Abstract
It is important that a pollution remediation system be able to cater for a variety of pollutant species present in the water to be treated. The aim of this study was to utilise a series of commercial zeolites (H-MOR, H-β, and H-ZSM5) for the concomitant adsorption and photodegradation of Cu2+ and tetracycline (TC) molecules. The adsorbent cum photocatalyst was characterised by SEM and FTIR. FTIR confirmed the key functional groups (Si-O-Si and Al-O-Si) in the series of zeolites, and H-β zeolite was demonstrated to be the most effective adsorbent cum photocatalyst for both adsorption and photodegradation of Cu2+ and TC molecules. These results were further corroborated from the pseudo-first-order rate constant values. Among the investigated zeolites, H-ZSM5 displayed the least adsorption and photodegradation performance for Cu2+ and TC molecules. The photolysis reaction confirms the significant role of zeolites in the photodegradation test, as low performance was recorded in the absence of the zeolites.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Industrialisation coupled with population growth has become a great concern and an overwhelming problem as environmental contamination caused by organic pollutants has become a problem all over the world (Chen et al. 2010; Ighalo et al. 2021c). Thus, there is a need to mitigate these emerging pollutants to avert the negative effects of their eco-toxicity(Ighalo et al. 2020a; Ighalo et al. 2020b). Over the years, adsorption (Ighalo et al. 2021a), ultra-filtration, coagulation (Ayazi et al. 2020), and other biological and physical treatment methods have been the techniques employed to remove organic pollutants from wastewater (Balarak et al. 2021; Ighalo et al. 2021b). However, a novel technique is required to chemically transform emerging anthropogenic organic pollutants especially those with very low concentration and high toxicity into non-hazardous compounds. These techniques should be cheap and efficient. That is, the used chemical reagents, catalyst, and energy source should be inexpensive, abundant, eco-friendly, and not produce secondary pollution (Chen et al. 2010).
Water contamination by compounds such as tetracycline (TC) is now a major environmental problem. Antibiotic, particularly tetracycline antibiotics (TCs), is one of the typical PPCPs. It mainly includes tetracycline hydrochloride, oxytetracycline hydrochloride, and chlortetracycline hydrochloride (Xiong et al. 2018). TC is emerging pollutants which due to abuse of pharmaceuticals are released into the environment, and even with more toxic by-products, they are difficult to degrade, thus posing serious threats to animals and human health (Dai et al. 2020). Over the years, it is used to prevent contagious diseases in plant and livestock farming and promotes growth rate (Xiong et al. 2018; Zhang et al. 2011); thus, its uses in many fields pose a lot of environmental issues as most TCs are absorbed in a small amount during metabolism, while the majority is excreted through faeces and urine in their unchanged form. TC residues have often been detected in soils, sediments, surface water, groundwater, and wastewater (Xiang et al. 2020; Zhang et al. 2011). Nevertheless, removing TCs from aqueous solutions is quite difficult owing to the complexity of their molecular structures, changeful occurrence state, and its low biodegradability; thus, high efficiency of its removal has become a great concern (Xiong et al. 2018).
Copper on the other hand regulates most body processes. Its minimum daily intake is said to be 0.5 mg/l. 1.3 mg/l is the maximum copper level that drinking water is expected to contain according to the USEPA. However, when the concentration is higher than the stated value, it can result in copper poisoning which in turn can lead to hypotension, jaundice, coma, haemostasis, and problems with the digestive system. With prolonged exposure to copper, the kidneys and liver may be damaged (Bandura et al. 2020; Shrivastava 2009). Membrane processes, solvent extraction, ion exchange, adsorption, reverse osmosis, precipitation, distillation, advanced oxidation, electrochemical oxidation, and biological degradation are different proposed purification methods to remove Cu2+ and TC from water (Bandura et al. 2020).
Many materials significantly degrade when exposed to ultraviolet radiation. Photo-oxidative degradation is caused by ultraviolet radiation, and this causes a breakdown of polymer chains, production of free radicals, loss in molecular weight, and deterioration of mechanical properties (Yousif and Haddad 2013). Photodegradation is very relevant in the areas of environmental and pharmaceutical, and it is found everywhere in nature (Mas et al. 2011). To comprehensively describe photodegradation processes, the photoproducts formed must be identified, and the process that governs it must be clarified. Zeolite use for most environmental application is gaining more interest as a result of its properties and significance in occurrence worldwide. Thus, the use of natural and synthetic zeolites as adsorbents, catalysts, ion exchangers, membranes, and molecular sieves for wastewater treatments has been realised and also remains a promising technique for environmental purification. Zeolites are cage-like in structure, hydrated aluminosilicate minerals, and it has up to several hundred square metres per gramme internal and external surface areas (Damjanović et al. 2010; Wang and Peng 2010). These outstanding characteristics make it a choice adsorbent for this work.
Some works have been previously done in this research area. Demircivi and Simsek (2019) investigated the photocatalytic activity of W-doped BaTiO3 for the degradation of tetracycline under UV-A light irradiation and visible light irradiation. It was discovered that the optimum pH for degradation of tetracycline was 10 due to OH− ions on the surface of the catalyst in an alkaline condition which by generating OH radicals enhanced the tetracycline degradation. Also, due to the production of OH radicals in solution, tetracycline degradation increased under visible light in the presence of H2O2. In their study, Qian et al. (2021) successfully synthesised Cu-TiO2/GO ternary composite through the hydrothermal-dipping method. They investigated the performance of Cu-TiO2/GO in the photodegradation process of TC under UV irradiation and found out that the Cu-TiO2/GO composite had a high photocatalytic performance with excellent stability and can be recommended for removing the antibiotic compounds. Bandura et al. (2020) worked on simultaneous removal of Cu(II) ions and phenol on zeolite NaP1 which was obtained from fly ash and NaP1 modified with chitosan at various environmental conditions to investigate the optimal condition for copper ion and phenol removal. Although work has been done on the adsorption of pollutants from water, the novelty of this work lays in the use of commercially purchased series of zeolites to remove targeted metal ion and organic pollutants (Cu2+ and TC) from aqueous solution by photodegradation.
This work aims to investigate the trend in the simultaneous removal of ions and photodegradation of organic pollutants using a series of zeolites. Within the exhaustive search of the authors, no work has been done in this research area using photodegradation to break down tetracycline (TC) and H-MOR, H-β, and H-ZSM5 zeolites in a competitive system with Cu(II). TC and Cu2+ solution was used as a model pollutant mixture as they represent species that would appear together in wastewater and effluents. The samples were characterised using SEM and FTIR, and the removal efficiency of the pollutants by series of zeolites was determined.
Methodology
Materials and reagents
Commercial H-MOR (SiO2/Al2O3 = 24), H-β (SiO2/Al2O3 = 25), and H-ZSM5 (SiO2/Al2O3 = 25) zeolites were purchased from Nankai University Catalyst Co., Ltd. The SiO2/Al2O3 molar ratios of H-MOR, H-β, and H-ZSM-5 are 24, 25, and 25, respectively. tetracycline hydrochloride (C22H24O8N2.HCl) with a purity ca. 95% manufactured by Yangzhou Pharmaceutical Company Limited was purchased from a local pharmacy. Copper (ii) sulphate pentahydrate (CuSO4.5H2O) salts were procured from Guangdong Guanghua Sci-Tech Co., Ltd. Shantou, Guangdong, China. The stock solution of 1000 mg/l Cu2+/TC was prepared by dissolving 1 g in 1000 ml of deionised water. Thereafter, lower concentrations were prepared by serial dilution. Freshly prepared deionised water was used throughout the experiment. Reagents were used as obtained without further purification, as they are of analytical grades. A photocatalytic reactor box with dimensions 16 × 16 × 26 inches comprising 8 W germicidal UV bulb and 78-1 magnetic stirrer hotplate was used in this study. The inner part of the box was painted black to minimise loss of light.
Characterisation of H-MOR, H-β, and H-ZSM5 zeolites
The morphology, bulk composition, and bandgap of H-MOR, H-β, and H-ZSM-5 zeolites were investigated using scanning electron microscopy (SEM) (Phenom ProX, Eindhoven Netherlands), electron diffraction spectroscopy (EDS), and UV–Vis spectrophotometer (JASCO V-550). Fourier transform infrared spectroscopy (FTIR)(Shimadzu, FTIR-8400S, Japan) technique was employed to study the prominent functional groups in the zeolite adsorbent. The FTIR analyses of the zeolite samples were performed at a resolution of 4 (cm−1).
Concomitant adsorption and photodegradation of Cu2+/TC using the zeolite series
The adsorption study was performed 10 mg/l Cu2+/TC solutions in the presence of the zeolites under magnetic stirring at room temperature for a period of 0.5–2 h. Photocatalytic degradation study was performed by illuminating ultraviolet (UV) light on a 10 mg/l Cu2+/TC solution (pH = 5.4) containing 25 mg of the zeolite for a duration of 0.5 to 2 h. During the photocatalytic reaction, the solution was magnetically stirred at room temperature. Before the illumination of UV light, the zeolite/Cu2+/TC mixture was stirred under dark conditions for 0.5 h to ensure homogenous distribution of Cu2+/TC molecules over the catalyst and vice versa. At the end of adsorption and photocatalytic reaction, an aliquot of the treated zeolite/Cu2+/TC solution was centrifuged, and the concentration of the supernatant (degraded Cu2+/TC solution) was measured using 752 W ultraviolet (UV-Vis) grating spectrophotometer at a wavelength of maximum absorbance of 356 nm and 272 nm for Cu2+ and TC, respectively. The adsorption capacity (qc, mg/g) and removal efficiency (qe, %) were calculated using Eqs. 1 and 2, respectively (Agarwal et al. 2016; Omorogie et al. 2019):
The extent of the degradation of Cu2+/TC was measured using Eq. 3
where C0, Ce, Ct, V, and m are the initial concentration, equilibrium concentration, concentration at the time, t, the volume of Cu2+/TC solution, and mass of zeolite, respectively. The rate constant (k) of degradation was calculated from the pseudo-first-order kinetic rate law (Villota-Zuleta et al. 2019) given in Eq. 4
Results and discussion
Zeolite photocatalyst characterisation
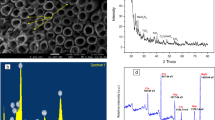
The morphology of H-MOR, H-β, and H-ZSM5 zeolites employed in the present study was investigated using the SEM technique. Figure 1 a, b, and c, respectively, show the morphology of H-β, H-MOR, and H-ZSM-5 zeolites. The morphology of H-MOR zeolites is observed to be comparatively finer. On the other hand, H-β and H-ZSM-5 are coarser with dispersed particles of irregular sizes. The order of increasing porosity in the zeolites may be considered thus H-MOR < H-ZSM-5< H-β. The photo-response of the zeolite samples was investigated using diffuse reflectance spectroscopy (DRS). H-β and H-ZSM5 zeolites were absorbed in the ultraviolet region (ca. 400 nm), while H-MOR displayed two absorption bands in the ultraviolet and visible region (Figure 1d–f). From the Tauc plots, the bandgaps of H-β, H-MOR, and Z-ZSM5 were calculated to be 3.30, 3.62, and 3.63 eV, respectively (inserts of Figure 1d–f).
The EDS technique was employed to study the bulk elemental composition of the zeolites. In this study, emphases were given to the main constituents of the zeolite samples (Al, Si, and O). The amounts of these key elements vary in the zeolite materials, as shown in Figure 2a–c. Comparatively, H-β zeolite contained the highest amount of O (43.5%) and Si (34.0%); meanwhile, it showed the lowest proportion of Al (0.5%). The highest level of Al (3.6%) was observed in H-MOR and contrastingly displayed the lowest amount of O (34.8%) and Si (20.7%). Subsequently, elemental mapping was performed to identify Al, O, and Si as depicted by the different colours in Figure 2 d, e, and d, respectively.
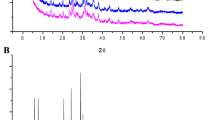
FTIR was carried out to confirm the key functional groups (Si-O-Si and Al-O-Si) in the series of zeolites (Figure 3). The figure shows the results for each zeolite adsorbent over a range of 438.58–4000 cm−1. H-β, H-MOR, and H-ZSM5 spectra display a symmetric stretching vibration of Si-O-Si groups at peaks 791.44, 800.01, and 801.44 cm−1, respectively. On the other hand, these groups’ asymmetric stretching vibrations are observed at 1037.16, 1087.16, and 1097.16 cm−1 for H-β, H-MOR, and H-ZSM5, respectively. The band at 455.72 cm−1 is for bending vibrations of Si-O-Si or Al-O-Si groups in H-β and H-MOR zeolites. H-ZSM5 shows a similar vibration at 438.58 cm−1.
Adsorption of Cu2+/TC using series of zeolite adsorbents
The adsorption capacity (qc, mg/g) and removal efficiency (qe, %) of H-MOR, H-β, and H-ZSM-5 zeolites were investigated using a 10 mg/l Cu2+/TC solution mixture. A concomitant adsorption study was performed by subjecting a solution of Cu2+ and TC to magnetic stirring for 2 h at room temperature. Results obtained show a direct relationship between qc and qe: adsorption capacity and adsorption efficiency increase over a duration of 2 h, as shown in Figure 4a–c. From Figure 4a, H-MOR zeolite showed better adsorption capacity and adsorption for Cu2+ than TC molecules. As observed from the SEM images, the smaller pore sizes enhance the adsorption of Cu2+ onto H-MOR compared to its zeolite counterparts.
On the contrary, from Figure 5b, better adsorption capacity and adsorption efficiency were observed for TC molecules using H-β zeolite; this may be associated to its higher porosity which is more favourable for the adsorption of a comparatively larger molecule like TC. This phenomenon may be attributed to the better affinity for cations by H-β zeolites compared to other samples. This behaviour indicates that the diffusion of ions with small size by the channels of natural zeolites leads to high adsorption (Hernández-Montoya et al. 2013). As for H-ZSM5 zeolite, no significant difference in adsorption capacity and adsorption efficiency for Cu2+ and TC molecules was noticed (see Figure 4c). Overall, H-MOR zeolite displayed the highest rate for concomitant adsorption of Cu2+ and TC molecules, while H-ZSM5 zeolite showed the lowest adsorption rate for Cu2+ and TC molecules.
Photodegradation of Cu2+ using series of zeolite adsorbents
Post adsorption studies of the zeolites, the effect of the presence of UV light on the concomitant photodegradation of Cu2+/TC was investigated using H-MOR, H-β, and H-ZSM5 adsorbents. The effect of light in the photodegradation process was examined by irradiating the Cu2+/TC solution in the absence of zeolite adsorbents (a process termed photolysis). The reaction conditions are similar to that of adsorption studies: 10 mg/l Cu2+/TC solution, pH = 5.4, and room temperature. Before illumination, a dark phase reaction was carried out before illumination: the adsorbents and Cu2+/TC were agitated for 0.5 h to ensure the equilibrium between the adsorbent and adsorbate mixture. Upon illumination of UV light on the reaction mixture for 2 h, among the zeolites understudy, H-β zeolite was observed to be superior in the degradation of Cu2+(as shown in Figure 5a); this outcome is consistent with the previous result on adsorption study. H-ZSM5 zeolite displayed the lowest degradation performance for Cu2+. Photolysis is shown to be the least effective approach for the degradation of Cu2+, which confirms the significance of the zeolites in the photodegradation reaction. The rate constants from kinetic studies (Figure 5b) of H-β, H-MOR, and H-ZSM5 zeolites were calculated as 0.294, 0.228, and 0.216 h−1, respectively.
Photodegradation of TC using series of zeolite adsorbents
Similarly, under the same experimental conditions, the concomitant photodegradation of TC molecules was performed using the zeolite adsorbents. From Figure 6a, the H-β zeolite adsorbents displayed TC molecules’ most effective degradation performance over 2-h photodegradation reactions. This finding is consistent with the previous result from the photodegradation of Cu2+. Again, among the investigated samples, the lowest degradation was displayed by H-ZSM5 zeolites. Predictably, photolysis showed the least degradation performance for TC molecules. The rate constants from kinetic studies (Figure 6b) of H-β, H-ZSM5, and H-MOR zeolites were calculated as 0.544, 0.378, and 0.358 h−1, respectively. The rate constants obtained are in the order of increasing bandgap of the zeolite samples; that is, the zeolite sample with the smallest bandgap (H-β: 3.30 eV) displayed the highest rate constant, while that with the largest bandgap (H-ZSM5: 3.63 eV) showed the lowest rate of pollutant degradation. The enhanced photodegradation of Cu2+ and TC using H-β zeolites is ascribed to its narrower bandgap, which suggests a better absorption of the irradiated light. Overall, based on the rate constant values, we can assert that the zeolites under investigation are more effective for removing TC molecules than Cu2+ ions.
Furthermore, a comparative study on the adsorption and photocatalytic degradation of TC/Cu2+ solution was performed to establish the role and impacts of these two processes. Under the same experimental conditions, unsurprisingly, photocatalytic degradation was observed to be more effective than the adsorption process, as shown in Figure 7a–c. It is worth mentioning that the adsorption process played a significant role in the overall performance of photocatalytic degradation, especially in H-MOR zeolites (Figure 7b). Similar to our previous result, all the zeolite samples demonstrated better adsorption and photocatalytic degradation for TC molecules than Cu2+.
The pH of a solution is a critical parameter that detects the effectiveness of a pollutant remediation process. For this reason, we investigated the influence of pH in the photocatalytic degradation of TC/Cu2+ solution under a highly acidic (pH 2), the pollutant medium (pH 5), and an alkaline medium (pH 10). From Figure 7 d and f, H-β and H-ZSM5 zeolites showed a similar trend in the removal of TC and Cu2+: photocatalytic degradation performance increased with an increase in the pH of the solution, which may be attributed to the increase in the number of hydroxyl groups (OH−) with a rise in pH (alkaline medium), which enhances the interaction between the positively charged pollutants (Cu2+ and TC+ (an ionic form of TC)) and the zeolites. Likewise, H-MOR zeolites displayed improved TC removal with an increase in the pH of the solution; however, on the contrary, the removal of Cu2+ was more effective in the pollutant medium pH (pH 5). The mechanism for the removal of these pollutants is briefly described in the following section.
Mechanism for adsorption and photodegradation of zeolites
The adsorption behaviour of the zeolites can be described by the following mechanisms, which can be of two different types for the positively charged adsorbate (Cu2+ and TC+): (1) ion exchange between the positively charged adsorbate species and the protons of surface hydroxyl groups of natural zeolite and (2) ion exchange between the positively charged adsorbate and the exchangeable cations (K+, Na+, Ca2+, etc.) that balance the negative charges of each AlO4 or SiO4 tetrahedron in the framework of zeolites. Eqs. 5–6 represent the two possible mechanisms for the adsorption process [4]:
We proposed the superior adsorption of H-MOR zeolite over its counterparts due to high affinity for the Cu2+ and TC molecule or weaker bond strength of its hydroxyl (-OH) and alkoxyl (–OK) group. The mechanism for the photocatalytic degradation of the binary mixture of TC/Cu2+ is presented in Figure 8. The heterojunction structure depicted in the figure is based on the commercial zeolites’ most prominent components: Al2O3 and SiO2. The role of such heterojunction in photocatalysis is well-known(Fatimah et al. 2019). Electrons and holes are generated upon illumination with a UV light source and then drifted to the nanoparticles’ surface. Photogenerated electrons tend to accumulate on the Al2O3 surface, possessing a more negative conduction band relative to SiO2, whereas holes are concentrated on the surface of SiO2 with a more positive valence band. As illustrated in Figure 8, the binary target pollutants are subjected to concomitant photodegradation via oxidation of TC on the surface of SiO2 and Cu2+ reduction to Cu metal at the Al2O3 surface.
Conclusion
Zeolites (H-MOR, H-β, and H-ZSM5) were utilised for the concomitant adsorption and photodegradation of Cu2+/TC molecules. Several important conclusions could be drawn from this study. H-β zeolite was demonstrated to be the most effective adsorbent for both adsorption and photodegradation of Cu2+ and TC molecules. The pseudo-first-order rate constant values further corroborated these results. The enhanced performance is attributed to its smaller bandgap, which makes it more photoresponsive to the illuminated light. On the other hand, H-ZSM5 displayed the least adsorption and photodegradation performance for Cu2+ and TC molecules. The photolysis reaction confirms the significant role of zeolites in the photodegradation test, as low performance was recorded in the absence of the zeolites. It can be concluded that zeolite is an effective catalyst for the simultaneous adsorption and photodegradation of Cu2+/TC molecules.
Availability of data and materials
Not applicable
References
Agarwal S, Sadegh H, Monajjemi M, Hamdy AS, Ali GA, Memar AO, Shahryari-ghoshekandi R, Tyagi I, Gupta VK (2016) Efficient removal of toxic bromothymol blue and methylene blue from wastewater by polyvinyl alcohol. J Mol Liq 218:191–197
Ayazi H, Akhavan O, Raoufi M, Varshochian R, Motlagh NSH, Atyabi F (2020) Graphene aerogel nanoparticles for in-situloading/pH sensitive releasing anticancer drugs. Colloids Surf B: Biointerfaces 186:110712
Balarak D, Zafariyan M, Igwegbe CA, Onyechi KK, Ighalo JO (2021) Adsorption of acid blue 92 dye from aqueous solutions by single-walled carbon nanotubes: isothermal, kinetic, and thermodynamic studies. Environ Proc 8:869–888. https://doi.org/10.1007/s40710-021-00505-3
Bandura L, Franus M, Madej J, Kołodyńska D, Hubicki Z (2020) Zeolites in phenol removal in the presence of Cu (II)ions—comparison of sorption properties after chitosan modification. Materials 13:643
Chen C, Ma W, Zhao J (2010)Semiconductor-mediated photodegradation of pollutants under visible-light irradiation. Chem Soc Rev 39:4206–4219
Dai J, Meng X, Zhang Y, Huang Y (2020) Effects of modification and magnetization of rice straw derived biochar on adsorption of tetracycline from water. Bioresour Technol 311:123455
Damjanović L, Rakić V, Rac V, Stošić D, Auroux A (2010) The investigation of phenol removal from aqueous solutions by zeolites as solid adsorbents. J Hazard Mater 184:477–484
Demircivi P, Simsek EB (2019)Visible-light-enhanced photoactivity of perovskite-type W-doped BaTiO3 photocatalyst for photodegradation of tetracycline. J Alloys Compd 774:795–802
Fatimah I, Prakoso NI, Sahroni I, Musawwa MM, Sim Y-L, Kooli F, Muraza O (2019) Physicochemical characteristics and photocatalytic performance of TiO2/SiO2 catalyst synthesized using biogenic silica from bamboo leaves. Heliyon 5:e02766
Hernández-Montoya V, Pérez-Cruz MA, Mendoza-Castillo DI, Moreno-Virgen M, Bonilla-Petriciolet A (2013) Competitive adsorption of dyes and heavy metals on zeolitic structures. J Environ Manag 116:213–221
Ighalo JO, Adeniyi AG, Adelodun AA (2020a) Recent advances on the adsorption of herbicides and pesticides from polluted waters: performance evaluation via physical attributes. J Ind Eng Chem
Ighalo JO, Ajala OJ, Adeniyi AG, Babatunde EO, Ajala MA (2021a) Ecotoxicology of glyphosate and recent advances in its mitigation by adsorption. Environ Sci Pollut Res 28:2655–2668. https://doi.org/10.1007/s11356-020-11521-5
Ighalo JO, Igwegbe CA, Adeniyi AG, Adeyanju CA, Ogunniyi S (2020b) Mitigation of metronidazole (Flagyl) pollution in aqueous media by adsorption: a review. Environ Technol Rev 9:137–148
Ighalo JO, Igwegbe CA, Aniagor CO, Oba SN (2021b) A review of methods for the removal of penicillins from water. J Water Process Eng 39:101886. https://doi.org/10.1016/j.jwpe.2020.101886
Ighalo JO, Sagboye PA, Umenweke G, Ajala JO, Omoarukhe FO, Adeyanju CA, Ogunniyi S, Adeniyi AG (2021c) CuO nanoparticles (CuO NPs) for water treatment: a review of recent advances Environmental Nanotechnology. Monit Manag 15:100443. https://doi.org/10.1016/j.enmm.2021.100443
Mas S, Tauler R, De Juan A (2011) Chromatographic and spectroscopic data fusion analysis for interpretation of photodegradation processes. J Chromatogr A 1218:9260–9268
Omorogie MO, Babalola JO, Olatunde AM, Alimi T, John KI, Adegboyega SA, Abesa SK (2019)Microwave-synthesized and Fenton-functionalized Pinus sylvestris bark activated carbon/metal oxides for the effective uptake of tetracycline and congo red dye. Biomass Conv Biorefinery 1-17 doi:https://doi.org/10.1007/s13399-019-00460-y
Qian S, Pu S, Zhang Y, Wang P, Bai Y, Lai B (2021) New insights on the enhanced non-hydroxyl radical contribution under copper promoted TiO2/GO for the photodegradation of tetracycline hydrochloride. J Environ Sci 100:99–109
Shrivastava A (2009) A review on copper pollution and its removal from water bodies by pollution control technologies. Indian J Environ Protect 29:552–560
Villota-Zuleta J, Rodríguez-Acosta J, Castilla-Acevedo S, Marriaga-Cabrales N, Machuca-Martínez F (2019) Experimental data on the photoelectrochemical oxidation of phenol: analysis of pH, potential and initial concentration. Data Brief 24:103949
Wang S, Peng Y (2010) Natural zeolites as effective adsorbents in water and wastewater treatment. Chem Eng J 156:11–24
Xiang W, Wan Y, Zhang X, Tan Z, Xia T, Zheng Y, Gao B (2020) Adsorption of tetracycline hydrochloride onto ball-milled biochar: governing factors and mechanisms. Chemosphere 255:127057
Xiong W, Zeng G, Yang Z, Zhou Y, Zhang C, Cheng M, Liu Y, Hu L, Wan J, Zhou C (2018) Adsorption of tetracycline antibiotics from aqueous solutions on nanocomposite multi-walled carbon nanotube functionalized MIL-53(Fe) as new adsorbent. Sci Total Environ 627:235–244
Yousif E, Haddad R (2013) Photodegradation and photostabilization of polymers, especially polystyrene. SpringerPlus 2:1–32
Zhang Z, Sun K, Gao B, Zhang G, Liu X, Zhao Y (2011) Adsorption of tetracycline on soil and sediment: effects of pH and the presence of Cu (II). J Hazard Mater 190:856–862
Acknowledgements
All authors whose work were cited are hereby acknowledged.
Author information
Authors and Affiliations
Contributions
Kingsley Igenepo John: Conceptualisation, data curation, methodology, investigation, writing-original draft, writing-review and editing, and validation
Aderemi Timothy Adeleye: Conceptualisation, data curation, methodology, investigation, writing-original draft, writing-review and editing, and validation
Comfort Abidemi Adeyanju: Writing-original draft and writing-review and editing
Samuel Ogunniyi: Writing-original draft and writing-review and editing
Joshua O. Ighalo: Conceptualisation, data curation, writing-review and editing, and validation
Adewale George Adeniyi: Conceptualisation, methodology, writing-review and editing, supervision, validation, and project administration
Corresponding author
Ethics declarations
Ethics approval
This article does not contain any studies involving human or animal subjects.
Consent to participate
All authors duly participated.
Consent for publication
All authors hereby consent to publish this manuscript.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible editor: Sami Rtimi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Concomitant adsorption and photodegradation of Cu2+/tetracycline was examined.
• H-MOR, H-β, and H-ZSM5 zeolites served as adsorbent cum photocatalyst.
• H-β zeolite was the most effective for both adsorption and photodegradation of Cu2+ and TC.
• The photolysis mechanism confirms the significant role of zeolites in the photodegradation test.
Rights and permissions
About this article
Cite this article
John, .I., Adeleye, A.T., Adeyanju, C.A. et al. Effect of light on concomitant sequestration of Cu(II) and photodegradation of tetracycline by H-MOR/H-β/H-ZSM5 zeolites. Environ Sci Pollut Res 29, 11756–11764 (2022). https://doi.org/10.1007/s11356-021-16556-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-16556-w