Abstract
Surface water used as an irrigation source can be a significant source of viral contamination of fresh produce. Enteric viruses such as hepatitis A virus (HAV) and human norovirus genogroup I (HNoV GI) and genogroup II (HNoV GII) can be transmitted to human via fresh produce when irrigated with contaminated water or when prepared by infected food handlers. In the current study, we investigated the presence of HAV, HNoV GI and GII in fresh produce and surface water used in cultivation of this produce using real-time PCR. Samples were collected from six different points in the Mansoura and Giza regions, Egypt. Our analysis showed that at least one virus was found in 41.6% (30/72) of surface water samples and 27% (13/48) of fresh produce samples. HAV (23/72) with a mean viral concentration = 4 × 106 genome copies/litre (GC/L) was the most frequently identified virus in surface water samples, followed by human norovirus genogroup II (HNoV GII) (15/72, with a mean concentration = 1.2 × 106 GC/L, and human noroviruses genogroup I (HNoV GI) (12/72, with a mean concentration = 1.4 × 104 GC/L). Additionally, HAV (10/48) with a mean concentration = 5.2 × 105 genome copies/gram (GC/g) was also the most frequently detected virus in the fresh produce samples, followed by HNoV GII (8/48, with a mean concentration = 1.7 × 104 GC/g); meanwhile, HNoV GI (6/48) was less detected virus with a mean concentration = 3 × 103 GC/g. This work suggests a wide prevalence of human enteric viruses in surface waters and fresh produce, which is of concern when the fresh produce is eaten raw. Thus, additional monitoring for viral pathogens in irrigation water and food is needed to increase the awareness of this issue to rise the control measures to reduce illness from contaminated food.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water and foodborne illness are two of the most serious public health issues, resulting in a considerable number of deaths in people each year (WHOa 2005). Waterborne illness is a global problem which is responsible for more than 2.2 million deaths per year (WHO 2005; Bitton 2014). Children account for around 1.4 million of these deaths (WHO 2015). In Egypt, the presence of enteric viruses, such as hepatitis A and E viruses, norovirus, rotavirus, adenovirus, human noroviruses, and human astrovirus has recently been reported in river water used in irrigation purpose (Elmahdy et al. 2020; Shaheen et al. 2018, 2019, 2020; Shaheen and Elmahdy 2019a, b). This underlines that irrigation water can play a role in transferring of these viruses to fresh produce.
Hepatitis A virus (HAV) and human noroviruses (HNoV) are recognized as major public health concerns where they are responsible for the majority of acute hepatitis or non-bacterial gastroenteritis which are occasionally fatal (Vaughan et al. 2014; Koo et al. 2010). HAV belong to the Picornaviridae family while HNoV is classified in the Caliciviridae. Both viruses possess a single-stranded and positive-sense RNA genome as they are small non-enveloped viruses. HNoV which was classified into ten genogroups (HNoV GI-GX) is responsible for about 20% of all cases of acute gastroenteritis, worldwide (Chhabra et al. 2019; Ahmed et al. 2014), with over 213,000 deaths and 677 million cases reported annually (Pires et al. 2015). Significant rates of morbidity and mortality due to HNoV infection are reported in risk groups comprising elderly, children, and immunocompromised individuals (Tian et al. 2014; Payne et al. 2013; Siebenga et al. 2009; Harris et al. 2008; Hutson et al. 2004). Also, HAV is a major cause of acute viral hepatitis, with nearly 1.4 million cases per year globally (Jacobsen 2009; Yong and Son 2009), and a high hepatitis A endemicity in areas with low sanitation (Osuolale and Okoh 2015; Gupta and Ballani 2014; Yong and Son 2009). They are primarily transmitted via the vomit-oral and faecal-oral routes, including contact with infected person, contact with contaminated surfaces, or by ingestion of polluted water and food: particularly vegetables, soft fruits, and shellfish (Kotwal and Cannon 2014; Matthews et al. 2012; Nainan et al. 2006).
Contamination of groundwater or surface water with these viruses represents a potential risk of infection through food or drinking water (Moreira and Bondelind 2017; Mathijs et al. 2012). The contamination of fresh produce can occur during pre-harvesting, due to contact with contaminated irrigation water or during post-harvesting due to an infected worker through handling of the product or by contaminated equipment through the production process (Leon-Felix et al. 2010; Seymour and Appleton 2001; Bidawid et al. 2000a, b). Viral contamination of irrigation, ground, surface water, and soil has been investigated in previous studies (Gerba and Choi 2006; Lodder and de Roda Husman 2005; Santamaria and Toranzos 2003). The aim of this study was to investigate the presence of HAV and HNoV in fresh produce and surface water used for irrigation.
Material and methods
Study area
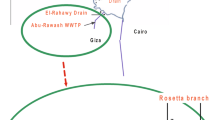
Dakahlia Governorate is located at the northeast of Nile Delta in Egypt. Mansoura (the capital of Dakahlia Governorate) is the most important cities in Dakahlia Governorate. Giza is located on the west bank of the Nile, 4.9 km southwest of central Cairo, and is a core city of the Greater Cairo Metropolis. The two regions have been chosen because of the fast rate of urbanization and very low studies on the simultaneous detection of these viruses in both fresh produce and irrigation water were made on them.
Sampling
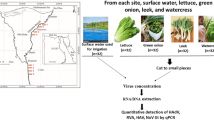
A total of 72 surface water samples and 48 fresh produce (24 green onions and 24 lettuces) samples were collected from three sites at two regions (Giza and Mansoura) in Egypt. As shown in Fig. 1, three water samples were collected monthly from each region during the period March 2019 to February 2020. Also, 3 fresh produce samples from each kind were collected monthly from the same sites at each region according to the harvest season (November 2019 to February 2020).
Determination of virus recovery from fresh produce and water samples
In this study, we used murine norovirus (MNV-1) as a process control virus to investigate the virus recovery in all virus detection assays. In brief, autoclaved surface water samples (5 L) were inoculated with 200 µL of MNV-1 suspension (4.7 × 108 GC/mL) prior to concentration. Also, leaves of green and lettuce were chopped into small pieces then 50 g of each sample was weighed and sanitized in chlorinated water for 20 min. After sanitation, the leaves of each fresh produce were divided into two equal parts and each part was placed into a sterile bag under aseptic conditions. One portion of the fresh produce sample was inoculated with the same amount of virus prior to concentration, while the other portion was left as blank. The efficiency of MNV-1 recovery from inoculated samples was assessed using the protocols described below. Furthermore, a representative sample was taken from green onion and lettuce collected during this study and inoculated with human MNV-1 as sample process control virus (SPCV) (previously tested negative for MNV-1 by RT-qPCR).
Virus concentration
Virus concentration from surface water samples was performed by using a protocol described by Katayama et al. (2002) with slight modification. In brief, 4 L of collected water sample was mixed with 40 mL of 2.5 M MgCl2 (5 mL of MgCl2/500 mL sample) then filtered through a negatively charged membrane (0.45 µm pore size and 142 mm diameter). Filter membrane was rinsed with 200 mL of 0.5 mM H2SO4 solution (pH 3.0). The membrane was removed and transferred to sterile glassware then eluted in 10 mL of 1 mM NaOH (pH = 10.5). After stirring for 10 min, the eluate was neutralized by adding 50 µL 1 mM Tris–EDTA buffer solution (pH 8.0) and 50 µL of 50 mM H2SO4 solution. After that, the neutralized eluate was ultra-filtrated by using a Centriprep Concentrator system then the filtrate was centrifuged at 1500 × g for 10 min at 4 °C. After removing the filtrate, the pellet was rinsed twice with 10 mL of double distilled water by the same procedure, and a final volume of 2 mL was obtained. The concentrated samples were stored at − 80 °C until the next analysis. On the other hand, virus elution from fresh produce samples was done according to ISO/TS 15,216–1 method (ISO 15216–1:2017 2017). Briefly, 25 g of green onion or lettuce in small pieces was mixed with 40 mL of Tris–glycine buffer (100 mM Tris–HCl, 50 mM glycine, and 1% beef extract, pH 9.5) in a sterile plastic bag. After 20 min at room temperature with constant rocking (approximately 70 oscillations/min) to elute the viruses from the surface of the fresh produce sample, the sample was distributed into clean centrifuge tubes. After centrifugation at 10,000 × g for 30 min at 4 °C, the vegetable matter was discarded and the eluate was transferred into clean centrifuge tubes then the pH was adjusted 7.2 ± 0.3 using 1.0 N HCl. For the PEG precipitation, 0.25 volume of 50% (w/v) polyethylene glycol 8000/1.5 M NaCl was added to the eluates and incubated on rocking at 120 rpm at 4 °C for 1 h. After additional centrifugation for 30 min at 10,000 × g at 4 °C, the resulting pellets were dissolved in 500 µL of 10 mM PBS then stored at − 20 °C until use.
RNA extraction and virus quantification
The final concentrates were used to extract viral RNA using a QIAamp MiniElute Virus Spin kit (Qiagen, Germany) according to the manufacturer’s instructions. Phosphate buffer saline as a negative control for nucleic acid extraction was applied. For molecular detection of HAV, HNoV GI and GII, and MNV-1, the qPCR was carried out according to Jothikumar et al. (2005), Kageyama et al. (2003), and Lee et al. (2015), respectively, using one-step Rotor-Gene Probe RT-PCR kit. All amplifications were conducted in duplicate using Rotor-Gene system (QIAGEN, Germany). For each assay, PCR amplicon for each positive control was generated by cloning the amplicon into a plasmid (pGEM-T Easy Vector (Promega) for HAV strain HM175 and pCR2.1-TOPO vector (Promega) for HNoV GI, GII, and MNV-1), and concentrations of a purified plasmid DNA were calculated using a Nano Drop spectrophotometer (Thermo Fisher Scientific, USA). Standard curves were prepared by ten-fold serial dilutions of the positive control plasmids. Ultra-pure water was used as negative controls in each assay to ensure that there was no cross-contamination in the assay. To increase PCR efficiency, serial ten-fold dilutions were prepared to viral nucleic acids to dilute the inhibitors (if found). Viral nucleic acid was amplified in a 25 µL real-time PCR mixture containing 5 µL of the RNA extract, 12.5 µL RT-PCR Master Mix (Qiagen, Germany), 400 nM each primer, 250 nM of probe, and nuclease-free water to complete the volume reaction up to 25 µL. This mixture was transferred into a 48-well microplate then loaded into the Rotor-Gene system. Fluorescence data were measured at the end of annealing step. All primers and probes used in this study, as well as the thermal cycling conditions of each virus, are shown in Table 1. The percentage of MNV-1 recovery rate from spiked water or fresh produce samples was calculated by using the following formula: amount of virus detected after spiking experiments/amount of viral inoculum × 100 (Hennechart-Collette et al. 2021).
Statistical analysis
Statistical analyses were performed using GraphPad Prism version 5.0 (USA) software. The critical P value for the test was set at < 0.05. The Pearson correlation was applied to evaluate the correlations between viral distributions and water/fresh produce samples. The mean viral load was compared between samples with a one-way analysis of variance. The detection limits of the qPCR were established according to the highest dilution where it was possible to virus quantification (the detection limits for HAV were 2.5 × 101 genome copies in 25 mg of fresh produce and in 10 L of water whereas the detection limit for both HNoV GI and HNoV GII was 2 × 101 genome copies in 25 mg of fresh produce and in 10 L of water).
Results
MNV-1 recovery assay
In the spiked surface water, green onion, and lettuce, the MNV-1 recoveries were 4.5 × 108 ± 3.7 × 108 GC/L (95.7%), 3.2 × 108 ± 2.4 × 108 GC/g (68%), and 2.7 × 108 ± 2.1 × 108 GC/g (57.4%), respectively.
Detection of HAV and HNoV in surface water samples
Of the 72 water samples, 23 samples (31.9%) were positive for HAV, 12 (16.7%) for HNoV GI, and 15 (20.8%) for HNoV GII. The viral load of HAV ranged from 1.2 × 102 to 8.3 × 107 GC/L, with a mean viral load of 4.3 × 106 GC/L. The viral load of HNoV GI ranged from 2.6 × 102 to 7.3 × 104 GC/L, with a mean viral load of 1.4 × 104 GC/L. The HNoV GII concentrations ranged from 8.1 × 102 to 7.8 × 106 GC/L, with a mean viral load of 1.2 × 106 GC/L. The most positive samples for HAV and HNoV were found in the samples collected from Mansoura region than those collected from Cairo region (Table 2). This variation was statistically significant (P ≤ 0.01).
Detection of HAV and HNoV in fresh produce samples
The prevalence of foodborne HAV and HNoV is given in Table 3. Of the 24 green onion samples, 9 samples were found to be contaminated with at least one virus. HAV, HNoV GI, and HNoV GII were detected in 6/24 (25%), 3/24 (12.5%), and 5/24 (20.8%), respectively. The HAV load ranged from 1.2 × 102 to 1.9 × 105 GC/g, with a mean viral load of 4.5 × 104 GC/g. The HNoV GI concentration ranged from 7.7 × 102 to 7.2 × 103 GC/g with a mean viral load of 2.9 × 103 GC/g. The HNoV GII load ranged from 2.2 × 102 to 7.2 × 103 GC/g with a mean viral load of 3.1 × 103 GC/g. Of the 24 lettuce samples, 7 samples were found to contain at least one of the viruses. HAV, HNoV GI, and HNoV GII were detected in 4/24 (16.6%), 3 (12.5%), and 3 (12.5%), respectively. The HAV load ranged from 4.9 × 103 to 4.8 × 106 GC/g, with a mean viral load of 1.2 × 106 GC/g. The HNoV GI load ranged from 2.4 × 102 to 8.2 × 103 GC/g, with a mean viral load of 3.1 × 103 GC/g. The HNoV GII load ranged from 7.2 × 103 to 2.9 × 104 GC/g, with a mean viral load of 4 × 104 GC/g.
Distribution of single and multiple viral agents in the positive water samples
As shown in Table 4, a single viral agent was found in 46.6% (14/30) of the positive samples, with HAV (9/72) being the most frequently found virus in samples contained only one viral agent. HNoV GI was found in 3 (10%) samples as a single viral agent whereas 2 (6.6%) samples contained only HNoV GII. Contamination with two viruses was detected in 40% (12/30) of positive samples: 6.6% (2/30) of positive samples contained both HNoV GI and GII, 10% (3/30) contained both HNoV GI and HAV, and 23.3% (7/30) contained both HNoV GII and HAV. The three viral agents were detected in 4 (13.3%) of positive samples.
Distribution of single and multiple viral agents in the positive fresh produce samples
In the green onion samples, single viral agent was detected in 25% (6/24) of positive samples. HAV, HNoV GI, and HNoV GII were detected in 50% (3/6), 16.6% (1/6), and 33.3% (2/6) as a single viral agent in the positive samples, respectively. Only one positive sample contained two viral agents (HAV + HNoV GII) while two samples contained the three viral agents (HAV + HNoV GI + HNoV GII). In the lettuce samples, contamination with single viral agent was found in 20.8% (5/24) of the positive samples. HAV (3/5) was the most frequently found alone in these samples, followed by HNoV GI (1/5) and by HNoV GII (1/5). Contamination with two viruses was detected only in one positive sample that contained both HNoV GI and HNoV GII. Also, combination of the three viral was detected in 14.3% (1/7) of the positive samples (Tables 5, and 6).
Discussion
Enteric viruses can be transferred to fresh produce by various routes during growth, harvest, packaging, transportation, and preparation of foods. Several viral outbreaks associated with fresh produce consumption have been identified or suspected to have originated from direct contact with virus-contaminated irrigation water (Shaheen et al. 2019; Prez et al. 2018; Gerba et al. 2006). Human HNoV and HAV are major etiological contributors to foodborne illness outbreaks. A systematic review published in 2018 stated that HNoV and HAV caused 48.7% and 46.1% of all viral outbreaks linked to fresh produce consumption, respectively, worldwide (Chatziprodromidou et al. 2018). Unfortunately, in Egypt, there is currently no official reporting system focus on foodborne viral illness outbreaks, and there is no active surveillance system for foodborne viral infections. Hence, information on the involvement of Egyptian foods in enteric illness outbreaks is limited (Shaheen et al. 2019; Zaher et al. 2008).
There are several previous studies from Egypt reported the detection of enteric viruses in surface water used as an irrigation source (Shaheen et al. 2020, 2018; Shaheen and Elmahdy 2019a, b). However, very low studies focused on the simultaneous detection of these viruses in both fresh produce and irrigation water (Shaheen et al. 2019). Indeed, monitoring these viruses in both the fresh produce and irrigation water can provide a useful tool to reduce the risk of foodborne disease linked to the occurrence of these viruses. In this context, the aim of this study was to investigate the presence of HAV and HNoV in surface waters and fresh produce irrigated by this water and collected from two regions (Giza and Mansoura) in Egypt.
Overall, in both matrices (n = 120), HAV showed a relatively higher prevalence rate (27.5%, 33/120 samples). HNoV was detected in 25% (30/120) of both matrices where HNoV GII (19.2%, 23/120 samples) was higher than HNoV GI (15%, 18/120 samples). In contrast, the detection rate of HNoV GI was higher than HNoV GII genogroup identified both in irrigation water and vegetable samples (Emilse et al. 2021). In a recent study from Egypt, HAV was detected in 11/32(34.4%), 6/32 (18.7%), 8/32 (25%), 9/32 (28%), and 10/32 (31%) of surface water, leek, green onion, watercress, and lettuce samples, respectively (Shaheen et al. 2019). Khan et al. (2014) detected HAV in all cultivated vegetables due to exposure to faecally contaminated irrigation water. In addition, several HAV outbreaks associated with green onion consumption at several restaurants were documented in 2003 in Monaca, Pennsylvania; in 2000 in Kentucky and Florida; in 2003 in Georgia, and North Carolina; and in 1999 in Ohio (Amon et al. 2005; Wheeler et al. 2005; Datta et al. 2001; Dentinger et al. 2001). Indeed, Bidawid et al. (2000a, b) reported that 9.2% of contaminated lettuce by infectious virus particles comes from contaminated hands. In the farm and during the growing stage, food can become contaminated by contact with sewage, contaminated fertilizers, or the use of contaminated irrigation water (Directorate 2002).
In Egyptian study, Shaheen et al. (2018) detected HNoV GI in 10/32 (31%), 11/32 (34.4%), 13/32 (40.6%), and 9/32 (28%) of lettuce, watercress, green onion, and leek samples, respectively, while the virus was detected in 10/32 (31.2%) of surface water used as an irrigation source. A series of outbreaks of HNoV GI and HNoV GII gastroenteritis related to the consumption of lettuce between 18 and 20 January 2010 were documented to Danish authorities (Ethelberg et al. 2010). In Canada and Malaysia, HNoV GI was found in 3.33%, 25%, and 13.33% of red onions, strawberries, and green onions, respectively (Brassard et al. 2012; Hidayah et al. 2011). Various prevalence rates of HNoV in leafy greens were found in several countries: 2.9% in Italy (Purpari et al. 2019), 12.4–50% in France (Loutreul et al. 2014; Baert et al. 2011), 5.3% in UK (Cook et al. 2019), and 33.3% in Belgium (Baert et al. 2011).
In this study, HAV detection in both matrices was higher than HNoV prevalence. This finding is similar to our previous study conducted on the same two regions (Shaheen et al. 2019), suggesting that surface irrigation water could be the source of fresh produce contamination. Since irrigation water can transmit the viral contamination for fresh produce, thus primary products must be produced only in regions where an appropriate water quality is used for irrigation purposes. If the quality control of irrigation water is not controlled, prior consumption, it is suggested to immerse the fresh produce with drinking water containing sodium hypochlorite (15–20 ppm free chlorine levels) for ˃ 2 min to reduce viral loads on the vegetable surfaces (Bosch et al. 2018).
This is the first report of HNoV GII detection in fresh produce in Egypt. However, this study has some limitations to not investigate the viral genotypes in the positive samples. Difficulties in HAV and HNoVs sequencing were due to the small amount of PCR product obtained from the RT-qPCR and cannot be used for that purpose. This fact has also been reported in other studies (Felix-Valenzuela et al. 2012; Baert et al. 2011). A major limitation in this study is that RT − qPCR technique used in the detection of viruses cannot differentiate between the infections and non-infectious virus particles in the positive samples. Molecular assays may pick up both non-infectious and infectious viral particles. In this way, detection of viral nucleic acid in a sample does not necessarily mean the presence of infectious virus particles in that sample (Hamza et al. 2009). Thus, detection of enteric viruses by the molecular technique cannot confirm the role of fresh produce as transmitting routes of infectious enteric viruses.
An additional limitation in this study is the absence of clinical data. However, previous Egyptian studies have reported the detection of HAV and HNoV among the population in both regions. Zaki and El Kheir (2017) identified HNoV (GI and GII) in 30% of diarrheal specimens collected from Mansura City. A recent study from Mansura region, HNoV was also detected in 70.5% of diarrheal samples with HNoV GII as the prevalent genotype (Zaki et al. 2019). This finding agrees with our study that HNoV GII is the most prevalent genotype. In Cairo region, HNoV (GI and GII) and HAV were also identified in samples collected from patients with severe diarrhea or hepatitis-related symptoms (Kamel et al. 2009, 2010, 2011). The HNoV genotypes as well as HAV detected in the clinical samples are also detected in fresh produce, suggesting that viral contamination of the fresh produce could be originated from contact with contaminated irrigation water.
In the current study, the viral load of HAV was higher than HNoV in the surface water and fresh produce samples. Comparatively, HAV had high variability in the viral loads, where some fresh produce samples had as low as 1.1 × 102 GC/g up to 8.3 × 107 GC/mL detected in one of the surface water samples tested. In surface water samples, most of the positive samples had viral concentrations between 104 and 105 GC/L while between 103 and 104 GC/g in fresh produce samples. High variability in the viral loads was also observed for HNoV where some fresh produce samples had as low as 2.2 × 102 GC/g which increased to 7.8 × 106 GC/mL detected in one of the surface water samples tested. Most of the positive surface water samples had HNoV concentrations between 103 and 104 GC/L. However, the HNoV in fresh produce samples had low variability with viral loads ranged from 2.2 × 102 to 8.2 × 104 GC/g. The low prevalence of HAV and HNoV with low concentrations in fresh produce than surface water may be due to their direct exposure on fresh produce surfaces to the ultraviolet radiation emitted from the sun.
The low viral concentrations found in the positive fresh produce and surface samples are by far higher than the infective dose required to induce disease by most of the enteric viruses, ranging between 10 and 100 viral particles for human rotavirus and HAV and even less for human HNoV (Yezli and Otter 2011). Thus, this low infective dose represents a potential risk for a viral outbreak if these fresh produce reach the consumers. In comparison, another study from Mexico found that the mean viral loads of HAV ranged from 2.8 × 102 to 2.4 × 103 GC/g while HNoV loads ranged from 2.1 × 102 to 1.3 × 103 GC/g (Felix-Valenzuela et al. 2012), which is lower than viral concentrations detected in this study. All precautions were applied in this study to prevent cross-contamination in the RT-qPCR reactions, and no amplification was found in negative controls.
In conclusion, this study shows the presence of human enteric viruses in surface water and fresh produce samples collected from different sites in Egypt. In addition, the results obtained in this work highlight the importance of viral surveillance programme for fresh produce and irrigation source to prevent cross contamination between the water environment and fresh produce.
Availability of data and materials
Not applicable.
References
Ahmed SM, Hall AJ, Robinson AE, Verhoef L, Premkumar P, Parashar UD, Koopmans M, Lopman BA (2014) Global prevalence of norovirus in cases of gastroenteritis: a systematic review and meta-analysis. Lancet Infect Dis 14:725–730
Amon JJ, Devasia R, Xia G, Nainan OV, Hall S, Lawson B, Wolthuis JS, Macdonald PD, Shepard CW, Williams IT, Armstrong GL, Gabel JA, Erwin P, Sheeler L, Kuhnert W, Patel P, Vaughan G, Weltman A, Craig AS, Bell BP, Fiore A (2005) Molecular epidemiology of foodborne hepatitis a outbreaks in the United States, 2003. J Infect Dis 192(8):1323–1330
Baert L, Mattison K, Loisy-Hamon F, Harlow J, Martyres A, Lebeau B, Stals A, Van Coillie E, Herman L, Uyttendaele M (2011) Review: norovirus prevalence in Belgian, Canadian and French fresh: a threat to human health? Int J Food Microbiol 151:261–269
Bidawid S, Farber JM, Sattar SA (2000a) Contamination of foods by food handlers: experiments on HAV transfer to food and its interruption. Appl Environ Microbiol 7:2759–2763
Bidawid S, Farber JM, Sattar SA (2000b) Contamination of foods by food handlers: experiments on hepatitis a virus transfer to food and its interruption. Appl Environ Microbiol 66(7):2759–2763
Bitton G (2014) Microbiology of drinking water: production and distribution. John Wiley & Sons
Bosch A, Gkogka E, Le Guyader FS, Loisy-Hamon F, Lee A, van Lieshout L, Marthi B, Myrmel M, Sansom A, Schultz AC, Winkler A, Zuber S, Phister T (2018) Foodborne viruses: detection, risk assessment, and control options in food processing. Int J Food Microbiol 285:110–128
Brassard J, Gagné MJ, Généreux M, Côté C (2012) Detection of human food-borne and zoonotic viruses on irrigated, field-grown strawberries. Appl Environ Microbiol 78(10):3763–3766
Chatziprodromidou IP, Bellou M, Vantarakis G, Vantarakis A (2018) Viral outbreaks linked to fresh produce consumption: a systematic review. J Appl Microbiol 124(4):932–942
Chhabra P, de Graaf M, Parra GI, Chan MC, Green K, Martella V, Wang Q, White PA, Katayama K, Vennema H, Koopmans MPG, Vinjé J (2019) Updated classification of norovirus genogroups and genotypes. J Gen Virol 100(10):1393–1406
Cook N, Williams L, D’Agostino M (2019) Prevalence of Norovirus in produce sold at retail in the United Kingdom. Food Microbiol 79:85–89
Datta SD, Traeger MS, Nainan OV (2001) Identification of a multi-state outbreak of hepatitis A associated with green onions using a novel molecular epidemiologic technique [abstract 896]. InProgram and abstracts of the 39th Annual Meeting of the Infectious Diseases Society of America (Vol. 192). Alexandra, VA: Infectious Diseases Society of America 2001
Dentinger CM, Bower WA, Nainan OV, Cotter SM, Myers G, Dubusky LM, Fowler S, Salehi ED, Bell BP (2001) An outbreak of hepatitis A associated with green onions. J Infect Dis 183:1273–1276
Directorate-General for Agriculture European Commission (2002) Economic impacts of genetically modified crops on the agri-food sector. A synthesis. p. 18
Elmahdy EM, Shaheen MN, Rizk NM, Saad-Hussein A (2020) Quantitative detection of human adenovirus and Human Rotavirus Group A in wastewater and el-Rahawy drainage canal influencing River Nile in the north of Giza, Egypt. Food Environ Virol 12(3):218–225
Emilse PV, Matías V, Cecilia ML, Oscar GM, Gisela M, Guadalupe D, Elizabethab RV, Victorioa PJ, Rodneyc C, Vivianaa NS, Angélica BP (2021) Enteric virus presence in green vegetables and associated irrigation waters in a rural area from Argentina. A quantitative microbial risk assessment. LWT 144:111201
Ethelberg S, Lisby M, Böttiger B, Schultz AC, Villif A, Jensen T, Olsen KE, Scheutz F, Kjelsø C, Muller L (2010) Outbreaks of gastroenteritis linked to lettuce, Denmark, January 2010. Eurosurveillance 15(6):19484
Felix-Valenzuela L, Resendiz-Sandoval M, Burgara-Estrella A, Hernández J, Mata-Haro V (2012) Quantitative detection of hepatitis A, rotavirus and genogroup I norovirus by RT-qPCR in fresh produce from packinghouse facilities. J Food Saf 32(4):467–473
Gerba CP, Choi CY (2006) Role of irrigation water in crop contamination by viruses. In: Goyal SM (ed) Viruses in Foods, 1st edn. Springer, New York, pp 257–263
Gerba PC, Choi YP, Goyal SM (2006) Role of irrigation water in crop contamination by viruses. Viruses in foods. Springer, Boston, MA, pp 257–263
Gupta E, Ballani N (2014) State of the globe: hepatitis A virus—return of a water devil. J Glob Infect Dis 6:57–58
Hamza IA, Jurzik L, Stang A, Sure K, Uberla K, Wilhelm M (2009) Detection of human viruses in rivers of a densly−populated area in Germany using a virus adsorption elution method optimized for PCR analyses. Water Res 43(10):2657–2668
Harris JP, Edmunds WJ, Pebody R, Brown DW, Lopman BA (2008) Deaths from norovirus among the elderly, England and Wales. Emerg Infect Dis 14:1546–1552
Hennechart-Collette C, Dehan O, Laurentie M, Fraisse A, Martin-Latil S, Perelle S (2021) Detection of norovirus, hepatitis A and hepatitis E viruses in multicomponent foodstuffs. Int J Food Microbiol 337:108931
Hidayah N, Tuan Zainazor C, Pui CF, Noorlis A, Noor Eliza MR, Naziehah MD, Ghazali FM, Cheah YK, Nakaguchi Y, Nishibuchi M, Son R (2011) Occurrence of Norovirus GI in green and red onion. Int Food Res J 18(2):677–681
Hutson AM, Atmar RL, Estes MK (2004) Norovirus disease: changing epidemiology and host susceptibility factors. Trends Microbiol 12:279–287
ISO 15216–1:2017 (2017) Microbiology of food and animal stuffs— horizontal method for detection of hepatis A virus and norovirus in food using real-time RT-PCR. International Organization for Standardization
Jacobsen KH (2009) The global prevalence of hepatitis A virus infection and susceptibility: a systematic review (No. WHO/IVB/10.01); WHO Press, Geneva, Switzerland
Jothikumar N, Cromeans TL, Sobsey MD, Robertson H (2005) Development and evaluation of a broadly reactive TaqMan assay for rapid detection of hepatitis A virus. Appl Environ Microbiol 71:3359–3363
Kageyama T, Kojima S, Shinohara M, Uchida K, Fukushi S, Hoshino FB, Takeda N, Katayama K (2003) Broadly reactive and highly sensitive assay for Norwalk-like viruses based on real-time quantitative reverse transcription-PCR. J Clin Microbiol 41:1548–1557
Kamel AH, AliMA E-N, De Rougemont A, Pothier P, Belliot G (2009) Predominance and circulation of enteric viruses in the region of Greater Cairo, Egypt. J Clin Microbiol 47(4):1037–1045
Kamel AH, Ali MA, El-Nady HG, Aho S, Pothier P, Belliot G (2010) Evidence of the co-circulation of enteric viruses in sewage and in the population of Greater Cairo. J Appl Microbiol 108(5):1620–1629
Kamel AH, Ali MA, El-Nady HG, Deraz A, Aho S, Pothier P, Belliot G (2011) Presence of enteric hepatitis viruses in the sewage and population of Greater Cairo. Clin Microbiol Infect 17(8):1182–1185
Katayama H, Shimasaki A, Ohgaki S (2002) Development of a virus concentration method and its application to detection of enterovirus and Norwalk virus from coastal seawater. Appl Environ Microbiol 68:1033–1039
Khan W, Irshad M, Rehman G, Hussain A (2014) HAV in fresh vegetables: a hidden health risk in district Mardan. Pakistan. Springerplus 3(1):1–5
Koo HL, Ajami N, Atmar RL, DuPont HL (2010) Noroviruses: the leading cause of gastroenteritis worldwide. Discov Med 10:61–70
Kotwal G, Cannon JL (2014) Environmental persistence and transfer of enteric viruses. Curr Opin Virol 4C:37–43
Lee M, Seo DJ, Seo J, Oh H, Jeon SB, Ha SD, Myoung J, Choi IS, Choi C (2015) Detection of viable murine norovirus using the plaque assay and propidium-monoazide-combined real-time reverse transcription-polymerase chain reaction. J Virol Methods 221:57–61
Leon-Felix J, Martinez-Bustillos RA, Baez-Sañudo M, Peraza-Garay F, Chaidez C (2010) Norovirus contamination of bell pepper from handling during harvesting and packing. Food Environ Virol 2:211–217
Lodder WJ, de RodaHusman AM (2005) Presence of noroviruses and other enteric viruses in sewage and surface waters in the Netherlands. Appl Environ Microbiol 71:1453–1461
Loutreul J, Cazeaux C, Levert D, Nicolas A, Vautier S, Le Sauvage AL, Perelle S, Morin T (2014) Prevalence of human noroviruses in frozen marketed shellfish, red fruits and fresh vegetables. Food Environ Virol 6(3):157–168
Mathijs E, Stals A, Baert L, Botteldoorn N, Denayer S, Mauroy A, Scipioni A, Daube G, Dierick K, Herman L, Van Coillie E, Uyttendaele M, Thiry E (2012) A review of known and hypothetical transmission routes for noroviruses. Food Environ Virol 4(4):131–152
Matthews JE, Dickey BW, Miller RD, Felzer JR, Dawson BP, Lee AS, Rocks JJ, Kiel J, Montes JS, Moe CL, Eisenberg JN, Leon JS (2012) The epidemiology of published norovirus outbreaks: a review of risk factors associated with attack rate and genogroup. Epidemiol Infect 140:1161–1172
Moreira NA, Bondelind M (2017) Safe drinking water and waterborne outbreaks. J Water Health 15(1):83–96
Nainan OV, Xia G, Vaughan G, Margolis HS (2006) Diagnosis of hepatitis a virus infection: a molecular approach. Clin Microbiol Rev 19:63–79
Osuolale O, Okoh A (2015) Incidence of human adenoviruses and hepatitis A virus in the final e_uent of selected wastewater treatment plants in Eastern Cape Province, South Africa. Virol J 12:1–8
Payne DC, Vinjé J, Szilagyi PG, Edwards KM, Staat MA, Weinberg GA, Hall CB, Chappell J, Bernstein DI, Curns AT, Wikswo M, Shirley SH, Hall AJ, Lopman B, Parashar UD (2013) Norovirus and medically attended gastroenteritis in U.S. children. N Engl J Med 368:1121–1130
Pires SM, Fischer-Walker CL, Lanata CF, Devleesschauwer B, Hall AJ, Kirk MD, Duarte ASR, Black RE, Angulo FJ (2015) Aetiology-specific estimates of the global and regional incidence and mortality of diarrhoeal diseases commonly transmitted through food. PLoS ONE 10:e0142927
Prez VE, Martinez LC, Victoria M, Giordano MO, Masachessi G, Re VE, Pavan JV, Colina R, Barril PA, Nates SV (2018) Tracking enteric viruses in green vegetables from central Argentina: potential association with viral contamination of irrigation waters. Sci Total Environ 637–638:665–671
Purpari G, Macaluso G, Di Bella S, Gucciardi F, Mira F, Di Marco P, Lastra A, Petersen E, La Rosa G, Guercio A (2019) Molecular characterization of human enteric viruses in food, water samples, and surface swabs in Sicily. Int J Infect Dis 1(80):66–72
Santamaria J, Toranzos GA (2003) Enteric pathogens and soil: a short review. Int Microbiol 6:5–9
Seymour IJ, Appleton H (2001) Foodborne viruses and fresh produce. J Appl Microbiol 91:759–773
Shaheen MN, Elmahdy EM (2019a) Environmental monitoring of astrovirus and norovirus in the Rosetta branch of the River Nile and the El-Rahawy drain, Egypt. Water Supply 19(5):1381–1387
Shaheen MN, Elmahdy EM (2019b) Molecular detection of group C rotavirus in environmental samples in Giza, Egypt. Asian J Water Environ Pollut 16(4):17–22
Shaheen MN, Abd El-Daim SE, Ahmed NI, Elmahdy EM (2018) Molecular detection of three gastroenteritis viruses in urban sewage treatment plant and river water in Egypt. J Aquat Biol Fish 22(5):615–627
Shaheen MN, Elmahdy EM, Chawla-Sarkar M (2019) Quantitative PCR-based identification of enteric viruses contaminating fresh produce and surface water used for irrigation in Egypt. Environ Sci Pollut Res 26(21):21619–21628
Shaheen MN, Abd El-Daim SE, Ahmed NI, Elmahdy EM (2020) Environmental monitoring of Aichi virus and human bocavirus in samples from wastewater treatment plant, drain, and River Nile in Egypt. J Water Health 18(1):30–37
Siebenga JJ, Vennema H, Zheng DP, Vinjé J, Lee BE, Pang XL, Ho EC, Lim W, Choudekar A, Broor S, Halperin T, Rasool NB, Hewitt J, Greening GE, Jin M, Duan ZJ, Lucero Y, O’Ryan M, Hoehne M, Schreier E, Ratcliff RM, White PA, Iritani N, Reuter G, Koopmans M (2009) Norovirus illness is a global problem: emergence and spread of norovirus GII.4 Variants, 2001–2007. J Infect Dis 200:802–812
Tian G, Jin M, Li H, Li Q, Wang J, Duan ZJ (2014) Clinical characteristics and genetic diversity of noroviruses in adults with acute gastroenteritis in Beijing, China in 2008–2009. J Med Virol 86:1235–1242
Vaughan G, Goncalves Rossi LM, Forbi JC, de Paula VS, Purdy MA, Xia G, Khudyakov YE (2014) Hepatitis A virus: host interactions, molecular epidemiology and evolution. Infect Genet Evol 21C:227–243
Wheeler C, Vogt TM, Armstrong GL, Vaughan G, Weltman A, Nainan OV, Dato V, Xia G, Waller K, Amon J, Lee TM (2005) An outbreak of hepatitis A associated with green onions. N Engl J Med 353(9):890–897
World Health Organization (2005) Acute water diarrhea outbreaks. Wkly Morb Mortal Rep 1(6)
World Health Organization, WHO/UNICEF Joint Water Supply, Sanitation Monitoring Programme (2015) Progress on sanitation and drinking water: 2015 update and MDG assessment. World Health Organization
Yezlis, Otter JA (2011) Minimum infective dose of the major human respiratory and enteric viruses transmitted through food and the environment. Food Environ Virol 3:1–30
Yong HT, Son R (2009) Review article hepatitis A virus—a general overview. Int Food Res J 467:455–467
Zaher KS, Ahmed WM, Syame SM, El-Hewairy HM (2008) Detection of health hazard-food born viruses in animal products anticipated for human consumption. Global Veterinaria 2(4):192–197
Zaki ME, Abdel-Rahaman EA, AshryAY El, Al-Kasaby NM (2019) Molecular study of norovirus in pediatric patients with gastroenteritis. Open Microbiol J 13(1):324–329
Zaki ME, El Kheir NA (2017) Molecular study of astrovirus, adenovirus and norovirus in community acquired diarrhea in children: One Egyptian center study. Asian Pac J Trop Biomed 7(11):987–990
Author information
Authors and Affiliations
Contributions
MNFS was the contributor in the plane design, performing RNA extraction and real time PCR, and data analysis as he was a major contributor in writing the manuscript. EME was the contributor in the plane design, performing RNA extraction and real time PCR, and data analysis. LHIM was the contributor in the sample collection, virus concentration, and in performing RNA extraction and real time PCR. IAH was the contributor in the plane design and data analysis as she revised the manuscript. ERSS was the contributor in the plane design, performing real time test, and data analysis as she revised the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Not applicable.
Additional information
Responsible Editor: Mohamed M. Abdel-Daim
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shaheen, M.N.F., Elmahdy, E.M., Mahmoud, L.H.I. et al. Quantitative RT-PCR detection of human noroviruses and hepatitis A virus in fresh produce and surface water used for irrigation in the Mansoura and Giza regions, Egypt. Environ Sci Pollut Res 29, 28139–28148 (2022). https://doi.org/10.1007/s11356-021-18412-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-18412-3