Abstract
This study’s hypothesis is that carbofuran and copper sulfate have a synergistic harmful impact on the fertility of male Nile tilapia. Hence, this study was designed to assess the toxic reproductive outcome of carbofuran, copper sulfate, and their mixture in male Nile tilapia. Sixty male Nile tilapia fishes were separated into four groups (15 fish/group). The control group; carbofuran group, was given dechlorinated tap water containing 0.02 mg/L (1/10 dose of LC50) carbofuran; copper group was given dechlorinated tap water containing 4.0 mg/L (1/10 dose of LC50) copper sulfate; carbofuran + copper sulfate group received dechlorinated tap water containing 0.02 mg/L carbofuran plus 4.0 mg/L copper sulfate. After 6 weeks, results revealed a significant rise in testicular malondialdehyde levels and a significant decrease in testicular reduced glutathione contents among all experimental groups compared to the control group. Testicular testosterone levels were significantly declined in copper and combined groups compared to the control. The seminal evaluation using computer-assisted sperm analysis showed a significant decline in the progressive motility percentage, motile ratio percentage, sperm concentration, curvilinear velocity, straight-line velocity, average path velocity, and wobble in all intoxicated groups, particularly, the combined group. The histopathology of testes in all intoxicated groups revealed a detachment of the basal membrane of some seminiferous tubules, and others were free from spermatogonia and spermatozoa with interstitial eosinophilic granular cell infiltration. Testicular lesions were more severe in the combined group. Finally, it was concluded that carbofuran and copper sulfate exerted a negative effect on the reproductive function of male Nile tilapia, and they have a synergistic harmful impact on the fertility of male Nile tilapia.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recently, growing interest was directed towards manufacturing and agricultural contaminants in the aquatic environment and the probable side effects of such contamination on both human health and animal welfare (Guo et al. 2018; Mohamed et al. 2019). In developing countries, pesticide misuse and lack of legislation relevant to their usage are two of the most common causes of pesticide toxicity (Konradsen et al. 2003). Carbofuran is among the significant carbamate insect repellent used to eliminate a broad of insect pests in cultivated sectors. It is frequently scattered directly on soil and plants to control pests (EPA 1995). Although carbofuran is a highly hazardous pesticide as classified by the World Health Organization (WHO) and consequently banned in the USA and Europe (USEPA 2006), it is still used in Egypt. Shalaby and Abdou (2020) revealed that 48, 76, and 84% of the farmers, pesticide dealers, and spraying workers, respectively, in the Dakahlia Governorate, Egypt, had residues of 11 pesticides in their blood. Among these pesticides, carbofuran was found in blood samples of farmers, pesticide dealers, and spraying workers with an average of 0.02, 0.01, and 0.02 mg/kg BW, respectively. However, these concentrations were lower than the no observable adverse effect levels (NOAEL) (20 mg/kg BW); there was a direct relationship between the accumulation of pesticide residues and exposure time.
Copper is a vital bioactive trace metal in marine aquaculture and an essential micronutrient for various aquatic species (Zitoun 2019). Recently, different types of copper have diverse and specific uses; for example, copper sulfate is a well-known insecticide used to reduce algae growth in lakes and ponds. The free cupric ions, “the most toxic forms of copper”, are used to stop fungal spores from germination through deactivating fungal enzymes (Malhotra et al. 2020). However, copper at concentrations in water is toxic to aquatic organisms (Abdel-Khalek et al. 2015). Aqueous contamination with trace metals distresses numerous physiological functions in fish, like breeding and growth. Furthermore, trace metal toxicity depends on their take-up and accumulation by fish, leading to metal-associated disorders in both histoarchitecture and function of different organs (Jezierska and Witeska 2001). In the Nile River, Egypt’s main source of water, metals are the most common pollutant with contamination originating from various anthropogenic sources, such as industrial, agricultural, and home effluents. Because of its potential toxicity to humans and the environment, metal poisoning of surface water is a severe ecological hazard. The copper concentration in the Nile River varies between 5 and 51 µg/L according to the season and river flow (Abdel-Satar et al. 2017).
The Nile tilapia (Oreochromis niloticus) is an economically important tropical freshwater fish (Tunçsoy and Erdem 2018). Because of its fast growth rate, great tolerance to environmental stress, ease of reproduction, and high market demand, this species is an appropriate model to be used as an indicator species in biomonitoring programs (Gadagbui et al. 1996).
Fish reproduction is one of the most important biological aspects that allow species to survive and continue (Aly and Abouelfadl 2020). Therefore, reproduction is a crucial criterion for studying the effects of various toxic substances. To express the harmful effects of insecticides on reproduction, alterations in reproductive capacity, fertility, reproductive period, progeny viability, secretion of hormones, and genitals structure should be evaluated (Colborn et al. 1993; Kumar 2004). Computer-assisted sperm analysis (CASA) aids in estimating parameters of sperm motility that the human eye cannot judge. This technique is accomplished by tracking movement parameters of numerous spermatozoa by objective, sensitive, and accurate analyses per sample in an objective and repetitious manner. CASA analyses rest on various images and video micrographic procedures of spermatozoa pathways through an outfitted computer with imaging programming (Rurangwa et al. 2004).
In fact, fish exposure to mixtures of toxic chemical compounds can induce marked changes in their vital organs (Harabawy and Ibrahim 2014; Abe et al. 2019; Abdel-Khalek et al. 2020). However, there are no published data on whether there are synergistic, antagonistic, or no reactions between carbofuran and copper sulfate toxicities in male Nile tilapia. Therefore, it was hypothesized that carbofuran and copper sulfate has a synergistic harmful impact on the fertility of male Nile tilapia. So, this work studied the toxic effects of carbofuran and copper sulfate on the fertility of male Nile tilapia based on the assessment of oxidative/antioxidant status, hormonal estimation, seminal evaluation, and histopathological examination of the testis. To our knowledge, this is the first study in Egypt evaluating Nile tilapia fish semen using CASA.
Materials and methods
Chemicals
Carbofuran was presented as 10% granular form (Furadan 10% G® FMC Chemical, SPRL). Copper sulfate pent-hydrate (CuSO4.5H2O) was obtained from El-Nasr Pharmaceutical Chemical Company, Egypt. The remaining chemicals and reagents used for biochemical estimations were of analytical grade.
Fish
One hundred and eighty apparently healthy male Nile tilapia fishes (Oreochromis niloticus) (110 ± 5 g. BW) were obtained from a private fish ranch. Fish were shipped alive to the Faculty of Veterinary Medicine, Alexandria University, in metal tanks containing water supplemented with oxygen. The fish were reserved in hygienic glass aquaria (90 × 50 × 35 cm) provided with dechlorinated tap water. The oxygen supply was sustained in each aquarium using an electric aerator pump. Water temperature was preserved at 21.5 ± 2 °C all over the experimental period. The fish diet contained 25% crude protein. The diet was provided daily at 3% of body weight, and the daily amount of feed was offered on two accessions over the day. Before the start of the experiment, fishes were adapted for at least 2 weeks.
Determination of LC50 for carbofuran in male Nile tilapia fish for 96 h
Sixty male Nile tilapia fish were separated into six equal groups and were exposed to water containing five different concentrations of carbofuran (0.05, 0.1, 0.2, 0.4, 0.8 mg/L). Ten fish were kept as the control group.
Determination of LC50 for copper sulfate in male Nile tilapia fish for 96 h
Sixty male Nile tilapia fish were separated into six equal groups and exposed to water containing five different concentrations of copper sulfate (10, 20, 40, 80, 160 mg/ L). Ten fishes were kept as the control group.
In both LC50 experiments, the time was 96 h. LC50 was calculated according to Stephan’s method (1977) as follows: LC50 = (AB) ½, A = the minimum concentration of the tested substance that causes a 100% mortality, and B = the maximum concentration of the tested substance that causes a 100% viability.
Experimental design
A total number of 60 male Nile tilapia fish were separated into four groups (15 fish/group) as the following: control group, fish supplied with dechlorinated tap water free from any chemical; carbofuran group, fish supplied with 0.02 mg/L (1/10 dose of LC50) carbofuran; copper group, fish supplied with 4.0 mg/L (1/10 dose of LC50) copper sulfate; and carbofuran + copper sulfate group, fish supplied with 0.02 mg/L carbofuran plus 4.0 mg/L copper sulfate. Water and chemicals in aquaria were renewed every 3 days, and the experimental period was 6 weeks.
Malondialdehyde (MDA), reduced glutathione (GSH), and testicular testosterone estimation
Testicular specimens were collected from fish of each group during the 6th week of the experiment. Before dissection, tissue specimens were immersed in phosphate-buffered saline (PBS) solution (pH 7.4) holding 0.16 mg/mL heparin to eliminate any blood clots. The tissue homogenization was accomplished in 5–10 mL cold buffer (50 mM potassium phosphates, pH 7.5, 1 Mm EDTA)/g tissue and then centrifuged at 10,000 × g at 4 °C for 15 min. The supernatant was collected and stored frozen at − 70 °C for assay. Kits for MDA and GSH estimation were purchased from (Bio-diagnostic, Egypt). MDA was enumerated as described by Ohkawa et al. (1979) , and GSH level was assessed spectrophotometrically (Sedlak and Lindsay 1968). Testicular testosterone was estimated according to Demetrious (1987) using testosterone Elisa kit (Life span Biosciences, Inc.).
Seminal evaluation
The semen was collected from three fish of each group during the 6th week of the experiment under slight abdominal pressure. Semen samples were applied to capture video for assessment in open-source CASA software (Sanches et al. 2010). First, sperm cells were counted in a Neubauer hematometric chamber to estimate sperm concentration (Wirtz and Steinmann 2006). Next, the semen was diluted with distilled water at a ratio of 1:3. Next, 10 µL of the mixture placed in a Neubauer chamber to capture videos using a fixed (2 megapixel) digital camera to a trinocular microscope. Different configurations were tested to increase the capacity of CASA application for the identification of motile and immotile spermatozoa. Sperm motility was graded according to Wilson-Leedy and Ingermann (2007) as follows: class A (rapid progressive motility ≥ 25 µm/s at 37° C) exhibited a green track, class B (slow or sluggish progressive motility) exhibited a blue track, and class C (non-progressive motility ≤ 25 µm/s) exhibited a yellow track, while class D (immotile) exhibited a red track. Analyses of sperm motility were directly performed after the start of sperm activation. Semen samples were tested for progressive motility percentage (class A + class B), motile ratio percentage (class A + class B + class C), curvilinear velocity (VCL): velocity between each frame evaluated, average path velocity (VAP): velocity between each frame using an average path at a rate of 1/6 of the frame rate used, straight line velocity (VSL): velocity between the first and the last frame analyzed, the linearity (LIN): obtained by division of VSL by VAP, and wobble (WOB): obtained by division of VAP by VCL.
Histopathological studies
Testes of fish (three fish/group) during the 6th week of the experiment were collected and rapidly fixed in 10% neutral buffered formalin. The fixed specimens were dehydrated using ascending concentrations of ethanol, cleared in xylene, and embedded in paraffin wax at 60 °C. Five-micron thick paraffin sections were prepared. These sections were stained using hematoxylin and eosin (HE) (Bancroft and Gamble 2013).
Statistical analysis
The variables were reported as mean ± standard error of the mean. Data were analyzed by one-way analysis of variance (ANOVA). To satisfy the assumptions of ANOVA, percent data (e.g., sperm motility) were arcsine-transformed, and data of sperm velocity and sperm concentration were log-transformed to reduce skewing with back-transformation for reporting results. Each variable’s distribution was assessed using the Shapiro–Wilk’s test, and variance homogeneity among groups was checked using the Levene’s test. When the parametric assumptions were met, the parametric ANOVA with Tukey’s post-hoc for pairwise comparison was used. Otherwise, the non-parametric Kruskal–Wallis test and Dunn’s post-hoc test were the alternatives. A probability (P) value ≤ 0.05 was considered statistically significant. Statistical Analysis System software (SAS v9.1, SAS Institute Inc., Cary, NC, USA) was used to run all analyses.
Results
Testicular malondialdehyde (MDA), reduced glutathione (GSH), and testosterone estimation
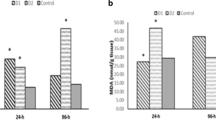
Testicular MDA levels showed a significant increase among all experimental groups compared to the control group. The greater elevation in testicular MDA levels was recorded in the copper sulfate fish group. While, testicular GSH levels showed a significant reduction among the experimental groups compared to control (Table 1), a marked decrease in testicular GSH levels was observed in the copper sulfate fish group. In comparison to the control group, the treatment of fish with carbofuran alone did not induce any significant difference in testicular testosterone. Simultaneously, there was a significant decrease in copper and combination groups (Table 1).
Seminal evaluation
Table 2 illustrates the seminal evaluation in all intoxicated groups. The intoxicated groups showed a significant decline in the progressive motility percentage, motile ratio percentage, VCL, VSL, VAP, LIN, and WOB compared to the control group. The sperm concentration showed a significant reduction in copper and combination groups. At the same time, it did not induce any significant difference in carbofuran alone compared to the control one. The straightness percentage showed a significant decrease in the carbofuran and copper group but did not show any significant difference in the combination group compared to the control one.
Histopathological findings
Table 3 summarizes the severity of histopathological lesions of testes of all intoxicated groups. Histopathological examination of testes of Nile tilapia fish of the control group showed a typical histological structure of the seminiferous tubules (Fig. 1a). While testes of Nile tilapia fish treated with carbofuran revealed a detachment of the basal membrane of some seminiferous tubules (Fig. 1b), the presence of oocyte inside the testicular tissue (Fig. 1c, d) besides hyalinization of the luminal content of some seminiferous tubules (Fig. 1e) and most of the seminiferous tubules had low sperm density (Fig. 1f). Moreover, testes of Nile tilapia fish treated with copper sulfate showed diffuse detachment of the basal membrane of seminiferous tubules (Fig. 2a) and congestion in the interstitial blood vessels with eosinophilic granular cells (EGCs) infiltration (Fig. 2b) in the interstitium besides most of the seminiferous tubules had few dark blue spermatozoa (Fig. 2c). Furthermore, the testis of Nile tilapia fish of the combination group showed detachment with an irregular basal membrane of some seminiferous tubules (Fig. 2d) with eosinophilic proteinaceous material or hyalinization of the luminal content of some seminiferous tubules (Fig. 2e) besides partial loss of some spermatogenic cell layers and a reduction in spermatogenesis as most of the seminiferous tubules were devoid or had little spermatogonia and spermatozoa (Fig. 2f).
Photomicrograph of Nile tilapia testis stained with HE (Bar = 100 µm), a and d (Bar = 50 µm). (a) Control group showing the normal histological structure of the seminiferous tubules. (b, c, d, e, and f). Carbofuran group showing detachment of the basal membrane of some seminiferous tubules (arrows), presence of oocyte inside the testicular tissue (arrowheads) besides hyalinization of the luminal content of some seminiferous tubules (asterisks) and most of seminiferous tubules had low sperm density
Photomicrograph of Nile tilapia testis stained with HE (Bar = 100 µm). (a, b, and c) Copper group showing diffuse detachment of the basal membrane of seminiferous tubules (arrows) and congestion in the interstitial blood vessels (stars) with EGCs infiltration (blue arrowheads) in the interstitial besides most of seminiferous tubules had few dark blue spermatozoa (d, e, and f) Combination group showing detachment (arrows) with irregular (short arrow) and detachment (arrows) basal membrane of some seminiferous tubules with eosinophilic proteinaceous material or hyalinization of the luminal content of some seminiferous tubules (asterisks) besides most of seminiferous tubules were devoid or had little spermatogonia and spermatozoa
Discussion
Owing to the growing contamination, levels of diverse chemical compounds have been amplified in aquatic environments. The possibility of reproductive toxic effects of carbofuran, copper sulfate, and their interaction in male Nile tilapia was evaluated. Previous studies have demonstrated the synergistic interaction between chemical pollutants. Barbieri et al. (2019) evidenced that when multiwalled carbon nanotubes were combined with carbofuran, they markedly reduced oxygen consumption and ammonia excretion, influencing the Astyanax ribeirae fish physiologically. Abdel-Hamid et al. (2021) reported that in Nile tilapia, a combination of copper sulfate and jasper herbicide caused more severe toxicity than each one individually. Similar findings were shown in this investigation, which found that combining carbofuran with copper sulfate had a negative impact on the fertility in male Nile tilapia. The toxicity of various contaminants to aquatic organisms is correlated with oxidative stress due to the production of reactive oxygen species (ROS). The augmentation of ROS production is involved in male infertility owing to lipid peroxidation, effect on sperm movement, and DNA damage that may enhance apoptosis of germinal cells, consequently a decline in sperm count (Ong et al. 2002; Agarwal et al. 2003; Baker and Aitken 2004). There was a significant rise in MDA with a significant reduction in GSH levels in testicular tissue of all intoxicated fish groups in this study; however, statistically, marked changes were observed in the copper sulfate alone. These results were parallel to those of Kirici et al. (2017), who recorded a significant elevation in MDA and a significant decline in GSH levels in all tissues of acpoeta umbla fish treated with copper sulfate. In contrast, Shaw et al. (2012) did not show any significant change in the GSH level of rainbow trout fish treated with copper sulfate. Also, Ibrahim and Harabawy (2014) recorded decreased levels of GSH in all tissues of African catfish treated with carbofuran. A recent study by Hamed et al. (2021) revealed that MDA levels in the liver and kidneys of carbofuran-exposed fish increased, while GSH levels decreased.
The primary goal of animal reproductive system development is to manufacture fertile gametes. As a result, male gametes (sperm) quality plays a critical role in fish reproductive success (Mishu et al. 2020). Fertilization and hatching processes depend on the sperm quality plus the capability of sperm to move sufficiently fast to meet the egg in the water next to their release (Kholodnyy et al. 2020).
Generally, fish spermatozoa are characterized by short-lived motility. Since the minimal time accessible for fertilization after sperm arrival into water (15 s), any harmful impact on sperm motility may drastically diminish fertilization achievement (Gage et al. 2004). Sperm motility features are susceptible and consistent pointers of aquatic pollution (Kime et al. 2001; Jobling et al. 2002; Lahnsteiner et al. 2004; Abascal et al. 2007).
These results showed a marked reduction in sperm motility parameters (progressive motility, motile ratio, VCL, VSL, VAP, LIN, and WOB %) in all intoxicated fish groups; a marked reduction was in the combined group. These findings are comparable to those of Zhou et al. (2006), who documented the significant decrease in sperm motility parameters (VCL, VSL, and VAP) after in vitro exposure to duroquinone. Lately, Mishu et al. (2020) reported that the incubation of semen of silver barb (Barbonymus gonionotus) with quinalphos for 2 h had a significant adverse effect on sperm motility parameters (VCL, VSL, motility ratio, and LIN). A decrease in sperm motility due to copper sulfate treatment was reported by Bombardelli et al. (2016) in silver catfish. Also, a marked reduction in VSL, VCL, VAP, and LIN was reported after exposure of sea bass fish to copper sulfate (Abascal et al. 2007). Moreover, these findings of straightness percentage and wobble percentage values in the copper sulfate group were parallel to those reported in sea bass by Abascal et al. (2007) and in Isochrysis galbana in a dose-dependent manner post-copper treatment (Liu et al. 2011). While in Tetraselmis chui, insignificant alterations in straightness percentage and wobble percentage were observed after exposure to different copper concentrations (Liu et al. 2011). In this study, sperm count showed a significant reduction in copper sulfate and the combined groups parallel with a significant decrease in testicular testosterone. The marked reduction was noticed in the combined group, referring to the synergistic action of both compounds. This is understandable because testosterone is required for spermatogenesis and sperm viability (Di Guardo et al. 2020). In the same way, histopathological examination of testis showed depletion in spermatogonial cell number and empty seminiferous tubules from spermatogonia and spermatozoa in fish treated with copper sulfate alone and carbofuran + copper sulfate. Marked feminization phenomena were noticed and represented by the formation of mature oocytes inside the testicular tissue and within the seminiferous tubules in fish intoxicated with carbofuran only. Afonso et al. (2001) recorded the presence of oocytes in the central testicular tissue in the Nile tilapia fish treated with 50 mg fadrozole/kg for 30 days.
Finally, it was concluded that carbofuran and copper sulfate exerted a negative effect on the reproductive function of the male Nile tilapia, and they have a synergistic harmful impact on the fertility of male Nile tilapia. Further studies are required to mitigate the harmful effect of environmental pollution using different co-contaminants, including pesticides and other chemical compounds like copper sulfate.
Data availability
All data generated and analyzed during our study are included in this article.
References
Abascal FJ, Cosson J, Fauvel C (2007) Characterization of sperm motility in sea bass: the effect of heavy metals and physicochemical variables on sperm motility. J Fish Biol 70:509–522
Abdel-Hamid EAA, Hashem SH, Ghareib AA (2021) The effect of a mixture of environmental pollutants on some biomarkers in the Nile tilapia (Oreochromis niloticus). Egypt J Aquat Biol Fish 25:659–675
Abdel-Khalek AA, Badran SR, Marie MS (2020) The efficient role of rice husk in reducing the toxicity of iron and aluminum oxides nanoparticles in Oreochromis niloticus: hematological, bioaccumulation, and histological endpoints. Water Air Soil Pollut 231:1–10
Abdel-Khalek AA, Kadry MAM, Badran SR, Marie MS (2015) Comparative toxicity of copper oxide bulk and nano particles in Nile tilapia; Oreochromis niloticus:biochemical and oxidative stress. J Basic Appl Sci 72:43–57
Abdel-Satar AM, Ali MH, Goher ME (2017) Indices of water quality and metal pollution of Nile River. Egypt Egypt J Aquat Res 43:21–29
Abe FR, Machado AA, Coleone AC, Cruz C, Machado-Neto JG (2019) Toxicity of diflubenzuron and temephos on freshwater fishes: ecotoxicological assays with Oreochromis niloticus and Hyphessobrycon eques. Water Air Soil Pollut 230:1–10
Afonso LO, Wassermann GJ, De Oliveira RT (2001) Sex reversal in Nile tilapia (Oreochromis niloticus) using a nonsteroidal aromatase inhibitor. J Exp Zool 290:177–181
Agarwal A, Saleh RA, Bedaiwy MA (2003) Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril 79:829–843
Aly W, Abouelfadl KY (2020) Impact of low-level water pollution on some biological aspects of redbelly tilapia (Coptodon zillii) in River Nile. Egypt Egypt J Aquat Res 46:273–279
Baker MA, Aitken RJ (2004) The importance of redox regulated pathways in sperm cell biology. Mol Cell Endocrinol 216:47–54
Bancroft JD, Gamble M (2013) Theory and practice of histological techniques, 7th edn. Churchill Livingstone, Edinburgh, p 252
Barbieri E, Ferrarini AMT, Rezende KFO, Martinez DST, Alves OL (2019) Effects of multiwalled carbon nanotubes and carbofuran on metabolism in Astyanax ribeirae, a native species. Fish Physiol Biochem 45:417–426
Bombardelli RA, Neumann G, Toledo CP, Sanches EA, Bastos DN, Oliveira JD (2016) Sperm motility, fertilization, and larval development of silver catfish (Rhamdia quelen) in copper-contaminated water. Semin Cienc Agrar 37:1667–1678
Colborn T, vom Saal FS, Soto AM (1993) Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ Health Perspect 101:378–384
Demetrious JA (1987) Testosterone in methods. In: Methods in clinical chemistry, Pesce AJ, Kaplan LA (Eds.). Mosby, Los Angles, p 268
Di Guardo F, Vloeberghs V, Bardhi E, Blockeel C, Verheyen G, Tournaye H, Drakopoulos P (2020) Low testosterone and semen parameters in male partners of infertile couples undergoing IVF with a total sperm count greater than 5 million. J Clin Med 9:3824
EPA US (1995) Carbofuran. Office of Water, U.S. Environmental Protection Agency, Washington, D.C.
Gadagbui BKM, Addy M, Goksøyr A (1996) Species characteristics of hepatic biotransformation enzymes in two tropical freshwater teleosts, tilapia (Oreochromis niloticus) and mudfish (Clarias anguillaris). Comp Biochem Physiol 114C:201–211
Gage MJ, Macfarlane CP, Yeates S, Ward RG, Searle JB, Parker GA (2004) Spermatozoal traits and sperm competition in Atlantic salmon: relative sperm velocity is the primary determinant of fertilization success. Curr Biol 14:44–47
Guo SN, Zheng JL, Yuan SS, Zhu QL (2018) Effects of heat and cadmium exposure on stress-related responses in the liver of female zebrafish: heat increases cadmium toxicity. Sci Total Environ 618:1363–1370
Hamed HS, Ismal SM, Faggio C (2021) Effect of allicin on antioxidant defense system, and immune response after carbofuran exposure in Nile tilapia, Oreochromis niloticus. Biochem Physiol C Toxicol Pharmacol 240:108919
Harabawy ASA, Ibrahim ATA (2014) Sublethal toxicity of carbofuran pesticide on the African catfish Clarias gariepinus (Burchell, 1822): hematological, biochemical and cytogenetic response. Ecotoxicol Environ Saf 103:61–67
Ibrahim AT, Harabawy AS (2014) Sublethal toxicity of carbofuran on the African catfish (Clarias gariepinus): hormonal, enzymatic and antioxidant responses. Ecotoxicol Environ Saf 106:33–39
Jezierska B, Witeska M (2001) Metal toxicity to fish. University of Podlasie Publisher, Siedlce, p 318
Jobling S, Coey S, Whitmore JG, Kime DE, Van Look KJ, McAllister BG, Beresford N, Henshaw AC, Brighty G, Tyler CR, Sumpter JP (2002) Wild intersex roach (Rutilus rutilus) have reduced fertility. Biol Reprod 67:515–524
Kholodnyy V, Gadêlha H, Cosson J, Boryshpolets S (2020) How do freshwater fish sperm find the egg? The physicochemical factors guiding the gamete encounters of externally fertilizing freshwater fish. Rev Aquacult 12:1165–1192
Kime DE, Van Looka KJ, McAllister BG, Huyskensb G, Rurangwab E, Ollevier F (2001) Computer-assisted sperm analysis (CASA) as a tool for monitoring sperm quality in fish. Comp Biochem Phys C 130:425–433
Kirici M, Turk C, Caglayan C, Kirici M (2017) Toxic effects of copper sulphate pentahydrate on antioxidant enzyme activities and lipid peroxidation of freshwater fish acpoeta umbla (Heckel, 1843) tissues. Appl Ecol Environ Res 15:1685–1696
Konradsen F, Van der Hoek W, Cole DC, Hutchinson G, Daisley H, Singh S, Eddleston M (2003) Reducing acute poisoning in developing countries-options for restricting the availability of pesticides. Toxicol 192:249–261
Kumar S (2004) Occupational exposure associated with reproductive dysfunction. J Occup Health 46:1–19
Lahnsteiner F, Mansour N, Berger B (2004) The effect of inorganic and organic pollutants on sperm motility of some freshwater teleosts. J Fish Biol 65:1283–1297
Liu G, Chai X, Shao Y, Hu L, Xie Q, Wu H (2011) Toxicity of copper, lead, and cadmium on the motility of two marine microalgae Isochrysis galbana and Tetraselmis chui. JBES 23:330–335
Malhotra N, Ger T, Uapipatanakul B, Huang J, Chen K, Hsiao C (2020) Review of copper and copper nanoparticle toxicity in fish. Nanomaterials 10:1126
Mishu AM, Mostakim GM, Khatun MtM, Rahman MdK, Shahjahan Md, Islam MS (2020) Sperm movement and morphological changes in the silver barb (Barbonymus gonionotus) exposed to quinalphos. Environ Sustainab Indic 8:100083
Mohamed WA, El-Houseiny W, Ibrahim RE, Abd-Elhakim YM (2019) Palliative effects of zinc sulfate against the immunosuppressive, hepato- and nephrotoxic impacts of nonylphenol in Nile tilapia (Oreochromis niloticus). Aquac 504:227–238
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Ong CN, Shen HM, Chia SE (2002) Biomarkers for male reproductive health hazards: are they available? Toxicol Lett 134:17–30
Rurangwa E, Kime DE, Ollevier F, Nash JP (2004) The measurement of sperm motility and factors affecting sperm quality in cultured fish. Aquac 234:1–28
Sanches EA, Bombardelli RA, Marcos RM, Neumann G, Rebechi de Toledo CP, Romagosa E (2010) Sperm motility of Rhamdia quelen studied using computer-assisted analysis by open-source software. Aquac Res 42:153–156
Sedlak J, Lindsay RH (1968) Estimation of total protein-bound and nonprotein sulfhygroups in tissue with Ellman’s reagent. Anal Biochem 25:192–205
Shalaby SEM, Abdou GY (2020) Assessment of pesticide residues in blood samples of agricultural workers in Egypt. J Plant Prot Res 60:369–376
Shaw BJ, Al-Bairuty G, Handy RD (2012) Effects of waterborne copper nanoparticles and copper sulphate on rainbow trout (Oncorhynchus mykiss): physiology and accumulation. Aquat Toxicol 116–117:90–101
Stephan C (1977) Methods for calculating an LC50. In: Mayer F, Hamelink J (eds) Aquatic toxicology and hazard evaluation. ASTM International, West Conshohocken, pp 65–84
Tunçsoy M, Erdem C (2018) Copper accumulation in tissues of Oreochromis niloticus exposed to copper oxide nanoparticles and copper sulphate with their effect on antioxidant enzyme activities in liver. Water Air Soil Pollut 229:269
USEPA (2006) Interim reregistration eligibility decision–carbofuran. U.S. Environmental Protection Agency, Washington DC EPA- 738-R: 706–031
Wilson-Leedy JG, Ingermann RL (2007) Development of a novel CASA system based on open source software for characterization of zebra fish sperm motility parameters. Theriogenol 67:661–672
Wirtz S, Steinmann P (2006) Sperm characteristics in perch Perca fluviatilis L. J Fish Biol 68:1896–1902
Zhou B, Liu W, Siu WH, Toole DO, Lam PK, Wu RS (2006) Exposure of spermatozoa to duroquinone may impair reproduction of the common carp (Cyprinus carpio) through oxidative stress. Aquat Toxicol 77:136–142
Zitoun R (2019) Copper speciation in different marine ecosystems around New Zealand (Thesis, Doctor of Philosophy) University of Otago, Dunedin
Acknowledgements
We would thank Professor Dr. Mahmoud M. A. Elmaghraby for performing the statistical analyses.
Author information
Authors and Affiliations
Contributions
Conceptualization and methodology: Tohamy HG and Abou-Srag MA. Investigation and data collection were performed by Abou-Srag MA, Oda SS, and Tohamy HG. Figures were made by Tohamy HG. Oda SS and Tohamy HG wrote the manuscript. The supervision was by El-Manakhly EM. All the authors read and agreed the final manuscript. Oda SS is the corresponding author.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate and consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by Bruno Nunes.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Oda, S.S., El-Manakhly, ES.M., Abou-Srag, M.A. et al. Assessment of reproductive toxicity of carbofuran and copper sulfate in male Nile tilapia (Oreochromis niloticus). Environ Sci Pollut Res 29, 15896–15904 (2022). https://doi.org/10.1007/s11356-021-16965-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-16965-x