Abstract
To know about the reasons leading to variations in dust control efficiency of the surfactant solution spray on coal dust (from the same coal source) with different diameters, the changes of coal dust surface features (specific surface area, pore volume, gas adsorption, and surface potential) with crush degrees and their effects on the wettability were investigated. The experimental results indicated that the surface characteristics of coal dust showed remarkably positive correlations with the crush degree. For example, dust size was reduced from 114.96 to 18.71 μm, the pore volume and gas adsorption of coal dust surface was enhanced by 75%, 104.5%, respectively. It made gas film around dust particles more easily been generated, hindering the contact between dust particles and droplets. The adsorption rate of the surfactant molecules on the coal dust surface was significantly reduced with the dust size decreased, increasing the difficulty of capturing coal dust by surfactant solution. Additionally, based on the linear fitting analysis between surface features and the dust control efficiency, it was indicated that the increased gas adsorption and pore structures on the dust surface was the key factors weakening the dust removal efficiency of the surfactant solution from the perspective of the physical features of coal dust. This study was conducive to optimizing the surfactant-aided dust control technology to better capture coal dust with small size.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coal dust used as a product of coal mining process has seriously threatened production safety of the coal industry, especially in terms of pneumoconiosis (Liu et al. 2020; Yin et al. 2019). With the increase of coal mining strength, the crush degree of coal body was significantly enhanced, leading to the rapid increase of coal dust (< 40 μm) production (Zhou et al. 2019, 2020). Statistically, the number of coal workers diagnosed with pneumoconiosis increased by tens of thousands in China every year (Chang et al. 2021; Yin et al. 2021). Recently, dust control technologies using coal seam water injection and solution spray were widely used to reduce coal dust concentration, some scholars conducted many investigations on these technologies. Wang proposed an innovative method for comprehensively characterizing coal structure and seepage dynamic process by combining CT 3D reconstruction and NUCLEAR magnetic resonance experiment, effectively revealing the influence of the distribution characteristics of pores and fissures in coal body on coal seam water injection (Wang et al. 2020). However, dust control effect of coal seam water injection was poor due to the high hydrophobicity of coal dust and low permeability of coal. Therefore, dust removal technologies using solution spray were the main methods to suppress dust particles in the underground coal mines. Nevertheless, their dust control efficiencies were significantly reduced with the increased proportion of respirable coal dust (Wang et al. 2019a). To better develop the effective dust control techniques using solution spray, it was necessary to analyze the reasons why coal dust removal efficiency of the solution spray was changed with the crush degree increased.
Recently, around the wettability of coal dust, some scholars mainly investigated the effects of coal metamorphic grade on the dust hydrophobicity (Li et al. 2013; Wang et al. 2017; Zhang et al. 2020). It was found that coal dust particles were more hardly wetted by aqueous solution with the increase of coal metamorphic grade. Xu investigated the relationships between the proximate analysis parameters and coal dust wettability, found that the moisture content of coal dust showed the positive correlation with its wettability; the fixed carbon content, ash content, and volatile matter content showed the unconspicuous effect relationship with coal wettability (Xu et al. 2017). However, segmental scholars found that the moisture content of coal dust did not have the significant effects on coal dust wettability, and the volatile matter of coal sample was the main factor affecting its hydrophilicity (Huang et al. 2017; W.M et al. 2017). Therefore, through the comparisons among some investigations, it was indicated that the proximate analysis parameters could not be used as the important parameters to evaluate the wettability of coal dust. In addition, lots of investigations were conducted to study the functional group structures of different coal bodies and their effects on the wettability of coal dust. Cheng and Xu found that coal dust become more hydrophobic with the increase of the hydrophobic functional groups such as aliphatic hydrocarbons and aromatic hydrocarbons (represented by the aromatic ring C=C and substituted benzenes) (Cheng et al. 2011). In order to capture hydrophobic coal dust efficiently, the surfactant was widely applied to improve the dust control ability of water spray (Wang et al. 2019b; Xu et al. 2020). However, the application optimization of the surfactant-aided coal dust suppression technologies was mainly based on the surface tension and contact angle parameters of the solution. It ignored the adsorption ability between surfactant molecules and coal dust, which might lead to the unreasonable application of the surfactant (Dou and Xu 2017; Tessum et al. 2014). Meanwhile, many investigations about coal dust characteristics were conducted around different kinds of coal (Cheng et al. 2014; Xu et al. 2018). Little research had been studied on the surface feature changes of coal dust (from the same coal sample) with crush degrees and their effects on dust control ability of the surfactant solution spray.
Coal dust was more easily flowed along the wind with the particle size decreased in underground coal mines, resulting in the contact time between coal dust and droplets becoming shorter. Zhou and Li found that the fractal dimension of coal dust significantly increased with the reduction of particle size, making the wettability of coal dust become poorer (Li et al. 2013; Zhou et al. 2018a). Meanwhile, some researchers investigated the surface structures of coal dust particles through the scanning electron microscope and specific surface and pore size analyzer, found that coal dust surface was crude meanwhile having many pore structures (Ni et al. 2021; Sang et al. 2020). Additionally, the wetting characteristics of ultra-fine coal dust were investigated, and it was found that the ultra-fine coal dust exhibited stronger hydrophobicity (Dong et al. 2004; Xia et al. 2018; Zhou et al. 2017). It was because that new surface of coal dust was constantly exposed in the crushing and refining process, which enhanced its specific surface area and surface-free energy. However, although some studies had been conducted to investigate the surface structures of coal dust, the surface feature changes of coal dust (from the same coal sample) with different diameters and its effects on coal dust wettability were not systematically analyzed. Additionally, the surfactant-aided coal dust control technologies were widely applied to control dust particles, but the key factors affecting the dust control ability of the surfactant solution spray were not deeply studied from the perspective of coal dust surface features. It was not conducive to direct the effective application of the surfactant-aided dust control techniques.
To know about the key factors weakening the dust suppression ability of the surfactant-aided dust control technology, the changes of physical properties of coal dust with crush degrees and their effects on dust control ability of the surfactant solution were investigated. Firstly, the pore structures of coal dust particles (from the same coal sample) with different diameters were qualitatively analyzed by SEM. Then, the pore diameter, gas adsorption capacity, specific surface area, and surface potential on the coal dust surface were quantitatively analyzed. Meanwhile, the adsorption relationships between the surfactant molecules and dust particles with different sizes were studied. Additionally, the linear correlation between surface features and the dust control efficiency was analyzed. Through the above investigations, the key factors affecting the dust removal efficiency of the surfactant solution were revealed, which was of great significance for developing the effective surfactant-aided dust control technology.
Experimental
Experimental materials
Coal sample was acquired from the Luwa coal mine, Jining City, China, which is 1/3 coke mine. Coal body was crushed and ground into dust particles with different diameters by ball grinding mill (PBM-1A, Changsha Zhongjing Chemical Machinery Co. Ltd., China). The dust size distribution of coal dust particles was measured by the laser particle analyzer (Bettersize 2600, Dandong Baxter Instrument Co. Ltd., Liaoning Province, China). The nonionic surfactant was acquired from the Lin Yi Green Chemical Co. Ltd., China, of which active matter content was more than 99%.
Experimental methods and devices
-
(1)
As shown in the previous research (Zhou et al. 2018a, b), it was verified that there were many pore structures existing on the coal dust surface. To more deeply investigate the effects of crush degree on coal dust surface structures, the surface features of coal dust with D50=33.75 μm and D50=18.71 μm were quantitatively observed by scanning electron microscope (SEM) (Quanta 250, FEI, Hillsboro, Oregon, USA). Due to the non-conductive material of coal dust, the dust particles should be translated into the conductive ones using the gold sputtering method before carrying out the SEM experiments.
-
(2)
The crude degree and gas adsorption ability of coal dust surface had the important effects on the dust control efficiency of the solution spray. To more systematically analyze the changes of coal dust surface features with crush degrees, this paper used the specific surface and pore size analyzer (JW-BK122W, Beijing JWGB Sci & Tech Co., Ltd., China) to quantitatively study the changes of specific surface area, pore volume, and gas adsorption of coal dust with different diameters (114.96 μm, 33.75 μm, 18.71 μm, 7.89 μm). The operation methods of this device had been shown in the previous research (Zhou et al. 2018a). Every experimental condition was measured three times (achieving a repeatability error < 4%).
-
(3)
The zeta potential will be generated on the coal dust surface during the crush process of coal, which hindered the condensation between dust particles. Thus, the zeta potential of coal dust surface was quantitatively measured by electrophoresis apparatus (JS94H, Shanghai Zhongchen Digital Technology Equipment Co. Ltd., China). Firstly, 1 mg experimental sample was put into the deionized water. Then, the mixed solution was dispersed by ultrasonic disperser for 5 min. Finally, the 0.5 ml measured solution was put into the experimental device. Through the electrode discharge of the device, the location of dust particles would be changed, of which displacement would be converted into the zeta potential. To guarantee the accuracy of experimental results, every experimental condition was measured three times (achieving a repeatability error < 4%).
-
(4)
In order to better improve the dust control ability of the surfactant solution, this paper deeply investigated the adsorption features between the surfactant and coal dust particles with different diameters (114.96 μm, 33.75 μm, 18.71 μm, 7.89 μm), further more scientifically directing the application of the surfactant-aided coal dust suppression technologies. The adsorption quantity of surfactants on the coal dust surface was measured by ultraviolet spectrophotometer (UV-1800PC, Shanghai Mepi instrument Co., Ltd., China). Firstly, the standard curve between the absorbance and surfactant concentration was acquired. Secondly, 0.5 g coal powder was put into the surfactant solution with the mass fraction of W and then the new mass fraction w of surfactant solution was measured by experimental device and standard curve. Finally, the surfactant adsorption capacity was calculated based on the surfactant mass fraction changes before and after adding coal dust. Every experimental condition was measured three times (achieving a repeatability error < 4%).
$$Q=\frac{\left(W-w\right)\times {M}_{1}}{{m}_{1}}$$(1)Here, Q was the adsorption quantity of surfactants on the coal dust surface, mg/g. W, w was the surfactant mass fraction changes before and after adding coal dust, %. M1 was the mass of the surfactant solution, mg. m1 was the mass of the coal powder, g.
-
(5)
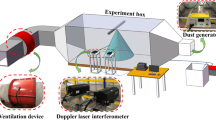
To analyze the relationship between the dust control efficiency of the surfactant solution spray and physical properties of coal dust with different crush degrees, the experimental system used to simulate a spray for dust suppression was constructed in the laboratory (Fig. 1), including a dust feeder, a turbulator, a simulated roadway, an air velocity transducer, a porous steady plate, a dust sampler, and an extractor fan. The simulated roadway was an enclosed chamber (9.5 × 0.5 × 0.5 m), constructed of the acrylic plate and steel. The wind velocity was 1.5 m/s in the simulated roadway while its value could be also changed. The dust concentration in the simulated roadway was 110-140 mg/m3. The dust sampler was used to collect dust particles before and after using the dust control methods. The dust concentration and dust control efficiency (η) were calculated using Eq. (2) and Eq. (3), respectively. Every experimental condition was measured three times (achieving a repeatability error < 4%).
$$C=\frac{M- m}{V}$$(2)$$\eta =\frac{C-c}{C}\times 100\%$$(3)Here, C was dust concentration, mg/m3; M, m was the dust mass on the sampling filter membrane before and after using dust control technologies, mg; V was the swept volume of dust sampler, m3.
Results and discussion
Surface morphology contrast of coal dust with different diameters
As shown in Fig. 2, it was found that coal dust surface was very crude containing many pore structures. The crude degree of coal dust with the diameter of 18.71 μm was significantly larger than that of coal dust with the diameter of 33.75 μm. It might because more pore structures were exposed on the coal dust surface in the crushing process. Meanwhile, the increased crude degree made spray droplets more difficultly spread on the coal dust surface, leading to the contact time between dust particles and droplets becoming short. It hindered the adsorption of surfactant molecules on the surface of coal dust while weakening the dust control efficiency of the surfactant solution spray. To quantitatively analyze the changes of coal dust surface features with crush degrees and their effects on the collision coagulation between the surfactant droplets and dust, these experimental phenomena implied by the SEM investigations should be further studied.
Effects of crush degree on the surface features of coal dust
From Fig. 3, it was seen that the specific surface area was remarkably affected by crush degree. When coal dust size was reduced from 114.96 to 7.89 μm, the specific surface area of coal dust was augmented by 263.758%, from 0.745 to 2.71 m2/g. This indicated that coal dust particles with small size had larger contact area with air under the same dust mass condition. It might lead to the gas film around dust particles being more easily generated, which affected the contact between the surfactant droplets and coal dust.
As shown in Fig. 3, the pore volume and gas adsorption of coal dust were also significantly enhanced with the dust diameter decreased. For example, dust size was reduced from 114.96 to 18.71 μm, the pore volume and gas adsorption of coal dust surface was increased by 75%, 104.5%, respectively. Because more pore structures on the surface of coal dust were exposed with the crush degree increased (Yang et al. 2014). The changes of the above parameters further verified that dust particles with small diameter (especially respirable dust) would more easily adsorb gas, leading to the gas film around dust surface being generated, which enhanced the difficulties of coal dust suppression using a spray field (Yang et al. 2010).
In addition, the surface potential of coal dust particles could weaken the aggregation among them. As shown in the previous research, the surface charge of dust particles was generated during the crushing process of coal, of which value was generally negative (Jin et al. 2020). As shown in Fig. 4, the surface potential of dust particles with the diameter of 114.96 μm was −45.82 mv, which was consistent with the relative research. In addition, it was also seen that the electronegativity of coal dust surface was significantly increased with the decrease of particle sizes. For example, when coal dust size decreased from 33.75 to 7.89 μm, the surface potential of coal dust was reduced by 8.9%, from −54.21 to −59.02 mv. The increased electronegativity led to the repulsive force among coal dust particles being enhanced, making the aggregation ability among them becomes weakened, which was not conducive to dust settlement (Tian et al. 2019).
Based on the above investigations, it was found that the surface feature changes could significantly enhance the crude degree and gas adsorption of coal dust surface, reducing the spreading ability of droplets on coal dust surface. Additionally, some surfactants added into the aqueous solution could remarkably improve the wettability of the spray field, but the adsorption action between the surfactant molecules and coal dust might be changed with the changes of coal dust surface features, such as the surface potential, pore volume. Thus, it was necessary to investigate on the relationships between the surfactant adsorption and coal dust surface features.
The changes of surfactant adsorption with crush degree and surface features
The surfactant can greatly reduce the surface tension of the aqueous solution by forming the dense isolation layer between the air and solution surface based on its hydrophilic and lipophilic groups. This made the spreading ability of droplets on the dust surface significantly enhanced. In addition, the lipophilic groups of the surfactant are the non-polar ones, making them have the strong affinity with the hydrophobic groups such as aromatic hydrocarbon and aliphatic hydrocarbon in the coal dust. When the lipophilic groups reached out into the air, they would adsorb coal dust particles into the aqueous solution. Therefore, the adsorption ability between coal dust particles and surfactant molecules played an important role in the dust control process of the surfactant solution spray.
As shown in Fig. 5, for the same surfactant, the equilibrium adsorption capacity was significantly increased with the dust size decreased. This mainly because the specific surface area and pore volume of coal dust was significantly increased with the dust diameter decreased, providing more adsorption space and sites for surfactant molecules. For example, the equilibrium adsorption capacity was improved by 12.89% when the dust diameter was reduced from 18.71 to 7.89 μm. This indicated that smaller dust particles need more contact time to adsorb the surfactant molecules in the dust control process. From Fig. 5, it can be seen that the sink time of coal dust particles with small size was remarkably enhanced than that of dust with large diameter, which further verified that the coagulation between the surfactant solution and coal dust particles needed more time with the crush degree increased. For example, when coal dust diameter was reduced from 18.71 to 7.89 μm, the sink time of coal dust particles was significantly enhanced by 79.09%. Meanwhile, this also indicated that coal dust particles with small diameter were more difficultly captured by surfactant solution droplets.
As shown in the “Effects of crush degree on the surface features of coal dust” section, the surface features of coal dust particles were significantly affected by the changes of coal dust diameters, which might affect the adsorption ability of surfactants on the coal dust surface. Therefore, the correlated relationships between the equilibrium adsorption capacity and different surface features were fitted by origin 9.1, of which fitting results were shown in Fig. 6.
From Fig. 6, it was seen that the surface features (specific surface area, pore volume, gas adsorption, and surface potential) of coal dust particles showed the strong relationships (R2 > 0.9) with the equilibrium adsorption capacity of surfactants. This indicated that the changes of coal dust surface features could significantly affect the surfactant adsorption.
To better show the changes of the surfactant adsorption on the coal dust surface with the contact time, and to more scientifically direct the surfactant-aided dust control technologies for effectively capturing coal dust, the dynamic adsorption capacity between surfactants and coal dust particles with different diameters was analyzed based on the following equations.
The quasi-first-order kinetic equation (4) was used to fit the dynamic adsorption curve of the surfactant on the coal dust surface (Ahmadi and Shadizadeh 2013; Liu et al. 2021).
Where, \(\theta\) represents the adsorption capacity at the time t, \({\theta }_{e}\) represents the equilibrium adsorption capacity, and k is the rate constant. Equation (4) could be transformed into Eq. (5).
As shown in Fig. 7, the adsorbing capacity of coal dust particles increased rapidly and then leveled off with the increase of the adsorption time. For example, the adsorbing capacity of dust particles with diameter of 114.96 μm was enhanced from 4.49 to 8.13 mg/g when the adsorption time increased from 1 min to 15 min; thereafter, its adsorption capacity showed the small changes in the range of 8.13-8.47 mg/g. This because a large number of polar oxygen-containing functional groups were exposed on the surface of coal particles at the initial stage of adsorption, providing many adsorbed sites for surfactant molecules, which made the adsorbing capacity rapidly increase. With the increased number of surfactant molecules on the coal dust surface, the adsorbed sites decreased and then the surfactant adsorption of coal dust would become saturated.
In addition, from Fig. 7, it can also see that the surfactant adsorption ability of coal dust with different diameters was various. With the dust diameter decreased, the adsorbing quantity was significantly augmented, indicating that coal dust particles with small size need more time to be settled by surfactant solution. For example, the adsorbing quantity of coal dust particles with the diameter (D50) of 7.89 μm was enhanced by 24.58% than that of coal dust particles with the diameter (D50) of 18.71 μm, of which sink time in the surfactant solution was 165.21 s, 92.25 s, respectively. It was verified that coal dust with small size was more hardly settled by surfactant solution. This might because the specific surface area of coal dust was significantly increased with the crush degree increased, making the same quality of coal dust expose more adsorbed sites for surfactant molecules.
However, as shown in Fig. 3, the specific surface area and pore volume of coal dust were significantly increased with the dust diameter decreased, leading to coal dust particles with small size having larger contact area and room to adsorb gas. It made the gas film around dust particle more easily generated, hindering the contact between the surfactant and coal dust surface, which led to the reduction of the surfactant adsorption rate. Through compared the fitting equations (Fig. 7), it was found that “k” values were reduced with the decrease of dust diameters, verifying that the adsorption rate of the surfactant on the coal dust surface decreased with dust size decreased. As shown in Fig. 7, the time required to reach equilibrium adsorption capacity of coal dust with the diameter (D50) of 7.89 μm was significantly longer than that of coal dust with the diameter (D50) of 114.96 μm. Therefore, coal dust with small diameters (especially respirable dust) was more hardly captured using the surfactant-aided dust control technologies.
Effects of surface feature changes on dust control efficiency
To further investigate the relationship between the physical properties of coal dust and dust control efficiency of the surfactant solution, and to reveal the key factors affecting the dust removal efficiency of the surfactant solution, the dust suppression experiments using the solution spray were conducted by self-constructed system (Fig. 1). However, the self-weight of coal dust with different sizes is different, which might affect the dust suppression effect of the surfactant solution. Therefore, it was necessary to analyze effects of self-weight on the dust control efficiency before conducting the dust control experiments of the surfactant solution.
When coal dust drifts in the air, its main forces include the gravity, buoyancy, and air resistance. The relative calculation formulas were showed as follow (Wang 2009):
Here, Fs, Fa, and Fc were the gravity, buoyancy force, and frictional drag, respectively. ρs is the dust density, 1350 kg/m3. ρa is the air density, 1.293 kg/m3. d is dust diameter, m. g is the gravitational acceleration, m/s2. μa is the dynamic viscosity coefficient of air, N▪s/m2, μa = 1.8 × 10−5. ut is the settling velocity, m/s.
As coal dust settles faster, its speed increases until the resistance equals to the settling force. At this time, dust particles reach their maximum settling speed, and then settle at a constant velocity.
According to Eq. 11, the ut was 53.91 cm/s, 4.64 cm/s, 1.43 cm/s, 0.25 cm/s, respectively when the d was 114.96 μm, 33.75 μm, 18.71 μm, and 7.89 μm, respectively.
Because the distance between the spray point and dust tested point was 5 m, while the dust measuring point was located in the center of the roadway, the dust tested point was 25 cm away from all sides of the roadway. To prevent the measured dust concentration from being affected by dust sedimentation, the settling distance should not exceed 25 cm when dust particles level run 5 m. According to Eq. 12, the sedimentation distance of coal dust with different diameter was shown in Table 1 when dust particles level run 5 m.
Here, t was the mobile time, s. L was the distance between the spray point and dust tested point, L = 5 m. v was the horizontal migration velocity of coal dust, v = 1.5 m/s. H was the settling distance, m.
As shown in Table 1, the settling distance of dust with different diameters was very different in the same deposition time. With the decrease of dust size, the dust settling distance was significantly reduced. It indicated that increased dust weight by improving the condensation capacity between droplets and dust particles played an important role in enhancing dust control efficiency. As shown in Table 1, dust particles with 114.96 μm were settled before reaching the dust measured point, which could not be used to investigate on effects of surface features on dust control efficiency. But in contrast, the settling distance of dust with 33.75 μm, 18.71 μm, and 7.89 μm was small, indicating that their self-weight did not affect the test results of dust concentration measured points.
As shown in Fig. 8, the dust control efficiency of the surfactant solution spray was significantly reduced with the dust diameter decreased. Because the self-weight of coal dust did not play the important role in the dust control process, the surface feature changes might affect dust control efficiency when dust particle size decreased. As shown in the “Effects of crush degree on the surface features of coal dust” section, the gas adsorption was augmented by 49.22%, from 4.47 to 6.67 cm3/g, when the dust diameter decreased from 33.75 to 7.89 μm. It led to gas film around particle more easily generated, which hindered the contact between droplets and dust particles. To further analyze effects of surface features on dust suppression efficiency of the surfactant solution spray, the influence relationships between them were fitted, shown in Fig. 9.
From Fig. 9, it was seen that the correlations between the dust control efficiency and gas adsorption was the best (R2=0.92), which further verified that the increased gas adsorption remarkably weakened dust control efficiency of the surfactant solution. Meanwhile, the pore volume, surface potential, specific surface area, and equilibrium adsorption ability showed good correlations with dust control efficiency, of which R2 was 0.91, 0.90, 0.90, and 0.83, respectively. These indicated that the changes of surface features could affect the dust suppression efficiency of the surfactant solution. Combined with the analysis about Fig. 6, it was verified that the increased gas adsorption and pore structures on the coal dust surface was the main factors (from the perspective of the physical features of coal dust) that led to the low dust removal efficiency of the surfactant solution.
Based on the above experimental results and analysis, to improve the dust control efficiency, it was necessary that the contact time and chances between coal dust particles and the surfactant droplets should be improved through some measurements, such as enhancing the number density of droplets, improving the coverage of a spray field for the dust source.
Conclusions
-
(1)
The surface features (specific surface area, pore volume, and gas adsorption) of coal dust were remarkably enhanced with the crush degree increased. As coal dust decreased, the pore volume and gas adsorption of coal dust surface significantly increased, which might lead to the poorer wettability due to the easier formation of a gas film around the dust particle. For example, dust size was reduced from 114.96 to 18.71 μm, the pore volume and gas adsorption of coal dust surface was augmented by 75%, 104.5%, respectively.
-
(2)
From the perspective of the physical features of coal dust, the increased gas adsorption and pore structures on the dust surface were the main factors that led to the low dust removal efficiency of the surfactant solution. With the increase of the gas adsorption and pore structures, more gas might enter into the pore structures, hindering the contact between the surfactant molecules and coal dust surface. It not only weakened the adsorption rate of surfactants on the dust surface but also significantly reduced the dust control efficiency of the surfactant solution.
Availability of data and materials
Not applicable.
References
Ahmadi MA, Shadizadeh SR (2013) Experimental investigation of adsorption of a new nonionic surfactant on carbonate minerals. Fuel 104:462–467
Chang P, Xu G, Chen YP, Ghosh A, Moridi M (2021) Improving coal powder wettability using electrolyte assisted surfactant solution. Colloids Surf A Physicochem Eng Asp 613:126042
Cheng WM, Xu CC, Zhou F (2011) Evolution law of carbon and oxygen groups on coal surface with increasing metamorphic grade and its effect on wettability. J Fuel Chem Technol 44:295–304
Cheng WM, Xue J, Zhou G, Nie W (2014) Study of coal dust wettability based on FTIR. J China Coal Soc 39:2256–2262
Dong P, Shan ZJ, Li Z (2004) Study on the surface wet characteristic of ultrafine coal powder. J China Coal Soc 29:346–349
Dou GL, Xu CH (2017) Comparison of effects of sodium carboxymethylcellulose and superabsorbent polymer on coal dust wettability by surfactants. J Dispers Sci Technol 38:1542–1546
Huang WM, Tang EX, Qian ZX (2017) Study on relationship between coal dust composition and water wettability. Coal Technol 36:159–161
Jin H, Zhang YS, Chen K, Niu K, Wu GG, Wei XR, Wang HW (2020) Preparation and characterization of a composite dust suppressant for coal mines. Polymers 12:2942
Li Q, Lin B, Zhao S, Dai H (2013) Surface physical properties and its effects on the wetting behaviors of respirable coal mine dust. Powder Technol 233:137–145
Liu RL, Zhou G, Wang CM, Jiang WJ, Wei X (2020): Preparation and performance characteristics of an environmentally-friendly agglomerant to improve the dry dust removal effect for filter material. J Hazard Mater 397
Liu ZL, Zhao G, Lv QC, Sudhölter ER (2021) Comprehensive review on surfactant adsorption on mineral surfaces in chemical enhanced oil recovery. Adv Colloid Interface Sci 294:102467
Ni GH, Wang H, Nie BS, Wang Y, Dou HR, Lu SQ, Wang G (2021) Research of wetting selectivity and wetting effect of imidazole ionic liquids on coal. Fuel 286
Sang FY, Yan S, Wang G, Ma ZJ, Li JZ, Ju S (2020) The effect of microemulsion on coal wetting characteristics and physicochemical structure. Colloid Interface Sci Commun 39
Tessum MW, Raynor PC, Keating-Klika L (2014) Factors influencing the airborne capture of respirable charged particles by surfactants in water sprays. J Occup Environ Hyg 11:571–582
Tian JL, Ruan DY, Zhou JH (2019) Study on influencing factors of phosphate ore flotation on factors affecting flotation by zeta potential method. Guangzhou Chem Ind 47:57–59
W.M H, Tang EX, Qian ZX (2017): Study on relationship between coal dust composition and water wettability. Coal Technol 36:159–161
Wang D (2009) Mine ventilation and safety. China University of Mining and Technology Press, Xuzhou, China
Wang G, Han DY, Qin XJ, Liu Z, Liu JF (2020) A comprehensive method for studying pore structure and seepage characteristics of coal mass based on 3D CT reconstruction and NMR. Fuel 281:118735
Wang H, Zhang L, Wang D, He X (2017) Experimental investigation on the wettability of respirable coal dust based on infrared spectroscopy and contact angle analysis. Adv Powder Technol 28:3130–3139
Wang X, Yuan S, Jiang B (2019a) Wetting process and adsorption mechanism of surfactant solutions on coal dust surface. J Chem 2019
Wang XN, Yuan SJ, Li X, Jiang BY (2019) Synergistic effect of surfactant compounding on improving dust suppression in a coal mine in Erdos, China. Powder Technol 344:561–569
Xia WC, Li YJ, Nguyen AV (2018) Improving coal flotation using the mixture of candle soot and hydrocarbon oil as a novel flotation collector. J Clean Prod 195:1183–1189
Xu C, Wang D, Wang H, Xin H (2017) Effects of chemical properties of coal dust on its wettability. Powder Technol 318:33–39
Xu C, Wang H, Wang D, Zhu X, Zhu Y, Bai X, Yang Q (2020) Improvement of foaming ability of surfactant solutions by water-soluble polymers: experiment and molecular dynamics simulation. Polymers 12
Xu G, Chen YP, Eksteen J, Xu JL (2018) Surfactant-aided coal dust suppression: a review of evaluation methods and influencing factors. Sci Total Environ 639:1060–1076
Yang JW, Xiukun, Gao Jianguang, Li, Gaiping (2010) Surface characteristics and wetting mechanism of respirable coal dust. Min Sci Technol 20:0365–0371
Yang JX, Hui, Gao Jianguang, Liu Dandan, Wang Yingfeng (2014) Influence of particle size on surface characteristic and wetting mechanism of coal dust. Saf Coal Mines 45:140-143
Yin W, Zhou G, Gao D (2019) Simulation analysis and engineering application of distribution characteristics about multi-stage atomization field for cutting dust in fully mechanized mining face. Adv Powder Technol 30:2600–2615
Yin W, Zhou G, Liu D, Meng Q, Zhang Q, Jiang T (2021) Numerical simulation and application of entrainment dust collector for fully mechanized mining support based on orthogonal test method. Powder Technol 380:553–566
Zhang QT, Zhou G, Hu YY, Wang WJ (2020) Experimental investigation on wetting mechanism for coal dust with different metamorphic degree based on infrared spectrum and C-13-NMR. Surf Interface Anal 52:470–485
Zhou F, Yan CJ, Wang HQ, Zhou S, Liang H (2017) The result of surfactants on froth flotation of unburned carbon from coal fly ash. Fuel 190:182–188
Zhou Q, Qin B, Wang J, Wang H, Wang F (2018) Experimental investigation on the changes of the wettability and surface characteristics of coal dust with different fractal dimensions. Colloids Surf A Physicochem Eng Asp 551:148–157
Zhou Q, Qin B, Wang J, Wang H, Wang F (2018) Effects of preparation parameters on the wetting features of surfactant-magnetized water for dust control in Luwa mine, China. Powder Technol 326:7–15
Zhou Q, Qin BT, Wang F, Wang HT, Hou J, Wang ZR (2019) Effects of droplet formation patterns on the atomization characteristics of a dust removal spray in a coal cutter. Powder Technol 344:570–580
Zhou Q, Xu G, Chen YP, Qin BT, Zhao ZD, Guo CW (2020) The development of an optimized evaluation system for improving coal dust suppression efficiency using aqueous solution sprays. Colloids Surf A Physicochem Eng Asp 602
Funding
This work was supported by the National Natural Science Foundation of China (52004275, 51774273), China Postdoctoral Science Foundation (2021T140706, 2021M693406), and Postdoctoral Research Program of Jiangsu Province (2020Z048).
Author information
Authors and Affiliations
Contributions
All the authors contributed to the study conception and design. Qun Zhou: conceptualization, writing-original draft, writing-review, and editing. Botao Qin: supervision, project administration. Huizhen Li: investigation, formal analysis. Jin Hou: data curation.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhou, Q., Qin, B., Li, H. et al. Changes of physical properties of coal dust with crush degrees and their effects on dust control ability of the surfactant solution spray. Environ Sci Pollut Res 29, 33785–33795 (2022). https://doi.org/10.1007/s11356-021-17832-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-17832-5