Abstract
The process of composting has been proposed as a biological alternative to improve the quality of tannery sludge (TS) by the action of microbial communities. However, there is limited knowledge about the dynamic of these microbial communities during the composting process. This study assessed the responses of bacterial and archaeal communities during TS composting using the 16S rRNA sequencing. The composting process occurred within 90 days, and samples of compost were collected on day 7 (d7; mesophilic stage), 30 (d30; thermophilic stage), 60 (d60; cooling stage), and 90 (d90; maturation stage). The results showed a succession of microbial phyla during the composting with enrichment of Synergistetes, WS1, and Euryarchaeota at the mesophilic stage, while at the thermophilic stage, there was an enrichment of Hydrogenedentes, WPS-2, Chloroflexi, and Deinococcus-Thermus. At the cooling stage, there was an enrichment of Kiritimatiellaeota, and at the maturation stage, there was an enrichment of Entotheonellaeota, Dadabacteria, Nitrospirae, Dependiatiae, and Fibrobacteres. When analyzing the drivers influencing microbial communities, Cr and pH presented more negative correlations with general phyla. In contrast, S, C, K, temperature, and N presented more positive correlations, while Ni, Cd, and P showed fewer correlations. According to niche occupancy, we observed a decreased proportion of generalists with a consequently increased proportion of specialists following the composting process. This study showed that different stages of the composting present a specific microbial community structure and dynamics, which are related to some specific composting characteristics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Solid wastes generating from tannery industries have been increased worldwide, being estimated at ~50,000 tons per year. This solid waste is commonly known as tannery sludge (TS) and presents high content of organic and inorganic elements, mainly chromium (Cr) (Miranda et al. 2019). The composition of TS has stimulated its potential use in agriculture as a soil conditioner improving soil pH and fertility (Araujo et al. 2020). However, the high content of Cr found in TS brings an environmental issue due to the possible contamination in the long term (Araujo et al. 2020). Recently, the process of composting has been suggested as an effective strategy to treat industrial solid wastes, such as textile and tannery sludge (Araujo et al. 2007; Santos et al. 2011). Indeed, composting is a suitable biological alternative to improve the quality of TS and potentially decrease its possible toxicity before using it in agriculture (Miranda et al. 2019).
As a known biological process, composting occurs by the action of several different microbial communities, mainly bacteria and archaea (Meng et al. 2019). During the composting process, the microbial communities act, through enzymatic activity, on the degradation of organic matter, i.e., proteins, lipids, cellulose, and lignin (Ren et al. 2016), with a consequent increase in inorganic nutrients, being a key component to improve the quality of the compost. Interestingly, along the process, there is a microbial succession where different groups of bacteria and archaea are more or less abundant as influenced by the abiotic factors found in the composting pile, such as temperature, moisture, pH, and chemical compounds (Meng et al. 2019). For instance, the main driver of the microbial shifting during the composting is the temperature, which varies significantly across the three main phases of composting, i.e., mesophilic (30–40°C), thermophilic (60–65°C), and maturation (30 days at 25–30°C) (Chandna et al. 2013).
Although the composting process has been widely studied, there is limited knowledge, in terms of technology and high-throughput sequencing, on how the structure, diversity, and composition of microbial communities change during the composting process (Mendes et al. 2017). Some previous studies have used high-throughput sequencing to assess the microbial communities during the composting of cow manure and corn straw (Meng et al. 2019), vegetable wastes and cow dung (Varma et al. 2018), and even sewage sludge (Piceno et al. 2017). However, there are no studies about the structure, diversity, and composition of microbial communities during TS composting. Considering that TS presents a high amount of Cr, it is important to understand its influence on microbial communities during the composting.
Therefore, this study assessed the responses of microbial communities during TS composting using the 16S rRNA sequencing. In addition, we evaluated what are the main drivers influencing the responses of the microbial communities’ structure, diversity, and composition.
Material and methods
Composting process, sampling, and chemical analysis
The composting pile (2.0 m length × 1.0 m width × 1.0 m height) was prepared with a mixture of TS, sugarcane straw, and cattle manure (1:3:1, v:v:v). The composting process occurred within 90 days under artificial turnings at 7, 15, 30, 60, and 90 days. The moisture content was kept at 50–60% by adding water. The samples of compost were collected from four different points of the composting pile on day 7 (d7; mesophilic stage), 30 (d30; thermophilic stage), 60 (d60; cooling stage), and 90 (d90; maturation stage). The collected samples were stored at −20°C for further DNA extraction. The remaining samples were dried, and the chemical properties (pH, C, N, P, K, S, and Cr) were analyzed according to USEPA (1996). Temperature levels at the four points of sampling were measured by a digital thermometer. These chemical properties are shown in Table 1.
DNA extraction and sequencing
DNA was extracted from 0.5 g (total humid weight) of compost using the PowerLyzer PowerSoil DNA Isolation Kit (MoBIO Laboratories, Carlsbad, CA, USA), according to the manufacturer’s instructions. The DNA extraction was performed in triplicate for each soil sample. The quality and concentration of the extracted DNA were determined using the NanoDrop 2000 spectrophotometer (Thermo Scientific, Waltham, USA).
The V4 region of the 16S rRNA gene was amplified with region-specific primers (515F/806R) (Caporaso et al. 2011). Each 25-μL PCR reaction contained the following: 12.25 μL of nuclease-free water (Certified Nuclease-free, Promega, Madison, WI, USA), 5.0 μL of buffer solution 5× (MgCl2 2Mm), 0.75 μL of a solution of dNTPs (10 mM ), 0.75 μL of each primer (515 YF 40 μM e 806 R 10 μM), 1.0 unit of Platinum Taq polymerase High Fidelity in a concentration of 0.5 μL (Invitrogen, Carlsbad, CA, USA), and 2.0 μL of template DNA. Moreover, a control reaction was performed by adding water instead of DNA. The conditions for PCR were as follows: 95°C for 3 min to denature the DNA, with 35 cycles at 98°C for 20 s, 55 °C for 20 s, and 72°C for 30 s, with a final extension of 3 min at 72°C to ensure complete elongation.
After indexing, the PCR products were cleaned up using Agencourt AMPure XP PCR purification beads (Beckman Coulter, Brea, CA, USA), according to the manufacturer’s manual, and quantified using the dsDNA BR assay kit (Invitrogen, Carlsbad, CA, USA) on a Qubit 2.0 fluorometer (Invitrogen, Carlsbad, CA, USA). Once quantified, equimolar concentrations of each library were pooled into a single tube. After quantification, the molarity of the pool was determined and diluted to 2 nM, denatured, and then diluted to a final concentration of 8.0 pM with a 20% PhiX (Illumina, San Diego, CA, USA) spike for loading into the Illumina MiSeq sequencing machine (Illumina, San Diego, CA, USA).
Sequence data were processed using QIIME 2 version 2019.10. Firstly, the sequences were demultiplexed, and quality control was carried out using DADA2 (Callahan et al. 2016), using the consensus method to remove any remaining chimeric and low-quality sequences. Afterward, samples were rarefied to 93,000 sequences, following the number of the lowest sample, and singletons and doubletons were removed. The taxonomic affiliation was performed at 97% similarity using the Silva database v. 132 (Quast et al. 2013), and the generated matrix was further used for statistical analyses. The sequences are submitted to the NCBI Sequence Read Archive under the identification PRJNA714913.
Data analysis
The statistical analyses were performed comparing the four different points of the composting pile (i.e., on days 7, 30, 60, and 90). Initially, the data were checked for normal distribution using the Shapiro-Wilk test and homogeneity of variance using Levene’s test, which indicated normal distribution. Thus, to evaluate the bacterial and archaeal communities’ structure and correlate with composting chemical parameters, we used redundancy analysis (RDA). To verify the significance of the composting chemical parameters upon the microbial community, we used forward selection (FS) followed by the Monte Carlo permutation test with 1000 random permutations. Then, to test whether the sample categories harbor significantly different bacterial communities, we used the permutational multivariate analysis of variance (PERMANOVA) (Anderson 2001). Richness and Shannon’s diversity index were calculated based on the taxonomic matrix at the OTU level and compared based on Tukey’s HSD test. RDA analysis was conducted using Canoco 4.5 (Biometrics, Wageningen, The Netherlands), and PERMANOVA and diversity measurements were calculated using PAST 4.01 software (Hammer et al. 2001).
To compare the composition of the microbial communities among the treatments, we used the Statistical Analysis of Metagenomic Profile (STAMP) software (Parks et al. 2014). For this, the OTU table at the phylum and genus level was used as input. P values were calculated using a two-sided Tukey-Kramer test, and the correction was made using the Benjamini-Hochberg false discovery rate (Benjamini and Hochberg 1995). For visualization, a heatmap was constructed based on z-score transformed phylum abundance using the “pheatmap” package in R (R Development Core Team). To further predict the relevant potential functions of the community, we performed a functional annotation using the FAPROTAX database (Louca et al. 2016), using a table of frequency of taxa at the genus level as input and converted into a putative functional table.
To explore the relationship between the relative abundance of microbial groups at the phylum level and soil properties, we calculated Spearman’s rank correlation coefficients using the “multtest” package in R, and the correction was made using Benjamini-Hochberg FDR. For visualization, a heatmap was constructed using the R package “corrplot.” We also correlated the composting chemical parameters with the microbial diversity using the R package “ggpubr.”
Then, the microbial community dynamic was assessed by comparing the four different time points. First, the niche occupancy was verified by the multinomial species classification method using the “vegan” package and the function “clamtest” in R, with individual test alpha of 0.05 and a coverage limit of 10. This test classifies the microbes into specialists, generalists, and too rare (Pedrinho et al. 2020). Also, to assess the complexity of interactions between taxa, we performed network analysis using the python module “SparCC” (Friedman and Alm 2012). For this, a table of frequency of OTUs with >50 sequences was included, which represent >95% of the total sequences. The SparCC correlations were calculated, and only strong (SparCC >0.9 or < −0.9) and highly significant (P < 0.01) were selected. The networks were created with the software Gephi (Bastian et al. 2009), and the comparison was based on a set of measurements, including the number of nodes and edges, modularity, number of communities, average node connectivity, average degree, diameter, and average path length.
Results
The temperature and some chemical parameters shifted during the composting process (Table 1). The temperature increased from the beginning of composting (d7) to the thermophilic phase (d30) and decreased to maturation (d90). The pH decreased, while C, N, P, K, and S increased during the composting. Interestingly, Cr content decreased significantly from the beginning to the end of composting.
The redundancy analysis (RDA) compared the structure of the prokaryotic communities and how they relate to the TS characteristics during the composting (Figure 1A). Thus, RDA analysis clustered the samples according to the stage of composting (PERMANOVA F = 5.37, P = 0.0001), and the first two axes of the graph explained 49.5% of the total variation. Axis 1 explained the highest variation and separated d7 and d30 from d60 and d90. According to RDA, the microbial communities found during the mesophilic (d7) and thermophilic stages (d30) were closely correlated with pH and temperature, respectively, and both stages presented a correlation with a high Cr rate. In contrast, the microbial communities of the cooling (d60) and maturation stages (d90) were correlated with high rates of P. The composting process also increased the microbial richness and diversity (Fig. 1B, C). Specifically, the lowest and highest microbial richness and diversity were found in d7 and d90, respectively.
Structure and diversity of microbial communities in four stages of tannery sludge composting process based on the 16S rRNA gene. A Redundancy analysis of microbial community structure and chemical properties. Arrows indicate correlation between microbial profile and chemical parameters. The significance of these correlations was evaluated via the Monte Carlo permutation test (P < 0.05). B Taxonomic diversity and richness based on OTU level at 97% similarity. Error bars represent the standard deviation, and different lowercase letters indicate significant differences between the stages based on Tukey’s HSD test (P < 0.05). Cr, chromium; P, phosphorus
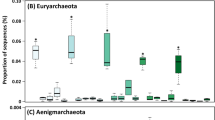
The microbial communities comprised 39 phyla, being dominated by Proteobacteria (22.6% of the total sequences), followed by Firmicutes (16.7%), Chloroflexi (16.1%), Actinobacteria (16%), Bacteroidetes (7.8%), Gemmatimonadetes 5%), Planctomycetes (4%), unclassified Bacteria (3.5%), Euryarchaeota (2.1%), Patescibacteria (2%%), and Acidobacteria (1.6%), all of them with a relative abundance higher than 1% (Fig. 2). The results showed a succession of microbial phyla during the composting with enrichment of groups in the beginning (d7), being significantly enriched the phyla Synergistetes, WS1, and Euryarchaeota. At the thermophilic stage (d30), there was an enrichment of Hydrogenedentes, WPS-2, Chloroflexi, and Deinococcus-Thermus, while at the cooling stage (d60), there was an enrichment of Kiritimatiellaeota. Finally, at the maturation stage (d90), there was an enrichment of Entotheonellaeota, Dadabacteria, Nitrospirae, Dependiatiae, and Fibrobacteres.
Heatmap showing the differential abundance of microbial phyla along four stages of tannery sludge composting process. The color key relates the heatmap colors to the standard score (z-score), i.e., the deviation from row mean in units of standard deviation above or below the mean. Asterisks indicate significantly different phylum abundances (two-sided Tukey-Kramer test with BH correction, P < 0.05), and upper and down arrows indicate increased or decreased relative abundance, respectively. Circles are proportional to the relative abundance of each group in all samples
We highlighted the 10 most abundant OTUs found in the samples (Figure 3A) and observed a decrease of Bacillaceae, Methanobacterium, Rhizobium, and Nocardia from the d7 to other stages. On the other hand, there was an increase of the OTUs affiliated to Chryseolinea, Anaerolineae, Rhizobiales, Chloroflexaceae, Acrinomarinales, and Hyphomicrobiaceae. Since the microbial communities found at the beginning stage of composting were significantly distinct from the other stages, we compared the microbial communities at OTU level in d7 against others (d30, d60, and d90) (Fig. 3B). Therefore, there was an increase in several groups, highlighting Rhizobiaceae, Microscillaceae, Pirellulaceae, Alphaproteobacteria, and Devosia, among others, which can explain the increased diversity at these stages.
Differential abundance of microbial OTUs along four stages of tannery sludge composting process. A Top 10 most abundant OTUs. B Scatter-plot showing the differential abundance of OTUs comparing d7 with the other stages. The differences are based on Welch’s t-test with Benajmini-Hochberg correction calculated in STAMP (P < 0.05)
Spearman’s rank was calculated to address the correlation between individual phyla and characteristics of the compost (Figure 4A). Cr and pH presented more negative correlations with general phyla. In contrast, S, C, K, temperature, and N presented more positive correlations, while Ni, Cd, and P showed fewer correlations. As important contrasts, Acidobacteria, Planctomycetes, WS2, and Nanoarchaeaeota correlated negatively with Cr and pH, while presented positive correlations with S, C, K, and N. Dadabacteria and Entotheonellaeota correlated negatively with Cr and positively with S, C, K, and N. Interestingly, temperature correlates positively with Deinococcus-Thermus, Bacteroidetes, Verrucomicrobia, Hydrogenedentes, BRC1, Chloroflexi, WPS-2, and Patescibacteria. In addition, we evaluated how the characteristics drove microbial diversity during composting (Figure 4B). Thus, Cr and pH presented a negative influence on microbial diversity, while S, K, N, and C correlated positively with microbial diversity.
A Heatmap showing the Spearman’s rank correlation coefficients and statistical significance between microbial phyla abundance and composting chemical parameters. Blue and red colors indicate significant positive and negative correlations, respectively (P < 0.05). B Spearman correlation between microbial diversity and composting chemical parameters (P < 0.05). Cr, chromium; S, sulfur; C, carbon; K, potassium; N, nitrogen; Ni, nickel; Cd, cadmium; P, phosphorus
We then observed the niche occupancy and found a variation of the proportion between specialists and generalists during each stage of composting (Figure 5A). In general, we observed a decreased proportion of generalists with a consequently increased proportion of specialists following the composting process. More specifically, when comparing only the specialists, d7 presents 20.8% against 22.9% in d30, 28.5% in d60, and 33.7% in d90. The co-occurrence network analysis showed shifts in the complexity of connections in the microbial community during the composting (Figure 5B and Table 2). Each stage of composting showed distinct network compositional and topological features. Therefore, the microbial community in d7 (mesophilic stage) exhibited more complexity (617 nodes, 6996 edges, and average degree 22.67). Then, there was a decrease in complexity following thermophilic (d30; 510 nodes, 2996 edges, and average degree 11.74) and cooling (d60; 484 nodes, 2047 edges, and average degree 8.45) stages. The complexity increased again in the maturation stage (d90; 584 nodes, 4234 edges, and average degree 14.5). Interestingly, the modularity and the number of communities within the network increased from d7 to d90 (Table 2). This result revealed changes in the community dynamics along the composting process, with temperature and pH playing a major role.
A Multinomial species classification method (CLAM) for the niche occupancy test based on pairwise comparison. The generalists (gray), specialists (blue tones), and rare (black) are indicated with their respective percentages. B Network co-occurrence analysis of the microbial communities in soils along four stages of tannery sludge composting process based on the 16S rRNA gene. A connection stands for SparCC correlation with magnitude > 0.9 (positive correlation—blue edges) or < − 0.9 (negative correlation—red edges) and statistically significant (P ≤ 0.01). Each node represents taxa at OTU level, and the size of node is proportional to the number of connections (i.e., degree). The color of the nodes is based on the betweenness centrality, where red colors indicated higher values. The network construction was made using Gephi software
Finally, we used the FAPROTAX database to predict the potential functions of the microbial community along each stage of the composting process. Thus, 14 functional groups changed according to the stages of composting (Fig. 6). At the beginning of composting (d7), there was an increase of microbial groups related to methylotrophy, dark hydrogen oxidation, and methanogenesis, while at the end of composting (d90), there was an increase of microbial groups related to aerobic nitrite oxidation, nitrification, aerobic ammonia oxidation, and aromatic compound degradation (P < 0.05).
Heatmap showing the differential abundance of predicted functional categories along four stages of tannery sludge composting process based on FAPROTAX database. The color key relates the heatmap colors to the standard score (z-score), i.e., the deviation from row mean in units of standard deviation above or below the mean. Upper and down arrows indicate increased or decreased relative abundance, respectively (two-sided Tukey-Kramer test with BH correction, P < 0.05)
Discussion
The chemical properties and temperature changed during composting, and it is resulting from microbial action, through degradation of organic sources and mineralization of nutrients (Hills et al. 2020). The concentration of Cr was significantly reduced through composting, suggesting a potential effect of microbial communities on Cr detoxification. However, it is unknown how Cr drives the microbial communities inside the composting process. In our study, the Cr presented a negative correlation with 11 phyla, which could explain the lower diversity in the first phases of the composting. Indeed, Cr in soils presents an impact on the composition and structure of microbial communities (Miranda et al. 2019). Some phyla presented a positive correlation with Cr concentration, such as Synergistetes. This phylum was linked to the digestion of organic residues in a tannery solid waste anaerobic digestion, with a role in the production of methane (Agustini et al. 2020). Interestingly, our metabolic prediction revealed a high abundance of methanogenesis at the beginning of the composting process, matching with the higher abundance of this phylum. However, it has been shown that methane production decreases with increasing the chromium concentration (Akyol et al. 2015). Thus, the increase of microbial groups associated with methanogenesis at the beginning of the composting process could be associated with other TS parameters, such as organic elements and microbial groups not directly affected by Cr.
In this study, Proteobacteria, Firmicutes, Actinobacteria, and Chloroflexi were the most abundant phyla found during the composting process as also reported in previous studies (Yin et al. 2017; Zhou et al. 2018; Meng et al. 2019). It suggests that these microbial groups are the most important during the degradation of organic materials. Indeed, Awasthi et al. (2017) found these phyla as the main players during the composting of sewage sludge. However, distinct microbial communities presented high abundance in each phase during composting, suggesting that each microbial group is more adapted to shifts in composting parameters. For example, Proteobacteria increased during the composting, while Actinobacteria decreased in d30 and d60 and recovered in d90. The increase of Proteobacteria could be linked to the increase of nutrients available during the composting since members of this phylum participate in the cycling of carbon, nitrogen, and sulfur (Pedrinho et al. 2019). This fact can be also observed in the increase of sequences related to nitrogen metabolism during the composting process, such as nitrification, aerobic ammonia oxidation, and aerobic nitrite oxidation (Figure 6). Also, at the beginning of composting (d7), the higher abundance of Synergistetes and Euryarchaeota can suggest that the initial composting process seems to produce methane, as discussed above since both phyla are associated with initial degradation of wastes contributing to methane emission (Lee et al. 2010). Although our composting process is aerobic, the first turning was done after the compost sampling at d7. It could explain the high abundance of methanogenic microbes (e.g., Synergistetes and Euryarchaeota) and the increase of microbe groups related to methylotrophy, dark hydrogen oxidation, and methanogenesis at d7 (Figure 6). On the other hand, as the temperature of compost increased at d30, we observed an enrichment of Hydrogenedentes, WPS-2, Chloroflexi, and Deinococcus-Thermus. This thermophilic phase may select thermotolerant microbial groups, some of which are highly tolerant of hazardous compounds, such as ultraviolet radiation, like Deinococcus-Thermus (Griffiths and Gupta 2007). For instance, the genus Truepera (Deinococcus-Thermus) plays an important role in the thermophilic phase of the composting due to their functions on cellulose degradation (Bishop et al. 2016; Zhang et al. 2014). Chloroflexi were also abundant in the thermophilic phase of aerated static pile composting (Neher et al. 2013). However, the ecological attributes of Chloroflexi members during composting are not well-known due to the difficulty to culture under controlled conditions. Interestingly, at the cooling stage (d60), there was an enrichment of Kiritimatiellaeota, and, at d90, Entotheonellaeota, Dadabacteria, Nitrospirae, Dependiatiae, and Fibrobacteres were increased. This result indicates that these groups can be related to nitrification metabolism since the final phase of composting has greater mineral compounds than organic fractions. For instance, Jarvis et al. (2009) and Maeda et al. (2010) also found Nitrospirae in the maturation stage in household waste and cattle manure composting, respectively. This bacterial group generates energy from the hydroxylamine oxidation route, using ATP to fix CO2 as C-source, indicating that these bacteria should be active on ammonium oxidation in the composting process (i.e., converting NH4+ into NO3−) (Maeda et al. 2011). This observation is also corroborated by the higher abundance of sequences linked to nitrification, aerobic ammonia oxidation, and aerobic nitrite oxidation at d90 (Figure 6).
When analyzing in a lower taxonomic level (OTU), at the beginning of composting (d7), we observed a high abundance of Nocardia, Rhizobium, Methanobacterium, and Bacillaceae. Nocardia is a group of bacteria recognized as lignocellulose degraders (Varma et al. 2018), and thus these groups could be acting in the first stage of composting. The genus Rhizobium grows preferentially at alkaline pH, but considering that the pH was alkaline along all stages of the compost, its higher abundance in d7 can be due to the lower temperature found in this stage as compared to other stages. In contrast, the enrichment of Anaerolineae could be linked to increased temperature. Anaerolinea (Chloroflexi) was found to be a facultative aerobic bacterium that presents an optimum growth at a temperature around 55 to 65°C, being recognized as a chemo-organoheterotroph on several carbohydrates (Sekiguchi et al. 2003). Thus, these traits found in this bacterium could explain its high abundance during the thermophilic phase in this study.
Our results demonstrated that each phase of composting harbors different microbial communities, which were significantly influenced by temperature, pH, Cr, and P. Indeed, the temperature is an important factor driving the behavior of microbial communities in the composting process, mainly during the thermophilic phase (Antunes et al. 2016). In addition, during the composting, the organic materials are degraded, and there is a shift in chemical parameters that contribute to changes in microbial communities. Similar patterns found on microbial communities during composting were reported by Meng et al. (2019), who evaluated four phases of composting on the structure of microbial communities. For instance, pH influenced microbial groups in the d7 phase, where the pH value was higher (pH = 9.4) than the other composting phases. However, in the final phases of composting, the pH reduced and contributed to the increase in microbial diversity. Generally, the reduction in pH during composting is attributed to the production of organic acids delivered by both bacterial and fungi communities to degrade recalcitrant material (Zakarya et al. 2018). In general, several studies have reported the pH as the main factor structuring community composition, and this correlation may be a result of the integration of pH with other chemical parameters (Fierer and Jackson 2006; Mendes and Tsai 2018). Indeed, the decrease of the pH to close to 7.0 increases nutrient availability (N, P, and S) in compost, promoting a more suitable environment for the microbial establishment. It partially explains the significant correlation between P and groups at d60 and d90 (Figure 1a), the continuous increment of microbial diversity along with composting phases (Figure 1b), and the strong positive relationship between Shannon diversity with K, N, S, and C contents (Figure 4).
The Spearman rank correlation test showed a higher number of negative correlations comparing microbial phyla with pH and Cd contents. Contrary, S, C, K, and N contents presented a higher number of positive correlations with microbial phyla. It seems to be coherent since the same pattern was found comparing these attributes with Shannon diversity. Generally, it could be related to the improvement of compost quality in terms of chemical parameters, such as increased nutrients and decreased Cr. In addition, as the composting is processed naturally, there is an increase in microbial richness and diversity (Meng et al. 2019). Also, Acidobacteria, Planctomycetes, Nanoarchaeota, and Entotheonellaeota positively correlate with S, C, K, and N during composting. Acidobacteria and Planctomycetes were positively correlated with total C during urban green waste composting, suggesting that feeding niches of these groups play a key role in changes of organic matter and, consequently, nutrient content (Tong et al. 2018). Indeed, our niche occupancy analysis showed an increased proportion of specialists along the composting process, revealing that specific microbial groups are responding to the increased nutrient availability. The archaeal community (through amoA gene studies) was abundant throughout agricultural wastes composting dominated NH4+ oxidation during the thermophilic and cooling stages (Zeng et al. 2011). Some reports showed that ammonia-oxidizing archaea can be relatively more abundant than ammonia-oxidizing bacteria in composts of tropical agricultural wastes (de Gannes et al. 2012).
Finally, we used co-occurrence networks analysis to understand the microbial community dynamics and compare the complexity of interaction between the microbes in each composting phase. Our analysis showed that the complexity of connections was higher at the beginning of composting (d7). In the initial phase of composting, the raw material presents a more diversified source of organic compounds. Therefore, it can result in a higher functional redundancy in microbial communities (Allison and Martiny 2009) in the initial phase of tannery sludge composting, resulting in higher generalist microbes and more complex communities. Since our sludge presented higher Cr contents, an increased functional redundancy in the initial phase, with a wide range of potential metabolic cooperation between microbes, is extremely important for maintaining efficient compost degradation. In accordance, the final phases are characterized by more recalcitrant substrates, needing thereby highly specialized microbial communities to promote their transformations (Buresova et al. 2019; Fukami 2015; Purahong et al. 2014). In this sense, the increased proportion of specialist microbes was followed by a decrease in the community complexity in d30, following an increase in the last two phases. Interestingly, the modularity and the number of communities within the network increased in the later stage (d90). Networks with a modular structure are characterized by the presence of different groups of nodes with a high number of interconnections within, suggesting diversity in species roles and functionality, increasing niche overlap, and a high abundance of negative interactions within the community (Peipoch et al. 2019). This can be observed by the increased diversity in the last stage of the composting, followed by an increased proportion of specialists and a higher proportion of negative interactions. Thus, our network analysis indicates that the composting process, along with changes in chemical parameters, shapes the community dynamics and co-occurrence of microbial taxa.
This study has shown the dynamics of the bacterial and archaeal community during composting of tannery sludge, and this knowledge is important to verify the role of bacteria in converting industrial solid waste into a potential stable and safe compost. The final quality of compost depends on the substrate, environmental conditions, and the interactions between microbes (Lu et al. 2018). Therefore, we found a bacterial succession during the composting with the predominance of Proteobacteria, Firmicutes, and Actinobacteria, which are known as important degraders of organic wastes (Awasthi et al. 2017). This bacterial succession and complex interactions positively influence the increased nutrients in the final compost and can contribute to improving soil properties (Rashid et al. 2016). Indeed, Zhao et al. (2019) have reported that the interactions of microbial communities influenced positively the stability, safety, and quality of composted sewage sludge. On the other hand, some bacterial groups positively correlated significantly with Cr, which suggests the potential for Cr degradation and then a reduction in the toxicity of composted tannery sludge.
Conclusion
In this study, different stages of the composting present a specific microbial community structure and dynamics, which are related to some specific composting characteristics, such as pH, temperature, chromium, and P content. The composting process increased the microbial diversity and the proportion of specialist microbes, which led to a decrease in Cr content and an increase in nutrients. These changes in the chemical properties are related to changes in specific microbial groups and functions. Although the process of composting improves the quality and stability of organic wastes with the contribution of microbes, composted tannery sludge presented yet high Cr concentration and prevents its use in agricultural soils. Therefore, the monitoring of Cr concentration in composted tannery sludge should be done to verify its suitability to application in soils.
Data availability
All sequences are deposited in the NCBI Sequence Read Archive under the identification PRJNA714913.
References
Agustini CB, Costa M, Gutterres M (2020) Biogas from tannery solid waste anaerobic digestion is driven by the association of the bacterial order bacteroidales and archaeal family methanosaetaceae. Appl Biochem Biotechnol 192:482–493
Akyol Ç, Demirel B, Onay TT (2015) Recovery of methane from tannery sludge: the effect of inoculum to substrate ratio and solids content. J Mater Cycles Waste Manag 17:808–815
Allison SD, Martiny JBH (2009) Resistance, resilience, and redundancy in microbial communities. Light Evol 2:149–166
Anderson MJ (2001) A new method for non parametric multivariate analysis of variance. Austral Ecol 26:32–46
Antunes LP, Martins LF, Pereira RV, Thomas AM, Barbosa D, Lemos LN, Silva GMM, Moura LMS, Epamino GWC, Digiampietri LA, Lombardi KC, Ramos PL, Quaggio RB, De Oliveira JCF, Pascon RC, Da Cruz JB, Da Silva AM, Setubal JC (2016) Microbial community structure and dynamics in thermophilic composting viewed through metagenomics and metatranscriptomics. Sci Rep 6:38915
Araujo ASF, Monteiro RTR, Carvalho EMS (2007) Effect of composted textile sludge on growth, nodulation and nitrogen fixation of soybean and cowpea. Bioresour Technol 98:1028–1032
Araujo ASF, de Melo WJ, Araujo FF, Van den Brink PJ (2020) Long-term effect of composted tannery sludge on soil chemical and biological parameters. Environ Sci Pollut Res 27:41885–41892
Awasthi MK, Zhang Z, Wang Q, Shen F, Li R, Li DS et al (2017) New insight with the effects of biochar amendment on bacterial diversity as indicators of biomarkers support the thermophilic phase during sewage sludge composting. Bioresour Technol 238:589–601
Bastian M, Heymann S, Jacomy M (2009) Gephi: An open source software for exploring and manipulating networks. In Proceedings of the Third International ICWSM Conference. California, USA. 361–362
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B 57:289–300
Bishop EL, Pecchia JA, Wilkinson V, Albert I, Royse DJ (2016) Effects of spent mushroom compost (SMC) as an ingredient in phase I compost on production of Agaricus bisporus. Compost Sci Util 24:246–258
Buresova A, Kopecky J, Hrdinkova V, Kamenik Z, Omelka M, Sagova-Mareckova M (2019) Succession of microbial decomposers is determined by litter type, but site conditions drive decomposition rates. Appl Environ Microbiol 85:1–16
Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP (2016) DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods 13:581–583
Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Lozupone CA, Turnbaugh PJ, Fierer N, Knight R (2011) Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci 108:4516–4522
Chandna P, Nain L, Singh S, Kuhad RC (2013) Assessment of bacterial diversity during composting of agricultural byproducts. BMC Microbiol 13:99
de Gannes V, Eudoxie G, Dyer DH, Hickey WJ (2012) Diversity and abundance of ammonia oxidizing archaea in tropical compost systems. Front Microbiol 3:1–16
Fierer N, Jackson RB (2006) The diversity and biogeography of soil bacterial communities. Proc Natl Acad Sci U S A 103:626–631
Friedman J, Alm EJ (2012) Inferring correlation networks from genomic survey data. PLoS Comput Biol e1002687
Fukami T (2015) Historical contingency in community assembly: integrating niches, species pools, and priority effects. Annu Rev Ecol Evol Syst 46:1–23
Griffiths E, Gupta RS (2007) Identification of signature proteins that are distinctive of the Deinococcus-Thermus phylum. Int Microbiol Off J Spanish Soc Microbiol 10:201–208
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: Paleontological Statistics Software Package for education and data analysis. Palaeontol Electron 4:1–9
Hills CD, Tripathi N, Carey PJ (2020) Mineralization technology for carbon capture, utilization, and storage. Front Energy Res 8:1–14
Jarvis Å, Sundberg C, Milenkovski S, Pell M, Smårs S, Lindgren PE, Hallin S (2009) Activity and composition of ammonia oxidizing bacterial communities and emission dynamics of NH3 and N2O in a compost reactor treating organic household waste. J Appl Microbiol 106:1502–1511
Lee YH, Kim SK, Kim YH, Jeong YS, Yun MG, Cho JJ, Kim JM, Yun HD, Kim H (2010) Archaeal diversity during composting of pig manure and mushroom cultural waste based on 16S rRNA sequence. J Korean Soc Appl Biol Chem 53:230–236
Louca S, Parfrey LW, Doebeli M (2016) Decoupling function and taxonomy in the global ocean microbiome. Science 353:1272–1277
Lu Q, Zhao Y, Gao X, Wu J, Zhou H, Tang P, Wei Q, Wei Z (2018) Effect of tricarboxylic acid cycle regulator on carbon retention and organic component transformation during food waste composting. Bioresour Technol 256:128–136
Maeda K, Hanajima D, Morioka R, Osada T (2010) Characterization and spatial distribution of bacterial communities within passively aerated cattle manure composting piles. Bioresour Technol 101:9631–9637
Maeda K, Hanajima D, Toyoda S, Yoshida N, Morioka R, Osada T (2011) Microbiology of nitrogen cycle in animal manure compost. Microb Biotechnol 4:700–709
Mendes LW, Tsai SM (2018) Distinct taxonomic and functional composition of soil microbiomes along the gradient forest-restinga-mangrove in southeastern Brazil. Anto van Leew 111:101–114
Mendes LW, Braga LPP, Navarrete AA, Souza DG, Silva GGZ, Tsai SM (2017) Using metagenomics to connect microbial community biodiversity and functions. Curr Issues Mol Biol 24:103–118
Meng Q, Yang W, Men M, Bello A, Xu X, Xu B, Deng L, Jiang X, Sheng S, Wu X, Han Y, Zhu H (2019) Microbial community succession and response to environmental variables during cow manure and corn straw composting. Front Microbiol 10:529
Miranda ARL, Mendes LW, Lemos LN, Antunes JEL, Amorim MR, Melo VMM, Melo WJ, van Den Brink PJ, Araujo ASF (2019) Dynamics of archaeal community in soil with application of composted tannery sludge. Sci Rep 9:e7347
Neher DA, Weicht TR, Bates ST, Leff JW, Fierer N (2013) Changes in bacterial and fungal communities across compost recipes, preparation methods, and composting times. PLoS One 8:e79512
Parks DH, Tyson GW, Hugenholtz P, Beiko RG (2014) STAMP: statistical analysis of taxonomic and functional profiles. Bioinformatics 30:3123–3124
Pedrinho A, Mendes LW, Merloti LF, Fonseca MC, Cannavan FS, Tsai SM (2019) Forest-to pasture conversion and recovery based on assessment of microbial communities in Eastern Amazon rainforest. FEMS Microbiol Ecol 95:fiy236
Pedrinho A, Mendes LW, Merloti LF, Andreote FD, Tsai SM (2020) The natural recovery of soil microbial community and nitrogen functions after pasture abandonment in the Amazon region. FEMS Microbiol Ecol 96:fiaa149
Peipoch M, Miller SR, Antao TR, et al (2019) Niche partitioning of microbial communities in riverine floodplains. Sci Rep 9:16384
Piceno YM, Pecora-Black G, Kramer S, Roy M, Reid FC, Dubinsky EA, Andersen GL (2017) Bacterial community structure transformed after thermophilically composting human waste in Haiti. PLoS One 12(6):e0177626
Purahong W, Schloter M, Pecyna MJ, Kapturska D, Däumlich V, Mital S, Buscot F, Hofrichter M, Gutknecht JLM, Krüger D (2014) Uncoupling of microbial community structure and function in decomposing litter across beech forest ecosystems in Central Europe. Sci Rep 4:1–7
Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO (2013) The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41:590–596
Rashid MI, Mujawar LH, Shahzad T, Almeelbi T, Ismail IMI, Oves M (2016) Bacteria and fungi can contribute to nutrients bioavailability and aggregate formation in degraded soils. Microbiol Res 183:26–41
Ren G, Xu X, Qu J, Zhu L, Wang T (2016) Evaluation of microbial population dynamics in the co–composting of cow manure and rice straw using high throughput sequencing analysis. World J Microbiol Biotechnol 32:1–11
Santos JA, Nunes LAPL, Melo WJ, Araujo ASF (2011) Tannery sludge compost amendment rates on soil microbial biomass of two different soils. Eur J Soil Biol 47:146–151
Sekiguchi Y, Yamada T, Hanada S, Hanada S, Ohashi A, Harada H, et al (2003) Anaerolinea thermophila gen. nov., sp. nov. and Caldilinea aerophila gen. nov., sp. nov., novel filamentous thermophiles that represent a previously uncultured lineage of the domain Bacteria at the subphylum level. Int J Syst Evol Microbiol 53:1843–1851
Tong J, Sun X, Li S, Qu B, Wan L (2018) Reutilization of green waste as compost for soil improvement in the afforested land of the Beijing Plain. Sustain. 10:1–17
Varma VS, Dhamodharan K, Kalamdhad AS (2018) Characterization of bacterial community structure during in-vessel composting of agricultural waste by 16S rRNA sequencing. Biotech 8:301
Yin Y, Gu J, Wang X, Tuo MX, Zhang K, Zhang ML et al (2017) Effects of copper on the composition and diversity of microbial communities in laboratory-scale swine manure composting. Can J Microbiol 64:409–419
Zakarya IA, Khalib SNB, Mohd Ramzi N (2018) Effect of pH, temperature and moisture content during composting of rice straw burning at different temperature with food waste and effective microorganisms. E3S Web Conf 34:4–11
Zeng G, Zhang J, Chen Y, Yu Z, Yu M, Li H, Liu Z, Chen M, Lu L, Hu C (2011) Relative contributions of archaea and bacteria to microbial ammonia oxidation differ under different conditions during agricultural waste composting. Bioresour Technol 102:9026–9032
Zhang X, Zhong Y, Yang S, Zhang W, Xu M, Ma A, Zhuang G, Chen G, Liu W (2014) Diversity and dynamics of the microbial community on decomposing wheat straw during mushroom compost production. Bioresour Technol 170:183–195
Zhao X, Wei Y, Zhang F, Tan W, Fan Y, Xi B (2019) How do fungal communities and their interaction with bacterial communities influence dissolved organic matter on the stability and safety of sludge compost? Environ Sci Pollut Res 26:4141–4146
Zhou H, Gu W, Sun W, Hay AG (2018) A microbial community snapshot of windrows from a commercial composting facility. Appl Microb Biotech 102:8069–8077
Acknowledgements
The authors thank the Centro de Genetica e Bioinformatica (CeGenBio) from the Unit of Research (NPDM/UFC). Jadson Emanuel Lopes Antunes and Sandra Mara Barbosa Rocha thank CAPES for their fellowship. Ademir Sergio Ferreira de Araujo, Fabio Fernando Araujo, Wanderley José de Melo, and Vania Maria Maciel Melo thank CNPq for their fellowship of research.
Funding
This study was funded by Conselho Nacional de Desenvolvimento Científico e Tecnologico (CNPq) (grant 305069/2018-1).
Author information
Authors and Affiliations
Contributions
ASFA and WJM conceived this study. JELA, LMSO, SMBR, MRA, and VMMM designed and conducted the composting, collected samples, and proceeded molecular sequencing. LWM and APAP provided bioinformatic and the 16S rRNA gene data. LWM and FFA performed the statistical, network analyses and generate the results. ASFA, LWM, VMMM, and FFA interpreted the results, elaborated the main arguments, and wrote the first draft. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate
Not applicable
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Diane Purchase
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Araujo, A.S.F., de Pereira, A.P., Antunes, J.E.L. et al. Dynamics of bacterial and archaeal communities along the composting of tannery sludge. Environ Sci Pollut Res 28, 64295–64306 (2021). https://doi.org/10.1007/s11356-021-15585-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-15585-9