0.22, 0.87, P = 0.249). At the same time, we present scatter plot results of ALT and AST fitting and Cd fitting for boys (Fig. 1). Previous reports have suggested that Cd may have a nonlinear dose–response relationship (Sun et al. 2019). Our results suggest that Cd does not have a nonlinear relationship with ALT (Supplementary Fig. S1, P-nonlinearity = 0.155, and P-overall = 0.0005) or AST (Supplementary Fig. S2, P-nonlinearity = 0.559, and P-overall = 0.0009) in boys.
Association of heavy metal Cd with blood lipids and inflammatory indicators
Next, we discussed the associations of Cd with blood lipids and inflammatory indicators. Table 4 shows that after adjusting for all covariates, Cd was found to be associated with increased blood C-reactive protein (0.04 mg/dL, P = 0.006) and decreased HDL cholesterol (− 0.53 mg/dL, P = 0.035). There were no significant changes in triglycerides, LDL cholesterol, or total cholesterol.
Discussion
The present study found that the heavy metal Cd is associated with the risk of liver damage, and long-term Cd exposure (as assessed by urinary Cd levels) increases the risk of elevated serum ALT and AST concentrations in adolescents. In addition, Cd can increase CRP levels and decrease HDL cholesterol levels, which could be the molecular mechanism by which Cd causes elevated ALT and AST levels.
Our results showed that the increase in urinary Cd concentrations in the total population of adolescents was positively correlated with the increases in ALT and AST. Previous exposure studies in Koreans showed that Cd exposure was positively correlated with ALT and AST levels (Park et al. 2021) and urinary Cd was positively correlated with high serum AST and ALT concentrations (Kim et al. 2021). Additionally, no nonlinear associations of Cd with AST and ALT were found, similar to our results. However, these previous results were based on studies of adults and did not account for gender differences in the study’s continuum variables. In addition, the sample selection of this study was from 1999 to 2016, which is a longer span, so more accurate conclusions can be obtained.
The cross-sectional design of this study made it impossible to determine the causal relationships of urinary Cd concentration with elevated ALT and AST. We cannot explain the elevation of ALT and AST caused by urinary Cd concentration. It is also possible that people with high ALT and AST are more likely to have Cd accumulation. Table 4 shows the relationship between Cd and blood lipids and inflammatory indices. With increasing Cd concentrations, CRP increased and HDL decreased, while triglyceride, LDL, and total cholesterol did not change significantly. HDL is known to be a class of lipids related to anti-inflammatory and antioxidant activities.
Evidence from epidemiological studies demonstrated that the downregulation of HDL cholesterol might play a significant role in mediating Cd exposure-associated cardiovascular disease risk increases (Xu et al. 2021b). Correspondingly, a 3-month treatment of rats with 2.0 mg/l CdCl2 in drinking water resulted in a significant reduction in serum HDL cholesterol (Samarghandian et al. 2015). Additionally, findings from Ferguson et al. suggested that intraperitoneal injection of 1 mg/kg body weight CdCl2 for 21 days also resulted in a significant 29% reduction in HDL-C levels compared to the control values (Mantur et al. 2014), which supports our study findings. In some previous animal studies, Cd exposure was found to increase ALT and AST levels. Adolescent mice were given a standard diet supplemented with 5 mg/kg bw CdCl2 in normal saline by intragastric administration, resulting in elevated serum ALT and AST levels, accompanied by the activation of inflammatory factors (Li et al. 2021b). Moreover, in carp exposed to at least 100 μg L/1 Cd for 30 days, ALT and AST levels increased and the activity of AChE in plasma decreased, while oxidative stress directly inhibited the activity of AChE (Banaee et al. 2019). The decrease in antioxidant enzymes can increase oxidative stress, which leads to an increase in liver injury (Salama et al. 2019). The above evidence indicates that Cd can cause hepatotoxicity, which is manifested as elevated ALT and AST concentrations. The mechanism might be related to the inflammatory response and the decrease in HDL-C.
Our results showed that Cd-induced increases in ALT and AST were statistically significant and positively correlated in boys. However, there was no significant difference between girls, suggesting that Cd-induced liver damage might differ by sex. Previous epidemiological studies have found that men exposed to Cd are more likely to develop thyroid hormone abnormalities (Chung et al. 2019) and autism spectrum disorders (Dickerson et al. 2017). The results of this study suggested that men might be more susceptible to Cd toxicity. One possible explanation is that men exposed to Cd show more overall hypermethylation, while women show hypomethylation (Young &Cai 2020). Exposure to Cd induced malignant transformation associated with global DNA hypermethylation (Arita &Costa 2009). Another study also showed that cadmium exposure can increase Klotho methylation levels, affecting the ability of the liver to synthesize proteins (Yu et al. 2020). Another reason could be differences in gene expression; many genes are upregulated in males and downregulated in females, and misfolded proteins increase reactive oxygen species production (Ba et al. 2017). In addition, genes responsible for Cd metabolism could be mostly downregulated in males, allowing Cd to accumulate in the body (Kadiene et al. 2020). In vivo, an environmental dose of cadmium at the early stages of life caused gut microbiota alterations, accelerated hepatic lipid metabolism, and resulted in life-long metabolic consequences in a sex-dependent manner (Ba et al. 2017). Moreover, Cd has also been reported to interact with sex hormones (Nagata et al. 2005). These findings may partially explain the sex differences in the associations between urinary cadmium and liver function in adolescents.
Our study has some advantages. First, it is the first to focus on the potential effects of Cd on liver function in adolescents. Second, we included a wide range of liver parameters and adjusted for factors that might influence Cd exposure or liver parameters in adolescents. Third, from the perspective of epidemiology, the relationships among urinary Cd concentration, inflammatory markers, and blood lipids in the human body were discussed, which could provide a possible molecular mechanism explaining the phenomenon of elevated ALT and AST induced by Cd.
There are also some limitations to this study. First, the cross-sectional design could not determine the causal relationship between urinary Cd concentrations and elevated ALT and AST. However, the results from animal experiments support the hypothesis that elevated Cd concentrations increase ALT and AST levels. Second, compared with the clinical diagnosis of nonalcoholic fatty liver disease and other diseases, the clinical significance of elevated ALT is not very clear and it is also affected by other factors. Although some covariates were adjusted, there were still some unknowns that were not considered. Third, we were unable to assess the effect of coexposure to environmental toxicants on liver damage. In a previous study, Cd and lead had both synergistic and antagonistic effects on rat livers (Andjelkovic et al. 2019). Other covariates were not adjusted, such as genetic factors. Genetic factors have a great impact on liver function (Middelberg et al. 2007). Future research needs to consider the interaction between environmental factors and genetic factors. Fourth, mixed chemical exposure also needs to be considered. A variety of chemicals, such as polycyclic aromatic hydrocarbons, are reported to be associated with liver function (Xu et al. 2021a). The combined effects of a variety of chemicals need to be considered in future research.
In summary, in this study, based on NHANES data, we found that increasing levels of urinary Cd were positively associated with elevated ALT and AST after adjustment for various covariates. We also performed linear regression of associations of urinary cadmium with elevated ALT and AST levels in boys. The increased CRP level and decreased HDL cholesterol level could be the intermediate pathways for ALT and AST increases caused by Cd. An important consideration, however, is that the sample in this study was from the USA, where concentrations of Cd are lower than in developing countries, such as China (Sun et al. 2016), suggesting that even comparatively low concentrations of Cd might affect liver function in adolescents. Further studies, such as longitudinal studies, are needed to confirm the exact relationship between Cd and liver function and the underlying mechanisms.
Data Availability
The datasets used and analyzed during the current study are available from https://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm.
References
Ahluwalia N, Dwyer J, Terry A, Moshfegh A, Johnson C (2016) Update on NHANES dietary data: focus on collection, release, analytical considerations, and uses to inform public policy. Adv Nutr 7:121–134
Andjelkovic M, Buha Djordjevic A, Antonijevic E, Antonijevic B, Stanic M, Kotur-Stevuljevic J, Spasojevic-Kalimanovska V, Jovanovic M, Boricic N, Wallace D, Bulat Z (2019): Toxic effect of acute cadmium and lead exposure in rat blood, liver, and kidney. Int J Environ Res Public Health 16
Arita A, Costa M (2009) Epigenetics in metal carcinogenesis: nickel, arsenic, chromium and cadmium. Metallomics 1:222–228
Ba Q, Li M, Chen P, Huang C, Duan X, Lu L, Li J, Chu R, Xie D, Song H, Wu Y, Ying H, Jia X, Wang H (2017) Sex-dependent effects of cadmium exposure in early life on gut microbiota and fat accumulation in mice. Environ Health Perspect 125:437–446
Banaee M, Soltanian S, Sureda A, Gholamhosseini A, Haghi BN, Akhlaghi M, Derikvandy A (2019): Evaluation of single and combined effects of cadmium and micro-plastic particles on biochemical and immunological parameters of common carp (Cyprinus carpio). Chemosphere 236, 124335
Bussler S, Vogel M, Pietzner D, Harms K, Buzek T, Penke M, Handel N, Korner A, Baumann U, Kiess W, Flemming G (2018) New pediatric percentiles of liver enzyme serum levels (alanine aminotransferase, aspartate aminotransferase, gamma-glutamyltransferase): effects of age, sex, body mass index, and pubertal stage. Hepatology 68:1319–1330
Chung SM, Moon JS, Yoon JS, Won KC, Lee HW (2019) Sex-specific effects of blood cadmium on thyroid hormones and thyroid function status: Korean nationwide cross-sectional study. J Trace Elem Med Biol 53:55–61
Dennis KK, Liu KH, Uppal K, Go YM, Jones DP (2021): Distribution of phytochelatins, metal-binding compounds, in plant foods: a survey of commonly consumed fruits, vegetables, grains and legumes. Food Chem 339, 128051
Dickerson AS, Rotem RS, Christian MA, Nguyen VT, Specht AJ (2017) Potential sex differences relative to autism spectrum disorder and metals. Curr Environ Health Rep 4:405–414
Gallo V, Leonardi G, Genser B, Lopez-Espinosa MJ, Frisbee SJ, Karlsson L, Ducatman AM, Fletcher T (2012) Serum perfluorooctanoate (PFOA) and perfluorooctane sulfonate (PFOS) concentrations and liver function biomarkers in a population with elevated PFOA exposure. Environ Health Perspect 120:655–660
Gao M, Yang Y, Lv M, Song W, Song Z (2018) Oxidative stress and DNA damage in zebrafish liver due to hydroxyapatite nanoparticles-loaded cadmium. Chemosphere 202:498–505
Goldner D, Lavine JE (2020): Nonalcoholic fatty liver disease in children: unique considerations and challenges. Gastroenterology 158, 1967–1983 e1
Hadzic N, Baumann U, McKiernan P, McLin V, Nobili V (2017) Long-term challenges and perspectives of pre-adolescent liver disease. Lancet Gastroenterol Hepatol 2:435–445
Hagedoorn IJM, Gant CM, Huizen SV, Maatman R, Navis G, Bakker SJL, Laverman GD (2020): Lifestyle-related exposure to cadmium and lead is associated with diabetic kidney disease. J Clin Med 9
Hu W, Liu Z, Hao HR, Yu WN, Wang XQ, Shao XJ, Wu XJ, Wen SR, Fan YQ, Ni YJ (2020) Correlation between income and non-alcoholic fatty liver disease in a Chinese population. Ann Endocrinol (paris) 81:561–566
Kadiene EU, Ouddane B, Gong HY, Kim MS, Lee JS, Pan YJ, Hwang JS, Souissi S (2020): Differential gene expression profile of male and female copepods in response to cadmium exposure. Ecotoxicol Environ Saf 204, 111048
Kim DW, Ock J, Moon KW, Park CH (2021): Association between Pb, Cd, and Hg exposure and liver injury among Korean adults. Int J Environ Res Public Health 18
Kim J, Garcia-Esquinas E, Navas-Acien A, Choi YH (2018) Blood and urine cadmium concentrations and walking speed in middle-aged and older U.S. adults. Environ Pollut 232:97–104
Lazo M, Hernaez R, Eberhardt MS, Bonekamp S, Kamel I, Guallar E, Koteish A, Brancati FL, Clark JM (2013) Prevalence of nonalcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988–1994. Am J Epidemiol 178:38–45
Le Dare B, Lagente V, Gicquel T (2019) Ethanol and its metabolites: update on toxicity, benefits, and focus on immunomodulatory effects. Drug Metab Rev 51:545–561
Li D, Lin H, Zhang M, Meng J, Hu L, Yu B (2021a): Urine cadmium as a risk factor for osteoporosis and osteopenia: a meta-analysis. Front Med (Lausanne) 8, 648902
Li H, Fagerberg B, Sallsten G, Borne Y, Hedblad B, Engstrom G, Barregard L, Andersson EM (2019) Smoking-induced risk of future cardiovascular disease is partly mediated by cadmium in tobacco: malmo diet and cancer cohort study. Environ Health 18:56
Li J, Wang X, Zhao G, Chen C, Chai Z, Alsaedi A, Hayat T, Wang X (2018) Metal-organic framework-based materials: superior adsorbents for the capture of toxic and radioactive metal ions. Chem Soc Rev 47:2322–2356
Li W, Homer K, Hull S, Boomla K, Robson J, Alazawi W (2020) Obesity predicts liver function testing and abnormal liver results. Obesity (silver Spring) 28:132–138
Li X, Li H, Cai D, Li P, Jin J, Jiang X, Li Z, Tian L, Chen G, Sun J, Bai W (2021b): Chronic oral exposure to cadmium causes liver inflammation by NLRP3 inflammasome activation in pubertal mice. Food Chem Toxicol 148, 111944
Liu Z, Que S, Xu J, Peng T (2014) Alanine aminotransferase-old biomarker and new concept: a review. Int J Med Sci 11:925–935
Ma Y, Ran D, Zhao H, Song R, Zou H, Gu J, Yuan Y, Bian J, Zhu J, Liu Z (2021): Cadmium exposure triggers osteoporosis in duck via P2X7/PI3K/AKT-mediated osteoblast and osteoclast differentiation. Sci Total Environ 750, 141638
Mantur VS, Somannavarib MS, Yendigeri S, Das KK, Goudar SS (2014) Ameliorating effect of black tea extract on cadmium chloride-induced alteration of serum lipid profile and liver histopathology in rats. Indian J Physiol Pharmacol 58:128–132
Middelberg RP, Medland SE, Martin NG, Whitfield JB (2007) A longitudinal genetic study of uric acid and liver enzymes in adolescent twins. Twin Res Hum Genet 10:757–764
Nagata C, Nagao Y, Shibuya C, Kashiki Y, Shimizu H (2005) Urinary cadmium and serum levels of estrogens and androgens in postmenopausal Japanese women. Cancer Epidemiol Biomarkers Prev 14:705–708
Noor N, Zong G, Seely EW, Weisskopf M, James-Todd T (2018) Urinary cadmium concentrations and metabolic syndrome in U.S. adults: The National Health and Nutrition Examination Survey 2001–2014. Environ Int 121:349–356
Park E, Kim J, Kim B, Park EY (2021): Association between environmental exposure to cadmium and risk of suspected non-alcoholic fatty liver disease. Chemosphere 266, 128947
Rinella ME (2015) Nonalcoholic fatty liver disease: a systematic review. JAMA 313:2263–2273
Salama SA, Arab HH, Hassan MH, Al Robaian MM, Maghrabi IA (2019) Cadmium-induced hepatocellular injury: modulatory effects of gamma-glutamyl cysteine on the biomarkers of inflammation, DNA damage, and apoptotic cell death. J Trace Elem Med Biol 52:74–82
Sall ML, Diaw AKD, Gningue-Sall D, Efremova Aaron S, Aaron JJ (2020) Toxic heavy metals: impact on the environment and human health, and treatment with conducting organic polymers, a review. Environ Sci Pollut Res Int 27:29927–29942
Samarghandian S, Azimi-Nezhad M, Shabestari MM, Azad FJ, Farkhondeh T, Bafandeh F (2015) Effect of chronic exposure to cadmium on serum lipid, lipoprotein and oxidative stress indices in male rats. Interdiscip Toxicol 8:151–154
Sun H, Wang D, Zhou Z, Ding Z, Chen X, Xu Y, Huang L, Tang D (2016) Association of cadmium in urine and blood with age in a general population with low environmental exposure. Chemosphere 156:392–397
Sun Y, Zhou Q, Zheng J (2019): Nephrotoxic metals of cadmium, lead, mercury and arsenic and the odds of kidney stones in adults: an exposure-response analysis of NHANES 2007–2016. Environ Int 132, 105115
Wang L, Zhang S, Wang Z, Xu M, Yuan L, Cui J, Liu S (2017) A protective role of Heme-regulated eIF2alpha kinase in cadmium-induced liver and kidney injuries. Chemosphere 185:284–289
Wannamethee SG, Shaper AG (2010) Cigarette smoking and serum liver enzymes: the role of alcohol and inflammation. Ann Clin Biochem 47:321–326
Weng Z, Xu C, Zhang X, Pang L, Xu J, Liu Q, Zhang L, Xu S, Gu A (2020): Autophagy mediates perfluorooctanoic acid-induced lipid metabolism disorder and NLRP3 inflammasome activation in hepatocytes. Environ Pollut 267, 115655
Williams R et al (2015) Implementation of the Lancet Standing Commission on liver disease in the UK. Lancet 386:2098–2111
Wu Y, Pang H, Liu Y, Wang X, Yu S, Fu D, Chen J, Wang X (2019) Environmental remediation of heavy metal ions by novel-nanomaterials: A review. Environ Pollut 246:608–620
Xiong X, Zhang Y, Xing H, Xu S (2020) Ameliorative effect of selenomethionine on cadmium-induced hepatocyte apoptosis via regulating PI3K/AKT pathway in chickens. Biol Trace Elem Res 195:559–568
Xu C, Liu Q, Liang J, Weng Z, Xu J, Jiang Z, Gu A (2021a): Urinary biomarkers of polycyclic aromatic hydrocarbons and their associations with liver function in adolescents. Environ Pollut 278, 116842
Xu C, Weng Z, Zhang L, Xu J, Dahal M, Basnet TB, Gu A (2021b): HDL cholesterol: a potential mediator of the association between urinary cadmium concentration and cardiovascular disease risk. Ecotoxicol Environ Saf 208, 111433
Young JL, Cai L (2020): Implications for prenatal cadmium exposure and adverse health outcomes in adulthood. Toxicol Appl Pharmacol 403, 115161
Younossi Z, Tacke F, Arrese M, Chander Sharma B, Mostafa I, Bugianesi E, Wai-Sun Wong V, Yilmaz Y, George J, Fan J, Vos MB (2019) Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology 69:2672–2682
Yu D, Zhang L, Yu G, Nong C, Lei M, Tang J, Chen Q, Cai J, Chen S, Wei Y, Xu X, Tang X, Zou Y, Qin J (2020) Association of liver and kidney functions with Klotho gene methylation in a population environment exposed to cadmium in China. Int J Environ Health Res 30:38–48
Yu L, Yang M, Cheng M, Fan L, Wang X, Xu T, Wang B, Chen W (2021): Associations between urinary phthalate metabolite concentrations and markers of liver injury in the US adult population. Environ Int 155, 106608
Zhao M, Xu Y, Zhang C, Rong H, Zeng G (2016) New trends in removing heavy metals from wastewater. Appl Microbiol Biotechnol 100:6509–6518
Zhao Y, Yan Y, Xie L, Wang L, He Y, Wan X, Xue Q (2020): Long-term environmental exposure to microcystins increases the risk of nonalcoholic fatty liver disease in humans: a combined fisher-based investigation and murine model study. Environ Int 138, 105648
Zhu MK, Li HY, Bai LH, Wang LS, Zou XT (2020) Histological changes, lipid metabolism, and oxidative and endoplasmic reticulum stress in the liver of laying hens exposed to cadmium concentrations. Poult Sci 99:3215–3228
Funding
This work was supported by funding from the National Science Foundation of China (81900281) and the China Postdoctoral Science Foundation (2021M691641).
Author information
Authors and Affiliations
Contributions
C.X.: conceptualization, methodology. J.J.L., and Q.L.: data curation, project administration. Z.N.X. and Z.K.W.: writing—original draft preparation and editing. C.X. and A.H.G.: supervision, investigation. X.Z. and J.X.: validation. C.X.: writing—review and editing.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The consent form was signed by the survey participants, and the participants consented to storing specimens of their blood for future research. The CDC/NCHS Ethics Review Board approved the NHANES study and gave approval for public dissemination.
Consent for publication
Not applicable. There are no individual-level data in our publication.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Lotfi Aleya
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zining Xu and Zhenkun Weng contributed equally to this paper
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xu, Z., Weng, Z., Liang, J. et al. Association between urinary cadmium concentrations and liver function in adolescents. Environ Sci Pollut Res 29, 39768–39776 (2022). https://doi.org/10.1007/s11356-022-18950-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-18950-4