Abstract
Exposure to lead among children, as well as adults, is a major global health issue. With diverse routes of exposure (e.g., food, air, and water) either environmentally or occupationally, lead among children can cause mild, moderate, to severe health complications in the later stages of life. The average blood lead level reported by CDC in 2021 is 3.5 µg/dL, and the level of blood lead toxicity is ≥ 10 μg/dL. In this study, we planned to systematically analyze the association between blood lead levels (BLLs) (≥ 10 μg/dL and < 10 μg/dL) and the risk of anemia (hemoglobin level < 11 g/dL) among Indian children aged between ≥ 1 and ≤ 18 years. An online literature search of 5 databases, PubMed, Ovid, EMBASE, Web of Science, and Google Scholar was accomplished with a search updated until 8th March 2021. Study designs included cohort, cross-sectional, and case–control studies that have evaluated the association of lead toxicity or exposure with anemia (Hb < 11 g/dL) reported in urban and/or rural Indian children. Meta-analysis was performed among a total of 864 children from 4 cross-sectional studies. The association between lead toxicity (BLLs ≥ 10 μg/dL) and the risk of being anemic was not statistically significant (RR = 1.15 (95% CI: 0.86–1.55, I2 = 77%). The risks of bias in all included studies were low according to the Newcastle Ottawa Scale. Increased blood lead levels did not appear to be the major contributor to anemia in Indian Children. We need to focus primarily on improving the nutritional quality, fortified food supplements, and a balanced diet for children to reduce the anemia burden in India. Lead toxicity should be sought as an etiological factor only in areas of high environmental risk factors which were leaded paints, leaded batteries, a house near major road/traffic areas, and pesticide exposure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lead is considered to be heavy metal and constitutes one of the major environmental pollutants and quantitative occurrence of lead has been reported in water, soil, air, and other organisms (Kiani et al. 2021) which is a major toxic component of the food chain. Varied average blood lead level (BLL) among infants, children, patients, ordinary people, pregnant women, industrial workers, and disease-specific patients has been reported in population (Kiani et al. 2021; Mahmoudi et al. 2018). The use of lead in the past has resulted in its widespread distribution in the present environment (Tong et al. 2000). Environmental exposure to lead among children at an early stage has many serious health consequences causing neurobehavioral, cognitive, physical impairments (Nussbaumer-Streit et al. 2020), organ damage, seizures, and anemia (Tong et al. 2000; Mason et al. 2014). In a study, lead toxicity has been linked with the socioeconomic status of the family, with impoverished children being more prone to exposure (Attina Teresa M. and Trasande Leonardo 2013; Bellinger 2008; GBD Risk Factor Collaborators 2018). In Europe, evironmental lead causes 3% of the total DALYs (disability-adjusted life years) in children (Rojas Rueda 2019). Water pipes, soldering, consumer products, soil, a house near a major road, leaded paints, and gasoline were reported as major exposure sources of lead (Tong et al. 2000; Miranda 2011).
In a recently published study, an association between iron deficiency anemia (SMD = 2.40; 95% CI 0.93–3.87; p = 0.0014) and higher blood lead levels (> 10 µg/dL) has been reported among children (Farkhondeh et al. 2021). Similarly, another systematic review reported prevalence rates of iron deficiency (27.7%) and iron deficiency anemia (18.2%) among children (Akbari et al. 2017; Nazari et al. 2019). An association between higher blood and erythrocyte lead levels, and iron deficiency among children has been reported (Saghazadeh and Rezaei 2017).
Blood lead levels in Indian children
In India, lead toxicity accounts for ~ 0.6% of the global burden of disease (National Health Portal 2017). The Ministry of Environment, Forest and Climate Change, Government of India passed a notification in November 2016 entitled “Regulation on Lead contents in Household and Decorative Paints Rules, 2016)” prohibiting/limiting the content below 90 ppm (parts per million) (National Health Portal 2017). According to the UNICEF report, over 275 million children in India are still suffering mild to severe side effects associated with lead poisoning (UNICEF and Pure Earth 2020). Although lead has been phased out in India since 2001 (Roy et al. 2009a, b), still lead toxicity has been linked with the nutritional status among Indian children (Srinivasa Reddy et al. 2011). Other Indian studies have also reported a higher prevalence of lead among children at all stages of the ages (Srinivasa Reddy et al. 2011; Kalra et al. 2003; Arlappa et al. 2008; Dhingra et al. 2009; Jain et al. 2005). Exposure either environmentally or occupationally has always been a serious issue globally, which is a result of industrialization (Tong et al. 2000). Countries, e.g., China, Argentina, India, Tunisia, Malaysia, Ethiopia, and Ghana, are still selling lead-based paints in markets (Kessler 2014). Children are more prone to get the exposure as compared to the adults, being active in playing and accessibility (soil and dust); less awareness makes children a carrier of lead (Bellinger 2008). In a study, dust from houses has been reported as a major contributor to blood lead toxicity among children (Nussbaumer-Streit et al. 2020).
The Centers for Disease Control and Prevention (CDC) in 1991 defined a cutoff (BLL ≥ 10 μg/dL) as lead toxicity among children (CDC Prevention Guidelines Database 2016); however, even lower levels of BLLs among children have been reported with the cognitive function supporting the no safe limit of BLLs for children (Canfield et al. 2003; Lanphear 2007; Grandjean et al. 2010). Once lead enters the body, it causes irreversible neurological manifestations (Tong et al. 2000) which can be improved by introducing chelation agents; current evidence shows the reduction in the mortality of severe acute lead encephalopathy among children with BLL > 45 μg/dL from the bone (O’Flaherty 1995). The mean half-life of blood lead among children (between 8 and 38 months) is higher as compared to adults (15 days) and bone was found to be the major source of lead deposition (Manton et al. 2000). Prevention of lead contamination in the environment as well as exposure to the children to reduce the body burden and further clinical manifestations was emphasized (Chisolm 2001).
This study aimed to assess the association of blood lead levels with the risk of anemia among Indian children. Despite multiple health outcomes until now, no safe limit of blood lead levels among children and adults has been established. Finding the association between blood lead levels and the risk of anemia in Indian children will help policymakers to formulate mitigation strategies to reduce lead exposure.
Methods
Protocol registration
This review followed the recommendations established in the MOOSE statement and has been registered in the PROSPERO database (registration number CRD42021245080).
Data sources and search strategy
PRISMA guidelines were followed to report the review (Liberati et al. 2009). Boolean operators ((((“environmental lead”) OR ((lead exposure) OR (Pb exposure) OR (lead Pb) OR (“Lead”[Mesh]) OR (lead toxicity) OR (lead toxic) OR (lead toxicology) OR (blood lead level))) AND ((deficiency anaemia) OR (anaemia) OR (“Anemia”[Mesh]) OR (Anemias))) AND ((child) OR (children) OR (preschool children) OR (infant) OR (adolescent) OR (pediatric))) AND (India) were used for the PubMed, Ovid, EMBASE, Google Scholar, and Web of Science. Additionally, we have also searched Indian database (Indianjournals.com). The study included published literature until 8th March 2021.
Inclusion and exclusion criteria
Average blood lead toxicity level of ≥ 10 µg/dL was considered (CDC) and compared with the risk of anemia. The present study included cross-sectional studies for quantitative analysis (meta-analysis); other study designs cohort and case–control were also included in the review part which reported the blood lead levels and its association with risk of anemia among Indian children. All studies published in the English language were included in the current systematic review and meta-analysis.
Data collection and study selection
In the initial search, 949 articles were obtained for the title and abstract screening; after screening the articles as per the inclusion criteria, finally 8 articles for qualitative (systematic review) and 4 articles for quantitative (meta-analysis) were selected. Details of authors, city, year, study type, setting, exposure, sample size, age range (years), blood lead level cutoffs, and anemia cutoffs among Indian children were extracted and recorded. Data screening (VSM, PP), data extraction (VSM, KS), and risk of bias assessment (VSM, MaS, HS) were accomplished by two authors independently. Any discrepancy was resolved by joint conversation (MaS, AC, AA).
Study quality evaluation
The Newcastle Ottawa scale for cross-sectional (5), cohort (2), and case–control (1) studies were used for the quality evaluation in the current study (Malik et al. 2020).
Data synthesis and statistical analysis
After extracting the results, the studies were pooled to evaluate the association of lead toxicity (BLLs ≥ 10 µg/dL) with the occurrence of anemia (Hb < 11 g/dL). The meta-analysis was done in RevMan V5.4 software using the random effect model as there was significant heterogeneity. The heterogeneity between studies was assessed using the I2-statistic based on the cutoffs mentioned in the Cochrane handbook. Heterogeneity above 50% was considered substantial whereas above 75% was considered considerable heterogeneity. Outcomes were presented with 95% CI as suggested by Higgins and colleagues (Malik et al. 2020; Krüger et al. 2016; Lasselin et al. 2016).
Results
Study characteristics
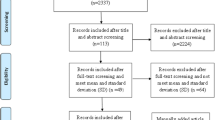
A total of 949 published research papers were retrieved by the search strategy, of which 780 remained after deleting the duplicate studies. After screening the abstracts and titles, 683 records were excluded and 97 articles were included for full-text assessment. Among them, 85 were excluded with reasons (adult data, animal models, outcome or intervention not reported, book chapter, editorial, studies conducted out of India, review articles, and randomized controlled trials) reported in the PRISMA chart (shown in Fig. 1). For the final qualitative assessment, we have included a total of 8 studies having a total sample size of 2138 children. The main characteristics of the studies included were summarized (Table 1). All observational studies were from Chennai, Hyderabad, Lucknow, Nagpur, New Delhi, and Vellore. Four studies were pooled to calculate the odds of developing anemia in children with lead toxicity. Four studies were not included in the meta-analysis because studies have not collected, reported, and analyzed the data as per inclusion and exclusion criteria required to analyze for the current systematic review and meta-analysis (Table 1).
Quality evaluation
The Newcastle Ottawa Scale was applied on cross-sectional, cohort, and case–control studies separately (Malik et al. 2020) as shown (Table 1). All studies were judged to be of good quality (Supplementary Table 2).
Characteristics of the included studies
The current systematic review reported data from 8 studies with 2138 participants. Out of them, 1045 were anemic and 1093 were non-anemic children. Among anemic, higher blood lead level (≥ 10 μg/dL) was observed in 730 and < 10 μg/dL in 315 Indian children. Similarly, among non-anemic out of 1093 children, higher BLLs (≥ 10 μg/dL) were observed in 760 and < 10 μg/dL in 333 children. For quantitative synthesis (meta-analysis), only cross-sectional studies were pooled that have reported the prevalence of anemia (Hb < 11 g/dL) with reported BLLs cutoffs (≥ 10 μg/dL and < 10 μg/dL).
Meta-analysis
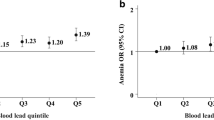
A random-effect model was used while pooling 4 studies (Fig. 2). The RR of developing anemia was 1.15 (95% CI: 0.86–1.55) in children with lead toxicity compared to those without. This difference was not statistically significant.
Publication bias.
The funnel plot (standard error) of anemic children showed no obvious publication bias (Fig. 3).
Discussion
Current systematic review summarizes the association between lead toxicity and the risk of being anemic in Indian children. To the best of our knowledge, this is the first Indian study summarizing the data on the association of lead exposure and anemia in children. Previous studies have reported a similar association with BLLs with risk of being anemic. In an Indian RCT, a reduction in 33% of median BLLs among iron-fortified food receiving group was reported, supporting iron-fortified as a community intervention especially for those living near industrial sites and lead-exposed areas (Zimmermann et al. 2006). High blood lead levels among children residing in Mumbai (Dharavi slum areas) were found to be associated with heavy vehicular traffic and industrial activities around the area (Lal et al. 1991). In China and Bangladesh, children residing in the urban areas have a high prevalence of lead toxicity, where prevalence of BLLs (≥ 10 μg/dL) is > 60%. In the USA, the prevalence of BLLs between 10 and 20 μg/dL among children aged between 4 and 12 years is 20% (Kaiser et al. 2001; Mathee et al. 2002). Even after phasing out of leaded gasoline, industrial exposure in children of low socioeconomic status continues to be a major source of lead toxicity (Roy et al. 2009a, b). In an urban study, there was a significant prevalence of high blood lead levels (≥ 10 μg/dL) in school children which was found even after the prohibition of leaded gasoline (Kalra et al. 2013). National Family Health Survey (NFHS) report showed a significant increase in the number of children having elevated blood lead levels with nutritional deficiencies among those living near the industries and tidal creeks in Mumbai city (Niranjan 2006). A study by Bergdahl et al. showed a negative correlation of BLLs with Hb among children (Bergdahl et al. 1999). Elevated blood lead levels (≥ 10 μg/dL) were significantly associated with the risk of anemia and also influenced by the status of essential trace elements (Ahamed et al. 2007). In a study lead, exposer was found to be linked with the lower survival time of erythrocytes which increases the fragility lacking the potassium in the cell (Jacob et al. 2000). Copper deficiency has been observed among lead-exposed children with anemia which might be linked with the inhibition of iron metabolism (Klauder and Petering 1975).
In Nagpur, BLL among children with encephalopathy was seen, where higher BLLs have been significantly (p < 0.05) associated with Surma use, wasting, anemia, and paint removal from the house (A. Patel and Athawale 2009). In a population-based survey among pre-children residing in Nagpur city, various exposure sources (e.g., paint, pencils, crayons, and clay) revealed higher lead levels (≥ 10 μg/dL) among 67.7% of the children (A. B. Patel et al. 2001).
In the community-based study, trends of iron deficiency and lead toxicity in early childhood can affect child development and school entry cognition (Mohan et al. 2014). Similarly, in Vellore, elevated blood lead levels are commonly seen in children residing in the slums and were associated with poor cognition and anemia among pre-school children (Koshy et al. 2020).
Heterogeneity
In the current study, we observed 77% of heterogeneity which might be due to the wide age difference (> 1 to ≤ 18 years), residential variation (cities), and the difference in the socio-economic status of the family.
Strength of the study
In this study, a meticulous review process, thoroughly searching of articles was accomplished. We included children from India only having age range (> 1 to ≤ 18 years) residing in the urban locality and environmentally exposed areas.
Limitations
Age, gender, exposure duration, dietary exposure source, body composition, and nutrients could be the major limitation of the current study. We have not analyzed the other heavy metals, exposure duration, sources, and nutritional status of the children, which may have a bearing on the lead levels, as well as the prevalence of anemia.
Conclusion
In previous studies, lead toxicity has been linked with mostly cognitive and neurobehavioral impairments. Systematic monitoring of hemoglobin, iron, and lead should be done among children, especially those living in the urban areas as well as in the vicinity of heavy traffic and industrial sites. Increased blood lead levels did not appear to be the major contributor to anemia in Indian children. We need to focus primarily on improving the nutritional quality, fortified food supplements, and a balanced diet for children to reduce the anemia burden in Indian. Lead toxicity should be sought as an etiological factor only in areas of high environmental risk factors which were leaded paints, leaded batteries, a house near major road/traffic areas, and pesticide exposure. In India, as nutritional anemia is very common, the effect of lead levels on anemia prevalence is less clear. We recommend further studies are necessary to determine the role of blood lead levels in anemia burden among children in developing countries.
Data availability
The data that support the findings of this study are available in the supplementary material of this article and raw data is available with the authors.
Abbreviations
- Pb:
-
Lead
- BLL:
-
Blood lead level
- CI:
-
Confidence interval
- μg/dL:
-
Microgram/deciliter
- Hb:
-
Hemoglobin
- I 2 :
-
Heterogeneity
References
Ahamed M, Akhtar MJ, Verma S, Kumar A, Siddiqui MKJ (2011) Environmental lead exposure as a risk for childhood aplastic anemia. Biosci Trends 5(1):38–43. https://doi.org/10.5582/bst.2011.v5.1.38
Ahamed M, Singh S, Behari JR, Kumar A, Siddiqui MKJ (2007) Interaction of lead with some essential trace metals in the blood of anemic children from Lucknow, India Clinica Chimica Acta. International Journal of Clinical Chemistry 377(1–2):92–97. https://doi.org/10.1016/j.cca.2006.08.032
Akbari M, Moosazadeh M, Tabrizi R, Khatibi SR, Khodadost M, Heydari ST, Tahami AN, Lankarani KB (2017) Estimation of iron deficiency anemia in Iranian children and adolescents: a systematic review and meta-analysis. Hematology 22:231–239
Arlappa N, Laxmaiah A, Balakrishna N, Harikumar R, Brahmam GNV (2008) Clinical and sub-clinical vitamin a deficiency among rural pre-school children of Maharashtra, India. Ann Hum Biol 35(6):606–614. https://doi.org/10.1080/03014460802380778
Attina TM, Leonardo T (2013) Economic costs of childhood lead exposure in low- and middle-income countries. Environ Health Perspect 121(9):1097–1102. https://doi.org/10.1289/ehp.1206424
Bellinger David C (2008) Very low lead exposures and children’s neurodevelopment. Current Opinion in Pediatrics 20(2):172–77. https://doi.org/10.1097/MOP.0b013e3282f4f97b
Bergdahl IA, Vahter M, Counter SA, Schütz A, Buchanan LH, Ortega F, Laurell G, Skerfving S (1999) Lead in plasma and whole blood from lead-exposed children. Environ Res 80(1):25–33. https://doi.org/10.1006/enrs.1998.3880
Canfield RL, Henderson CR, Cory-Slechta DA, Cox C, Jusko TA, Lanphear BP (2003) Intellectual impairment in children with blood lead concentrations below 10 microg per deciliter. N Engl J Med 348(16):1517–1526. https://doi.org/10.1056/NEJMoa022848
CDC Prevention Guidelines Database. 2016. “Preventing lead poisoning in young children.” https://wonder.cdc.gov/wonder/prevguid/p0000029/p0000029.asp. Accessed Oct 2021
Chisolm JJ (2001) The road to primary prevention of lead toxicity in children. Pediatrics 107(3):581–83. https://doi.org/10.1542/peds.107.3.581
Dhingra U, Hiremath G, Menon VP, Dhingra P, Sarkar A, Sazawal S (2009) Zinc deficiency: descriptive epidemiology and morbidity among preschool children in peri-urban population in Delhi, India. J Health Popul Nutr 27(5):632–639. https://doi.org/10.3329/jhpn.v27i5.3639
Farkhondeh T, Mansouri B, Binkowski LJ, Błaszczyk M, Pirsaheb M, Azadi NA, Słoboda M, Amirabadizadeh A, Javadmoosavi SY (2021) Blood lead concentrations in children with iron deficiency anemia: a systematic review and meta-analysis. Environ Sci Pollut Res Int. https://doi.org/10.1007/s11356-021-17301-z
GBD Risk Factor Collaborators (2018) “Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017-The Lancet.” November 10, 2018. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(18)32225-6/fulltext. Accessed Oct 2021
Grandjean P, Satoh H, Murata K, Eto K (2010) Adverse effects of methylmercury: environmental health research implications. Environ Health Perspect 118(8):1137–1145. https://doi.org/10.1289/ehp.0901757
Jacob B, Ritz B, Heinrich J, Hoelscher B, Wichmann HE (2000) The effect of low-level blood lead on hematologic parameters in children. Environ Res 82(2):150–159. https://doi.org/10.1006/enrs.1999.4011
Jain NB, Laden F, Guller U, Shankar A, Kazani S, Garshick E (2005) Relation between blood lead levels and childhood anemia in India. Am J Epidemiol 161(10):968–973. https://doi.org/10.1093/aje/kwi126
Kaiser R, Henderson AK, Daley WR, Naughton M, Khan MH, Rahman M, Kieszak S, Rubin CH (2001) Blood lead levels of primary school children in Dhaka, Bangladesh. Environ Health Perspect 109(6):563–566. https://doi.org/10.1289/ehp.01109563
Kalra V, Chitralekha KT, Tarun Dua RM, Pandey, and Yogesh Gupta. (2003) Blood lead levels and risk factors for lead toxicity in children from schools and an urban slum in Delhi. J Trop Pediatr 49(2):121–123. https://doi.org/10.1093/tropej/49.2.121
Kalra V, Sahu JK, Bedi P, Pandey RM (2013) Blood lead levels among school children after phasing-out of leaded petrol in Delhi, India. Indian J Pediatr 80(8):636–640. https://doi.org/10.1007/s12098-013-0999-6
Kessler R (2014) Lead-based decorative paints: where are they still sold-and why? Environ Health Perspect 122(4):A96-103. https://doi.org/10.1289/ehp.122-A96
Kiani B, Hashemi Amin F, Bagheri N, Bergquist R, Mohammadi AA, Yousefi M, Faraji H, Roshandel G, Beirami S, Rahimzadeh H, Hoseini B (2021) Association between heavy metals and colon cancer: an ecological study based on geographical information systems in North-Eastern Iran. BMC Cancer 21:414
Klauder DS, Petering HG (1975) Protective value of dietary copper and iron against some toxic effects of lead in rats. Environ Health Perspect 12(December):77–80. https://doi.org/10.1289/ehp.751277
Koshy B, Srinivasan M, Zachariah SM, Karthikeyan AS, Roshan R, Bose A, Mohan VR et al (2020) Body iron and lead status in early childhood and its effects on development and cognition: a longitudinal study from urban Vellore. Public Health Nutr 23(11):1896–1906. https://doi.org/10.1017/S1368980019004622
Krüger K, Mooren F-C, Pilat C (2016) The immunomodulatory effects of physical activity. Curr Pharm Des 22(24):3730–3748. https://doi.org/10.2174/1381612822666160322145107
Lal M, Joseph D, Choudhury RK, Bajpai HN, Gauba I, Lokeshwar MR, Wagle CS (1991) Studies of blood lead levels in children by proton-induced X-ray emission (PIXE). The Science of the Total Environment 103(2–3):209–214. https://doi.org/10.1016/0048-9697(91)90146-6
Lanphear BP (2007) The conquest of lead poisoning: a pyrrhic victory. Environ Health Perspect 115(10):A484–A485. https://doi.org/10.1289/ehp.10871
Lasselin J, Alvarez-Salas E, Grigoleit J-S (2016) Well-being and immune response: a multi-system perspective. Curr Opin Pharmacol 29(August):34–41. https://doi.org/10.1016/j.coph.2016.05.003
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 62(10):e1–e34. https://doi.org/10.1016/j.jclinepi.2009.06.006
Mahmoudi N, Latifi AM, Amani MA, Masoumbeigi H, Ghanizadeh G (2018) Data on the environmental exposure to lead in Iran. Data Brief 20:1133–1141
Malik VS, Ravindra K, Attri SV, Bhadada SK, Singh M (2020) Higher body mass index is an important risk factor in COVID-19 patients: a systematic review and meta-analysis. Environ Sci Pollut Res Int 27(33):42115–42123. https://doi.org/10.1007/s11356-020-10132-4
Manton WI, Angle CR, Stanek KL, Reese YR, Kuehnemann TJ (2000) Acquisition and retention of lead by young children. Environ Res 82(1):60–80. https://doi.org/10.1006/enrs.1999.4003
Mason LH, Harp JP, Han DY (2014) Pb neurotoxicity: neuropsychological effects of lead toxicity. Biomed Res Int 2014:840547. https://doi.org/10.1155/2014/840547
Mathee A, von Schirnding YER, Levin J, Ismail A, Huntley R, Cantrell A (2002) A survey of blood lead levels among young Johannesburg school children. Environ Res 90(3):181–184. https://doi.org/10.1016/s0013-9351(02)00010-5
Miranda, Marie Lynn. 2011. “A geospatial analysis of the effects of aviation gasoline on childhood blood lead levels | Environmental Health Perspectives | Vol. 119, No. 10.” October 1, 2011. https://doi.org/10.1289/ehp.1003231.
Mohan VR, Sharma S, Ramanujam K, Babji S, Koshy B, Bondu JD, John SM, Kang G (2014) Effects of elevated blood lead levels in preschool children in urban Vellore. Indian Pediatr 51(8):621–625. https://doi.org/10.1007/s13312-014-0464-2
National Health Portal. 2017. “Lead poisoning.” https://www.nhp.gov.in/disease/non-communicable-disease/lead-poisoning. Accessed Nov 2021
Nazari M, Mohammadnejad E, Dalvand S, Ghanei Gheshlagh R (2019) Prevalence of iron deficiency anemia in Iranian children under 6 years of age: a systematic review and meta-analysis. J Blood Med 10:111–117
Niranjan, S. 2006. “Spatial variations in elevated blood lead levels among young children in Mumbai, India - Niranjan - 2006 - Population, Space and Place - Wiley Online Library.” 2006. https://doi.org/10.1002/psp.411.
Nussbaumer-Streit, Barbara, Verena Mayr, Andreea Iulia Dobrescu, Gernot Wagner, Andrea Chapman, Lisa M. Pfadenhauer, Szimonetta Lohner, Stefan K. Lhachimi, Laura K. Busert, and Gerald Gartlehner. 2020. “Household interventions for secondary prevention of domestic lead exposure in children.” Cochrane Database of Systematic Reviews, no. 10. https://doi.org/10.1002/14651858.CD006047.pub6.
O’Flaherty EJ (1995) Physiologically based models for bone-seeking elements. V. Lead absorption and disposition in childhood. Toxicology and Applied Pharmacology 131(2):297–308. https://doi.org/10.1006/taap.1995.1072
Patel AB, Williams SV, Frumkin H, Kondawar VK, Glick H, Ganju AK (2001) Blood lead in children and its determinants in Nagpur, India. Int J Occup Environ Health 7(2):119–126. https://doi.org/10.1179/107735201800339498
Patel A, Athawale A (2009) Blood lead levels in children with encephalopathy. Indian Pediatr 46(10):845–848
Raviraja A, Babu GNV, Bijoor AR, Menezes G, Venkatesh T (2008) Lead toxicity in a family as a result of occupational exposure. Arh Hig Rada Toksikol 59(2):127–133. https://doi.org/10.2478/10004-1254-59-2008-1861
Rojas-Rueda D, Nieuwenhuijsen MJ, Gascon M, Perez-Leon D, Mudu P (2019) Green spaces and mortality: a systematic review and meta-analysis of cohort studies. Lancet Planet Health 3(11):e469-e477. https://doi.org/10.1016/S2542-5196(19)30215-3. Erratum in: Lancet Planet Health. 2021 Aug;5(8):e504. PMID: 31777338; PMCID: PMC6873641
Roy A, Howard Hu, Bellinger DC, Palaniapan K, Wright RO, Schwartz J, Balakrishnan K (2009a) Predictors of blood lead in children in Chennai, India (2005–2006). Int J Occup Environ Health 15(4):351–359. https://doi.org/10.1179/oeh.2009.15.4.351
Roy A, Howard Hu, Bellinger DC, Palaniapan K, Wright RO, Schwartz J, Balakrishnan K (2009b) Predictors of blood lead in children in Chennai, India (2005–2006). Int J Occup Environ Health 15:351–359
Saghazadeh A, Rezaei N (2017) Systematic review and meta-analysis links autism and toxic metals and highlights the impact of country development status: Higher blood and erythrocyte levels for mercury and lead, and higher hair antimony, cadmium, lead, and mercury. Prog Neuropsychopharmacol Biol Psychiatry 79:340–368
Singhi S, Ravishanker R, Singhi P, Nath R (2003) Low plasma zinc and iron in pica. Indian J Pediatr 70(2):139–143. https://doi.org/10.1007/BF02723740
Srinivasa Reddy Y, Raghu Pullakhandam KV, Radha Krishna P, Kumar U, Dinesh Kumar B (2011) Lead and essential trace element levels in school children: a cross-sectional study. Ann Hum Biol 38(3):372–377. https://doi.org/10.3109/03014460.2010.536166
Tong S, von Schirnding YE, Prapamontol T (2000) Environmental lead exposure: a public health problem of global dimensions. Bull World Health Organ 78(9):1068–1077
UNICEF and Pure Earth. 2020. “The toxic truth: children’s exposure to lead pollution undermines a generation of future potential.” https://www.unicef.org/sites/default/files/2020-07/The-toxic-truth-children%E2%80%99s-exposure-to-lead-pollution-2020.pdf. Accessed Oct 2021
Zimmermann MB, Muthayya S, Moretti D, Kurpad A, Hurrell RF (2006) Iron fortification reduces blood lead levels in children in Bangalore, India. Pediatrics 117(6):2014–2021. https://doi.org/10.1542/peds.2005-2440
Acknowledgements
We would like to thank Indian Council of Medical Research (ICMR), New Delhi, and ICMR, Advanced Centre for Evidence-Based Child Health (ACEBCH), Department of Pediatrics, PGIMER Chandigarh.
Funding
This study was supported by the Indian Council of Medical Research, New Delhi, India.
Author information
Authors and Affiliations
Contributions
Data curation: Vivek Singh Malik (VSM), Pranita Pradhan (PP), Kusum Singal (KS).
Formal analysis: Vivek Singh Malik (VSM), Manvi Singh (MS), Anil Chauhan (AC).
Investigations: Vivek Singh Malik (VSM), Manvi Singh (MS), Anil Chauhan (AC).
Methodology: Manvi Singh (MaS), Anil Chauhan (AC), Amit Agarwal (AA), Harnoor Sra (HS).
Project administration: Meenu Singh (MeS*).
Supervision: Meenu Singh (MeS*).
Visualization: Meenu Singh (MeS*), Vivek Singh Malik (VSM).
Writing–original draft: Vivek Singh Malik (VSM), Manvi Singh (MaS).
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Lotfi Aleya
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Malik, V.S., Singh, M., Pradhan, P. et al. Role of environmental lead in the occurrence of anemia in Indian children: a systematic review and meta-analysis. Environ Sci Pollut Res 29, 37556–37564 (2022). https://doi.org/10.1007/s11356-021-18199-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-18199-3