Abstract
2,4,6-Tribromophenol (TBP, CAS No. 118-79-6), the most widely produced brominated phenol, is frequently detected in environmental components. The detection of TBP in human bodies has earned great concerns about its adverse effects on human beings, especially for early embryonic development. Here, we optimized the mouse embryo in vitro culture (IVC) system for early post-implantation embryos and employed it to determine the embryotoxicity of TBP. With this new research model, we revealed the dose-dependent toxic effects of TBP on mouse embryos from peri-implantation to egg cylinder stages. Furthermore, TBP exposure inhibited the differentiation and survival of epiblast (EPI) cells and extraembryonic endoderm (ExEn) cells, while those of extraembryonic ectoderm (ExEc) cells were not influenced. These results implied that TBP might inhibit embryonic development by influencing the generation of three primary germ layers and fetal membranes (the amnion, chorionic disk, umbilical cord, and yolk sac). In summary, we showed a proof of concept for applying mouse embryo IVC system as a novel research model for studying mammalian embryonic toxicology of environmental pollutants. This study also demonstrated the toxicity of TBP on early embryonic development of mammals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
2,4,6-tribromophenol (TBP) is a brominated phenol that widely used as a brominated flame retardant (BFR), intermediate to produce other BFRs, and fungicide for wood preservative (Koch and Sures 2018). In 2001, the production volume of TBP approximately reached 9500 t/year worldwide (Agency 2016). In addition to the industrial origins, TBP can also be produced and secreted by a huge variety of marine organisms like algae, bryozoans, and polychaetes to defend against predators and biofouling (Flodin and Whitfield 1999; Whitfield et al. 1999). Furthermore, food processing and water treatment also facilitate the production of TBP as a by-product(Boudjellaba et al. 2016; Mizukawa et al. 2017; Richardson and Postigo 2015; Yang et al. 2014; Zhai and Zhang 2011). Given the multiple sources, TBP has been detected in various environmental components like soil (Ronen et al. 2005), house dust (15–620 ng/g) (Suzuki et al. 2008; Takigami et al. 2009), indoor (220–690 pg/m3) and outdoor air (49–73 pg/m3) (Takigami et al. 2009), surface freshwater (up to 0.32 μg/L) (Xiong et al. 2015) and groundwater (Blythe et al. 2006), sewage and sludge (Oberg et al. 2002), and drinking water (up to 56.9ng/L) (Pan et al. 2016).
Daily food and water ingestion, and exposure to the environmental components containing TBP provide the primary routes for TBP to enter human bodies (Koch and Sures 2018). Recent studies have revealed that TBP can be found in human serum (up to 19.2 ng/g) (Butt et al. 2016; Dufour et al. 2017), breast milk (0.59 ng/g) (Fujii et al. 2018), and urine (5.57 μg/L) (Feng et al. 2016). Furthermore, it has been reported that the concentration of TBP in fetal blood was roughly sixfold higher than that of the maternal blood (Qiu et al. 2009), and approximately 1.31–316 ng/g of TBP was also detected in human placental tissues (Leonetti et al. 2016), which was expected to contribute to prenatal exposures. Therefore, whether TBP is capable of bioaccumulation and toxic to human embryonic development has earned more and more concerns.
Although the neurological, reproductive, and developmental toxicity of TBP has been well studied in aquatic animals (Deng et al. 2010; Folle et al. 2020; Haldén et al. 2010; Liu et al. 2011; Kammann et al. 2006), it is just the beginning in mammals. Rodents have been widely used as animal models to study the process of mammalian development, the initiation and progression of diseases, and the toxicity of chemicals or environmental pollutants. In pregnant rats, TBP could be detected in placenta and fetuses just 24 h after a single maternal exposure (Knudsen et al. 2020). It has been reported that TBP exposure could compromise the function of the blood-brain barrier in both rats and mice by decreasing the activity of protective efflux transporter (Trexler et al. 2019), and similar effects might also exist in the placenta (Leonetti et al. 2016). Therefore, the protective roles of the placenta for the developing fetus might not be complete. Long-term exposure of pregnant rats to aerosolized TBP resulted in both pre-implantation and post-implantation embryo losses in a dose-dependent manner, and the offspring also exhibited skeletal malformations and visceral abnormalities (Lyubimov et al. 1998). However, whether the toxic effects on the development of conceptus (including extraembryonic tissues as well as the fetus) were caused by TBP itself remains to be determined. Furthermore, whether the TBP dosimetry–embryotoxicity relationships also differ with embryonic developmental stages remains to be unraveled.
The rodent whole embryo culture (WEC) technique, a method by which post-gastrulation embryos are isolated and cultured in an artificial womb, has enabled detailed investigations into the regulation of normal embryonic development as well as mechanisms underlying embryotoxicity induced by a wide range of reagents ex uterus (Ellis-Hutchings and Carney 2010). Andrew et al. had employed the WEC technique to demonstrate that combined exposure of multiple disinfection by-products(DBPs) to transferred gestational day 9.5 (GD9.5) rat embryos significantly increased the incidence of tail development delay (Andrews et al. 2004). When embryonic day 8.0 (E8.0) mouse embryos were isolated for in vitro culture, exposure to dichloroacetic acid, dibromoacetic acid, and bromochloroacetic acid would destroy the subsequent embryogenesis (Hunter 3rd et al. 2006). However, since only a brief period of embryogenesis from pre-somite to the limb bud stages can be recaptured with the WEC technique, it is not applicable to toxicological studies focusing on early developmental stages.
Although the in vitro culture (IVC) system for post-implantation mouse embryos has been established since 1971 (Bedzhov et al. 2014; Hsu 1971; Morris et al. 2012; Wu et al. 1981), its application in embryotoxicity studies was not reported until recently. Gordeeva et al. employed IVC system to assess the toxic and teratogenic effects of 5-hydroxytryptophan(5-HTP) on mouse embryos at early developmental stages (Gordeeva and Gordeev 2021). However, due to the asynchronous development of mouse embryos and unpredictable development endpoints, they only selected stage and morphology synchronized embryos for further testing at the pre-implantation, peri-implantation, and early post-implantation stages, separately (Gordeeva and Gordeev 2021). Therefore, the establishment of validated protocols for continuous testing through multiple embryonic development stages will facilitate the application of the IVC system in embryonic toxicology and teratology.
In this study, we modified the mouse embryo IVC system by embedding the E3.5 blastocysts in three-dimensional(3D) Matrigel drops instead of seeding in the tissue culture plate directly. Furthermore, we set new selection standards for the embryos with high developmental potential to develop into the egg cylinder stage in culture. With these modifications, we improved the repeatability of mouse embryo IVC system and made it suitable for toxic evaluation. Finally, by combining the new 3D culture system and non-invasive and non-destructive assays like immunofluorescence imaging, we determined the toxic effects of TBP on cell survival and lineage specification in early post-implantation mouse embryos.
Materials and methods
Chemical
The stocking solution of TBP (TCI Shanghai, T0349) was prepared in DMSO (Solarbio, D8371) at the concentration of 400 mM and detailed information about TBP was provided in Table S1 of supplemental materials.
Mouse embryo recovery
ICR mice (6–8 weeks) were obtained from the Animal Core Facility of Nanjing Medical University (Nanjing, China). Animal maintenance and experiments were approved by the Ethics Committee of the Animal Core Facility of Nanjing Medical University (Nanjing, China).
Superovulation was carried out by intraperitoneal injection of 10 IU of pregnant mare serum gonadotrophin (PMSG) (Ningbo Second Hormone Factory, 181108) followed by 10 IU of human chorionic gonadotrophin (HCG) (Ningbo Second Hormone Factory, 180428) 46–48 h later. To obtain the embryos, the female mice were mated with males overnight and vaginal plugs were checked on the following morning. The day of plug detection was counted as E0.5.
To collect the E3.5 embryos, female mice were sacrificed and their uterus were dissected and flushed with prewarmed DMEM/F12 (Thermo Fisher Scientific, C11330500BT) supplemented with 1% FBS (Thermo Fisher Scientific,10270106). The E3.5 embryos were treated with Tyrode’s solution, Acidic (Sigma-Aldrich, T1788) for 1–2 min to remove the zona pellucida and transferred for further culture.
To collect the E5.5 and E6.5 embryos which were developed in vivo, the decidua and outside membrane layers including Reichert’s membrane were removed with forceps under a surgical microscope (Nikon SMZ1270, Japan). Then, these in vivo developed E5.5 and E6.5 embryos were fixed immediately with ice-cold 4% paraformaldehyde (PFA, Ding Guo, AR-0211) for further immunofluorescence analysis.
To collect the IVC cultured E5.5- and E6.5-like embryos, we washed embryos briefly with ice-cold PBS for 2 min to remove Matrigel for further analysis.
Embryo culture and toxicity evaluation
Freshly isolated E3.5 embryos were directly seeded into 24-well tissue culture plates (Corning, 3526) or embedded in Matrigel drops to reach the pre-egg cylinder or egg cylinder stage (equivalent to in vivo developed embryos at E5.5 and E6.5, respectively).
Embryos cultured in tissue culture plates were added 250 μL of IVC1 medium to each well of the 24-well tissue culture plates, and the embryos were transferred into the wells by pipette. Then, the plates were incubated in the 5% CO2 incubator at 37°C. After all the embryos attached stably to the bottom of the well, the IVC1 medium was replaced with 250-μL equilibrated IVC2 medium. Thereafter, changed the IVC2 medium every 48 h.
Embryos cultured with Matrigel drops were given deposited drops (15 μL) of ice-cold Growth Factor-Reduced Matrigel (Corning, 354230) to the bottom of each well of the 24-well plate and embedded 3–4 embryos in each drop. Then, the plates were incubated in a 5% CO2 incubator at 37°C for about 15–20 min to allow the matrix to solidify, then filled each well with 350 μL IVC1 medium to cover the drops. After 48 h, aspirated the IVC1 medium and refed the embryos with IVC2 medium. Thereafter, changed the IVC2 medium every 48 h. The embryos were cultured in a 5% CO2 incubator at 37°C until further analysis.
To evaluate the embryonic toxicity of TBP, the E3.5 embryos were embedded in Matrigel drops and fed with IVC1 medium for the first 48 h, and changed into IVC2 medium supplemented with TBP at the concentrations of 0, 25, 50, and 100 μM until further analysis.
The formulas of IVC1 and IVC2 medium were described in Text S1 of supplemental materials in detail.
Morphological evaluation of embryos
The morphology of cultured mouse embryos was assessed with an inverted phase-contrast microscopy (Nikon Ts2R, Japan). Image processing and the measurement of pixel area of mouse embryos were performed with FIJI software (V2.0.0, National Institutes of Health, USA).
Immunofluorescence assay
The embryos freshly isolated from female mice or cultured in Matrigel drops were fixed with ice-cold 4% PFA (Ding Guo, AR-0211) for 15 min at room temperature (RT) and rinsed three times with PBS (Thermo Fisher Scientific, C10010500BT). Then the samples were permeabilized with PBS containing 0.2% (vol/vol) Triton X-100 (Beyotime, P0096) at RT for 25-30 min, and blocked with PBS containing 2.5% (wt/vol) normal donkey serum (Jackson ImmunoResearch, 017-000-121), 0.1% (vol/vol) Tween-20 (Beyotime, ST825), and 0.1% (wt/vol) bovine serum albumin (Beyotime, ST023) (hereafter, blocking buffer) at RT for 45 min. Primary antibodies diluted in blocking buffer were applied to the embryos and incubated at 4°C overnight. The samples were rinsed for three times with PBS and followed by incubating with fluorescence-conjugated secondary antibodies diluted in PBS containing 2.5% normal donkey serum at RT for 1 h in the dark. The nuclei were stained with 1 μg/mL 4′,6-diamidi-no-2-phenylindole (DAPI) (YIFEIXUE BIO TECH, YD0020-10). At last, all these samples were imaged by a confocal microscope (ZEISS LSM700, Germany) and processed with ZEN 2012 software (ZEISS, Germany).
The antibodies used here were listed in Table S2 of supplemental materials.
Total RNA extraction and real-time quantitative PCR analysis
The embryos of TBP-treated and control groups, which had been cultured in Matrigel drops for 96 h (pre-egg cylinder stage) or 120 h (egg cylinder stage) separately, were collected and washed briefly with PBS for 2 min to remove Matrigel. Then all the embryos were suspended in a lysis buffer and RNA was extracted with RNeasy Plus Micro Kit (QIAGEN, USA). The first-strand cDNA was synthesized with EasyScript® One-Step gDNA Removal and cDNA Synthesis SuperMix (TransGene, China) and random primers according to manuals. PCR reactions were performed in an ABI 2720 Thermal Cycler (Thermo Scientific, USA).
Real-time quantitative PCR were carried out with StepOneTM Real-Time PCR System (Thermo Scientific, USA) using ChamQ Universal SYBR qPCR Master Mix (Vazyme, China). The expression of GAPDH was used as an internal standard for normalization with the ΔΔCt method. The primers used in this assay were listed in Supplementary materials Table S3.
Caspase-3/7 activation assay
To assess the effect of TBP on cell apoptosis in cultured mouse embryos, the caspase-3/7 activation analysis was performed. Briefly, CellEventTM Caspase-3/7 Green Detection Reagent (Thermo Fisher Scientific, C10723) was added into the embryo culture medium to a final concentration of 1 μM and then incubated at 37 °C for 30 min in the dark. Then the embryos were imaged with an inverted fluorescent microscope (Nikon Ts2R, Japan). Detailed information about this reagent was described in Text S2 of supplemental materials.
Statistical analysis
Statistical analysis was performed using the GraphPad Prism program (V8.2, GraphPad Software, San Diego, CA, USA). Embryos were randomly allocated to control and experimental groups. Investigators were not blinded to group allocation. Values were expressed as the means ± S.D. from three independent experiments and evaluated by using a two-sided unpaired Student’s t-test at a statistical significance level of p < 0.05.
Results
Mouse embryos developed beyond the blastocyst stage in Matrigel drops
To determine the toxicity of environmental hazards on mouse embryonic development between pre-implantation and early post-implantation stages in vitro, we must build a reproducible IVC system for mouse embryos, which would permit continuous, real-time tracking of the transition from the pre-implantation blastocyst to the post-implantation egg cylinder.
We first tried to obtain mouse embryos at the egg cylinder stage by seeding E3.5 blastocysts into tissue culture plates and feeding them with IVC1 and IVC2 medium in sequence. However, only about 20% of the blastocysts developed into egg cylinders successfully (Fig. S1a, S1b), which limited their applications in embryonic toxicology. The low egg cylinder formation efficiency in our hands might result from the random attachment of blastocysts to the substrate since the initial positioning of the inner cell mass (ICM) at the time of attachment greatly influences egg cylinder formation in vitro (Morris et al. 2012; Wu et al. 1981). Considering the application of 3D Matrigel drops culture system in obtaining elongated cylindrical architectures resembling post-implantation embryos from mouse embryonic stem cells (ESCs) and trophoblast stem cells (TSCs)(Harrison et al. 2017; Harrison et al. 2018), we hypothesized that a 3D scaffold of extracellular matrix in Matrigel would promote the efficiency of egg cylinder formation from blastocysts in culture.
To validate this hypothesis, we embedded E3.5 blastocysts into Matrigel drops instead of seeding directly into tissue culture plates for further culture (Fig.1a) and only a small portion of embryos reached the egg cylinder stage (Fig. S2a). The appearance of disorganized embryos at 120 h in culture (Fig. S2a) implied that the implanting blastocysts with poor developmental potential should be excluded. Therefore, we traced the whole development process from E3.5 blastocysts into egg cylinders and found that the embryos at 48 h could be classified into two major types (Fig. S2a, S2b): the trophoblast giant cells (TGCs) in type B embryos (~17.91%) spread out, while those in type A embryos do not (~82.09 %). At 120 h in culture, about 50–75% type B embryos developed into egg cylinders, while the efficiency of type A embryos was about 25–35% (Fig. S2c). These results suggested that successfully spreading out of TGCs was critical for the blastocysts cultured in vitro to reach the egg cylinder stage, in accordance with the study of Bedzhov et al. (Bedzhov et al. 2014). Besides, we found that the type A embryos with larger volume at 48 h in culture seemed to develop into egg cylinders more likely (Fig. S2a). Therefore, we calculated the volume of each type A embryo based on its pixel area and found that no embryos formed egg cylinders when the area of embryos was below 500 pixels, and the efficiency of egg cylinder formation increased with increasing area of the embryos, while all embryos formed egg cylinder when the area was beyond 900 pixels (Fig. S2d). Hereafter, we defined the embryos in type A group with the area less than 500 pixels as type A1 (~45.45% in type A), and those with equal to or more than 500 pixels as type A2 (~54.55% in type A) (Fig. S2e). None of type A1 embryos developed into egg cylinders, while the efficiency of type A2 embryos was about 40–60% (Fig. S2f). When type A1 embryos (about 37.3 % of total) were excluded, the efficiency of egg cylinder formation could be improved from 28–42% to 50–67% with high repeatability (Fig. S2g).
The in vitro cultured mouse embryos resembled those developed in vivo. a Scheme of mouse blastocyst (E3.5) recovery and in vitro development into egg cylinders in Matrigel drops. The cells from early embryonic lineages were distinguished by different colors and the expression of lineage-specific markers were annotated following the abbreviations. TGCs, trophoblast giant cells; EPI, epiblast; TE, trophectoderm; ExEc, extraembryonic ectoderm; ExEn, extraembryonic endoderm. b Representative brightfield images of in vivo developed mouse embryos at pre-egg cylinder (E5.5) and egg cylinder (E6.5) stages, and in vitro cultured mouse embryos at 96 h and 120 h (96 h IVC and 120 h IVC). c Representative images of the expression of early embryonic lineage-specific markers, OCT4 (red) and EOMES (green), in in vivo developed pre-egg cylinder embryos (E5.5) and 96 h in vitro cultured embryos (96 h IVC). The nuclei were stained with DAPI (blue). d Representative images of the expression of early embryonic lineage-specific markers, OCT4 (red), GATA6 (gray), and EOMES (green), in in vivo developed egg cylinder embryos (E6.5) and 120 h in vitro cultured embryos (120 h IVC). The nuclei were stained with DAPI (blue). Pseudo-colors were used here. Scale bars, 50 μm.
Thus, the application of 3D Matrigel drop-based mouse embryo IVC system significantly facilitated mouse blastocysts reaching egg cylinder stage in vitro and exclusion of embryos with poor developmental potential (type A1) further made such mouse embryo IVC system suitable for toxicological studies.
Correct lineage differentiation in ex vivo mouse embryos
At the end point of pre-implantation stage, blastocysts consist of three distinct cell types: the trophectoderm (TE) which gives rise to TGCs and extraembryonic ectoderm (ExEc) cells, the pluripotent epiblast (EPI), and the primitive endoderm (PrE) cells. The latter will differentiate into extraembryonic endoderm (ExEn) lineages including visceral endoderm (VE) and parietal endoderm (PE) at the post-implantation stage, comprising egg cylinder together with ExEc and EPI lineage cells (Bassalert et al. 2018; Chazaud and Yamanaka 2016). Detailed information about mouse early embryonic development was provided in Text S3 of supplemental materials.
Therefore, we determined whether the embryos that developed in a progressive sequence of spatial and temporal morphogenetic steps are consistent with normal embryogenesis in vivo. At 96 h of culture, the ExEc localized at the opposite side of the EPI as the initiation of egg cylinder formation (pre-egg cylinder), comparable to E5.5 embryos that developed in utero (Fig. 1b). At 120 h of culture, mature egg cylinders were formed, in which the EPI formed the shape of a cup and the ExEn enveloped both the embryonic and extraembryonic regions, reminiscent of the normal embryos at E6.5 (Fig. 1b). To further evaluate the extent to which IVC embryos resembled that in vivo, we examined the expression of lineage-specific markers in embryos recovered from both in vitro and in vivo. Consistent with E5.5 embryos in vivo, the embryos cultured for 96 h correctly established the proximal-distal(P-D) axis as the OCT4+ EPI and EOMES+ ExEc located at opposite ends of the embryos, and the initiation of egg cylinder formation was identified by the lumens formed in OCT4+ EPI (Fig. 1c). At 120 h of culture, both the OCT4+ EPI and EOMES+/GATA6- ExEc expanded, and the EOMES+/GATA6+ ExEn were observed enveloping the whole embryos, comparable to E6.5 embryos (Fig. 1d). These results indicated that the embryos developed in Matrigel drops recaptured the sequential morphogenetic steps and lineage specification that happened during embryogenesis in vivo.
Thus, the 3D Matrigel drops-based mouse embryo IVC system could be applied to evaluate the embryotoxicity of hazards in vitro by analyzing the 3D architecture of embryos and lineage differentiation.
TBP-induced developmental arrest of mouse embryos
For the evaluation of developmental toxicity using the mouse embryos in culture, only type A2 embryos (with a pixel area not less than 500 pixels) and type B embryos (with TGCs spreading out) were chosen for further analysis to rule out the negative influence of mouse embryos with poor developmental potential.
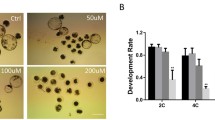
To assess the toxic effects of TBP, the mouse embryos pre-cultured for 48 h were exposed to TBP for the next 72 h, and the developmental dynamics and morphological changes were studied during the period of drug exposure (Fig. 2a). Based on our previous studies on the toxic effects of TBP on human-extended pluripotent stem cells in culture (Liu et al. 2021), the experiments with mouse embryos in culture were performed at a range of 25–100 μM. The egg cylinder formation efficiency decreased with increasing concentration of TBP, and almost no blastocysts reached the egg cylinder stage when exposed to 100 μM TBP (Fig. 2b, c). Despite of growth inhibition slightly, nearly 25–57% embryos in 25 μM TBP-treated group remained normal morphology reminiscent of those in untreated control groups and reached the egg cylinder stage (Fig. 2b, c). However, when exposed to TBP at high concentrations (50 and 100 μM), the embryos started to lose their intact 3D architecture since 72 h (24 h exposure), while the integrity of ExEc was maintained (Fig. 2b). These data implied that in comparison with ExEc, TBP was more toxic to EPI of mouse embryos in culture. To further quantify the inhibitory effects of TBP on the growth of mouse embryos in culture, we measured the total pixel area of each embryo and found that the values decreased with increasing TBP concentration (Fig. 2d). Although no significant differences were observed between the control group and 25 μM TBP-treated group, the volume of embryos in both 50 μM and 100 μM TBP-treated groups decreased significantly. These results demonstrated that TBP was toxic to the viability and development of mouse embryos in a dose-dependent manner.
TBP exposure inhibited egg cylinder formation of cultured mouse embryos. a Experimental design for evaluating the toxic effects of TBP on mouse embryonic development from peri-implantation to egg cylinder stage. b Representative brightfield images of the dynamic morphological change of in vitro cultured mouse embryos in the control and the groups exposed to TBP (25, 50, 100 μM) from 48 h (peri-implantation) to 120 h (egg cylinder) culture. Scale bar, 50 μm. c The efficiency of egg cylinder formation of embryos in the control (n1 = 4, n2 = 4, n3 = 4), 25 μM (n1 = 7, n2 = 6, n3 = 4), 50 μM (n1 = 3, n2 = 4, n3 = 6), and 100 μM (n1 = 4, n2 = 3, n3 = 11) TBP-treated groups. d Serial changes of the volume of cultured mouse embryos in the control and TBP-treated groups. n = 12 in each group. The volume of embryos was calculated with pixel area and the data was presented as the means ± SD. Two-sided unpaired Student’s t-test was used. Given the very large number of significant mean comparisons, no significant comparisons within a timepoint across treatments are not indicated. Asterisks indicated significant differences with 50 μM TBP-treated group compared to the untreated group at the same time point across treatments: **P<0.002, ****P<0.0001. Pound signs indicated significant differences with 100 μM TBP-treated group compared to the untreated group at the same time point across treatments: ####P<0.0001. px, pixels
The effects of TBP on lineage differentiation in mouse embryos
Our previous study had shown that pretreated human-extended pluripotent stem cells with TBP would affect their further differentiation potential into later embryonic lineages, i.e., three primary embryonic germ layers including definitive endoderm, mesoderm, and ectoderm (Liu et al. 2021). However, whether TBP has toxic effects on early embryonic lineages (EPI, ExEc, and ExEn) remains to be evaluated. Therefore, the expressions of lineage-specific gene markers (OCT4, EOMES, and GATA6) in untreated and TBP-treated mouse embryos were determined by immunofluorescence analysis.
At the pre-egg cylinder stage (96 h in culture), the embryos in 25 μM TBP-treated group and untreated control group exhibited similar expression patterns of transcriptional factors OCT4 and EOMES (Fig. 3a). With the increasing concentrations of TBP (50 μM and 100 μM), no specific spatial localizations of OCT4+ EPI cells and EOMES+ ExEc cells were observed (Fig. 3a), reminding us of the deconstruction of embryonic structures after TBP exposure (Fig. 2b). Furthermore, we counted the number of OCT4+ cells and EOMES+ cells to quantify the toxic effects of TBP on the differentiation of EPI and ExEc in mouse embryos, respectively. The number of OCT4+ EPI cells decreased in a dose-dependent manner when exposed to TBP and a significantly smaller number of OCT4+ cells were observed in 50 μM and 100 μM TBP-treated groups (Fig. 3b). In contrast, there was no significant difference in the number of the EOMES+ ExEc cells among all four groups (Fig. 3c). These data demonstrated that TBP was more toxic to pluripotent EPI in comparison with ExEc.
The effects of 48-h TBP exposure on mouse peri-implantation embryos developed into pre-egg cylinder stage in vitro. a Representative images of the expression of early embryonic lineage-specific markers, OCT4 (red) and EOMES (green), in the embryos from the control and TBP-treated groups. The nuclei were stained with DAPI (blue). Pseudo-colors were used here. Scale bar, 50 μm. b, c The average number of OCT4+ (b) and EOMES+ (c) cells at the maximum cross section of embryos in each group (n = 4). Two-sided unpaired Student’s t-test was used. The data was presented as the means ± SD. Asterisks indicated significant differences with TBP-treated groups compared to the untreated group: ***P<0.001; ns, no significance
At the egg cylinder stage (120 h in culture), when exposed to 25 μM TBP, no obvious difference was observed in the distribution of OCT4+ EPI cells, EOMES+/GATA6- ExEc cells, or EOMES+/GATA6+ ExEn cells in the embryos comparing with those from the control group (Fig. 4a). Furthermore, the EOMES+/GATA6- ExEc cells also spread out in both groups (Fig. 4a). However, when the concentration of TBP was increased to 50 μM, almost all OCT4+ cells and GATA6+ cells were lost, and the migration of EOMES+ cells was also inhibited (Fig. 4a). As expected, the number of OCT4+ cells and GATA6+ cells in the embryos declined significantly when exposed to TBP in a dose-dependent manner (Fig. 4b, c), while that of EOMES+ cells remained similar among all groups (Fig. 4d). Additionally, the more significant inhibitory effects of 25 μM TBP on OCT4+ EPI cell differentiation in mouse embryos at 120 h of culture (72 h treatment) showed here indicated that the toxic effects of TBP at low concentration would be accumulated with prolonged exposure (Figs. 3b and 4b).
The effects of 72-h TBP exposure on mouse peri-implantation embryos developed into egg cylinder stage in vitro. a Representative images of the expression of early embryonic lineage-specific markers, OCT4 (red), GATA6 (gray), and EOMES (green), in the embryos from the control and TBP-treated groups. The nuclei were stained with DAPI (blue). Pseudo-colors were used here. Scale bar, 50 μm. b, d The average number of OCT4+ (b), GATA6+ (c), and EOMES+ (d) cells at the maximum cross section of embryos in each group (n = 6). Two-sided unpaired Student’s t-test was used. The data was presented as the means ± SD. Asterisks indicated significant differences with TBP-treated groups compared to the untreated group: ***P<0.001; *P<0.05; ns, no significance
Furthermore, we performed a RT-qPCR assay to confirm the mRNA expression levels of lineage-specific genes (Oct4, Eomes, and Gata6) in TBP-treated or non-treated embryos, which had been cultured in Matrigel for 96 h or 120 h, respectively (Fig. 5). As expected, the expression of Oct4 was inhibited after 48-h treatment of TBP in a dose-dependent manner, with the mRNA level of 50 μM and 100 μM TBP-treated embryos significantly declined (Fig. 5a). By contrast, the mRNA level of Eomes remained no obvious variation (Fig. 5b). These results were in accordance with the change trends of OCT4- and EOMES-positive cell numbers (Fig. 3b, c).
A RT-qPCR assay to confirm the mRNA expression levels of lineage-specific genes. TBP affected the transcription of Oct4, Gata6, and Eomes genes in embryos. The mRNA levels of these genes in 48 h (a, b) and 72 h (c–e) TBP-treated groups were analyzed and normalized to those in the control group. Data are expressed as the means ± S.D. (n = 2). Two batches of independent experiments were performed.
Surprisingly, after being treated for 72 h, Oct4 mRNA expression levels of 25 μM and 50 μM TBP-treated embryos increased slightly compared to that of untreated embryos, which was opposite to the variation tendency of OCT4-positive cell numbers at 72-h treatment (Fig. 4b). This disagreement might be caused by analysis of the whole embryos instead of detecting EPI, ExEn, and ExEc parts separately, while the counting results of OCT4-positive cells were performed with the location information. Additionally, the results of RT-qPCR can only reflect the average expression level of Oct4 in all cells, being not able to exclude some external cells with extremely high or low Oct4 mRNA expression. However, the inhibitory effects of TBP at 72-h exposure on the expression level of Gata6 was obvious even when the concentration of TBP was 25 μM, indicating that TBP was toxic to the ExEn lineage (Fig. 5d). The Eomes mRNA expression level remained similar among the non-treated and TBP-treated groups, which was in agreement with the counting data for EOMES-positive cells (Fig. 4d).
In summary, these results demonstrated that TBP was more toxic to the lineages derived from the ICM (EPI and ExEn) when comparing with that from TE (ExEc)(Figs. 3, 4 and 5).
TBP-induced cell apoptosis in the mouse embryos
The growth inhibition and deconstruction of 3D architecture of mouse embryos induced by TBP informed us that cell apoptosis might be involved in this process. To validate this hypothesis, we assessed the caspase activities in cultured embryos with a caspase-3/7-specific cell-permeant fluorogenic substrate fluorochrome dye (green) at 72, 96, and 120 h of culture. Basal level of activated Caspase-3/7 was detected in the embryos from untreated control group at each time point (Fig. 6), reminiscent of the important role of cell apoptosis in embryonic development. The number of caspase-3/7-positive cells increased slightly after exposed to TBP at low dosage (25 μM) since 72 h of culture (Fig. 6), in accordance with the slight growth inhibition effects of TBP on embryos at this concentration (Fig. 3b, d). However, significant increment of caspase-3/7-positive cells was observed in the embryos treated with 50 and 100 μM TBP, especially at 120 h of culture (Fig. 6). Furthermore, the accumulation of caspase-3/7-positive cells was mainly detected in EPI cells at 72 and 96 h of culture, while that in ExEc cells were only observed at 120 h (Fig. 6). These results were consistent with the observation that the destruction of intact 3D architecture was mainly detected in the EPI when exposed to high concentrations of TBP (Fig. 6). Thus, the developmental arrest and abnormal lineage differentiation in mouse embryos exposed to TBP mainly resulted from TBP-induced apoptosis in EPI.
Conclusively, the application of 3D Matrigel drops and proposing of new embryonic selection standards made the IVC platform suitable for embryonic toxicological studies. With this, we revealed the dose-dependent embryotoxicity of TBP on growth and differentiation potential, as well as egg cylinder formation (Fig. 7).
Discussion
Considering the wide distribution of TBP in the environment, its potential adverse effects on health have attracted great attention. Although the toxic effects of TBP on the reproduction and development of aquatic animals have been well studied, it is still far from complete for our knowledge on the reproductive and developmental toxicity in mammals, particularly regarding early embryonic development from peri-implantation to early post-implantation stages. In this study, we optimized the mouse embryo IVC system and evaluated the adverse effects of TBP on mouse embryos from peri-implantation to egg cylinder stages.
In vitro models for embryonic toxicological studies
Despite the wide acceptance in embryonic toxicological studies, the rodent WEC technique is inapplicable for studying the embryos before gastrulation stage. The mouse embryo IVC system can recapture mouse embryonic development from pre-implantation to post-implantation stages in vitro. However, the lack of robust IVC system-based protocols for toxicological studies has hindered their applications for decades. Recently, by manually selecting embryos synchronized by stage and morphology, Gordeeva et al. evaluated the adverse effects of 5-HTP on mouse embryos at three separate stages, including the pre-implantation, peri-implantation, and early post-implantation stages (Gordeeva and Gordeev 2021). Although it represented a great beginning, their protocols cannot be applied to determine the bioaccumulation effects of chemicals, which was mainly limited by the unpredictable developmental outcomes. Here, after being combined with Matrigel drops based 3D culture system, about 33–42% blastocysts developed into egg cylinders, comparing with the efficiency of 20% when the blastocysts were directly seeded into tissue culture plates in our hands. These results were in consistent with the study conducted by Xiang et al., in which they found that the inclusion of 10% Matrigel in the IVC medium enabled 23.5% of human blastocysts to develop into pre-gastrulation stage with normal embryonic structures (Xiang et al. 2020). Both studies indicated the critical roles of extracellular matrix in normal embryonic development. Detailed evaluation of the concentration of Matrigel in our mouse embryo IVC system might further improve the potential of mouse blastocysts to develop into egg cylinders with normal embryonic structures and made it more suitable for embryotoxic studies. On the other hand, by tracking the whole developmental process of mouse embryos in culture, we identified the mouse embryos least likely to develop into egg cylinders, which were characterized by no TGCs spreading out and with an embryo area less than 500 pixels at 48 h in culture. After the exclusion of these embryos, about 50–67% blastocysts developed into egg cylinders with high repeatability. These modifications made it possible to continuously evaluate the toxic effects of chemicals on mouse embryos from pre-implantation to post-implantation stage in culture. Nonetheless, the post-blastocyst stage (0 h in culture) might not be suitable as a start point for chemical exposure in our culture system and more efforts should be made in the future.
Comparing with the widely used cell lines like 3T3-L1 and HEK 293 (Chen et al. 2019; Zhang et al. 2019), pluripotent stem cells (PSCs) have provided promising alternatives since they can generate various somatic and germ cells. Various PSC-based procedures have been developed to determine the toxicity of environmental hazards since the proposal of the embryonic stem cell test (EST), an alternative solution validated by the European Center for the Validation of Alternative Methods (ECVAM) for reproductive and developmental toxicity animal tests (Faiola et al. 2015; Genschow et al. 2004; Marx-Stoelting et al. 2009). While directed differentiation of PSCs to certain cell lineages provides a useful platform for evaluating the effects of certain chemicals on specific cell types (Fu et al. 2019; Hu et al. 2020a, b; Yin et al. 2018), the application of embryoid body (EB) differentiation provides a complementary strategy to perform toxic analysis on multiple cell types from different germ layers at the same time (Liang et al. 2021; Liu et al. 2021; Yin et al. 2019). Furthermore, the inclusion of transcriptome analyses at population or single-cell level also enabled us to access the underlying molecular mechanisms (Guo et al. 2019). Recently, Gordeeva et al. conducted a comparative analysis of toxic effects of 5-HTP on early mouse embryos and mouse ESC-derived EBs (Gordeeva and Gordeev 2021). Although similar dose- and stage-dependent adverse effects were observed in both early mouse embryos and EBs, several inconsistencies remained. Since the EBs failed to generate ExEc cells, they cannot be applied to evaluate the toxicity of chemicals to the differentiation and survival of ExEc cells (Gordeeva and Gordeev 2021). On the other hand, unlike the mouse embryos, the EBs failed to form the bilaterally symmetric structures. Therefore, while the EB differentiation procedure represents a reliable, repeatable, and high-throughput technique for analyzing the developmental toxicity of various toxic substances, the rodent WEC and IVC techniques provide the necessary complement for toxicological studies in vitro.
The adverse effects of TBP on early mouse embryos in culture
There are three major lineages in early embryos, including EPI, ExEc, and ExEn. The fetus is mainly developed from the EPI cells. The ExEc cells will give rise to the placenta, while ExEn cells will develop into the yolk sac tissue and partially contribute to gut endoderm (Boss et al. 2018; Hemberger et al. 2020). Besides, the ExEn also play critical roles in patterning the underlying EPI to generate three primary germ layers and extraembryonic mesoderm (ExM) which gives rise to the amnion, chorionic disk, and umbilical cord (Boss et al. 2018; Hemberger et al. 2020). Any failure of early embryonic lineage differentiation or survival will result in the failure of egg cylinder formation and further embryonic development, which is one of the major causes of miscarriage. Great concerns have been raised about the toxic effects of TBP on human embryonic development since the presence of TBP in both human fetal blood and placental tissues.
In current study, we found that TBP treatment prohibited the mouse blastocysts from developing into post-implantation egg cylinders in a dose-dependent manner. Among the early embryonic lineages, ExEc cells were the most resistant ones to TBP exposure since no significant difference was observed for the number of EOMES+/GATA6− cells between the control and TBP-treated groups. These results suggest that the placental tissues play protective roles in TBP exposure of the fetus, since up to 316 ng/g of TBP was detected in human placental tissues (Leonetti et al. 2016). However, whether any embryonic toxicity of TBP mediated by the placental tissues remains to be determined. On the contrary, the survival of both OCT4+ EPI cells and EOMES+/GATA6+ ExEn cells were greatly inhibited in the embryos exposed to TBP even at low concentration (25μM). The toxicity of TBP to OCT4+ EPI cells implies that TBP exposure will influence the specification of three primary germ layers including. First, definitive endoderm that gives rise to the organs like the liver and pancreas. Second, the mesoderm generates the organs like the heart and muscle. Third, ectoderm that develops into the organs like the neural system and skin. Previously, we have demonstrated that pretreating human-extended pluripotent stem cells with TBP will not only inhibit the maintenance of pluripotency, but also influence the differentiation of embryonic lineages in EB differentiation assay (Liu et al. 2021). Furthermore, we had also shown that the specification of both definitive endoderm and mesoderm was inhibited by TBP exposure, while that of ectoderm was promoted. Whether similar toxic effects of TBP will be observed in cultured mouse embryos remains to be evaluated and the prolonged culture of the mouse embryos into gastrulation stage in vitro is required first. On the other hand, the toxic effects of TBP on EOMES+/GATA6+ ExEn cells suggested that TBP exposure will not only disturb the development of yolk sac, but also indirectly influence the generation of amnion, chorionic disk, and umbilical cord due to abnormal ExM development. The developmental deficiency of yolk sac and ExM-derived tissues will result in the failure of fetal−maternal exchange of nutrients, wastes, and gases. Therefore, TBP exposure can interfere with the development of mouse embryos and might result in malformed embryos and miscarriage.
Conclusions
In conclusion, we develop a new model for in vitro evaluating the toxicity of chemicals and hazards on mammalian embryos at early developmental stages (from peri-implantation to egg cylinder stage). The application of 3D Matrigel drops-based mouse embryo IVC system promotes mouse blastocysts to develop into egg cylinders and standards proposed for selecting embryos with good developmental outcomes made the platform suitable for reproductive and embryonic toxicological studies. With the aid of this newly developed platform, we determined the adverse effects of TBP on mouse embryonic development and found that TBP exposure significantly inhibited the growth of mouse embryos, the differentiation of early embryonic lineages, and finally the formation of egg cylinders in a dose-dependent manner. The toxic effects of TBP mainly cause abnormal differentiation and poor survival of pluripotent EPI cells and ExEn cells that derived from the ICM, while the ExEc cells derived from the TE were resistant to TBP treatment. These results imply that long-term TBP exposure of pregnant women might lead to abnormal embryonic development of the fetus and fetal membranes. Therefore, our study here provides a valuable platform for evaluating the toxic effects of chemicals and hazards on mammalian embryos at early developmental stages.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Agency NE. (2016). Substance evaluation conclusion as required by REACH Article 48 and EVALUATION REPORT for 2,4,6- Tribromophenol. In: Agency EC, editor.
Andrews JE, Nichols HP, Schmid JE, Mole LM, Hunter ES 3rd, Klinefelter GR (2004) Developmental toxicity of mixtures: the water disinfection by-products dichloro-, dibromo- and bromochloro acetic acid in rat embryo culture. Reprod Toxicol (Elmsford, NY) 19(1):111–116. https://doi.org/10.1016/j.reprotox.2004.06.005
Bassalert C, Valverde-Estrella L, Chazaud C (2018) Primitive endoderm differentiation: from specification to epithelialization. Curr Top Dev Biol 128:81–104. https://doi.org/10.1016/bs.ctdb.2017.12.001
Bedzhov I, Leung CY, Bialecka M, Zernicka-Goetz M (2014) In vitro culture of mouse blastocysts beyond the implantation stages. Nat Protoc 9(12):2732–2739. https://doi.org/10.1038/nprot.2014.186
Blythe JW, Heitz A, Joll CA, Kagi RI (2006) Determination of trace concentrations of bromophenols in water using purge-and-trap after in situ acetylation. J Chromatogr A 1102(1-2):73–83. https://doi.org/10.1016/j.chroma.2005.10.042
Boss AL, Chamley LW, James JL (2018) Placental formation in early pregnancy: how is the centre of the placenta made? Hum Reprod Update 24(6):750–760. https://doi.org/10.1093/humupd/dmy030
Boudjellaba D, Dron J, Revenko G, Démelas C, Boudenne JL (2016) Chlorination by-product concentration levels in seawater and fish of an industrialised bay (Gulf of Fos, France) exposed to multiple chlorinated effluents. Sci Total Environ 541:391–399. https://doi.org/10.1016/j.scitotenv.2015.09.046
Butt CM, Miranda ML, Stapleton HM (2016) Development of an analytical method to quantify PBDEs, OH-BDEs, HBCDs, 2,4,6-TBP, EH-TBB, and BEH-TEBP in human serum. Anal Bioanal Chem 408(10):2449–2459. https://doi.org/10.1007/s00216-016-9340-3
Chazaud C, Yamanaka Y (2016) Lineage specification in the mouse preimplantation embryo. Development (Cambridge, England) 143(7):1063–1074. https://doi.org/10.1242/dev.128314
Chen Y, Xu T, Yang X, Chu W, Hu S, Yin D (2019) The toxic potentials and focus of disinfection byproducts based on the human embryonic kidney (HEK293) cell model. Sci Total Environ 664:948–957. https://doi.org/10.1016/j.scitotenv.2019.01.361
Deng J, Liu C, Yu L, Zhou B (2010) Chronic exposure to enviro-nmental levels of tribromophenol impairs zebrafish reproduction. Toxicol Appl Pharmacol 243(1):87–95. https://doi.org/10.1016/j.taap.2009.11.016
Dufour P, Pirard C, Charlier C (2017) Determination of phenolic organohalogens in human serum from a Belgian population and assessment of parameters affecting the human contamination. Sci Total Environ 599-600:1856–1866. https://doi.org/10.1016/j.scitotenv.2017.05.157
Ellis-Hutchings RG, Carney EW (2010) Whole embryo culture: a “new” technique that enabled decades of mechanistic discoveries. Birth Defects Res Part B, Dev Reprod Toxicol 89(4):304–312. https://doi.org/10.1002/bdrb.20263
Faiola F, Yin N, Yao X, Jiang G (2015) The rise of stem cell toxicology. Environ Sci Technol 49(10):5847–5848. https://doi.org/10.1021/acs.est.5b01549
Feng C, Xu Q, Jin Y, Lin Y, Qiu X, Lu D, Wang G (2016) Determination of urinary bromophenols (BrPs) as potential biomarkers for human exposure to polybrominated diphenyl ethers (PBDEs) using gas chromatography-tandem mass spectrometry (GC-MS/MS). J Chromatogr B Anal Technol Biomed Life Sci 1022:70–74. https://doi.org/10.1016/j.jchromb.2016.03.041
Flodin C, Whitfield FB (1999) Biosynthesis of bromophenols in marine algae. Water Sci Technol 40(6):53–58. https://doi: 10.1016/S0273-1223(99)00537-5
Folle N, Azevedo-Linhares M, Garcia J, Souza A, Grötzner SR, Oliveira EC, Paulin AF, Leite NF, Filipak Neto F, Oliveira Ribeiro CA (2020) Low concentration of 2,4,6-tribromophenol (TBP) represents a risk to South American silver catfish Ramdia quelen (Quoy and Gaimard, 1824) population. Ecotoxicol Environ Saf 187:109815. https://doi.org/10.1016/j.ecoenv.2019.109815
Fu H, Wang L, Wang J, Bennett BD, Li JL, Zhao B, Hu G (2019) Dioxin and AHR impairs mesoderm gene expression and cardiac differentiation in human embryonic stem cells. Sci Total Environ 651(Pt 1):1038–1046. https://doi.org/10.1016/j.scitotenv.2018.09.247
Fujii Y, Kato Y, Masuda N, Harada KH, Koizumi A, Haraguchi K (2018) Contamination trends and factors affecting the transfer of hexabromocyclododecane diastereomers, tetrabromobisphenol A, and 2,4,6-tribromophenol to breast milk in Japan. Environ Pollut (Barking, Essex: 1987) 237:936–943. https://doi.org/10.1016/j.envpol.2018.03.015
Genschow E, Spielmann H, Scholz G, Pohl I, Seiler A, Clemann N, Bremer S, Becker K (2004) Validation of the embryonic stem cell test in the international ECVAM validation study on three in vitro embryotoxicity tests. Altern Lab Anim: ATLA 32(3):209–244. https://doi.org/10.1177/026119290403200305
Gordeeva O, Gordeev A (2021) Comparative assessment of toxic responses in 3D embryoid body differentiation model and mouse early embryos treated with 5-hydroxytryptophan. Arch Toxicol 95(1):253–269. https://doi.org/10.1007/s00204-020-02909
Guo H, Tian L, Zhang JZ, Kitani T, Paik DT, Lee WH, Wu JC (2019)Single-cell RNA sequencing of human embryonic s-tem cell differentiation delineates adverse effects of nicotine on embryonic development. Stem Cell Rep 12(4):772–786. https://doi.org/10.1016/j.stemcr.2019.01.022
Haldén AN, Nyholm JR, Andersson PL, Holbech H, Norrgren L (2010) Oral exposure of adult zebrafish (Danio rerio) to 2,4,6-tribromophenol affects reproduction. Aquat Toxicol (Amsterdam, Netherlands) 100(1):30–37. https://doi.org/10.1016/j.aquatox.2010.07.010
Harrison SE, Sozen B, Christodoulou N, Kyprianou C, Zernicka-Goetz M (2017) Assembly of embryonic and extraembryonic stem cells to mimic embryogenesis in vitro. Science (New York, NY) 356(6334):eaal1810. https://doi.org/10.1126/science.aal1810
Harrison SE, Sozen B, Zernicka-Goetz M (2018) In vitro generati-on of mouse polarized embryo-like structures from embryonic and trophoblast stem cells. Nat Protoc 13(7):1586–1602. https://doi.org/10.1038/s41596-018-0005-x
Hemberger M, Hanna CW, Dean W (2020) Mechanisms of early placental development in mouse and humans. Nature reviews. Genetics 21(1):27–43. https://doi.org/10.1038/s41576-019-0169-4
Hsu YC (1971)Post-blastocyst differentiation in vitro. Nature 231(5298):100–102. https://doi.org/10.1038/231100a0
Hu B, Yang R, Cheng Z, Liang S, Liang S, Yin N, Faiola F (2020a)Non-cytotoxic silver nanoparticle levels perturb human embryonic stem cell-dependent specification of the cranial placode in part via FGF signaling. J Hazard Mater 393:122440. https://doi.org/10.1016/j.jhazmat.2020.122440
Hu B, Yin N, Yang R, Liang S, Liang S, Faiola F (2020b) Silver nanoparticles (AgNPs) and AgNO3 perturb the specification of hu-man hepatocyte-like cells and cardiomyocytes. Sci Total Environ 725:138433. https://doi.org/10.1016/j.scitotenv.2020.138433
Hunter ES 3rd, Blanton MR, Rogers EH, Leonard Mole M, Andrews J, Chernoff N (2006)Short-term exposures to dihaloacetic acids produce dysmorphogenesis in mouse conceptuses in vitro. Reprod Toxicol (Elmsford, NY) 22(3):443–448. https://doi.org/10.1016/j.reprotox.2006.01.001
Kammann U, Vobach M, Wosniok W (2006) Toxic effects of brominated indoles and phenols on zebrafish embryos. Arch Environ Contam Toxicol 51(1):97–102. https://doi.org/10.1007/s00244-005-0152-2
Knudsen GA, Chapman M, Trexler AW, Juberg CT, Birnbaum LS (2020) 2,4,6-Tribromophenol disposition and kinetics in pre-gnant and nursing Sprague Dawley rats. Toxicol Sci: a-n official journal of the Society of Toxicology 178(1):36–43. https://doi.org/10.1093/toxsci/kfaa133
Koch C, Sures B (2018) Environmental concentrations and toxicology of 2,4,6-tribromophenol (TBP). Environ Pollut (Barking, Essex: 1987) 233:706–713. https://doi.org/10.1016/j.envpol.2017.10.127
Leonetti C, Butt CM, Hoffman K, Miranda ML, Stapleton HM (2016) Concentrations of polybrominated diphenyl ethers (PBDEs) and 2,4,6-tribromophenol in human placental tissues. Environ Int 88:23–29. https://doi.org/10.1016/j.envint.2015.12.002
Liang X, Yang R, Yin N, Faiola F (2021) Evaluation of the effects of low nanomolar bisphenol A-like compounds’ levels on early human embryonic development and lipid metabolism with human embryonic stem cell in vitro differentiation models. J Hazard Mater 407:124387. https://doi.org/10.1016/j.jhazmat.2020.124387
Liu M, Hansen PE, Lin X (2011) Bromophenols in marine algae and their bioactivities. Mar Drugs 9(7):1273–1292. https://doi.org/10.3390/md9071273
Liu Y, Zhu D, Zhao Z, Zhou Q, Pan Y, Shi W, Qiu J, Yang Y (2021) Comparative cytotoxicity studies of halophenolic disinfection byproducts using human extended pluripotent stem cells. Chemosphere 263:127899. https://doi.org/10.1016/j.chemosphere.2020.127899
Lyubimov AV, Babin VV, Kartashov AI (1998) Developmental neurotoxicity and immunotoxicity of 2,4,6-tribromophenol in Wistar rats. Neurotoxicology 19(2):303–312
Marx-Stoelting, P., Adriaens, E., Ahr, H. J., Bremer, S., Garthoff, B., Gelbke, H. P., Piersma, A., Pellizzer, C., Reuter, U., Rogiers, V., Schenk, B., Schwengberg, S., Seiler, A., Spielmann, H., Steemans, M., Stedman, D. B., Vanparys, P., Vericat, J. A., Verwei, M., van der Water, F., … Schwarz, M. (2009). A review of the implementation of the embryonic stem cell test (EST). The report and recommendations of an ECVAM/ReProTect Workshop. Altern Lab Anim: ATLA, 37(3), 313–328. https://doi.org/10.1177/026119290903700314
Mizukawa H, Nomiyama K, Nakatsu S, Yamamoto M, Ishizuka M, Ikenaka Y, Nakayama S, Tanabe S (2017) Anthropogenic and naturally produced brominated phenols in pet blood and pet food in Japan. Environ Sci Technol 51(19):11354–11362. https://doi.org/10.1021/acs.est.7b01009
Morris SA, Grewal S, Barrios F, Patankar SN, Strauss B, Buttery L, Alexander M, Shakesheff KM, Zernicka-Goetz M (2012) Dynamics of anterior-posterior axis formation in the developing mouse embryo. Nat Commun 3:673. https://doi.org/10.1038/ncomms1671
Oberg K, Warman K, Oberg T (2002) Distribution and levels of brominated flame retardants in sewage sludge. Chemosphere 48(8):805–809. https://doi.org/10.1016/s0045-6535(02)00113-3
Pan Y, Li W, An H, Cui H, Wang Y (2016) Formation and occu-rrence of new polar iodinated disinfection byproducts in drinking water. Chemosphere 144:2312–2320. https://doi.org/10.1016/j.chemosphere.2015.11.012
Qiu X, Bigsby RM, Hites RA (2009) Hydroxylated metabolites of polybrominated diphenyl ethers in human blood samples from the United States. Environ Health Perspect 117(1):93–98. https://doi.org/10.1289/ehp.11660
Richardson SD, Postigo C. (2015). Formation of DBPs: state of the science. Recent Advances in Disinfection By-Products. 1190. Am Chem Soc pp. 189-214.
Ronen Z, Visnovsky S, Nejidat A (2005) Soil extracts and co-culture assist biodegradation of 2,4,6-tribromophenol in culture and soil by an auxotrophic achromobacter piechaudii strain tbpz. Soil Biol Biochem 37(9):1640–1647. https://doi.org/10.1016/j.soilbio.2005.02.001s
Suzuki G, Takigami H, Watanabe M, Takahashi S, Nose K, Asari M, Sakai S (2008) Identification of brominated and chlorinated -phenols as potential thyroid-disrupting compounds in indoor dust-s. Environ Sci Technol 42(5):1794–1800. https://doi.org/10.1021/es7021895
Takigami H, Suzuki G, Hirai Y, Sakai S (2009) Brominated flame retardants and other polyhalogenated compounds in indoor air and dust from two houses in Japan. Chemosphere 76:270–277. https://doi.org/10.1016/j.chemosphere.2009.03.006
Trexler AW, Knudsen GA, Nicklisch S, Birnbaum LS, Cannon RE (2019) 2,4,6-Tribromophenol exposure decreases P-glycoprotein transport at the blood-brain barrier. Toxicol Sci: an official journal of the Society of Toxicology 171(2):463–472. Advance online publication. https://doi.org/10.1093/toxsci/kfz155
Whitfield FB, Drew M, Helidoniotis F, Svoronos D (1999) Distribution of bromophenols in species of marine polychaetes and bryozoans from eastern Australia and the role of such animals in the flavor of edible ocean fish and prawns (shrimp). J Agric Food Chem 47(11):4756–4762. https://doi.org/10.1021/jf9904719
Wu TC, Wan YJ, Damjanov I (1981) Positioning of inner cell mass determines the development of mouse blastocysts in vitro. J Embryol Exp Morpholog 65:105–117
Xiang L, Yin Y, Zheng Y, Ma Y, Li Y, Zhao Z, Guo J, Ai Z, Niu Y, Duan K, He J, Ren S, Wu D, Bai Y, Shang Z, Dai X, Ji W, Li T (2020) A developmental landscape of 3D-cultured human pre-gastrulation embryos. Nature 577(7791):537–542. https://doi.org/10.1038/s41586-019-1875-y
Xiong J, An T, Zhang C, Li G (2015) Pollution profiles and risk assessment of PBDEs and phenolic brominated flame retardants in water environments within a typical electronic waste dismantling region. Environ Geochem Health 37(3):457–473. https://doi.org/10.1007/s10653-014-9658-8
Yang Y, Komaki Y, Kimura SY, Hu HY, Wagner ED, Mariñas BJ, Plewa MJ (2014) Toxic impact of bromide and iodide on drinking water disinfected with chlorine or chloramines. Environ Sci Technol 48(20):12362–12369. https://doi.org/10.1021/es503621e
Yin N, Liang S, Liang S, Yang R, Hu B, Qin Z, Liu A, Faiola F (2018) TBBPA and its alternatives disturb the early stages of neural development by interfering with the NOTCH and WNT pathways. Environ Sci Technol 52(9):5459–5468. https://doi.org/10.1021/acs.est.8b00414
Yin N, Liang X, Liang S, Liang S, Yang R, Hu B, Cheng Z, Liu S, Dong H, Liu S, Faiola F (2019) Embryonic stem cell- and transcriptomics-based in vitro analyses reveal that bisphenols A, F and S have similar and very complex potential developmental toxicities. Ecotoxicol Environ Saf 176:330–338. https://doi.org/10.1016/j.ecoenv.2019.03.115
Zhai H, Zhang X (2011) Formation and decomposition of new and unknown polar brominated disinfection byproducts during chlorination. Environ Sci Technol 45(6):2194–2201. https://doi.org/10.1021/es1034427
Zhang L, Sun W, Duan X, Duan Y, Sun H (2019) Promoting differentiation and lipid metabolism are the primary effects for DINP exposure on 3T3-L1 preadipocytes. Environ Pollut (Barking, Essex: 1987) 255(Pt 1):113154. https://doi.org/10.1016/j.envpol.2019.113154
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science Foundation of China (No. 32070804, 31801205), and Natural Science Foundation of Jiangsu Province, China (No. BK20180677, BK20200675). This work also received financial supports from the Six Talent Peaks Project in Jiangsu Province, China (No. WSW-035); China Postdoctoral Science Foundation (No. 2019M651894, 2019M651895); Jiangsu Postdoctoral Research Foundation (No. 2019Z376); High Level Youth Talents Cultivation Project of Nanjing Medical University (No. 2019NJMURC011); and Science and Technology Development Foundation of Nanjing Medical University (No. NMUB2019034).
Author information
Authors and Affiliations
Contributions
DCZ, QZ, and JFQ conceived of the presented idea. ZHZ carried out the experiment with the support of YJL, YLH, and WTZ. ZHZ and YJL performed data analysis. YY and DCZ wrote the manuscript after ZHZ writing the first draft. JFQ and CX participated in review and editing. YY, DCZ, and YLH secured funding. All authors discussed the results and contributed to the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate:
All procedures on animals were performed according to the protocols approved by the Ethics Committee of the Animal Core Facility of Nanjing Medical University (No. IACUC-1811061).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible editor: Mohamed M. Abdel-Daim
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 4664 kb)
Rights and permissions
About this article
Cite this article
Zhao, ., Zhu, D., Liu, Y. et al. Embryotoxic effects of tribromophenol on early post-implantation development of mouse embryos in vitro. Environ Sci Pollut Res 29, 12085–12099 (2022). https://doi.org/10.1007/s11356-021-16614-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-16614-3