Abstract
Activated coke-based catalysts have attracted extensive attention in denitration by selective catalytic reduction by NH3 (NH3-SCR), due to their excellent catalytic performance at low temperature. In the paper, the V2O5/AC catalyst was prepared by the impregnation method to investigate the effect of pre-oxidation process on its NH3-SCR activity. Activity test results show that the V2O5/AC catalyst with 4-h pre-oxidation exhibits the best NOx removal efficiency, which reaches the NOx conversion is over 75% in the range of 200–240 °C and exhibits an excellent resistance to SO2 and H2O. Characterization results demonstrate that the V4+ was oxidized by oxygen molecule during pre-oxidation process, which contributes to the formation of V5+ ions and surface-active oxygen species. The surface-active oxygen species are conducive to promoting the “fast SCR” reaction; thus, the pre-oxidized process can contribute to the superior NH3-SCR performance for V2O5/AC catalyst at low temperature.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The use of fossil energy produces a large number of nitrogen oxides, which could cause acid rain, photochemical smog, and harm to human health (Hou et al. 2014; Jiang et al. 2016; Tang et al. 2016). With the implementation of ultra-low emission standard for atmospheric pollutants in China, the promotion and implement of flue gas denitrification (De-NOx) technologies will be forced and stressed (Xie et al. 2019). The selective catalytic reduction of NOx by NH3 (NH3-SCR) is a mature technology to solve NOx emissions. The catalyst is essential for NH3-SCR technology, and it determines the operating temperature and cost of the industrial SCR system. V2O5-WO3/TiO2 is extensively used as the SCR denitration catalyst due to its high catalytic activity and resistance to SO2 poisoning. However, the active window temperature of V2O5-WO3/TiO2 catalyst is higher (320–400 °C), and it is not suitable for low-temperature flue gas (below 150 °C) in iron and steel, cement, glass, and other industries (Yang et al. 2020). Therefore, it is important to research a highly efficient NH3-SCR catalyst at low temperature. Some catalysts show a high SCR activity in the range of 120–250 °C, such as MnOx/Al2O3, CuO/Al2O3, and Mn/TiO2 catalyst, but their catalytic activities easily are inhibited by SO2(Hou et al. 2014).
Developing a catalyst with high resistance to SO2 deactivation at low temperature is critical to the NH3-SCR process. Studies have shown that it is superior to TiO2 and SiO2 in terms of resistance to ammonium sulfate, activated carbon, or coke as a carrier (Han et al. 2018). Compared with the activated carbon, activated coke (AC) has the characteristics of low cost and high mechanical strength (Ye et al. 2020). AC has a large specific surface area, abundant surface functional groups, and strong adsorption capacity, which had been successfully applied to SO2 and Hg capture and NOx purification of flue gas in the steel industry (Liu and Liu 2013, Li et al. 2018, Li et al. 2019, Ye et al. 2020). Pure AC shows a low catalytic activity; thus, the method of introducing new active components (such as MnOx, CuO, or V2O5) is used to enhance its denitration activity (Marban et al. 2004; Boyano et al. 2008; Lazaro et al. 2009). Compared with these catalysts, AC-supported V2O5 (V2O5/AC) catalysts are more promising, because those still have good catalytic activity in the presence of low moisture and SO2 conditions (Sun et al. 2009; Wang et al. 2014). In order to further develop the V2O5/AC catalyst, the studies about NH3-SCR reaction mechanism and active site were carried, and the result has shown that the V2O5 species are the main sites for the adsorption and activation of NH3(Sun et al. 2009).
AC has characteristic of flammable at high temperature; thus, N2 atmosphere is used during loading V2O5 species (the decomposition of NH4VO3 into V2O5 occurs only when the temperature is over 500 °C). After loading V2O5 species, and calcined (pre-oxidized) under air atmosphere to activate the V2O5 and catalyst. According to the literature, pre-oxidized catalyst can enhance catalytic activity, compared with the non-pre-oxidized catalyst, and the pre-oxidized catalyst has better catalytic activity and stability (Zhu et al. 1999). However, there is no literature report on the relationship between pre-oxidation process and catalytic activity. In this work, catalysts were prepared by different pre-oxidation time and tested for their NH3-SCR performance at low temperature, and the SEM, BET, XPS, H2-TPR, and NH3-TPD characterizations were performed to explore the reasons for this phenomenon and construct the structure-activity relationship of the catalyst.
Experimental
Catalyst preparation
The V2O5/AC catalyst was prepared by the impregnation method. Generally, 5 g activated coke (40–80 mesh) and 0.3214 g NH4VO3(AR) were added into 100 mL deionized water under vigorously stirring for 1 h at room temperature. Subsequently, the mixture solution was stirred at 80 °C for 2 h followed by drying at 120 °C for 5 h and then calcined at 400 °C in a N2 atmosphere for 5 h to obtain a V2O5/AC catalyst. After that, the catalyst was pre-oxidized at 250 °C, the obtained V/AC catalysts was labeled as V/AC-X (X was pre-oxidization time, X=0, 3, 4, and 5). Among them, V/AC-0 means the preparation of the V2O5/AC catalyst without pre-oxidation.
Characterization
The Supplementary materials provide the particulars of characterization.
Catalyst activity tests
The efficiency of NOx catalytic removal was tested in a fixed-bed reactor (i.d. 7mm). The mass of catalyst was 0.7 g (40–80 mesh) in the test, the feeding gases were mixed by the gas mixer and then sent to the reactor, the total flow rate was 200 mL/min, and the inlet gas hourly space velocity (GHSV) was maintained at 12000 h−1. The inlet gas concentrations of NO and NH3 in the feeding gas both were 500 ppm, while O2 was 5%, and the N2 was balanced gas. Meanwhile, 5 vol % H2O (when used), 300 ppm SO2 (when used) was added in the test. The values of NOx removal efficiency can be calculated by following equation:
Results and discussion
Catalyst performance
Fig. 1 exhibited the NOx removal efficiency of AC and V/AC-X (X= 0, 3, 4, and 5) catalysts. The result indicated that the NOx conversion decreased in the following order: V/AC-4 > V/AC-3> V/AC-5> V/AC-0>AC. The AC exhibited a low activity, the NOx conversion at 120 °C was only 20%, and it reduced with the increase of reaction temperature. This result was similar with research previously (Jing et al. 2014). After loading V2O5 species, the NOx removal efficiency of the V/AC catalyst increased significantly, suggesting that V2O5 is the main active species of NH3-SCR reaction. After pre-oxidizing, the catalytic activities had been further improved; for the V/AC-0, the NOx conversion was 76% at 240 °C, while V/AC-X were 85% (V/AC-3) and 94% (V/AC-4). However, the NOx conversion of V/AC-5 was 83% at 240 °C, which was lower than V/AC-4. In summary, the pre-oxidation process can contribute to the superior NH3-SCR performance of V/AC-X catalyst, and pre-oxidation time is the most important influencing factor; the best pre-oxidize time is 4 h.
The long-term stability of V/AC-4 catalyst was investigated by testing the NH3-SCR performance of the catalyst at 240 °C for 60 h. The result was exhibited in Fig. S1a. The NOx removal efficiency of the catalyst was not decreased within 60 h, but increased a little (the NOx conversion has increased from 94.0 to 97.7%), suggesting that the stability of V/AC-4 catalyst was good. The effect of SO2 and water vapor on V/AC-4 catalyst was examined at 240 °C. As presented in Fig. S1b–c, when 300 ppm SO2 and 5 vol% H2O were added, respectively, the NOx removal efficiency of V/AC catalyst increases and keeps the increase trend slightly. After removing SO2 and H2O, the catalytic activity of V/AC-4 is still increased with time, indicating that the tolerance of SO2 and H2O over V/AC-4 catalyst was good. After removing SO2, the catalytic activity is still increased with time. The reason for the increased activity could be caused by the sulfate formed during test process, which enhanced the surface acidity of catalyst (Zhu et al. 2001). The more surface acidity of the catalyst could be beneficial to the adsorption and activation of ammonia, thereby increasing the NH3-SCR activity. In general, water vapor and reaction gas produce competitive adsorption on the catalyst surface (Hou et al. 2014; Lu et al. 2018), thereby inhibiting the adsorption of reactive species and reducing the NH3-SCR performance. Due to the hydrophobicity of the activated coke (Zhang et al. 2020), water vapor cannot adsorb on the catalyst surface, explaining why the V/AC-4 catalysts showed good H2O resistance.
Catalyst characterization
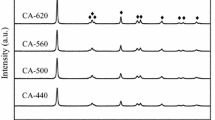
In order to explain the influence of pre-oxidation process on the crystal structures of the catalyst, the AC and V/AC-X catalysts were characterized by XRD, respectively. The results are presented in Fig. 2. Two broad diffraction peaks located at 20–30° and 40–50° are characteristic diffractions of activated coke graphite crystals (Han et al. 2018; Yang et al. 2020). The characteristic peaks of SiO2 (an additive substance used in the industrial production of activated coke) located at 20.8°, 26.6°, 36.6°, 44.0°, 50.1°, 60.0°, and 68.2° were observed (Ge et al. 2019). No peaks that belong to V2O5 species were detected in the patterns of V/AC catalyst, suggesting that vanadium species has extremely high dispersibility on the surface of AC carrier.
The physical structures of AC and V/AC-X catalysts are presented in Table 1. AC exhibited a specific surface area of 214 m2 g−1 and a total pore volume of 0.025 cm3 g−1 with an average pore diameter of 4.44 nm. Compared with AC catalyst, the BET surface area, pore volume, and average pore size of the V/AC-0 catalyst decreased. With the increase of the pre-oxidation time, the specific surface area, pore volume, and average pore size of the V/AC-3 and V/AC-4 catalysts both increased, while the V/AC-5 catalyst is greatly reduced, which is consistent with activity test results, which due to the structure of V/AC-X catalyst changed during the pre-oxidation process, thereby affecting the catalytic activity. Compared with the V/AC-0 catalyst, the surface area of V/AC-5 catalyst is smaller, while the catalytic activity is better, indicating that the specific surface area is not the main factor for the enhancement of the catalytic activity. The specific surface area of the V/AC-4 catalyst is similar to the V/AC-3 and V/AC-0 catalyst, while the pore volume and average pore size of V/AC-4 catalyst was the largest, and it had the best NH3-SCR performance, thus indicating that the new pore structure is formed to facilitate the NH3-SCR reaction. In order to further explore the impact of the pre-oxidation process on the catalyst structure, the sample morphology and particle size were analyzed by SEM, and the results are presented in Fig. S2. Compared with AC catalyst, the surface of V/AC-0 catalyst is uneven (Fig. S2a–b), and it has shown that the structure of the catalyst was changed after loading vanadium species. With the increase of the pre-oxidation time, there was agglomerative phenomenon accompanying with V/AC-3 and V/AC-4 catalyst (Fig. S2c–e), while the V/AC-5 catalyst presents a number of large block crystals, obviously, which is consistent with the previous BET results. This phenomenon can explain the reason for that the NOx removal efficiency of V/AC-5 catalyst is lower than that the V/AC-4 catalyst. Both results of SEM and BET tests have shown that the new pore structure formed in the catalyst surface during the pre-oxidation process, thus affecting their SCR performance at low temperature.
The surface elemental compositions of AC, V/AC-X, and V/AC-4-used (after 60-h stability test) catalysts were tested by XPS. The V2p1/2 XPS results are exhibited in Fig. 3a. The peaks at 515.7–517.0 eV and 517.0–518.0 eV were attributed to V4+ and V5+, respectively (Cha et al. 2013). The content of V4+ on the catalyst was calculated by the peak area V4+/(V4++V5+). As presented in Table 2, the content of V4+ on V/AC-0, V/AC-3, V/AC-4, and V/AC-5 catalysts was 60.6%, 43.3%, 30.6%, and 29.0%, respectively. With the increase of the pre-oxidation time, the V4+ content decreases, which indicated that the V4+ was oxidized to V5+ by the oxygen in air atmosphere (Zhu et al. 1999). After stability testing, the V4+ content of V/AC-4 sample increased from 30.6% (V/AC-4-fresh) to 32.4% (V/AC-4-used), which is due to the reduction of V5+ into V4+ by NH3 during NH3-SCR reaction.
The O1s XPS spectra are exhibited in Fig. 3b. The peaks at 529–530 eV, 531–533 eV, and 533–535 eV were attributed to lattice oxygen (marked as Oβ), surface chemical oxygen (marked as Oα), and the oxygen species in hydroxide groups (marked as Oγ), respectively (Jiang et al. 2019). The proportions of Oα/(Oα+ Oβ+ Oγ) in V/AC-0 sample was 20.88%. After pre-oxidizing, the content of Oα is significantly increased. The content of Oα of V/AC-3, V/AC-4, and V/AC-5 catalysts were 48.22%, 49.05%, and 48.32%. According to previous reports, chemically adsorbed oxygen can boost that the NO was oxidized to NO2, which can promote “fast SCR” reaction (Ma et al. 2011). After pre-oxidizing, the V4+ content decreased, and the Oα content increased, indicating that the V4+ was oxidized by oxygen molecule during pre-oxidation process, which contributes to the formation of V5+ ions and surface-active oxygen species, thereby improving the catalytic activity. Compared with the V/AC-4 catalyst, the Oα content and V4+ content of V/AC-4-used sample were both increased in the test, indicating that the Oα was formed during the V4+ and was oxidized to V5+, while V4+ was generated again in NH3-SCR reaction atmosphere, which can enhance the cycle of V4+/V5+, which explains why the catalytic activity increased gradually in the stability test. With the increase of the pre-oxidation time, the Oγ content increases, and this may be due to the formation of hydroxyl functional groups during the pre-oxidation process. The catalytic activity of the V/AC-3 catalyst is better than the V/AC-5 catalyst, which is different from the activity results, thereby the content of Oγ is not the influencing factor of the catalytic activity.
The redox property of catalyst is one of the important influencing factors in SCR reaction. Fig. 4 exhibited the H2-TPR curves of AC and V/AC-X catalysts. The peak located at 624 °C for AC catalyst was attributed to the vaporization peak of carbon carrier on activated coke (Zhang et al. 2020). The peaks located at 624 °C and 513 °C in the V/AC-0 catalyst were caused by the vaporization peak of carbon carrier and reduction peak of V5+, respectively (Han et al. 2018). Compared with the AC catalyst, the reduction peak (after pre-oxidation) of V/AC-X catalyst shifted to a lower temperature, indicating that the interaction between activated coke carrier and vanadium species could improve the redox property of catalysts (Lu et al. 2018). With the increase of pre-oxidation time, the intensity of the reduction peak of V5+ and the vaporization peak of the activated coke carrier of the V/AC-3 and V/AC-4 catalyst are obviously enhanced, indicating that the content of V5+ and the redox sites of the catalyst are both increases during the pre-oxidation progresses. However, the reduction peak area of the V/AC-5 catalyst is significantly reduced. Combined with the result of BET, it could be shown that the excessive pre-oxidation time causes the sintering of active species on V/AC-5 catalyst, thereby decreasing its catalytic activity.
NH3-TPD tests are performed to study the ammonia desorption over AC, V/AC-X, and V/AC-4-SO2 (after sulfur resistance test) samples. As shown in Fig. 5a, the AC curve showed only one peak at 147 °C, while the V/AC-X samples present two broad peaks; the desorption peaks located at 147 °C and 170 °C were the physical adsorption of ammonia and the adsorption of NH4+ at the Brønsted acid site (Yin et al. 2018). After loading vanadium species, the desorption peak area increased significantly, indicating that vanadium species can provide more acidic sites on the catalyst surface, which can promote the adsorption and activation of ammonia. Compared with V/AC-0 catalyst, the desorption peak areas of the V/AC-3 and V/AC-4 further increase, suggesting that the pre-oxidation process promotes the adsorption of ammonia and can promote the formation of V5+ on the catalyst surface (Xu et al. 2018), which is consistent with the H2-TPR results. However, the desorption peak area of the V/AC-5 catalyst is reduced, which is because catalytic activity species was agglomerated, and the adsorption of NH3 is inhibited. As shown in Fig. 5b, NH3-TPD patterns were performed among V/AC-4-SO2 (after sulfur resistance test) samples, the desorption peak area of V/AC-4-SO2 sample is larger, and this is because the sulfate formed during test process could improve the adsorption and activation of ammonia, thereby increasing the NH3-SCR activity.
Conclusion
The activity test results showed that the NOx catalytic removal efficiency of V2O5/AC catalyst is better than the AC catalyst, suggesting that the V2O5 is the active substance. The activity sequence of V/AC-X catalysts prepared by different pre-oxidation time at 120–240 °C is V/AC-4> V/AC-3> V/AC-5> V/AC-0. The results showed that the pre-oxidation process is beneficial to catalytic performance of V2O5/AC-X catalysts. Series of characterizations were used to explain the reasons for the phenomenon. The BET and SEM characterization results demonstrated that the specific surface area, pore volume, and average pore size of the catalyst increase with the increase of the pre-oxidation time, and the new pore structure is formed to facilitate the NH3-SCR reaction. During the pre-oxidation progress, the V4+ was oxidized by oxygen molecule, which contributes to the formation of V5+ ions and surface-active oxygen species. The surface-active oxygen species is conducive to promoting the “fast SCR.” In summary, the pre-oxidized V2O5/AC has the best catalytic activity when the pre-oxidation time is 4 h.
Data availability
Not applicable.
References
Boyano A, Iritia MC, Malpartida I, Larrubia MA, Alemany LJ, Moliner R, Lazaro MJ (2008) Vanadium-loaded carbon-based monoliths for on-board NO reduction: influence of nature and concentration of the oxidation agent on activity. Catal Today 137:222–227. https://doi.org/10.1016/j.cattod.2008.02.010
Cha W, Park E, Chin S, Yun ST, Jurng J (2013) Changes in the chemical composition of V2O5-loaded CVC-TiO2 materials with calcination temperatures for NH3-SCR of NOx. J Porous Mater 20:1069–1074. https://doi.org/10.1007/s10934-013-9688-0
Ge TT, Zhu BZ, Sun YL, Song WY, Fang QL, Zhong YX (2019) Investigation of low-temperature selective catalytic reduction of NOx with ammonia over Cr-promoted Fe/AC catalysts. Environ Sci Pollut Res 26:33067–33075. https://doi.org/10.1007/s11356-019-06301-9
Han F, Gao Y, Huo Q, Han L, Wang J, Bao W, Chang L (2018) Characteristics of vanadium-based coal gasification slag and the NH3-selective catalytic reduction of NO. Catalysts. 8(8):545. https://doi.org/10.3390/catal8080327
Hou YQ, Cai GQ, Huang ZG, Han XJ, Guo SJ (2014) Effect of HCl on V2O5/AC catalyst for NO reduction by NH3 at low temperatures. Chem Eng J 247:59–65. https://doi.org/10.1016/j.cej.2014.01.036
Jiang H, Wang Q, Wang H, Chen Y, Zhang M (2016) Temperature effect on the morphology and catalytic performance of Co-MOF-74 in low-temperature NH 3 -SCR process. Catal Commun 80:24–27. https://doi.org/10.1016/j.catcom.2016.03.013
Jiang LJ, Liu QC, Ran GJ, Kong M, Ren S, Yang J, Li JL (2019) V2O5-modified Mn-Ce/AC catalyst with high SO2 tolerance for low-temperature NH3-SCR of NO. Chem Eng J 370:810–821. https://doi.org/10.1016/j.cej.2019.03.225
Jing W, Guo QQ, Hou YQ, Ma GQ, Han XJ, Huang ZG (2014) Catalytic role of vanadium(V) sulfate on activated carbon for SO2 oxidation and NH3-SCR of NO at low temperatures. Catal Commun 56:23–26. https://doi.org/10.1016/j.catcom.2014.06.017
Lazaro MJ, Boyano A, Herrera C, Larrubia MA, Alemany LJ, Moliner R (2009) Vanadium loaded carbon-based monoliths for the on-board No reduction: influence of vanadia and tungsten loadings. Chem Eng J 155:68–75. https://doi.org/10.1016/j.cej.2009.06.033
Li Y, Li G, Lu Y, Hao W, Wei Z, Liu J, Zhang Y (2018) Denitrification performance of non-pitch coal-based activated coke by the introduction of MnO x –CeO x –M (FeO x , CoO x ) at low temperature. Mol Catal 445:21–28. https://doi.org/10.1016/j.mcat.2017.10.035
Li Y, Lin Y, Wang B, Ding S, Qi F, Zhu T (2019) Carbon consumption of activated coke in the thermal regeneration process for flue gas desulfurization and denitrification. J Clean Prod 228:1391–1400. https://doi.org/10.1016/j.jclepro.2019.04.225
Liu Q, Liu Z (2013) Carbon supported vanadia for multi-pollutants removal from flue gas. Fuel 108:149–158. https://doi.org/10.1016/j.fuel.2011.05.015
Lu P, Li R, Xing Y, Li YR, Zhu TY, Yue HF, Wu WR (2018) Low temperature selective catalytic reduction of NOX with NH3 by activated coke loaded with FexCoyCezOm: the enhanced activity, mechanism and kinetics. Fuel 233:188–199. https://doi.org/10.1016/j.fuel.2018.05.076
Ma L, Li J, Ke R, Fu L (2011) Catalytic performance, characterization, and mechanism study of Fe-2(SO4)(3)/TiO2 catalyst for selective catalytic reduction of NOx by ammonia. J Phys Chem C 115:7603–7612. https://doi.org/10.1021/jp200488p
Marban G, Valdes-Solis T, Fuertes AB (2004) Mechanism of low-temperature selective catalytic reduction of NO with NH3 over carbon-supported Mn3O4 - Role of surface NH3 species: SCR mechanism. J Catal 226:138–155. https://doi.org/10.1016/j.jcat.2004.05.022
Sun DK, Liu QY, Liu ZY, Gui GQ, Huang ZG (2009) An in situ DRIFTS study on SCR of NO with NH3 over V2O5/AC surface. Catal Lett 132:122–126. https://doi.org/10.1007/s10562-009-0080-2
Tang CJ, Zhang HL, Dong L (2016) Ceria-based catalysts for low-temperature selective catalytic reduction of NO with NH3. Catal Sci Technol 6:1248–1264. https://doi.org/10.1039/c5cy01487e
Wang JP, Yan Z, Liu LL, Chen Y, Zhang ZT, Wang XD (2014) In situ DRIFTS investigation on the SCR of NO with NH3 over V2O5 catalyst supported by activated semi-coke. Appl Surf Sci 313:660–669. https://doi.org/10.1016/j.apsusc.2014.06.043
Xie W, Liang D, Li L, Qu S, Wu T (2019) Surface chemical properties and pore structure of the activated coke and their effects on the denitrification activity of selective catalytic reduction. Int J Coal Sci Technol 6:595–602. https://doi.org/10.1007/s40789-019-00267-2
Xu JQ, Chen GR, Guo F, Xie JQ (2018) Development of wide-temperature vanadium-based catalysts for selective catalytic reducing of NOx with ammonia: review. Chem Eng J 353:507–518. https://doi.org/10.1016/j.cej.2018.05.047
Yang J, Ren S, Zhang T, Su Z, Long Z (2020) Iron doped effects on active sites formation over activated carbon supported Mn-Ce oxide catalysts for low-temperature SCR of NOx. Chem Eng J 379:122398. https://doi.org/10.1016/j.cej.2019.122398
Ye M, Cheng C, Li Y, Lin Y, Wang X, Chen G (2020) Enhancement of the denitrification efficiency over low-rank activated coke by doping with transition metal oxides. Can J Chem Eng 98:1390–1397. https://doi.org/10.1002/cjce.23698
Yin SL, Zhu BZ, Sun YL, Zi ZH, Fang QL, Li GB, Chen C, Xu TY, Li JX (2018) Effect of Mn addition on the low-temperature NH3-selective catalytic reduction of NOx over Fe2O3/activated coke catalysts: Experiment and mechanism. Asia Pac J Chem Eng 13:1–7. https://doi.org/10.1002/apj.2231
Zhang Y, Li C, Zhu Y, Du X, Lyu Y, Li S, Zhai Y (2020) Insight into the enhanced performance of toluene removal from simulated flue gas over Mn-Cu oxides modified activated coke. Fuel 276:118099. https://doi.org/10.1016/j.fuel.2020.118099
Zhu Z, Liu Z, Liu S, Niu H (1999) A novel carbon-supported vanadium oxide catalyst for NO reduction with NH3 at low temperatures. Appl Catal B Environ 23:229–233. https://doi.org/10.1016/S0926-3373(99)00085-5
Zhu ZP, Liu ZY, Niu HX, Liu SJ, Hu TD, Liu T, Xie YN (2001) Mechanism of SO2 promotion for NO reduction with NH3 over activated carbon-supported vanadium oxide catalyst. J Catal 197:6–16. https://doi.org/10.1006/jcat.2000.3052
Author information
Authors and Affiliations
Contributions
Jiahua Xie: Conceptualization, data curation, methodology, validation, writing - original draft. Mengyu Li: Conceptualization, data curation, methodology, validation, writing - original draft
Zihua Wu: Conceptualization, methodology, writing - review and editing
Yiqing Zeng: Conceptualization, methodology, writing - review and editing
Shule Zhang: Funding acquisition, project administration, resources, supervision
Jing Liu: Funding acquisition, project administration, resources, supervision
Qin Zhong: Funding acquisition, project administration, resources, supervision
Corresponding authors
Ethics declarations
The manuscript has not been submitted to any other journal for publication. The submitted work is original and it is not published in any form at any journal. Full work has been presented in this paper without splitting. Results are presented clearly, honestly, without fabrication, falsification, or inappropriate data manipulation. Also, sources used are properly cited.
Ethical approval and consent to participate
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Santiago V. Luis
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOC 1096 kb)
Rights and permissions
About this article
Cite this article
Xie, J., Li, M., Wu, Z. et al. Effect of pre-oxidation process on V2O5/AC catalyst for the selective catalytic reduction of NOx with NH3. Environ Sci Pollut Res 29, 13534–13540 (2022). https://doi.org/10.1007/s11356-021-16491-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-16491-w