Abstract
A fractured karst aquifer polluted by petroleum hydrocarbons (PH) for several decades was selected to study the influences of PH on the hydrochemical environment. The research was implemented using the hydrochemical indicators (Ca2+, Mg2+, Na++K+, HCO3−, NO3−, Cl−, F−, and SO42−) and PH with the help of GIS and origin platforms, statistical analyses, and graphical methods. Results showed that PH had significant influences on the hydrochemical environment over the last several decades. The main principle elements influencing the evolution processes of hydrochemical environment were carbonates dissolution, leaking wastewater, and biodegradation processes from 1977 to 2019, and hydrochemistry types changed from HCO3–Ca–Mg and HCO3–Ca to HCO3–Cl–Ca–Mg and HCO3-Cl-Ca. The contribution rate of PH biodegradation to the representative ion increased at first, then decreased over time, which has a close relationship with the variation characteristics of PH. The dynamic evolution processes of hydrochemical environment have significances for indentifying the influencing mechanisms of hydrogeochemical reactions, which could provide valuable scientific suggestions for the local administrators to take effective efforts to optimize and protect the karst groundwater environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater resources account for ~98% of the earth’s available fresh water (Marić et al. 2018), where karst groundwater almost provides drinkable water for 25% of the world’s population (Hillebrand et al. 2015). Karst aquifer systems in North China have enhanced carbonate networks and stable groundwater flow, whose groundwater resources have become the most important water supply sources for local inhabitants (Liang et al. 2018).
The karst hydrogeological system has high sensitivity and vulnerability with the complex dissolution processes of limestone and dolomite (Iván and Mádl-Szőnyi 2017). With the growth of population and the accelerating pace of economic development, petrochemical industries and wide utilization of petroleum products contributed to social development significantly (Liang et al. 2011). However, improper manual operation and old and damaged mining equipment have become the most common causes of groundwater pollution during the processes of mining, transportation, storage, and production (Tfincsics et al. 2010). Petroleum contamination is one of the most serious types of groundwater pollution, possibly becoming more widespread and more serious in the future as more freeways and more petrochemical plants are built. Many constituents of petroleum crude are recalcitrant and highly toxic (Meckenstock et al. 2016), volatile organic compounds (VOCs) are extremely harmful to humans (Souza et al. 2014), and polycyclic aromatic hydrocarbons (PAHs) have potential to induce malignant tumors (Desforges et al. 2016). Consequently, the petroleum contamination in karst groundwater sources is a global concern of protecting drinking water source in the world. Groundwater contaminated by petroleum hydrocarbons (PH) has become a major challenge in North China where the groundwater resources are in severe shortage. Being one of the most important groundwater supply sources, karst groundwater and its hydrochemical evolution characteristics have always been the focus of hydrogeological studies in North China (Wang et al. 2018).
It is notable that organic compounds start natural attenuation when they enter the aquatic environment (Borden et al. 1995; Varjani and Upasani 2017; Logeshwaran et al. 2018). Natural attenuation of PH in the karst groundwater system is one of the most popular strategies to remove the contaminants. As the most effective way of natural attenuation, bioremediation with a low energy consumption was defined as a process where microorganisms are capable, under favorable conditions, of utilizing these compounds as a metabolic carbon source (Marić et al. 2018; Zhang et al. 2017). Microorganisms are widely distributed in water, soil, and air, which adapt to the polluted environment rapidly (Varjani and Upasani 2017). Consequently, their subsequent generations would become hydrocarbon degraders in petroleum polluted areas. PH being natural energy-rich compounds, there are several hydrocarbon degrading/utilizing organisms available in nature (Varjani 2017). According to the thermodynamic concept, electron acceptors of the environment will be utilized in the following sequence: O2→ NO3−→ Mn4+→Fe3+→SO42− (Marić et al. 2018). Dissolved oxygen (DO) is supplied as an electron acceptor in aerobic conditions, and other electron acceptors (e.g., NO3−, Mn4+, Fe3+, SO42−) would be used by microorganisms in anaerobic conditions (Huang et al. 2018). Natural biodegradation is a series of oxidation and reduction reactions where the hydrocarbons become the electron donors, concentrations of electron acceptors and PH reduce, and biodegradation products (e.g., CO2, CH4, Cl−) would influence the characteristics of hydrochemical compounds, isotopic characteristics of related reactants, and resultants (hydrochemical indicators) in the groundwater system (Lv et al. 2019; Guo et al. 2020a). The long-term exposure of groundwater to PH would cause significant changes in water chemistries and isotopic compositions due to the biodegradation reactions in the groundwater system (Marić et al. 2018). Interrelationships among hydrochemical components and PH could interpret the dynamic variation characteristics of hydrochemical environment with the help of multivariate statistical analyses, graphic methods, mathematical model, and other approaches.
A typical karst groundwater source polluted by PH for several decades was chosen to study the variation characteristics of hydrochemical environment. PH was always one of the most important influencing factors in the evolution processes of karst groundwater environment (Liu et al. 1996; Xu et al. 2000; Li et al. 2014; Gao et al. 2019; Guo et al. 2020b). Oil sewage leakage of petrochemical company in the 1990s has caused hydrogeochemical changes comparing with the groundwater environmental background values in the study area (Liu et al. 1996). Groundwater quality of the study area was mainly polluted by trichloromethane, carbon tetrachloride, benzene, and methylbenzene in 1990s (Xu et al. 2000). The typical organic pollutants screened by potential damage risk index and comprehensive evaluation method were trichloromethane, trichloroethylene, tetrachloroethylene, carbon tetrachloride, and benzene in 2010. (Li et al. 2014). Gao et al. (2019) also reported that the main types of organic pollutants are chlorinated hydrocarbons and monocyclic aromatic hydrocarbons, and the over standard organic pollutants were mainly trichloromethane, trichloroethylene, carbon tetrachloride, and benzene using the monitoring data in 2017 and 2018. Contamination characteristics of PH and its influences on hydrochemical ions in the study area were mainly summarized from the monitoring data in a short period and the previous research results. However, the variation characteristics of groundwater chemistries influenced by PH during the last several decades were unclear. In this work, monitoring data of hydrochemical components and PH in the karst groundwater system were collected and analyzed to intepret the evolution mechanisms of hydrochemical environment influenced by organic contaminants during the past several decades. Overall, the objectives of the research were to (1) interpret variation characteristics of hydrochemical environment in the last several decades, (2) analyze the interrelationships among hydrochemical indicators, and (3) calculate the contribution rate of PH to the representative component. The evolution processes of hydrochemical environment influenced by PH were studied based on the biodegradation mechanisms of PH combining with the actual situations in the groundwater system, which have significances for the optimization of groundwater environment and remediation technologies development of petroleum hydrocarbons in the fractured karst environment.
Study area
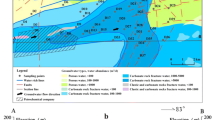
The study area, with an area of ~115 km2, is a rare super-large fractured karst water source in Zibo city, Shandong Province, North China (Fig. 1). The fractured network composed of faults, joints, and fissures is the main catchment space of the aquifer. The nine subareas in Fig. 1 a were divided based on the groundwater types and water abundances of the groundwater source. Generally, groundwater flow direction is from southwest to northeast; the hydrogeological profile map of A→B (Fig. 1a) was drawn in Fig. 1 b. The study area belongs to a warm temperate continental monsoon climate with an average annual temperature of 13.1 °C, rainfall of 630 mm, and relative humidity of 67%; the rainy season is from July to September for each year (Guo et al. 2016).
The hydrological boundaries of the groundwater source are composed of geological structures (Fig. 1a). The eastern boundary is the compressive and water-resistant graben. The western boundary is the water-transporting geological fault. Groundwater flows to the north where Carboniferous and Permian coal-bearing strata with relatively impermeable formed a groundwater barrier (Zhu et al. 1990). The karst aquifer locates in Ordovician strata with the lithologies of thick-bedded limestone, leopard limestone, dolomitic limestone, and dolomite (Fig. 1b); the formations fractured, karstified, and formed a uniform aquifer (Guo et al. 2018). Recharging sources of the karst aquifer are lateral runoff, precipitation infiltration, and surface water seepage. Groundwater resources can be discharged in ways of artificial exploitation, lateral discharge, land surface evaporation, and surface flow spilling water overflowing belt (Guo et al. 2016).
In the middle of 1980s, a petrochemical company was built up in the western area and had the ethylene engineering plant of 300,000 tons (including petrochemical industries, oil refining industries, ethylene chemical plants, alkene plants, and chlor-alkali plants) (Zhu et al. 2000) (Fig. 1a), which made it become the important petrochemical industry base in Zibo city, Shandong Province, China. The foundation in the southern company was directly placed on the Ordovician limestone, the thickness of Quaternary in the north part of the company is only 1–12 m, and many wastewater pipes were directly placed surrounding the Ordovician limestone (Zhu et al. 2000). Its oily wastewater including large amounts of oils, Na+, Ca2+, and Cl− was very corrosive (Liu et al. 1996), so that pipes were damaged, leaking wastewater entered the fractured karst aquifer, and polluted the karst groundwater (Zhu et al. 2000). These contamination areas were exactly located at the upstream of water-rich regions, putting the groundwater source into serious situations.
Methods and materials
Sample analyses
Karst aquifer in the groundwater source provides drinking water and domestic water for local inhabitants and industrial productions. Therefore, groundwater samples were collected in the karst aquifer and analyzed to describe the evolution mechanisms of hydrochemical environment. Hydrochemical ions were detected in all monitoring periods, and PH were detected since the 1990s.
There are ~18 monitoring wells arranged around the groundwater flow path (Fig. 1a). Different groundwater samples were collected in different periods due to natural or anthropogenic factors. HCO3− (mmol/L) was titrated in the field using a portable alkalinity meter (Merck KGaACo., Germany) with a precision of 0.05 mmol/L. Water samples were collected using injection syringes, filtered through 0.45-μm filter membranes for ion analyses, and stored in 500-mL high-density polyethylene (HDPE) bottles. Water samples for cations measurement were acidified to pH<2 using 1:1 HNO3. PH samples were collected in one 1000-mL and two 40-mL brown glass bottles with polytetrafluoroethylene (PTFE) caps to avoid the influence of light. All groundwater samples were kept on ice in the field and then at 4 °C in the laboratory, and they were analyzed in Key Laboratory of Karst Dynamics/Institute of Karst Geology, CAGS within 1 week after collection.
Major hydrochemial cations (e.g., Ca2+, Mg2+, Na+, K+) were measured by ICP-OES (IRIS Intrepid II XSP, Thermo Fisher Scientific, USA) based on EPA method 200.7. Major hydrochemial cations anions (e.g., HCO3−, NO3−, SO42−, Cl−, F−) were measured by the automated Dionex ICS-900 ion chromatograph based on APHA 2012 method. Estimated analytical errors were within ± 5%.
PH are composed of saturates (or alkanes), aromatics, resins and asphaltenes, and resins compounds are dissolved in oil, while asphaltenes are colloidally dispersed in crude oil (Varjani and Upasani 2017). Therefore, PH compounds existed in the groundwater system are alkanes, alkenes cycloalkanes, aromatic hydrocarbons, and other unsaturated hydrocarbons (Lv 2014). Seventy species of these hydrocarbon compounds were analyzed by LC-20A Liquid chromatography and Agilent 7890A-5975C gas chromatograph/mass spectrometer based on EPA 8260B method with the room temperature of 24°C and humidity of 56%, the detection range is 0.10~100 μg/L (groundwater sample with high concentration exceeding the maximum detection limit should be diluted to detect), estimated analytical errors were within ± 4.38%, all blank samples were below the minimum detection limit, and their average standard recovery rate is 97.15%.
Analyses methods
Geospatial analysis has been used in analyzing spatio-temporal patterns of groundwater variables successfully (Chien et al. 2013; Yu et al. 2015). Geographic Information System (ArcGIS 10.2) was used to graph the spatial distribution of groundwater environment information. Spatial distribution correlations of PH, Cl−, and petrochemical company were used to analyze the contamination characteristics of PH. The spatial variation characteristics of hydrochemistry types in the last several decades using inverse distance weighted (IDW) interpolation technique were used to evaluate the evolution processes of hydrochemical environment. Piper diagram was plotted to analyze the variation characteristics of hydrochemistry types influenced by PH with the help of Origin platform using the percentages of major ions (meq L−1, Ca2+, Mg2+, Na+, K+, CO32−, HCO3−, Cl−, and SO42−).
The evolution mechanisms of hydrochemical environment influenced by PH were multivariate problems. The hydro-bio-geochemical processes could be obscurely interpreted by multivariate statistical methods (Monjerezi et al. 2011). The Na+ normalized Cl− versus NO3− plot with the help of statistical analyses platform was used to evaluate the hydrochemical evolution processes. Descriptive statistical analyses of hydrochemical data were used in the research to reveal the influencing mechanisms of PH in the karst groundwater system.
Results and discussion
Contamination characteristics of PH
The petrochemical company started its production activities in 1987, and 1 year later, the dissolved components of PH have been detected in the groundwater monitoring wells (Zhu et al. 2000; Li et al. 2001). During the long-term monitoring periods, the highest concentration level of total PH was 78.23 mg/L (detected in 1994) (Zhu et al. 2000). The serious contamination of PH in the karst groundwater caught attentions of local administrators, and numerous remediation strategies were deployed in the 1990s (Liu and Dai 1996; Zhu et al. 1997; Chen and Zhu 1999; Li et al. 2001). Concentration levels of PH in the contaminated site followed the first-order decay model from 2017 to 2019 based on the monitoring data (Guo et al. 2021), suggesting that the contamination sources (e.g., leaking pipes and tanks) have been controlled. The groundwater flow patterns have recovered recently compared with that of the 1970s (in the natural situation) (Guo et al. 2021) due to the reasonable exploitation of groundwater resources. The industrial wastewater must meet the integrated wastewater discharge standard of China (GB8978-1996) since 1996; otherwise, production activities could not be carried out. Therefore, the entire groundwater system was expected to improve significantly over time since the 1990s. Concentration levels of PH in the contaminated sources decreased from > 20 mg/L in 1996 to 10–20 mg/L in 2009, and 1–5 mg/L in 2017 and 2018 (Guo et al. 2020a) due to the natural attenuation ability of the fractured karst aquifer.
Concentrations of total PH were mostly reported as the organic contamination characteristics before the 2000s, and PH components were detected since the 2000s to analyze their influence mechanisms on the hydrochemical environment. The detected dataset in the dry season of 2018 was analyzed to interpret the relationships among organic compounds with petrochemical company. Twenty organic compounds were detected from ten groundwater samples collected in the fractured karst aquifer, and their detection rates and ranges were summarized in Table 1. The detected chlorinated hydrocarbons are mostly C1–C5 chlorinated alkanes and alkenes (Table 1), which are reported to be petroleum hydrocarbon compounds in many contaminated sites caused by petrochemical companies (Giger and Schaffner 1981; Flathman et al. 1989; Deng 2010; Varjani 2017; Jiang 2020). Chlorinated hydrocarbons have been reported to come from the production activities of petrochemical company and the major compounds of PH in the study area (Li et al. 2014; Gao et al. 2019; Guo et al. 2020c), and the same conclusions were also concluded in other similar fractured karst aquifer (Wei 2015; Lu 2019) or porous aquifer (Jiang 2020) contaminated by petroleum hydrocarbons. It can be concluded that chlorinated hydrocarbons probably come from the production activities of petrochemical plants, chlor-alkali plants and olefin plants in the petrochemical company. D5 is the groundwater monitoring well at the contaminated site, which has the highest concentration of organic contaminants. All its detected organic compounds were chlorinated hydrocarbons, and its top seven chlorinated hydrocarbon compounds accounted for 95.54% of the total organic hydrocarbons, whose order from high to low is trichloromethane, 1,1,2-trichlorethane, 1,1-dichlorethane, carbon tetrachloride, trichloroethylene, tetrachloroethylene, and 1,2-dichloropropane.
The area of petrochemical company was delineated based on the spatial distribution of numerous plants. From the total spatial perspective, there are other petrochemical plants in the southwestern of the study area, which also have influences on the karst groundwater considering the groundwater flow direction. Cl− as the indicator of the intensity of human activities (Henson et al. 2017) could be used to describe the production activities of petrochemical company in the study area. Although the spatial distribution of PH and Cl− mapped using ArcGIS 10.2 did not perfectly depict the real field due to the non-homogeneous karst aquifer media and dispersed monitoring wells, the overall spatial distribution characteristics could be captured adequately. The delineated petrochemcial company had the area of 10.40 km2, and its sewage pipelines and leakage points were distributed dispersively. Even though the highest concentration zones of Cl− and PH did not overlap, the area where petrochemical company located had higher concentrations of Cl− and PH (Fig. 2). Concentrations of Cl− and PH both decreased along the groundwater flow direction and had the similar spatial variation distribution characteristics. Therefore, Cl− could be used to indicate the contamination characteristics of PH in the study area.
Variation characteristics of hydrochemical environment
Because the petrochemical company was built up in 1987, hydrochemical environment was in the natural evolution state before 1987. Anthropogenic influences on the karst groundwater environment increased with the rapid urbanization since 1987. The productions of petrochemical industries (alkene plants, alkali-chlorine plants, plastic plants, and others) brought a quite large amount of wastewater to the surroundings. The hydrochemical environment would be changed by the long-term productive and anthropogenic activities. Hydrochemical data in different periods (1977, 1988, 1999, 2009, and 2019) was used to interpret the dynamic variation characteristics of hydrochemical environment over time. Both NO3− and Cl− are indicators of the intensity of human activities (Henson et al. 2017; Squillace and Moran 2007), and the Na+ normalized Cl− vs NO3− map (Fig. 3) could interpret the variation characteristics of influencing factors during the last several decades. Spatial distributions of hydrochemistry types (Fig. 4) and the piper diagram of groundwater samples (Fig. 5) during the monitoring periods could reveal the evolution characteristics of hydrochemical environment.
It can be seen from Fig. 3 that Cl− came from the halite dissolution in 1977 without human activities, variation ranges of NO3−/Na+ and Cl−/Na+ became wider over time, and the influencing degrees of human activities became greater. The great increasement in the concentrations of Cl- made the groundwater had similar features of seawater. Therefore, hydrochemistry types also had great changes in the last four decades (Fig. 4) due to the human activities.
Even though the spatial distributions of hydrochemistry types based on the uneven monitoring groundwater wells could not perfectly describe the real situation, evolution characteristics of hydrochemical environment were captured adequately in the last several decades (Fig. 4). The piper diagram (Fig. 5) graphically represented the relative contents of major ions in groundwater samples, which could also be used to evaluate hydrochemical evolution processes (Huang et al. 2018). In the early stage (1970s), hydrochemical environment was mainly determined by the geological background (Fig. 3). The lithologies of the karst aquifer media are mainly calcite (CaCO3), dolomite (CaMg(CO3)2), and a small amount of gypsum (CaSO4) in the study area (Lan 1996). In 1977, hydrochemical ions come from the interactions among water and carbonates without the influences of human activities, hydrochemistry types were mainly HCO3–Ca–Mg and HCO3–Ca, only a few of groundwater monitoring points were HCO3–SO4–Ca–Mg where have the dominated lithologies of CaMg(CO3)2 and CaSO4 (Fig. 4a). The hydrochemical environment has been influenced by the petrochemical company since 1987 (Li et al. 2001), and the spatial distribution of hydrochemistry types in 1988 already had differences from 1977 (Fig. 4b). Long-term natural attenuation of PH in the karst groundwater system had made the great influences on the hydrochemistry types in the last several decades (Fig. 4c~e). With unobvious influences of PH in the beginning, spatial distributions of hydrochemistry types in 1999 changed in comparison with 1977 and 1988 (Fig. 4a~c). Groundwater samples collected in 1977, 1988, and 1999 mostly fell in area 5 (Fig. 5), indicating that the relative Ca2++Mg2+ and HCO3−+CO32− contents exceeded 50% of the total cation and anion contents, respectively. With respect to cations and anions, groundwater samples collected in 1977, 1988, and 1999 mostly fell in zones A (Ca2+ type) and E (HCO3− type) (Fig. 5), respectively. Hence, hydrochemistry types were mainly influenced by carbonates dissolution from 1977 to 1999. More than 90% of the PH are chlorinated hydrocarbons in the karst groundwater system (Guo et al. 2020a). Cl− is a conservative ion, and the increasement of Cl− in the groundwater system would come from the wastewater and the long-term natural degradation of chlorinated hydrocarbons (Guo et al. 2020a; Yang et al. 2019; Wu et al. 2020). Groundwater samples collected in 2009 and 2019 mostly fell in area 9 and zone B where had the mixed hydrochemistry type and no dominant cation or anion type (Huang et al. 2018) (Fig. 5). The first pattern (arrow I) showed that the percentages of anions have changed, and Cl− content had an obvious increasing trend from 1977 to 2019 (Fig. 5). It can be seen from the second pattern (arrow II) that Cl−+SO42− contents were almost equal to Cl− content, which had the similar percentage with HCO3−+CO32− contents, indicating that Cl− became the important anion in the karst groundwater system gradually over time. Consequently, the main hydrochemistry types have turned into HCO3–Cl–Ca–Mg and HCO3–Cl–Ca in 2009 and 2019 (Fig. 4d, e).
Evolution processes of the hydrochemical environment
Typical hydrochemical indicators
PH had obvious influences on the hydrochemical environment from 1999 to 2019 based on the foregoing analyses. In the groundwater system, Ca2+, Mg2+, and Na++K+ are common cations, and HCO3−+CO32−, SO42−, and Cl− are common anions. With respect to cations, groundwater samples of all monitoring periods fell in zone A (Ca2+ type) (Fig. 5), indicating that Ca2+ was always the main cation in the evolution processes of hydrochemical environment. NO3− and SO42− were the main electron acceptors in the natural biodegradation processes of PH (Guo et al. 2020a). Oxidation and reduction reactions of iron and manganese could be ignored by considering the background conditions in the fractured karst aquifer (Guo et al. 2020a; Lan 1996). As the reaction products, Cl− and HCO3− would be greatly influenced by the natural biodegradation of PH in the study area (Guo et al. 2020a).
Average values of typical hydrochemical indicators in 1977 (Liu 2013), 1988, 1999, and 2009 were summarized in Table 2. Since the petrochemical company was built in 1987, the chemical field was changed by its production activities from that time, and the variation evidences were showed in Table 2. Generally, the groundwater system has the continuous water table with a flow direction from southwest to northeast. Because the groundwater flow channels are composed of the fractured karst networks, there are some differences in the regional water flow direction. The hydrodynamic field is the carrier of hydrochemical field, so the heterogeneous dynamic variation of water tables would influence the water chemistries. Therefore, the summarized values of hydrochemical ions have higher standard deviations (Table 2), which is the characteristic of hydrochemical field in the fractured karst aquifer.
Concentrations of Cl− increased obviously so that hydrochemistry types have changed a lot. The interpretation of Cl− sources was very essential to obtain influencing mechanisms of PH. Correlations among F-, Na+, NO3−, and Cl− during the evaluation periods (Fig. 6) could interpret the variation characteristics of Cl−. Average values of Cl− in 1977 and 1988 were 36.54 mg/L and 31.73 mg/L, respectively. Average values of F− in 1977 and 1988 were 0.40 mg/L and 0.26 mg/L, respectively. Cl− and F− had little changes without the influences of petrochemical company. The lithologies are mainly carbonate rocks, so Cl− and F− accounted for about 12% and 1% of the main anion (HCO3−) before 1988, respectively. The strong linear correlations between Cl− and F− in 1977 and 1988 (Fig. 6a) suggested that they had the same source of halite dissolution without human activities. Wastewater discharged by petrochemical company and human daily activities includes Na+, Cl−, and NO3−. Variation ranges of F- had little changes in the last four decades, while Cl− became wider since 1988 (Table 2, Fig. 6a). The compound of human daily wastewater including Cl− is mainly NaCl. Sampling points collected in 1977 and 1988 fell on nearby the line of 1:1 (Fig. 6b), others fell below the line of 1:1, suggesting that Cl− had another source of petrochemical company besides the halite dissolution and human daily wastewater. NO3− is the hydrochemical indicator of human activities in the groundwater system and the priority electron acceptor in the biodegradaton processes of PH. The becoming stronger relationship between NO3− and Cl− over time (Fig. 6c) also indicated that the another source of Cl− is petrochemical company. Concentration of Cl− in 1977 is the background value (36.54 mg/L) without the influences of human activities. The difference value of Na+ and the background value of Cl− comes from the human daily wastewater. The difference value of Cl− and Na+ comes from the petrochemical company. The calculated concentrations of Cl− coming from the human daily wastewater in 1999, 2009, and 2019 were 15.54 mg/L, 31.12 mg/L, and 13.49 mg/L, respectively. The calculated concentrations of Cl− coming from the petrochemical company in 1999, 2009, and 2019 were 15.62 mg/L, 89.81 mg/L, and 56.01 mg/L, respectively. The percentages of Cl− coming from human daily wastewater and petrochemical company in total Cl− in 1999, 2009, and 2019 were 46.03%, 76.80%, and 65.54%, respectively. It could be concluded that petrochemical company and human activities had great contributions to the concentrations of Cl− in the groundwater system.
Interrelationships among hydrochemical indicators
Interrelationships among hydrochemical indicators during the evaluation periods could be used to interpret the influencing mechanisms of PH in the evolution processes of hydrochemical environment. Concentrations of PH and hydrochemical indicators in the contaminated site during the monitoring periods were mapped in Fig. 7, and relationships among these components could be captured from these variation curves.
Because of the higher natural attenuation rate in the fractured karst aquifer (Guo et al. 2020a), as well as the effective actions carried by administrators, PH had an obvious decreasing trend during the monitoring periods (Fig. 7a). Although there existed uncertainty due to the dynamic variations of hydrochemical ions, the variation trend could still be captured in the long-term monitoring periods. Ca2+, Mg2+, and Na++K+ had the similar variation features and the increasing trend over time (Fig. 7b~d), indicating that they had close interrelationships. The discharged wastewater including Na+ (Liu 2013) would increase the concentrations of Na++K+ in the groundwater wells, and Ca2+ and Mg2+ would also be increased under the influences of cation alternative adhesive action. After the built of petrochemical company, NO3− as the main electron acceptor was the typical hydrochemical indicator of human activities and had continuous recharing sources (Guo et al. 2020a) increasing from 1977 to 1988, while SO42− as another main electron acceptor had litte changes due to the different sources from 1977 to 1988. The highest concentration of PH was detected in 1994 during the long-term monitoring periods (Zhu et al. 2000). The decreasing of NO3− and SO42− from 1988 to 1999 (Fig. 7f~g) suggested that there existed strong anoxic or anaerobic natural biodegration of PH in this period. Therefore, HCO3− as the biodegradation product had the obvious increasing trend from 1988 and 1999. The increasing trends of SO42−, Na++K+, and Cl− were similar from 1999 to 2009 (Fig. 7d, f, h). The consumed amount of SO42− decreased with the decreasing concentrations of PH from 1999 to 2009. SO42− had the increasing trend in this period. Na++K+ probably came from the wasterwater, and the variation of Cl− was probably caused by the natural biodegradation of chlorinated hydrocarbons in PH besides wastewater by analyzing the relationship between Na+ and Cl−. Chlorinated hydrocarbons biodegradation was a long process, Cl− as the final biodegradation product had the obvious increasing trend from 1988 to 2009. Because of the decreasing concentrations of PH (Fig. 7a) and discharge amount of wastewater recently, HCO3− and Cl− decreased from 2009 to 2019 due to the weak biodegradation of PH and strong hydrodynamic conditions; Ca2+, Mg2+, Na++K+, and Cl− also decreased due to the small amount of leaking wastewater controlled by administrators.
The increasment of Na++K+ mainly came from wastewater, while Cl− had another source of biodegradation of chlorinated hydrocarbons besides wastewater. The differences in the slope values of variation curves of Na++K+ and Cl− over time could be used to calculate the contribution rates of PH to the chemical ion of Cl−. Contribution rates of biodegradation of PH to Cl− in 1988–1999, 1999–2009, and 2009–2019 were 53.84%, 82.64%, and 65.72%, respectively. The highest concentration of PH was detected in 1994; concentrations of PH in the groundwater had the variation trend of increased firstly after declining from 1988 to 2019, which has the similar variation trend of contribution rates of biodegradation of PH to Cl− over time.
Evolution mechanisms of the hydrochemical environment
Correlations among hydrochemical indicators can be provided by multivariate statistical analyses, which could be used to unveil the obscured relationships and elucidate the hydrochemical processes (Lv et al. 2019). Principal component analysis (PCA) could be used to identify the hydrochemical processes by analyzing the hydrochemical datasets (Lv et al. 2019). PCA could drill complex datasets down to a few factors based on the relationships among hydrochemical indicators, which could explain the monitoring indicators to reveal the underlying data structure and highlight the interrelationships among indicators. Two principal factors were extracted in different monitoring periods (Table 3) to evaluate the evolution mechanisms of the hydrochemical environment influenced by PH. KMO (Kaiser-Meyer-Olkin) test values of hydrochemical datasets were higher than 0.50, and cumulative variance contribution rates of the two principal factors were greater than 90% in all periods (Table 3), indicating that PCA could be used to analyze the hydrochemical characteristics and the extracted principal factors could represent almost all the hydrochemical information in the karst groundwater system.
Natural attenuation processes of PH and water-rock interactions could be evaluated by analyzing variation characteristics of principle factors using the long-term monitoring hydrochemical data. PH had little influences on the hydrochemical environment before 1988, and indicators of F1 from 1977 to 1988 were Ca2+, Mg2+, and HCO3− (Table 3) due to carbonates dissolution, which was consistent with the foregoing analyzed hydrochemistry types in 1977 and 1988. PH was always the main influencing element in F1 from 1999 to 2019, indicating that it has become the part of the hydrochemical environment since the production activities of the petrochemical company. The long-term natural attenuation processes of PH influenced the concentrations of hydrochemical indicators. Cl− in F1 from 1999 to 2009 was caused by biodegradation of PH and wastewater, while Na++K+ mainly came from the wastewater. Both Ca2+, Mg2+ and Cl−, Na++K+ belonged to the F1 in 2009, Cl− and Na++K+ exchanged with Ca2+ and Mg2+ in 2019, and the transitional processes of elements in F1 from 1999 to 2019 were caused by the increasing influences of cation alternative adhesive action over time. The degradation products decreased with the decreasing concentration levels of PH, Cl− as the biodegradation product of chlorinated hydrocarbons turned into F2 in 2019 due to the decreasing concentration of PH (Fig. 7a) and the decreasing discharged amount of wastewater controlled by administrators, which was consistent with the foregoing analysis. As the most common pollutant caused by human activities, NO3− had the continuous and various sources in the karst groundwater system and weak relationships with other elements. Therefore, NO3− was always in F2 from 1977 to 2019.
The evolution mechanisms of hydrochemical environment in the study area could be interpreted based on the dynamic variation components of principal factors in different monitoring periods. The evolution characteristics of hydrochemical environment were determined by the geological background conditions before 1988. Dissolution of carbonates and other minerals dominated the main hydrochemical ions in the karst groundwater system without the great influences of production activities of petrochemical company. PH has been the main influencing factor in the evolution processes of hydrochemcial environment since the production activities started. The highest concentration of PH was detected in 1990s. In 1999, Ca2+ and Mg2+ became the main components of F2, while Cl− became the component of F1. These phenomena indicated that the large volume of wastewater (including Na+, Cl−) leaking into the groundwater had relatively high contributions to the hydrochemical ions from 1988 to 1999. In 2009, contamination sources and wastewater were controlled to a certain extent. Ca2+ and Mg2+ became the components of F1, and Cl− as the final product of chlorinated hydrocarbons is still the component of F1, indicating that the biodegradation processes had great influences on the hydrochemical environment from 1999 to 2009. In 2019, the preventive measures of potential contamination sources and wastewater discharge were very well, and the obvious decreasing trend of PH in the karst groundwater system over time (Guo et al. 2020a) suggested that the contamination of PH in the fractured karst aquifer has been greatly improved. Therefore, Na++K+ and Cl− became the components of F2 again in 2019, which also indicated that the influences of PH on the hydrochemical environment declined with the decreasing concentration of PH over time.
Conclusions
The influencing mechanisms of PH on the karst groundwater environment in the last several decades were elucidated using hydrochemical indicators and PH. Spatio-temporal variation distributions of hydrochemistry types were mapped and analyzed from 1977 to 2019. Hydrochemistry types were HCO3–Ca–Mg and HCO3–Ca in the background conditions due to carbonates dissolution. HCO3–Cl–Ca–Mg and HCO3–Cl–Ca became the common hydrochemistry types in the karst groundwater system influenced by PH for several decades. The evolution characteristics of hydrochemical environment were influenced by carbonates dissolution, leaking wastewater, and biodegradation processes over time. Contribution rates of biodegradation of PH to the representative ion had a close relationship with the variation concentrations of PH during the long-term evaluation periods. The research showed that the long-term existence of PH in the karst groundwater system had siginificant influences on the evolution processes of hydrochemical environment. The influencing mechanisms of PH on the hydrochemical environment would help the administrators to take effective efforts to optimize and protect the karst groundwater environment.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Borden RC, Gomez CA, Becker MT (1995) Geochemical indicators of intrinsic bioremediation. Groundwater 33:180–189
Chen YD, Zhu XY (1999) Mechanisms of biodegradation of hydrocarbon contaminants in the groundwater in Dawu well field in Zibo. Guangxi Geol 12:31–34 (in Chinese)
Chien LC, Yu HL, Schootman M (2013) Efficient mapping and geographic disparities in breast cancer mortality at the county-level by race and age in the U.S. Spatial Spatio-temporal Epidemiol 5:27–37
Deng T (2010) Study on the migration and transformation of oil-contaminants in vadose zone. Changan University (in Chinese)
Desforges JW, Sonne C, Levin M, Siebert U, Guise SD, Dietz R (2016) Immunotoxic effects of environmental pollutants in marine mammals. Environ Int 86:126–139
Flathman PE, Jerger DE, Bottomley LS (1989) Remediation of contaminated groundwater using biological techniques. Groundwater Monit R 9:105–119
Gao ZJ, Sun JF, Lu TM, Wang XY, Yang LZ, Liu ZZ (2019) Types and assessment of organic pollutants in groundwater of Dawu source area in Zibo. J Shandong Univ Sci Technol 38:1–9 (in Chinese)
GB8978-1996 (1996) Integrated wastewater discharge standard. In: People’s Republic of China, Environmental Protection Agency, State Bureau of Technical Supervision
Giger W, Schaffner C (1981) Groundwater pollution by volatile organic chemicals. Stud Environ Sci 17:517–522
Guo YL, Zhai YZ, Wu Q, Teng YG, Jiang GH, Wang JS, Guo F, Tang QJ, Liu SH (2016) Proposed APLIE method for groundwater vulnerability assessment in karst-phreatic aquifer, Shandong Province, China: a case study. Environ Earth Sci 75:112
Guo YL, Wu Q, Li CS, Zhao ZH, Sun B, He SY, Jiang GH, Zhai YZ, Guo F (2018) Application of the risk-based early warning method in a fracture-karst water source, North China. Water Environ Res 90:206–219
Guo YL, Wen Z, Zhang C, Jakada H (2020a) Contamination and natural attenuation characteristics of petroleum hydrocarbons in a fractured karst aquifer, North China. Environ Sci Pollut Res 27(18):22780–22794
Guo YL, Quan XQ, Wang QG, Zhang C, Wu Q (2020b) Characteristics of groundwater chemistry in Dawu karst water source. South-to-North Water Transf Water Sci Technol 18(4):130–140 (in Chinese)
Guo YL, Quan XQ, Wu Q (2020c) Pollution characteristics and health risk assessment of volatile organic compounds of a typical karst groundwater source in North China. J Guangxi Normal Univ 38:102–113 (in Chinese)
Guo YL, Wen Z, Zhang C, Jakada H (2021) Contamination characteristics of chlorinated hydrocarbons in a fractured karst aquifer using TMVOC and hydro-chemical techniques. Sci Total Environ 794:148717
Henson WR, Huang L, Graham WD, Ogram A (2017) Nitrate reduction mechanisms and rates in an unconfined eogenetic karst aquifer in two sites with different redox potential. J Geophys Res Biogeosci 122:1062–1077
Hillebrand O, Nödler K, Sauter M, Licha T (2015) Multitracer experiment to evaluate the attenuation of selected organic micropollutants in a karst aquifer. Sci Total Environ 506:338–343
Huang XJ, Wang GC, Liang XY, Cui LF, Ma L, Xu QY (2018) Hydrochemical and stable isotope (δD and δ18O) characteristics of groundwater and hydrogeochemical processes in the Ningtiaota Coalfeld, Northwest China. Mine Water Environ 37:119–136
Iván V, Mádl-Szőnyi J (2017) State of the art of karst vulnerability assessment: overview, evaluation and outlook. Environ Earth Sci 76:112
Jiang WN (2020) Study on identification of natural attenuation of pollutants in groundwater in petrochemical contaminated site. Jilin University, Jilin (in Chinese)
Lan JK (1996) Simulation models of water-rock interaction in the aquifer of Linzi area, Zibo city, Shandong. J Guilin Inst Technol 16(4):410–414 (in Chinese)
Li D, Song XL, Niu PS (2001) A simulation of the groundwater environment and study on control of the petroleum pollution in water supply source of Dawu. Beijing Geol 13(4):20–24 (in Chinese)
Li MR, Wang WS, Ren SJ, Wang JS, Li J (2014) Screening typical pollutants by modified comprehensive evaluation method —— a case study of typical pollutants screening in groundwater of Dawu water source. Environ Pollut Control 36:72–77 (in Chinese)
Liang SH, Kao CM, Kuo YC (2011) In situ oxidation of petroleum hydrocarbon contaminated groundwater by using passive ISCO system. Water Res 45:2496–2506
Liang YP, Gao XB, Zhao CH, Tang CL, Shen HY, Wang ZH, Wang YX (2018) Review: Characterization, evolution, and environmental issues of karst water systems in Northern China. Hydrogeol J 26:1371–1385
Liu SL (2013) The analysis of water quality evaluation regular pattern and the pollution trend prediction of groundwater in Dawu well field, Zibo City. China University of Geosciences, Beijing postgraduate thesis
Liu XH, Dai QL (1996) Remediation programme study on oil pollution in groudwater system. J South China Univ Technol 24:188–192 (in Chinese)
Liu XH, Fu JM, Shen ZL, Zhong ZS (1996) Hydrogeochemical change induced by oil sewage leakage – a case of the groundwater source in Zibo city, Shandong province, China. Geochimica 25:331–338 (in Chinese)
Logeshwaran P, Megharaj M, Chadalavada S, Bowman M, Naidu R (2018) Petroleum hydrocarbons (PH) in groundwater aquifers: An overview of environmental fate, toxicity, microbial degradation and risk-based remediation approaches. Environ Technol Inno 10:175–193
Lu H (2019) Study on the early warning of groundwater system chlorinated hydrocarbons pollution in the eastern area of Jinan. University of Jinan (in Chinese)
Lv H (2014) Research on biodegradation of petroleum hydrocarbon contaminants and enhanced in-situ remediation in groundwater. Jilin University (in Chinese)
Lv H, Wang Y, Wang H (2019) Determination of major pollutant and biogeochemical processes in an oil-contaminated aquifer using human health risk assessment and multivariate statistical analysis. Hum Ecol Risk Assess 25(3):505–526
Marić N, Matić I, Papić P, Beškoski VP, Ilić M, Gojgić-Cvijović G, Miletić S, Nikić Z, Vrvić MM (2018) Natural attenuation of petroleum hydrocarbons—a study of biodegradation effects in groundwater (Vitanovac, Serbia). Environ Monit Assess 190:89
Meckenstock RU, Boll M, Mouttaki H, Koelschbach JS, Tarouco PC, Weyrauch P, Dong X, Himmelberg AM (2016) Anaerobic degradation of benzene and polycyclic aromatic hydrocarbons. J Mol Microbiol Biotechnol 26:92–118
Monjerezi M, Vogt RD, Aagaard P, Saka JDK (2011) Hydro-geochemical processes in an area with saline groundwater in lower Shire River valley, Malawi: an integrated application of hierarchical cluster and principal component analyses. Appl Geochem 26(8):1399–1413
Souza EC, Vessoni-Penna TC, Oliveira RPDS (2014) Biosurfactant-enhanced hydrocarbon bioremediation: an overview. Int Biodeterior Biodegrad 89:88–94
Squillace PJ, Moran MJ (2007) Factors associated with sources, transport, and fate of volatile organic compounds and their mixtures in aquifers of the United States. Environ Sci Technol 41(7):2123–2130
Tfincsics A, Szabo I, Bake E, Szoboszlay S, Kukolya J, Kriszt B, Marialigeti K (2010) Investigation of catechol 2, 3-dioxygenase and 16S rRNA gene diversity in hypoxic, petroleum hydrocarbon contaminated groundwater. Syst Appl Microbiol 33(7):398–406
Varjani SJ (2017) Microbial degradation of petroleum hydrocarbons. Bioresour Technol 223:277–286
Varjani SJ, Upasani VN (2017) A new look on factors affecting microbial degradation of petroleum hydrocarbon pollutants. Int Biodeterior Biodegrad 120:71–83
Wang YX, Ma R, Wang WK, Su XS (2018) Preface: Groundwater sustainability in fast-developing China. Hydrogeol J 26:1295–1300
Wei C (2015) The chlorinated hydrocarbon pollution mechanism of groundwater system in the eastern city. University of Jinan (in Chinese)
Wu NJ, Zhang W, Wei WX, Yang SC, Wang HJ, Sun ZP, Song Y, Li PZ, Wang Y (2020) Field study of chlorinated aliphatic hydrocarbon degradation in contaminated groundwater via micron zero-valent iron coupled with biostimulation. Chem Eng J 384:123349
Xu P, Song CQ, Ding SY (2000) Water environmental problems in Dawu water resource area and prevention countermeasures. Shandong Geol 16(3):36–40 (in Chinese)
Yang J, Meng L, Guo L (2019) In situ remediation of chlorinated solvent-contaminated groundwater using ZVI/organic carbon amendment in China: field pilot test and full-scale application. Environ Sci Pollut Res 25(6):5051–5062
Yu X, Ghasemizadeh R, Padilla I, Irizarry C, Kaeli D, Alshawabkeh A (2015) Spatiotemporal changes of CVOC concentrations in karst aquifers: analysis of three decades of data from Puerto Rico. Sci Total Environ 511:1–10
Zhang YL, Qian H, Wang JL, Si CQ, Chen ZY, Dang JY, Zhang ZY (2017) Variations in the environmental characteristics of groundwater slightly contaminated with petroleum: effects of enhanced bioremediation in northeast China. Environ Earth Sci 76:90
Zhu XY, Zhu GR, Liu YH, Xie XY (1990) Modeling of karst-fracture groundwater resource in Xidian area, China. Memoires of the 22nd Congress of IAH 56-65.
Zhu XY, Xu SH, Si JF (1997) Application of tracing test to the remediation of contaminated fracture-karst water in Zibo city. Carsolog Sin 16:131–144 (in Chinese)
Zhu XY, Liu JL, Zhu JJ, Chen YD (2000) Characteristics of distribution and transport of petroleum contaminants in frature-karst water in Zibo area, Shandong Province, China. Sci China 43(2):141–150
Acknowledgements
The authors would like to thank the editors and reviewers who read the draft of this paper for their constructive comments.
Funding
Financial support was provided by the National Key Research and Development Program of China (2020YFE0204700, 2021YFE0107100), Global Karst Resource Ecology United laboratory-Comparative study of classical karst areas between China and Slovenia (KY201802009), National Natural Science Foundation of Guangxi Province (2021GXNSFBA075013), Project (201501001) supported by Research Center of Groundwater Resources and Environment Engineering in Shandong province, Hydrological and Environmental Survey for Karst Groundwater Resources System of Fengshui Spring Basin in Shandong Province (12120113103900), and National Natural Science Foundation of China (41702277, 42022018) and UNESCO/IUGS (IGCP 661).
Author information
Authors and Affiliations
Contributions
Yuming Peng and Yongli Guo: conceptualization, methodology, investigation, and writing. Qing Wu, Huanliang Chen, Chao Ma, and Chuanlei Li: investigation, methodology, and editing. Wen Liu: conceptualization, methodology, and supervision.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible editor: Xianliang Yi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yuming Peng and Yongli Guo are the first author of this work.
Rights and permissions
About this article
Cite this article
Peng, ., Guo, Y., Wu, Q. et al. Hydrochemical environment of a fractured karst aquifer influenced by petroleum hydrocarbons. Environ Sci Pollut Res 29, 2244–2257 (2022). https://doi.org/10.1007/s11356-021-15661-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-15661-0