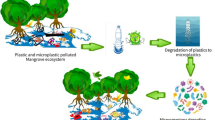
Abstract
Mangroves are one of the most productive ecosystems in the world harboring huge biological diversity. The prime ecological roles of mangroves are prevention of coastal erosion and shoreline protection. Mangroves face varying degrees of threats due to overexploitation, conversion of mangrove habitats for agriculture, settlement and industrial purposes, illegal encroachment, global warming, sea-level rise, El Nino, and pollution. Among them, microplastic (MP) pollution is a major concern threatening not only the mangroves per se but also the rich biodiversity that it shelters. In general, the microbial communities which are paramount to nutrient recycling and ecological dynamics undergo substantial changes upon MP exposure. If the MP pollution in the mangrove habitats continues unabated in the coming decades, there may be serious consequences on the already threatened mangrove ecosystems and the coastal communities. This review article attempts to consolidate MP pollution of mangrove wetlands, its impact on mangroves and associated microbiota, and the microbial solution for its remediation as a sustainable strategy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mangrove forests comprise plants from diverse families of angiosperms with unique morphological, anatomical, and physiological features (Duke 1995; Friess 2016). There are nearly 73 species of “true mangroves” which harbor intertidal regions of the tropics and subtropics of around 118 countries (Spalding et al. 2010). Mangroves are one of the most productive ecosystems and protect the shorelines from cyclones, tsunamis, storms, floods, and sea waves by virtue of their extensive root system (Pillai and Harilal 2018; Lovelock et al., 2015; Garcés-Ordóñez et al. 2019). Mangrove forests are also known as blue carbon ecosystems for their ability to store huge amounts of organic carbon (Bouillon et al., 2003; FAO 2007). Despite their huge economic and ecological roles, mangrove areas are cleared on a large scale for aquaculture and other non-mangrove activities and around 50% of the mangrove area has been lost since the 1950s causing species extinction (Feller et al. 2010). Mangrove species accounting for nearly 17% have been categorized as critically endangered, endangered, or vulnerable whereas 7% are near-threatened (Spalding et al. 2010; IUCN 2020; Kumar et al. 2021). The whole mangrove biome may disappear in the next century if the rate of biomass reduction continues unabated (Duke et al. 2007). In addition to land clearance, pollution-induced climatic changes, global warming, and the resultant rise in sea level are the key stressors reducing mangrove flourishing zones (Parida and Jha, 2010). Among them, pollution caused by plastics has been recognized as a major concern to the mangroves (Owuor et al. 2019). The plastic debris gets trapped by the sweeping root system of mangroves and directly suffocates the respiring roots (van Bijsterveldt et al. 2021; Luo et al. 2021).
Mangrove areas have already become huge sinks of plastic waste entering the ecosystem either from marine or terrestrial sources (Deng et al. 2021). Every year, open oceans are dumped with an estimated 4.8 to 12.7 million tons of plastics due to improper waste management strategies (Jambeck et al. 2015). The larger plastics in turn are fragmented by chemical reactions, UV (ultra-violet) radiations, wave action, and biodegradation to form small plastic pieces, termed microplastics (MPs; Thompson et al. 2004). They are of size < 5 mm, including nanoplastics (< 0.1 μm), and also produced directly as byproducts of manufacturing industries (Neff et al. 2018). The MPs are then carried over to coastal regions by tides, water currents, and wind (Galgani et al. 2015). They have been increasingly found to be a cause of concern for the coastal vegetation as well as marine flora and fauna. MPs accumulate in plant parts and hinder nutrient uptake (Khalid et al. 2020). MPs absorb and carry organic pollutants and heavy metals which enhances its toxicity (Jeong and Choi 2020). The ingestion or absorption of the MPs enriched with organic pollutants is potentially more hazardous to animals and plants than of MPs alone (Huang et al. 2021).
The remediation of this degradation-resistant pollutant was challenging until it was found that MPs are susceptible to microbial deterioration (Shah et al. 2008; Brooks et al. 2011). Biodegradation of MPs by microorganisms is driven by the inherent capability of microbes to get adapted to newer environments. The microbial remediation of MPs is highly encouraged as their deployment causes the least harm to the environment (Shah et al. 2008; Brooks et al. 2011). From early 2020, a few authors have reviewed microbial deterioration of MPs (Yuan et al. 2020; Othman et al. 2021) and the distribution and fate of MPs in mangrove habitats (Deng et al. 2020). Recently, it has been reported that mangrove areas accumulate more MPs compared to the unvegetated areas of wetlands (Huang et al. 2021). Increased accumulation of MPs in the mangrove habitats suggests vulnerabilities of the mangroves, microbiota, and other organisms inhabiting mangrove ecosystems to MPs. However, much information on the effect of MPs on mangrove trees and the rhizosphere microbiota is completely lacking. We do not know much as to how MPs behave in the mangrove habitats and how it affects animals, other plants, and microorganisms harboring these ecosystems. Thus, the current review is an attempt to consolidate and connect the information available on the MP pollution in the mangrove wetlands with the following: (i) its effects on mangroves itself and the microbial symbionts; (ii) the possible microbial solutions including in situ biodegradation using native mangrove-associated microorganisms; and (iii) the futuristic microbial solutions to manage and remediate MPs including use of biotechnological approaches such as genetic engineering and genome editing.
MP pollution in the mangrove habitats
MP en route to the mangrove ecosystem
Mangrove wetlands are polluted on a large scale due to anthropogenic activities. There are multiple and complex sources of mangrove MP pollution as presented in Fig. 1. MP reaches mangrove habitats either as primary or secondary MPs based on the source point (GESAMP 2015). Primary MPs are plastic particles synthesized to have < 5 mm size and accidentally released to the environment from various manufacturing activities (GESAMP 2015; Koelmans et al. 2014). They are the constituent particles of personal care products such as bath gel, facial cleansers, toothpaste, textiles, and cosmetics (Suardy et al. 2020). Primary MPs are also formed during pre-production of resin pellets, in the air-blasting industry (Andrady 2011). On the other hand, MPs generated from chemical (freeze–thaw cycle and UV radiations), physical (water disturbance, wave strike, and abrasion), and biological degradation of larger plastics are termed secondary MPs (Dong et al. 2021; Khalid et al. 2021).
Both the primary and secondary MPs are carried to the mangal (mangrove) region from the improperly managed terrestrial and marine waste streams (Deng et al. 2021). The terrestrial sources of plastic litter include agricultural runoff, sewage treatment systems, fishing activities, and industries located nearer to the streams feeding the mangrove habitats. The plastic waste dumped from coastal tourist spots can also contribute to MP pollution (Zhou et al. 2020). Other reasons for MP pollution include vast industrialization of coastal areas resulting in greater input of MP polluted water to mangrove habitats via drainage system as exemplified by the case study on Jinjiang estuary, China (Zhou et al. 2020). Greenhouse industries near coastal areas are also responsible for plastic waste accumulation in mangrove areas (de Stephanis et al. 2013). In Mediterranean basin countries, greenhouse cultivation uses plastic mulches, especially made of black plastics which subsequently accumulate as MPs and are eventually carried to nearby wetlands (de Stephanis et al. 2013). Marine-based sources of MP complement those from terrestrial sources where shipping and marine aquaculture are the major contributors of MP pollution in the mangrove ecosystem (Garcés-Ordóñez et al. 2019). Besides the external sources, in situ degradation of plastic debris retained by the mangrove root system by means of either chemical, physical, or biological forces discussed earlier can also contribute to wetland MPs (Mohamed Nor and Obbard 2014).
Evidence of MPs in mangrove wetlands around the world
As mentioned in the earlier section, plastic debris from a variety of sources are ultimately entrapped by the roots and branches of mangrove trees (Fig. 2). To assess the various polymer types and shapes of these trapped MPs within the mangrove sediments, several efforts have been made since 2014. Among them, the earliest reports on mangrove MP distribution came from Singaporean (Mohamed Nor and Obbard 2014), Indonesian (Hastuti et al. 2014), and Malaysian (Barasarathi et al. 2014) mangrove wetlands. Compared to other areas around the world, mangrove ecosystems spanning Chinese coastal regions are widely studied for the characteristics of MP pollution. Of which the very first study was conducted along seven mangrove habitats of Qinzhou Bay (Li et al. 2018). Afterwards, a total of 55 mangrove habitats of China including Futian, Maowei Sea, Pearl River Estuarine, Beibu Gulf, and Jinjiang Estuarine region were detected to be MP-contaminated (Li et al. 2019, 2020a, 2020b; Zhou et al. 2020; Zuo et al. 2020; Deng et al. 2020; Zhang et al. 2020; Duan et al. 2021). Though the dominant type of MPs varies from region to region, polypropylene (PP) contributes more towards the overall MP pollution of Chinese wetlands and fiber being the most abundant shape.
Only a few mangrove zones of other countries have been investigated for MP pollution leaving behind most of the estuarine regions of 118 mangrove possessing countries remaining untouched. Though Singaporean coastlines are one among the earliest geographical areas of the MP pollution research, no further notable works have been reported from Singapore. Similarly one report each is available from Panama (Feldberg, 2018), Colombia (Garcés-Ordóñez et al. 2019), and South Africa (Govender et al. 2020). The second and latest MP reports from the other two earliest geographical locations of MP pollution research; Malaysia (Hamid et al. 2020) and Indonesia (Cordova et al. 2021) came very recently after a long gap. A total of 11 mangrove habitats within the Persian Gulf of Iran were explored for MP distribution in 5 different studies (Naji et al. 2017a, 2017b, 2019; Nabizadeh et al. 2019; Maghsodian et al. 2020). The detailed MP distribution of individual mangrove areas of different countries specifically with the abundant type and shape are tabulated (Table 1). Taken altogether, the dominant polymer type and shape varies from place to place depending on the respective terrestrial as well as marine sources of MPs.
Impact of MP pollution on mangroves and associated microbiota
Mangrove devastation by (micro)plastics
Even though the MP invasion of the mangrove regions is evident, the extent of deterioration caused by this pollutant is still unclear. Correlative effects of plastic pollution on respiration by aerial roots, seedling survival, and photosynthesis of mangroves have been analyzed in few studies (Williams and Simmons 1996; Smith 2012; Suyadi and Manullang 2020). But the very first and only manipulative research to date is from Java, Indonesia (van Bijsterveldt et al. 2021). The study found that constant burial of plastic debris in the topmost layer of mangrove sediment results in anoxia and suffocation. To some extent, the mangrove trees withstand this stressed condition by enhancing pneumatophore (aerial root) growth and reducing canopy (van Bijsterveldt et al. 2021). Upon higher exposure to plastics (100% plastic cover), significant leaf loss occurs and the tree survival becomes risky (van Bijsterveldt et al. 2021). More supportive scientific evidence from the mangrove zones all over the world is necessary to completely understand the mechanism of mangrove devastation by (micro)plastics. The suffocation imparted by plastics may not be the deteriorative effect of smaller-sized MPs on mangroves. So, the responses of mangroves to different polymer types of MPs should also be evaluated together with the chances of adaptive regulations the mangroves may acquire during the course of interaction with various MPs.
Mangrove microbiota are better suited to the (micro)plastic environment?
Microorganisms utilize MPs as a surface for colonization. The colonization process is influenced by physicochemical and biological factors. After colonization, a novel niche of colonizing microbes termed “plastisphere” is formed over the plastic surface (Zettler et al. 2013). Here, we suggest a related term “mangrove plastisphere” for the microbial niche on the surface of plastic debris trapped within the mangrove rhizosphere. The inhabiting microbiota within a plastisphere may either get benefit or be adversely affected by the (micro)plastic substratum. In general, a positive response showing gradual adaptation of the microorganisms to the stressed environment caused by MPs is observed (Oberbeckmann and Labrenz 2020). The overall gene expression patterns and the cell behavior alter to facilitate cell adhesion to the surface of MPs and the mobility of the microbial population over the surface (Tuson and Weibel 2013). The other adaptive responses include deviations in growth and metabolic rate and the production of protective macro-molecules (NicAogáin and O’Byrne 2016; Guan and Liu 2020). The microbial stress-responsive pathways are regulated by enzyme switches (Cooper 2000; Winkel 2017).
The adapted microbes benefit from MP attachment with a better surface area for easy access to energy sources and nutrient accumulation, facilitating cell growth (Shen et al. 2019). The metal ions adhered to the MP surface are utilized by the microbiota for cellular activities involving electron receptors (Maret 2016). Biofilm formation upon the MP surface is another favorable adaptation microbes, especially bacteria acquire. It provides mechanical strength, protection from predators, and the diffusivity is also enhanced (Tuson and Weibel 2013). The negative aspects of microbial interactions with MPs are not well understood. Surprisingly, the interplay of “mangrove plastisphere” is not even focused to date. The response of mangrove microbiota to MP is still a mystery. This gap area invites scientific initiatives addressing adaptive responses and adhesive characteristics of MP-associated mangrove microbiota.
Microbial solutions for the reduction and management of mangrove MP pollution
Though MPs are more or less resistant to microbial invasions and degradation, they are the most transformed material upon microbial attack (Rujnić-Sokele and Pilipović 2017). The different types of plastic/MP degrading microorganisms and their mechanism of action have been specifically reviewed by (Yuan et al. 2020). The following subsections discuss the MP degrading native mangrove microbes as well as microbial enzymes/biofilms from mangrove and non-mangrove sources identified so far.
Prospecting native mangrove microbes with MP degradation potential
The mangrove ecosystem harbors a wide variety of microorganisms such as algae, bacteria, cyanobacteria, and fungi. Among the mangrove bacteria, methanogenic (e.g., Methanoccoides methylutens), phosphate solubilizing (e.g., Vibrio proteolyticus), N2-fixing (e.g., Rhizobium), and sulfate-reducing groups (e.g., Desulfotomaculum) are the most dominant. The fungal groups of mangrove areas broadly include proteolytic, amylolytic, pectinolytic, cellulolytic, and ligninolytic types (Kandasamy and Bingham 2001). Cyanophyta, Rhodophyta, Phaeophyta, Chrysophyta, and Chlorophyta are the notable algal groups of the mangrove ecosystems (Sen and Naskar 2003). The microbial richness of such an extremophilic habitat like mangroves is not effectively exploited for its efficacy in biodegradation of MPs either via in vitro or in situ incubation methods. The studies can be carried out to prospect the MP degrading microbes. In vitro analysis of MP degradation is one important strategy to identify the microbes with MP degradation potential.
Evidence of MP degradation potential of native mangrove microbiota through in vitro analysis
In general, several microbes with MP degrading potential have been identified, information on microbes from mangrove habitats is limited (Yuan et al. 2020; Othman et al. 2021). Despite slow progress, recent studies have attempted in vitro investigation of plastic degrading potential of microbes isolated from the mangrove habitats. The earliest in vitro analysis identified several heterotrophic bacteria such as Vibrio, Listeria, Micrococcus, Staphylococcus, and Bacillus from the mangrove soil of Suva, Fiji Island, with degradation potential of low- and high-density polyethylene (LDPE and HDPE; Kumar et al. 2007). Mangrove-associated bacteria, viz. Streptomyces sp., Pseudomonas aeruginosa, and Staphylococcus aureus, and fungi, viz. Aspergillus niger and Rhizopus sp. of Muthupet ecosystem, were found to be capable of effectively deteriorating plastic debris especially PE (Kannahi and Sudha 2013). Indigenous bacilli namely Bacillus mycoides and Bacillus subtilis of Niger Delta mangrove ecosystem were found to biodegrade PE following an initial abiotic degradation by sunlight (Ibiene et al. 2013). Some selected isolates and consortium of fungi such as Phialophora alba, Paecilomyces variotii, Eupenicillium hirayamae, Aspergillus terreus, Aspergillus caespitosus, and Alternaria alternata of mangrove habitat (Red sea coast, Saudi Arabia) were proven to exhibit LDPE digestibility (Ameen et al. 2015). The bacterial strains namely Bacillus gottheilii, Rhodococcus sp. (36), and Bacillus sp. (27) which degrade polypropylene (PP) were also isolated from the mangrove ecosystem (Auta et al. 2017, 2018). Later, it was proved that the bacterial strains of Pichavaram, India, mangrove wetland are able to digest HDPE (Sangeetha Devi et al. 2019). Among the 109 fungal isolates collected from the Avicennia marina rhizosphere (West Coast of India), a remarkable PE degrading ability was demonstrated by Aspergillus terreus (MANGF1/WL) and Aspergillus sydowii (PNPF15/TS) (Sangale et al. 2019). These in vitro studies followed a weight loss calculation of plastic substratum and a few with morphology characterization of plastic polymers. The observations highlight the fact that plastic debris gets trapped within the mangrove wetlands and is fragmented into respective MPs. The subsequent biodegradation of MPs into smaller particles is yet to be systematically analyzed. Whether the plastic degradation by the mangrove biota enhances the MP content of the mangrove plastisphere or the subsequent and prolonged degradation of MPs eventually reduces the MP concentration is still not fully understood. To better understand the fate of mangrove MP polymers, controlled in situ experimental MP degradation and monomer tracking are needed. Further studies must focus on the mechanisms of MP degradation by the microorganisms and how microbial degradation contributes to MP elimination.
In situ MP biodegradation
MP degradation by microorganisms is greatly affected by environmental factors and the interactions among them (Yuan et al 2020). Isolation of individual mangrove microorganisms and in vitro incubation with MPs may be good enough to explore the efficacy of MP degradation. But to reveal the MP degradation potential of native microorganisms within the mangrove habitats, in situ analysis is necessary where the environmental factors affecting the rate of MP degradation can also be examined. The in situ studies are even more important for practical reasons because it is easier to deploy the microbes in the MP-polluted mangrove ecosystems rather than doing in vitro degradation of the isolated MPs. The mangrove rhizosphere microorganisms are the nutrient houses of mangroves by virtue of the ability to decompose detritus material (Holguin et al. 2001). The utility of mangrove-associated microorganisms in in situ plastic degradation was recognized by Kathiresan (2003). The degradation of polymers within the mangrove rhizosphere is largely influenced by the microbe type, heat of mangrove soil, and moisture content (Kathiresan 2003). Microbes (bacteria: Pseudomonas, Moraxella, Staphylococcus, Micrococcus, and Streptococcus; fungi: Aspergillus niger and Aspergillus glaucus) of Rhizophora- and Avicennia-growing zones were found to be effectively degrading polyethylene bags and plastic cups. The bacterial and fungal candidates exhibiting the highest percentage of plastic degradation were Pseudomonas and Aspergillus glaucus respectively (Kathiresan 2003). After a long gap, a recent and unique research from Zhanjiang Mangrove National Nature Reserve, China, showed degradation of three MP substrata (LDPE, polyamide 6, and polyvinyl chloride) out of nine under in situ incubation initially for 1 month and extended up to 3 months (Xie et al. 2021). The microbial colonization in all the nine candidate MPs (others are acrylonitrile butadiene styrene (ABS), PET, PP, expanded polystyrene (EPS), PS, and polycarbonate (PC)) was specific to the polymer groups. This study also identified 1746 and 2077 unique and shared colonizing native microbes within the MP surface biofilm after one month and three months of incubation. Among them, the dominant mangrove microbes belonged to the orders Desulfobacterales, Cellvibrionales, Bacteroidales, Sphingomonadales, Steroidobacterales, Rhodobacterales, SBR1031, Campylobacterales, B2M28, Gamma proteobacteriales, Vibrionales, Clostridiales, Actinomarinales, Anaerolineales, Bacillales, and Flavobacteriales (Xie et al. 2021). These studies suggest that native microorganisms inhabiting the mangrove rhizosphere may be ideal candidates for the biodegradation of plastics. The identification of the native microbes and their deployment in the mangrove wetlands could help in the microbe-mediated remediation of the MP-polluted mangrove ecosystems. However, extensive studies are required to prospect.
Microbial enzymes for MP remediation
The polymeric MPs are specifically digested by microbial enzymes into component monomeric units which serve as a carbon source for the degrading microorganisms (Ganesh et al. 2020; Bollinger et al. 2020; Liu et al. 2021). The enzymatic mechanisms of microbial degradation of MP were reviewed in detail by Othman et al. (2021). Briefly, the microbial enzymes act via surface modification (Vertommen et al. 2005) or polymer degradation (Gómez-Méndez et al. 2018). Although several plastic degrading microbial enzymes have been identified from other environments, the microbial enzymes from mangrove ecosystems are not yet identified. Auta et al. (2018) have found enzymatic degradation of MPs; still, the identity of the plastic degrading enzymes is not yet established. The bacterial strains examined for polypropylene degradation were efficiently reducing the polymer mass (Auta et al. 2018). Table 2 summarizes the microbial enzymes, their sources, and the respective MP substrates. Similar enzymes might be existing in the microbial communities of the mangrove ecosystems. Further in-depth studies must be carried out to harvest the potential native microbial enzymes from the mangrove habitats.
Microbial biofilms and their role in MP degradation
Microbial consortia assemble and unite under varying settings to form wide varieties of biofilms which respond quickly to environmental cues (Hall-Stoodley et al. 2004). Microorganisms ranging from smaller viruses to larger fungi colonize and form biofilms over the MP surface (Oberbeckmann et al. 2015). The whole process of MP degradation by biofilms may occur in four stages. During the first stage, the microbes attach to the MP surface and mask surface properties like adhesive characteristics and hydrophilicity. In the second and third stages, MP degrading microbial enzymes are secreted out causing leaching of monomers and additives out of MP. In the fourth stage, deep penetration of MP by microbial filaments and further degradation takes place (Flemming 1998; Miao et al 2019). MP degradation mediated by bacterial biofilms and the polymer specificity of the degrading microorganisms is better studied in recent years as compared to fungal biofilms. For example, bacterial biofilms of Escherichia coli, Burkholderia cepacia, and Acinetobacter calcoaceticus specifically deteriorate PP (Hossain et al. 2019). On the other hand, MPs are supposed to selectively enrich the type of microorganism growing on its surface (Miao et al. 2019). The most favored biofilm-forming microbial families on the MP surfaces are Cyclobacteriaceae, Phycisphaerales, Pirellulaceae, and Roseococcus than those established on natural substrates (Miao et al. 2019). However, the complexity of mechanisms that lead to biodegradation specificity of MPs by biofilms is yet to be studied. There is no information available to date regarding the MP attached biofilms of mangrove microbiota and the subsequent MP degradation.
Futuristic microbe-mediated strategies for remediation of MPs from mangrove wetlands
Microbial biofilm traps: clear out already polluted mangrove zones
As evident from the earlier discussion, several studies have been done to find out suitable microbial enzymes and biofilms for MP degradation. As these microbial mechanisms specifically or preferably target individual MP types, removal of MP mixture containing different types and sizes of polymers is not possible (Liu et al. 2021). Moreover, the process of biodegradation is comparatively slow which leaves behind the MP traces for longer durations (Miao et al. 2019). Bioaggregation, which has been proven in the remediation process of pesticides and metalloids, is a potent and emerging strategy for the efficient removal of MP mixtures (Chua et al. 2015; Wang 2016). The major advantage of this biological trap is that it is possible to recycle the MP aggregates rather than opting for landfill disposition and incineration (Deshpande et al. 2020). Liu et al. (2021) engineered P. aeruginosa biofilm to trap and aggregate (“trap-and-release” bioaggregation mechanism) MPs within its sticky matrix which could be released and recycled later. The trap-and-release mechanism is independent of material type and size and easily removes the resultant MP clusters due to its aggregation at high concentrations (Liu et al. 2021). The removal of MP aggregates is also supported by lowering of cyclic diguanylate (c-di-GMP) levels (Chua et al. 2015). The native microflora constantly being exposed to the mangrove MPs might have adapted better potential to form biofilms on their surface and to serve as efficient MP traps. Future applied studies aimed at remediation of wetland MPs should also incorporate in situ biofilm traps made of mangrove microbiota to eliminate MP pollutants.
Bioplastics from mangrove microbes
Ordinary plastics and their MP counterparts are petroleum byproducts which are non-biodegradable. To save our environment from being a plastic sink, it is necessary to replace petroleum based plastics with biodegradable plastics (bioplastics). Bioplastics are synthesized from microbial degradation of renewable biomasses such as corn and sugarcane, or from microbes such as yeast (Alzubaidy et al. 2016; Hong et al. 2019). The use of mangrove microorganisms as reservoirs of a wide range of resources like antimicrobial substances, enzymes, medicines, and food is well discussed (Lin et al. 2001; Maria et al. 2005). But their contributions to bioplastic production are least documented. The biopolymer poly-beta-hydroxybutyrate (PHB) was isolated from the estuarine microorganisms long ago (Herron et al. 1978). Later, Alarfaj et al. (2015) extracted and characterized PHB from a mangrove bacterium (Bacillus thuringiensis KSADL127) of Saudi Arabia. A hydroxyvalerate additive of PHB was also characterized from a halotolerant mangrove Bacillus (Moorkoth and Nampoothiri 2016). Among the four mangrove rhizospheric bacteria isolated from Al-Marabi and Al-Madhaya, Erythrobacter aquimar accumulates greater concentrations of PHB (Mostafa et al. 2020). Taken together, the research exploring native mangrove microbes in bioplastic production is comparatively less and it requires screening more candidates for their capability to produce bioplastics in near future.
Biotechnological tools for microbe-mediated MP remediation
Microbial degradation is an eco-friendly strategy to overcome the after-effects of deadly MP pollution in the global mangrove wetlands. The characterization of the mangrove plastisphere microbiome is important to increase our understanding of the plastic-microbe interactions. Its understanding is important because MPs offer unique surfaces as substratum for the microbes to colonize leading to alteration of microbial composition/functioning within the microbial population (Bhagwat et al. 2021). The interactions between MPs and microorganisms are established mainly through enzymatic activities but most of them remain uncharacterized. The recent developments in omics technologies have revolutionized genomic analysis of environmental samples which facilitates identification of plastic degrading microbes from various ecosystems (Danso et al. 2019; Jaiswal et al. 2020). It is easier and cheaper to sequence the genomes of organisms including microorganisms to decipher the structure and functions of the genes (Loman et al. 2012). The combined application of genomic, proteomic, metabolomic, and ionomics approaches could help identify the gene-protein-metabolite and ion networks that are involved in the biodegradation of MPs (Wei et al. 2021). Genetic dissection of the enzymes and identification of other regulators including miRNAs, lncRNAs that mediate the functioning of the enzymes would be helpful in devising and deploying highly efficient microbes for bioremediation of the MPs (Nazarov et al. 2013; Deng et al. 2018). The multi-omics tools can also help identify and pinpoint the exact enzymes and the genes that encode them for easier manipulation using gene modification/editing tools (Jaiswal et al. 2020; Wei et al. 2021). The candidate genes encoding the most important enzymes can be easily identified through the multi-omics approaches (Blimkie et al. 2020). Moreover, the microbial interactions within a biofilm which might be influencing the rate of MP degradation process can also be elucidated (Yu et al. 2019). Meyer-Cifuentes et al. (2020) studied the biodegradation of copolyester blend (PBAT-based blend film, PF) by the microbial consortium from the marine samples of three different countries, viz. Germany, Greece, and Italy. Using multi-omics approaches such as meta-transcriptomics, metaproteomics, and metagenomics, this study further identified important genes encoding PF degrading enzymes such as PETase-like enzymes (Ples) and MHETase-like enzymes (Mles) which are crucial for the degradation of PF (Meyer-Cifuentes et al. 2020). One important candidate genes with MP degradation potential are characterized, biotechnological tools such as genetic engineering and gene editing can be applied to create over-expressing, genetically modified, or genetically edited microbes with enhanced biodegradability potential. The availability of limited information on the mangrove microbes and the MP degrading enzymes shows that mangrove microorganisms are the least exploited microbiota for their potential in bioremediation of MPs. The current review proposes several possible futuristic strategies utilizing mangrove microbiota to tackle mangrove MP pollution as depicted in Fig. 3.
Futuristic strategies involving incorporation of mangrove plastisphere microbes for microbe-mediated remediation of MPs from mangrove wetlands. The microbial inhabitants of the mangrove plastisphere may be either used directly or with genetic modifications for onsite biodegradation of MPs. Bioengineered biofilms from the plastisphere may serve as efficient traps for bio aggregation of wetland MPs. Finally, the efficiency of native or genome-edited plastisphere microorganisms may be analyzed for bioplastic production. The multi-omics approaches may contribute more towards the understanding of MP-biofilm interactions and prospection of new MP degrading enzymes
Conclusions
Considering the ecological and economic importance of the mangroves and the vulnerabilities due to multiple threats, it is essential to take concerted efforts to tackle various types of pollution affecting mangroves. MP pollution being one of the serious issues has recently received little attention from environmental scholars. Although not much literature is available from nearly 118 countries where mangroves are found, the limited studies from some of the mangrove-dominated areas point towards the seriousness of the MP pollution not just for the mangroves per se but for a number of other organisms that shelter in the mangrove habitats. It points towards how vulnerable mangrove ecosystems are. A future without mangroves is not imaginable especially for the coastal communities who are protected by the mangroves especially during cyclones, sea waves, floods, and tsunamis. The current review concludes that the mangrove habitats are contaminated with (micro)plastics dumped from marine as well as coastal sources. It is also evident that MP pollution results from the lack of basic sanitation and anthropogenic activities. The previous studies reporting global distribution and composition of MPs in the mangrove ecosystem are not balanced. Most of the data comes from the Chinese coastal regions with only a handful of reports from the other parts of the world. Of the various solutions to MP pollution, sooner or later microbial mitigation forms the key strategy for MP-free mangrove wetlands. Genomic investigation of multiple mechanisms regulating microbial degradation of MPs using multi-omics tools will increase our understanding of genetic regulation of microbe-mediated MP degradation. The genetic underpinnings of microbe-MP interactions will help in devising futuristic strategies aimed at the removal of MPs from the vulnerable mangrove wetlands.
Future perspectives
The current article emphasizes deployment of microbes, especially the native microbiota of “mangrove plastisphere” for bioremediation of increasing MPs in the mangrove ecosystem. The future applied research must take advantage of advancements in multi-omics, genetic engineering, and gene editing tools to bridge the key gaps in addressing the issue of wetland MP pollution. Other areas of research in the future surrounding biodegradation of MP pollution in the mangrove wetlands are presented in the following points:
-
Characterization of MP-polluted mangrove wetlands: It is high time to evaluate the abundance, sources, and types of MPs in the global mangrove inhabiting countries to get the actual picture of MP distribution which will guide us to design accurate prevention/management strategies.
-
Studies focusing on microbial degradation must consider aspects such as (i) identification of the potential microbes capable of MP degradation, (ii) identification of the genes, enzymes that aid MP degradation, and (iii) application of gene editing and engineering tools to increase MP degrading efficiency of the microbes.
-
As the mangrove ecosystems have already been reported as MP sinks, the mangrove microbiota might have acquired adaptive characteristics upon constant exposure which is yet to be studied and documented.
-
Though the microbial aspects of MP degradation have been discussed by many authors, the possibility of using mangrove microbiota for in situ MP biodegradation strategy is not widely adopted. The review invites wider attention of the scientific community towards this end.
-
Devising integrative strategies to mitigate MP pollution: Multi-omics studies should be encouraged to unravel genes, enzymes, and novel and efficient MP-biofilm adhesions. Multi-omics studies must be deployed to investigate the genes, enzymes important for MP biodegradation including deciphering the efficacy of biofilms in MP biodegradation.
-
The remarkable future prospect from the current review is the use of biofilm traps for addressing MP pollution. Using biofilm traps for bioremediation of MPs from the mangrove habitats is perceived as one of the promising strategies in the future.
-
Mangrove-associated microbes are expected to possess peculiar characteristic features due to the extremophilic habitat they share. This peculiarity might be an added advantage while utilizing mangrove microbiota for bioplastic production. Based on the studies reported so far, more scientific research is required to validate the efficiency of mangrove microorganisms in mass production of bioplastics and also tune their genome to enhance MP degradation efficacy and efficiency.
Data availability
All data generated or analyzed during the study are included in the published article(s) cited within the text and acknowledged in the reference sec.
Availability of data and materials
Not applicable.
References
Alarfaj AA, Mohammed A, Sholkamy EN, Munusamy MA (2015) Extraction and Characterization of polyhydroxybutyrates (PHB) from Bacillus thuringiensis KSADL127 isolated from mangrove environments of Saudi Arabia. Braz Arch Biol Technol 58:781–788. https://doi.org/10.1590/S1516-891320150500003
Alzubaidy H, Essack M, Malas TB et al (2016) Rhizosphere microbiome metagenomics of gray mangroves (Avicennia marina) in the Red Sea. Mar Genomics 576:626–636. https://doi.org/10.1016/j.gene.2015.10.032
Ameen F, Moslem M, Hadi S, Al-Sabri AE (2015) Biodegradation of low density polyethylene (LDPE) by mangrove fungi from the Red Sea Coast. Prog Rubber Plast Recycl Technol 31:125–143. https://doi.org/10.1177/147776061503100204
Andrady AL (2011) Microplastics in the marine environment. Mar Pollut Bull 62:1596–1605. https://doi.org/10.1016/j.marpolbul.2011.05.030
Auta HS, Emenike CU, Fauziah SH (2017) Screening of Bacillus strains isolated from mangrove ecosystems in Peninsular Malaysia for microplastic degradation. Environ Pollut 231:1552–1559. https://doi.org/10.1016/j.envpol.2017.09.043
Auta HS, Emenike CU, Jayanthi B, Fauziah SH (2018) Growth kinetics and biodeterioration of polypropylene microplastics by Bacillus sp. and Rhodococcus sp. isolated from mangrove sediment. Mar Pollut Bull 127:15–21. https://doi.org/10.1016/j.marpolbul.2017.11.036
Barasarathi J, Agamuthu P, Emenike CU, and Fauziah SH (2014) Microplastic abundance in selected mangrove forest in Malaysia. Proceedings of the ASEAN Conference on Science and Technology (Bogor, Indonesia)
Bhagwat G, Zhu Q, O’Connor W et al (2021) Exploring the composition and functions of plastic microbiome using whole-genome sequencing. Environ Sci Technol 55:4899–4913. https://doi.org/10.1021/acs.est.0c07952
Blimkie T, Lee AHY, Hancock RE (2020) MetaBridge: an integrative multi-omics tool for metabolite−enzyme mapping. Curr Protoc Bioinform 70:e98. https://doi.org/10.1002/cpbi.98
Bollinger A, Thies S, Knieps-Grünhagen E, Gertzen C, Kobus S, Höppner A, Ferrer M, Gohlke H, Smits SHJ, Jaeger KE (2020) A novel polyester hydrolase from the marine bacterium Pseudomonas aestusnigri –structural and functional insights. Front Microbiol 11:114. https://doi.org/10.3389/fmicb.2020.00114
Bouillon S, Dahdouh-Guebas F, Rao AVVS, Koedam N, Dehairs F (2003) Sources of organic carbon in mangrove sediments: variability and possible ecological implications. Hydrobiologia 495:33–39. https://doi.org/10.1023/A:1025411506526
Brooks AN, Turkarslan S, Beer KD et al (2011) Adaptation of cells to new environments. Wiley Interdiscip Rev Syst Biol Med 3:544–561. https://doi.org/10.1002/wsbm.136
Chatterjee S, Roy B, Roy D, Banerjee R (2010) Enzyme-mediated biodegradation of heat treated commercial polyethylene by Staphylococcal species. Polym Degrad Stab 95:195–200. https://doi.org/10.1016/j.polymdegradstab.2009.11.025
Chua SL, Sivakumar K, Rybtke M et al (2015) C-di-GMP regulates Pseudomonas aeruginosa stress response to tellurite during both planktonic and biofilm modes of growth. Sci Rep 5:10052. https://doi.org/10.1038/srep10052
Cooper GM (2000) The cell: a molecular approach, 2nd edn. Sinauer Associates, Sunderland, Massachusetts, United States
Cordova MR, Ulumuddin YI, Purbonegoro T, Shiomoto A (2021) Characterization of microplastics in mangrove sediment of Muara Angke Wildlife Reserve. Indonesia Mar Pollut Bull 163:112012. https://doi.org/10.1016/j.marpolbul.2021.112012
Da Costa AM, de Oliveira Lopes VR, Vidal L, Nicaud JM, de Castro AM, Coelho MAZ (2020) Poly(ethylene terephthalate) (PET) degradation by Yarrowia lipolytica: investigations on cell growth, enzyme production and monomers consumption. -Process Biochem 95:81-90. https://doi.org/10.1016/j.procbio.2020.04.001
Danso D, Chow J, Streit WR (2019) Plastics: environmental and biotechnologicalperspectives on microbial degradation. Appl Environ Microbiol 85(19):e01095-19. https://doi.org/10.1128/AEM.01095-19
Deng P, Liu S, Nie X, Weining S, Wu L (2018) Conservation analysis of long non-coding RNAs in plants. Sci China Life Sci 61:190–198. https://doi.org/10.1007/s11427-017-9174-9
de Stephanis R, Giménez J, Carpinelli E et al (2013) As main meal for sperm whales: plastics debris. Mar Pollut Bull 69:206–214. https://doi.org/10.1016/j.marpolbul.2013.01.033
Deng H, He J, Feng D et al (2021) Microplastics pollution in mangrove ecosystems: a critical review of current knowledge and future directions. Sci Total Environ 753:142041. https://doi.org/10.1016/j.scitotenv.2020.142041
Deng J, Guo P, Zhang X et al (2020) Microplastics and accumulated heavy metals in restored mangrove wetland surface sediments at Jinjiang Estuary (Fujian, China). Mar Pollut Bull 159:111482. https://doi.org/10.1016/j.marpolbul.2020.111482
Deshpande PC, Skaar C, Brattebø H, Fet AM (2020) Multi-criteria decision analysis (MCDA) method for assessing the sustainability of end-of-life alternatives for waste plastics: a case study of Norway. Sci Total Environ 719:137353. https://doi.org/10.1016/j.scitotenv.2020.137353
Dong H, Chen Y, Wang J et al (2021) Interactions of microplastics and antibiotic resistance genes and their effects on the aquaculture environments. J Hazard Mater 403:123961. https://doi.org/10.1016/j.jhazmat.2020.123961
Dou PC, Mai L, Bao LJ, Zeng EY (2021) Microplastics on beaches and mangrove sediments along the coast of South China. Mar Pollut Bull 172:112806. https://doi.org/10.1016/j.marpolbul.2021.112806
Duan J, Han J, Cheung SG et al (2021) How mangrove plants affect microplastic distribution in sediments of coastal wetlands: case study in Shenzhen Bay South China. Sci Total Environ 767:144695. https://doi.org/10.1016/j.scitotenv.2020.144695
Duke NC (1995) Genetic diversity, distributional barriers and rafting continents- more thoughts on the evolution of mangroves. In: Wong YS, Tam NFY (eds) Asia-Pacific symposium on mangrove ecosystems. Developments in Hydrobiology, vol 106. Springer, Dordrecht. pp 167–181. https://doi.org/10.1007/978-94-011-0289-6_21
Duke NC, Meynecke JO, Dittmann S et al (2007) A world without mangroves? Science 317:41–42. https://doi.org/10.1126/science.317.5834.41b
FAO (2007) The world’s mangroves 1980–2005. FAO. www.fao.org/documents/card/en/c/880053ed-9752-5939-b242-35fd7603a2ba/. Accessed 14 Nov 2021
Feldberg B (2018) Macro implications of microplastics: a comparative study of microplastic distribution in Bahía Almirante, Bocas del Toro, Panama. Independant study project (ISP) collection. 2943 https://core.ac.uk/download/pdf/232740798.pdf. Accessed 9 Jul 2021
Feller I, Lovelock C, Berger U, McKee KL, Joye SB, Ball MC (2010) Biocomplexity in mangrove ecosystems. Annu Rev Mar Sci 2:395–417. https://doi.org/10.1146/annurev.marine.010908.163809
Flemming HC (1998) Relevance of biofilms for the biodeterioration of surfaces ofpolymeric materials. Polym Degrad Stab 59(1–3):309–315. https://doi.org/10.1016/S0141-3910(97)00189-4
Friess DA (2016) Mangrove forests. Curr Biol 26:R746–R748. https://doi.org/10.1016/j.cub.2016.04.004
Galgani F, Hanke G, Maes T (2015) Global distribution, composition and abundance of marine litter. In: Bergmann M, Gutow L, Klages M (eds) Marine Anthropogenic Litter. Springer International Publishing, Cham, pp 29–56
Ganesh K, Anjana K, Sujitha M et al (2020) Review on plastic wastes in marine environment – biodegradation and biotechnological solutions. Mar Pollut Bull 150:110733. https://doi.org/10.1016/j.marpolbul.2019.110733
Garcés-Ordóñez O, Castillo-Olaya VA, Granados-Briceño AF et al (2019) Marine litter and microplastic pollution on mangrove soils of the Ciénaga Grande de Santa Marta, Colombian Caribbean. Mar Pollut Bull 145:455–462. https://doi.org/10.1016/j.marpolbul.2019.06.058
GESAMP (2015) Sources, fate and effects of microplastics in the marine environment: A global assessment. Rep stud GESAMP 90:1–96
Ghatge S, Yang Y, Ahn JH, Hur HG (2020) Biodegradation of polyethylene: a brief review. Appl Biol Chem 63:27. https://doi.org/10.1186/s13765-020-00511-3
Gómez-Méndez L, Moreno D, Poutou-Piñales R et al (2018) Biodeterioration of plasma pretreated LDPE sheets by Pleurotusostreatus. PLoS One 13:e0203786. https://doi.org/10.1371/journal.pone.0203786
Govender J, Naidoo T, Rajkaran A, Cebekhulu S, Bhugeloo A, Sershen (2020) Towards characterising microplastic abundance, typology and retention in mangrove-dominated estuaries. Water 12:2802. https://doi.org/10.3390/w12102802
Guan N, Liu L (2020) Microbial response to acid stress: mechanisms and applications. Appl Microbiol Biotechnol 104:1–15. https://doi.org/10.1007/s00253-019-10226-1
Hall-Stoodley L, Costerton J, Stoodley P (2004) Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol 2:95–108. https://doi.org/10.1038/nrmicro821
Hamid F, Jia W, Zakaria R (2020) Microplastics abundance and uptake by Meretrix lyrata (hard clam) in mangrove forest. J Eng Technol Sci 52:436–448
Hastuti A, Yulianda F, Wardiatno Y (2014) Spatial distribution of marine debris in mangrove ecosystem of Pantai Indah Kapuk, Jakarta. Bonorowo Wet 4:94–107. https://doi.org/10.13057/bonorowo/w040203
Herron J, King J, White D (1978) Recovery of poly-beta-hydroxybutyrate from estuarine microflora. Appl Env Microbiol 35:251–257. https://doi.org/10.1128/aem.35.2.251-257.1978
Holguin G, Vazquez P, Bashan Y (2001) The role of sediment microorganisms in the productivity, conservation, and rehabilitation of mangrove ecosystems: an overview. Biol Fertil Soils 33:265–278. https://doi.org/10.1007/s003740000319
Hong JW, Song HS, Moon YM et al (2019) Polyhydroxybutyrate production in halophilic marine bacteria Vibrio proteolyticus isolated from the Korean peninsula. Bioprocess Biosyst Eng 42:603–610. https://doi.org/10.1007/s00449-018-02066-6
Hossain MR, Jiang M, Wei Q, Leff LG (2019) Microplastic surface properties affect bacterial colonization in freshwater. J Basic Microbiol 59:54–61. https://doi.org/10.1002/jobm.201800174
Huang Y, Xiao X, Effiong K et al (2021) New insights into the microplastic enrichment in the blue carbon ecosystem: evidence from seagrass meadows and mangrove forests in coastal South China Sea. Env Sci Technol 55:4804–4812. https://doi.org/10.1021/acs.est.0c07289
Ibiene A, Stanley H, Immanuel O (2013) Biodegradation of polyethylene by Bacillus sp. indigenous to the Niger Delta mangrove swamp. Niger J Biotechnol 26:68–79
Iiyoshi Y, Tsutsumi Y, Nishida T (1998) Polyethylene degradation by lignin-degrading fungi and manganese peroxidase. J Wood Sci 44:222–229. https://doi.org/10.1007/BF00521967
IUCN (2020) IUCN red list of threatened species. In: IUCN Red List Threat. Species. https://www.iucnredlist.org/. Accessed 9 Jul 2021
Jabloune R, Khalil M, Moussa IEB, Simao-Beaunoir AM, Lerat S, Brzezinski R, Beaulieu C (2020) Enzymatic degradation of p-nitrophenyl esters, polyethylene terephthalate, cutin, and suberin by Sub1, a suberinase encoded by the plant pathogen Streptomyces scabies. Microbes Environ 35:1–7. https://doi.org/10.1264/jsme2.ME19086
Jaiswal S, Sharma B, Shukla P (2020) Integrated approaches in microbial degradation of plastics. Environ Technol Inno 17:100567. https://doi.org/10.1016/j.eti.2019.100567
Jambeck JR, Geyer R, Wilcox C et al (2015) Marine pollution. Plastic waste inputs from land into the ocean. Science 347:768–771. https://doi.org/10.1126/science.1260352
Jeon H, Kim M (2012) Isolation of a thermophilic bacterium capable of low-molecular-weight polyethylene degradation. Biodegradation 24:89–98. https://doi.org/10.1007/s10532-012-9560-y
Jeong J, Choi J (2020) Development of AOP relevant to microplastics based on toxicity mechanisms of chemical additives using ToxCastTM and deep learning models combined approach. Environ Int 137:105557. https://doi.org/10.1016/j.envint.2020.105557
Kandasamy K, Bingham B (2001) Biology of mangroves and mangrove ecosystems. Adv Mar Biol 40:81–251. https://doi.org/10.1016/S0065-2881(01)40003-4
Kannahi M, Sudha P (2013) Screening of polythene and plastic degrading microbes from Muthupet mangrove soil. J Chem Pharm Res 5:122–127
Kathiresan K (2003) Polythene and plastics-degrading microbes from the mangrove soil. Rev Biol Trop 51:629–633
Khalid N, Aqeel M, Noman A (2020) Microplastics could be a threat to plants in terrestrial systems directly or indirectly. Environ Pollut 267:115653. https://doi.org/10.1016/j.envpol.2020.115653
Khalid N, Aqeel M, Noman A et al (2021) Linking effects of microplastics to ecological impacts in marine environments. Chemosphere 264:128541. https://doi.org/10.1016/j.chemosphere.2020.128541
Kim HR, Lee HM, Yu HC et al (2020) Biodegradation of Polystyrene by Pseudomonas sp. isolated from the gut of superworms (Larvae of Zophobas atratus). Environ Sci Technol 54:6987–6996. https://doi.org/10.1021/acs.est.0c01495
Kim M, Kim C, Moon J et al (2017) Polymer film-based screening and isolation of polylactic acid (PLA)-degrading microorganisms. J Microbiol Biotechnol 27:342–349. https://doi.org/10.4014/jmb.1610.10015
Koelmans AA, Gouin T, Thompson R, Wallace N, Arthur C (2014) Plastics in the marine environment. Environ Toxicol Chem 33:5–10. https://doi.org/10.1002/etc.2426
Kumar A, Anju T, Archa V, et al (2021) Mangrove forests: Distribution, species diversity, roles, threatsand conservation strategies In: Sharma S and Singh P (eds) Wetlands conservation: current challenges and future strategies. John wiley & Sons Ltd, New Jersey, pp 229–271. https://doi.org/10.1002/9781119692621.ch12
Kumar S, Abdulla MH, Christi K (2007) Diversity and effectiveness of tropical mangrove soil microflora on the degradation of polythene carry bags. Rev Biol Trop 55:777–786. https://doi.org/10.15517/rbt.v55i3-4.5954
Li J, Zhang H, Zhang K et al (2018) Characterization, source, and retention of microplastic in sandy beaches and mangrove wetlands of the Qinzhou Bay, China. Mar Pollut Bull 136:401–406. https://doi.org/10.1016/j.marpolbul.2018.09.025
Li R, Yu L, Chai M et al (2020a) The distribution, characteristics and ecological risks of microplastics in the mangroves of Southern China. Sci Total Environ 708:135025. https://doi.org/10.1016/j.scitotenv.2019.135025
Li R, Zhang L, Xue B, Wang Y (2019) Abundance and characteristics of microplastics in the mangrove sediment of the semi-enclosed Maowei Sea of the south China sea: new implications for location, rhizosphere, and sediment compositions. Environ Pollut 244:685–692. https://doi.org/10.1016/j.envpol.2018.10.089
Li R, Zhang S, Zhang L et al (2020b) Field study of the microplastic pollution in sea snails (Ellobium chinense) from mangrove forest and their relationships with microplastics in water/sediment located on the north of Beibu Gulf. Environ Pollut 263:114368. https://doi.org/10.1016/j.envpol.2020.114368
Lin Y, Wu X, Feng S et al (2001) Five unique compounds: xyloketals from mangrove fungus Xylaria sp. from the South China Sea coast. J Org Chem 66:6252–6256. https://doi.org/10.1021/jo015522r
Liu SY, Leung MM-L, Fang JK-H, Chua SL (2021) Engineering a microbial ‘trap and release’ mechanism for microplastics removal. Chem Eng J 404:127079. https://doi.org/10.1016/j.cej.2020.127079
Lovelock CE, Cahoon DR, Friess DA et al (2015) The vulnerability of Indo-Pacific mangrove forests to sea-level rise. Nature 526:559–563. https://doi.org/10.1038/nature15538
Loman N, Constantinidou C, Chan J et al (2012) High-throughput bacterial genome sequencing: an embarrassment of choice, a world of opportunity. Nat Rev Microbiol 10:599–606. https://doi.org/10.1038/nrmicro2850
Luo YY, Not C, Cannicci S (2021) Mangroves as unique but understudied traps for anthropogenic marine debris: a review of present information and the way forward. Environ Pollut 271:116291. https://doi.org/10.1016/j.envpol.2020.116291
Maghsodian Z, Sanati AM, Ramavandi B, Ghasemi A, Sorial GA (2020) Microplastics accumulation in sediments and Periophthalmus waltoni fish, mangrove forests in southern Iran. Chemosphere 264:128543. https://doi.org/10.1016/j.chemosphere.2020.128543
Maret W (2016) The metals in the biological periodic system of the elements: concepts and conjectures. Int J Mol Sci 17:66. https://doi.org/10.3390/ijms17010066
Maria GL, Raviraja NS, Sridhar K (2005) Antimicrobial and enzyme activity of mangrove endophytic fungi of southwest coast of India. Int J Agric Technol 1:67–80. http://ijat-aatsea.com/pdf/Maria_Sridhar%20page%2067-80.pdf
Meyer-Cifuentes IE, Werner J, Jehmlich N et al (2020) Synergistic biodegradation of aromatic-aliphatic copolyester plastic by a marine microbial consortium. Nat Commun 11:5790. https://doi.org/10.1038/s41467-020-19583-2
Miao L, Wang P, Hou J et al (2019) Distinct community structure and microbial functions of biofilms colonizing microplastics. Sci Total Environ 650:2395–2402. https://doi.org/10.1016/j.scitotenv.2018.09.378
Mohamed Nor NH, Obbard JP (2014) Microplastics in Singapore’s coastal mangrove ecosystems. Mar Pollut Bull 79:278–283. https://doi.org/10.1016/j.marpolbul.2013.11.025
Moorkoth D, Nampoothiri KM (2016) Production and characterization of poly(3-hydroxy butyrate-co-3 hydroxyvalerate) (PHBV) by a novel halotolerant mangrove isolate. Bioresour Technol 201:253–260. https://doi.org/10.1016/j.biortech.2015.11.046
Mostafa YS, Alrumman SA, Otaif KA et al (2020) Production and characterization of bioplastic by polyhydroxybutyrate accumulating Erythrobacter aquimaris isolated from mangrove rhizosphere. Molecules 25:179. https://doi.org/10.3390/molecules25010179
Nabizadeh R, Sajadi M, Rastkari N, Yaghmaeian K (2019) Microplastic pollution on the Persian Gulf shoreline: a case study of Bandar Abbas city, Hormozgan Province. Iran Mar Pollut Bull 145:536–546. https://doi.org/10.1016/j.marpolbul.2019.06.048
Naji A, Esmaili Z, Khan FR (2017a) Plastic debris and microplastics along the beaches of the Strait of Hormuz, Persian Gulf. Mar Pollut Bull 114:1057–1062. https://doi.org/10.1016/j.marpolbul.2016.11.032
Naji A, Esmaili Z, Mason S, Vethaak A (2017b) The occurrence of microplastic contamination in littoral sediments of the Persian Gulf. Iran Environ Sci Pollut Res 24:20459–20468. https://doi.org/10.1007/s11356-017-9587-z
Naji A, Nuri M, Amiri P, Niyogi S (2019) Small microplastic particles (S-MPPs) in sediments of mangrove ecosystem on the northern coast of the Persian Gulf. Mar Pollut Bull 146:305–311. https://doi.org/10.1016/j.marpolbul.2019.06.033
Nakajima-Kambe T, Onuma F, Akutsu Y, Nakahara T (1997) Determination of the polyester polyurethane breakdown products and distribution of the polyurethane degrading enzyme of Comamonas acidovorans strain TB-35. J Ferment Bioeng 83:456–460. https://doi.org/10.1016/S0922-338X(97)83000-0
Nakamiya K, Sakasita G, Ooi T, Kinoshita S (1997) Enzymatic degradation of polystyrene by hydroquinone peroxidase of Azotobacter beijerinckii HM121. J Ferment Bioeng 84:480–482. https://doi.org/10.1016/S0922-338X(97)82013-2
Nazarov PV, Reinsbach SE, Muller A, Nicot N, Philippidou D, Vallar L, Kreis S (2013) Interplay of microRNAs, transcription factors and target genes: linking dynamic expression changes to function. Nucleic Acids Res 41:2817–2831. https://doi.org/10.1093/nar/gks1471
Neff H, Burger PH, Culleton BJ et al (2018) Izapa’s industrial hinterland: the eastern Soconusco mangrove zone during archaic and formative times. Anc Mesoam 29:395–411. https://doi.org/10.1017/S0956536118000299
NicAogáin K, O’Byrne CP (2016) The role of stress and stress adaptations in determining the fate of the bacterial pathogen Listeria monocytogenes in the food chain. Front Microbiol 7:1865. https://doi.org/10.3389/fmicb.2016.01865
Oberbeckmann S, Labrenz M (2020) Marine microbial assemblages on microplastics: diversity, adaptation, and role in degradation. Annu Rev Mar Sci 12:209–232. https://doi.org/10.1146/annurev-marine-010419-010633
Oberbeckmann S, Löder M, Labrenz M (2015) Marine microplastic-associated biofilms - a review. Environ Chem 12:551–562. https://doi.org/10.1071/EN15069
Othman A, Abu Hasan H, Muhamad M, et al (2021) Microbial degradation of microplastics by enzymatic processes: a review. Environ Chem Lett 19:3057–3073. https://doi.org/10.1007/s10311-021-01197-9
Owuor MA, Icely J, Newton A (2019) Community perceptions of the status and threats facing mangroves of Mida Creek, Kenya: implications for community based management. Ocean Coast Manag 175:172–179. https://doi.org/10.1016/j.ocecoaman.2019.03.027
Parida A, Jha B (2010) Salt tolerance mechanisms in mangroves: a review. Trees-Structure Funct 24:199–217. https://doi.org/10.1007/s00468-010-0417-x
Pillai N, Harilal CC (2018) Inventory on the diversity and distribution of mangroves from the coastal ecosystems of Kerala State, India. Int J Recent Sci Res 9:24002–24007. https://doi.org/10.24327/ijrsr.2018.0902.1579
Rujnić-Sokele M, Pilipović A (2017) Challenges and opportunities of biodegradable plastics: a mini review. Waste Manag Res 35:132–140. https://doi.org/10.1177/0734242X16683272
Sangale M, Shahnawaz M, Ade A (2019) Potential of fungi isolated from the dumping sites mangrove rhizosphere soil to degrade polythene. Sci Rep 9:1–11. https://doi.org/10.1038/s41598-019-41448-y
Sangeetha Devi R, Ramya R, Kannan K et al (2019) Investigation of biodegradation potentials of high density polyethylene degrading marine bacteria isolated from the coastal regions of Tamil Nadu, India. Mar Pollut Bull 138:549–560. https://doi.org/10.1016/j.marpolbul.2018.12.001
Sen N, Naskar K (2003) Algal flora of Sundarbans Mangal. Daya Publishing House, New Delhi, India
Shah AA, Hasan F, Hameed A, Ahmed S (2008) Biological degradation of plastics: a comprehensive review. Biotechnol Adv 26:246–265. https://doi.org/10.1016/j.biotechadv.2007.12.005
Shah Z, Krumholz L, Aktas DF et al (2013) Degradation of polyester polyurethane by a newly isolated soil bacterium, Bacillus subtilis strain MZA-75. Biodegradation 24:865–877. https://doi.org/10.1007/s10532-013-9634-5
Shen M, Zhu Y, Zhang Y et al (2019) Micro(nano)plastics: unignorable vectors for organisms. Mar Pollut Bull 139:328–331. https://doi.org/10.1016/j.marpolbul.2019.01.004
Smith SDA (2012) Marine debris: a proximate threat to marine sustainability in Bootless Bay, Papua New Guinea. Mar Pollut Bull 64:1880–1883. https://doi.org/10.1016/j.marpolbul.2012.06.013
Spalding M, Kainuma M, Collins L (2010) World Atlas of Mangroves, 1st edn. CRC Press, Taylor & Francis Group, London, UK
Suardy N, Abu Tahrim N, Ramli S (2020) Analysis and characterization of microplastic from personal care products and surface water in Bangi, Selangor. Sains Malays 49:2237–2249. https://doi.org/10.17576/jsm-2020-4909-21
Suyadi, Manullang CY (2020) Distribution of plastic debris pollution and its implications on mangrove vegetation. Mar Pollut Bull 160:111642. https://doi.org/10.1016/j.marpolbul.2020.111642
Teeraphatpornchai T, Nakajima T, Shigeno-Akutsu Y et al (2003) Isolation and characterization of a bacterium that degrades various polyester-based biodegradable plastics. Biotechnol Lett 25:23–28. https://doi.org/10.1023/A:1021713711160
Thompson RC, Olsen Y, Mitchell RP et al (2004) Lost at sea: where is all the plastic? Science 304:838. https://doi.org/10.1126/science.1094559
Tuson H, Weibel D (2013) Bacteria-surface interactions. Soft Matter 9:4368–4380. https://doi.org/10.1039/C3SM27705D
van Bijsterveldt CEJ, van Wesenbeeck BK, Ramadhani S et al (2021) Does plastic waste kill mangroves? A field experiment to assess the impact of macro plastics on mangrove growth, stress response and survival. Sci Total Environ 756:143826. https://doi.org/10.1016/j.scitotenv.2020.143826
Vertommen MAME, Nierstrasz VA, van der Veer M, Warmoeskerken MMCG (2005) Enzymatic surface modification of poly(ethylene terephthalate). J Biotechnol 120:376–386. https://doi.org/10.1016/j.jbiotec.2005.06.015
Wang WX (2016) Bioaccumulation and biomonitoring. In: Blasco J, Chapman PM, Campana O, Hampel M (eds) Marine Ecotoxicology: Current knowledge and future issues. Elsevier, New York, pp 99–119
Wei STS, Chen YL, Wu YW et al (2021) Integrated multi-omics investigations reveal the key role of synergistic microbial networks in removing plasticizer di-(2-ethylhexyl) phthalate from estuarine sediments. mSystems 6:e00358-21. https://doi.org/10.1128/mSystems.00358-21
Wilkes RA, Aristilde L (2017) Degradation and metabolism of synthetic plastics and associated products by Pseudomonas sp.: capabilities and challenges. J Appl Microbiol 123:582–593. https://doi.org/10.1111/jam.13472
Williams AT, Simmons SL (1996) The degradation of plastic litter in rivers: implications for beaches. J Coastal Conserv 2:63–72. https://doi.org/10.1007/BF02743038
Winkel BSJ (2017) When an enzyme isn’t just an enzyme anymore. J Exp Bot 68:1387–1389. https://doi.org/10.1093/jxb/erx080
Xie H, Chen J, Feng L et al (2021) Chemotaxis-selective colonization of mangrove rhizosphere microbes on nine different microplastics. Sci Total Environ 752:142223. https://doi.org/10.1016/j.scitotenv.2020.142223
Yang J, Yang Y, Wu W et al (2014) Evidence of polyethylene biodegradation by bacterial strains from the guts of plastic-eating waxworms. Environ Sci Technol 48:13776–13784. https://doi.org/10.1021/es504038a
Yoon MG, Jeon HJ, Kim MN (2012) Biodegradation of polyethylene by a soil bacterium and AlkB cloned recombinant cell. J Bioremed Biodegrad 3:1–8. https://doi.org/10.4172/2155-6199.1000145
Yoshida S, Hiraga K, Takehana T et al (2016) A bacterium that degrades and assimilates poly(ethylene terephthalate). Science 351:1196. https://doi.org/10.1126/science.aad6359
Yu K, Yi S, Li B et al (2019) An integrated meta-omics approach reveals substrates involved in synergistic interactions in a bisphenol A (BPA)-degrading microbial community. Microbiome 7:16. https://doi.org/10.1186/s40168-019-0634-5
Yuan J, Ma J, Sun Y et al (2020) Microbial degradation and other environmental aspects of microplastics/plastics. Sci Total Environ 715:136968. https://doi.org/10.1016/j.scitotenv.2020.136968
Zettler ER, Mincer TJ, Amaral-Zettler LA (2013) Life in the “plastisphere”: microbial communities on plastic marine debris. Environ Sci Technol 47:7137–7146. https://doi.org/10.1021/es401288x
Zhang L, Zhang S, Guo J et al (2020) Dynamic distribution of microplastics in mangrove sediments in Beibu Gulf, South China: implications of tidal current velocity and tidal range. J Hazard Mater 399:122849. https://doi.org/10.1016/j.jhazmat.2020.122849
Zhou Q, Tu C, Fu C et al (2020) Characteristics and distribution of microplastics in the coastal mangrove sediments of China. Sci Total Environ 703:134807. https://doi.org/10.1016/j.scitotenv.2019.134807
Zuo L, Sun Y, Li H et al (2020) Microplastics in mangrove sediments of the Pearl River Estuary, South China: correlation with halogenated flame retardants’ levels. Sci Total Environ 725:138344. https://doi.org/10.1016/j.scitotenv.2020.138344
Acknowledgements
A.K. gratefully acknowledges the funding support provided by Science and Engineering Research Board (SERB), Department of Science and Technology (DST) vide sanction order File No. EEQ/2019/000441 dated 2 December 2019, and the Central University of Kerala for extending other facilities. The authors gratefully acknowledge the three anonymous reviewers for their valuable comments and suggestions that helped improve this review.
Funding
The research on mangroves in the AK Lab is funded by the Science and Engineering Research Board (SERB), Department of Science and Technology (DST) vide sanction order File No. EEQ/2019/000441 dated 2 December 2019.
Author information
Authors and Affiliations
Contributions
S.P.M.: data curation, analysis, writing, review, editing, figure drafting, M.B.: initial draft, review, editing; A.N.: analysis, review, figures; A.K.: conceptualization and supervision, initial draft, figure drafting, editing, analysis, critical comments, and validation. All authors gave final approval of the version to be published.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Robert Duran.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Meera, S.P., Bhattacharyya, M., Nizam, A. et al. A review on microplastic pollution in the mangrove wetlands and microbial strategies for its remediation. Environ Sci Pollut Res 29, 4865–4879 (2022). https://doi.org/10.1007/s11356-021-17451-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-17451-0