Abstract
In this study, a magnetic metal–organic framework (MMOF) was synthesized and post-modified with poly(propyleneimine) dendrimer to fabricate a novel functional porous nanocomposite for adsorption and recovery of palladium (Pd(II)) from aqueous solution. The morphological and structural characteristics of the prepared material were identified by field emission scanning electron microscopy (FESEM), X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), Brunauer–Emmet–Teller (BET) isotherm, and vibrating sample magnetometer (VSM). The results confirmed the successful synthesis and post-modification of MMOF. Semispherical shape particles (20–50 nm) with appropriate magnetic properties and a high specific surface area of 120 m2/g were obtained. An experimental design approach was performed to show the effect of adsorption conditions on Pd(II) uptake efficiency of the dendrimer-modified magnetic adsorbent. The study showed that the Pd(II) uptake on dendrimer-modified MMOF was well described by the Langmuir isotherm model with the highest uptake capacity of 291 mg/g under optimal condition (adsorbent content of 12.5 mg, Pd ion concentration of 80 ppm, pH = 4, and contact time of 40 min). The adsorption kinetics of Pd(II) ions was suggested to be a pseudo-first-order model. The results revealed a faster adsorption rate and higher adsorption capacity (about 43%) for dendrimer-modified MMOF. Finally, the reusability of the provided adsorbent was evaluated. This work provides a valuable strategy for designing and developing efficient magnetic adsorbents based on MOFs for the adsorption and recovery of precious metals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, palladium (Pd) as a precious metal has received increasing attention due to high industrial demands for jewelry, medicine, electronics, fuel cell technology, and catalysts (Mergola et al. 2020). However, the high value of Pd and its low natural abundance make its recovery and reusability economically feasible. Hence, the development of cost-effective and efficient methods for the separation and extraction of Pd from industrial wastewater has become a big concern. So far, conventional methods such as membrane separation, ion exchange, solvent extraction, adsorption, and microbiological approaches have been widely used to recover or separate Pd ion (B. Zhang et al. 2018; Lim et al. 2020). However, most of these techniques have several drawbacks including high costs, complicated processes, and poor selectivity, which make their application rather questionable. Adsorption technique, as the most attractive and competitive approach, is a cost-effective, simple operation, highly efficient, and reliable approach (Molavi et al. 2018; El Salam and Zaki 2018; Kousha et al. 2012; Qiu et al. 2017; Hasanzadeh et al. 2019; Khan et al. 2019). Various adsorbents such as activated carbon (Di Natale et al. 2017), graphene (J. Li et al. 2019), biopolymers (Mincke et al. 2019; Elwakeel et al. 2021; Elwakeel et al. 2013; El-Shorbagy et al. 2021), magnetic composites (Donia et al. 2007), and nanofibers (Moawed et al. 2016) have been developed to efficiently recover the precious metals from dilute solutions.
Metal–organic frameworks (MOFs), as an emerging nanoporous crystalline material with fascinating physico-chemical features, have widely been considered for the adsorption process. They exhibit high porosity, tunable pore size, large specific surface area, and high thermal and chemical stability, which make them potentially suitable for many applications (Khiarak et al. 2020; Moghadam et al. 2020), especially for wastewater treatment (Hasanzadeh et al. 2020; Hasanzadeh et al. 2019) and precious metal recovery (Khiarak et al. 2020; Lin et al. 2018). Studies have shown the recovery of Pd(II) from acidic solution using MOFs with different structures (Lin et al. 2019). Incorporation of nanomaterials such as magnetic nanoparticles to MOF structures has shown further improvement and rapid extraction of adsorbent on wastewater treatment and metal recovery (Zhao et al. 2015; Abdi et al. 2019; Hamedi et al. 2019). For instance, magnetic copper-based MOF was successfully applied for the adsorption of palladium from different environmental samples (Bagheri et al. 2012).
The surface modification and functionalization of magnetic MOFs (MMOFs) are suggested which could provide more potential adsorbents with active functional groups to improve the adsorption efficiency (X. Zhang et al. 2020). Several approaches have been made for modification of MMOFs, such as mercaptoacetic acid (Huang et al. 2016), poly(4-vinylpyridine) (Li et al. 2020a, b), glutathione (Liu et al. 2018), and cetrimonium bromide (CTAB) (Li et al. 2020a, b). In a recent study, magnetic MOF composite was modified with β-cyclodextrin to enhance the adsorption selectivity toward triazole fungicides (Senosy et al. 2020). Dendrimers, as highly branched three-dimensional structures, possess a large number of functional end groups which make them appealing for adsorption-based applications. In recent years, great attention has been paid to the dendrimer modification of common adsorbents for metal uptake (Yen et al. 2017; Krawczyk et al. 2016; Kanani-Jazi et al. 2020) and wastewater treatment (Hayati et al. 2015; Yang et al. 2019).
In this work, we studied the post-modification of magnetic zirconium-based MOF nanocomposite with second-generation poly(propyleneimine) (PPI) dendrimer for removal of Pd(II) ions from aqueous solution. We have hypothesized that the unique characteristics of highly stable nanoporous zirconium-based MOF, as well as highly functional branched dendrimer, could contribute to an efficient and sustainable precious metal recovery. The magnetic property of Fe3O4 nanoparticles also offers a simple and effective way for the regeneration of adsorbents and recovery of metal ions. To our knowledge, there is no report on literature dealing with the adsorption of metal ions on such hybrid dendritic magnetic nanostructures. The Pd(II) uptake on dendrimer-modified MMOF (MMOF@PPI) was studied. The effects of four parameters including adsorbent content, Pd ion concentration, solution pH, and contact time were investigated through response surface methodology (RSM). Investigation of Pd(II) uptake capacity of MMOF@PPI was conducted through adsorption kinetics and isotherms models.
Experiments
Materials
Zirconium chloride (ZrCl4, purity ≥ 98%), terephthalic acid (purity ≥ 98%), N,N-dimethylformamide (DMF), acetic acid (HAc), ammonia (25%), palladium standard solution (1 g/L), thiourea, hydrochloric acid, ethanol, and methanol were received from Merck Co. (Germany). Iron chloride hexahydrate (FeCl3·6H2O, purity ≥ 99%) and iron chloride tetrahydrate (FeCl2·4H2O, purity ≥ 99%) were obtained from Sigma-Aldrich (USA). Second-generation PPI dendrimer was also purchased from SyMO-Chem (Netherlands). All the chemicals were analytical grade and were used without further purification.
Synthesis of magnetic MOF (MMOF)
MMOF adsorbent was synthesized according to the reported procedure (Far et al. 2020). First, Fe3O4 particles in spherical morphology were prepared by the coprecipitation method (Huang et al. 2015). Briefly, FeCl3·6H2O and FeCl2·4H2O in a 2:1 ratio were mixed with water under a nitrogen atmosphere. After stirring at 80°C for 3 h, 25% ammonia was added and stirring continued at the same temperature for 5 h. The product was magnetically separated and washed with water and dried at 70°C for 24 h. Then the MMOF material was synthesized solvothermally on as-prepared Fe3O4 particles. At first, a mixture of ZrCl4 (0.15 g), terephthalic acid (0.13 g), DMF (80 mL), acetic acid (4 mL), and newly synthesized Fe3O4 particles (0.2 g) was prepared (Wu et al. 2018). The mixture was transferred into a Teflon autoclave followed by solvothermal treatment at 130°C for 12 h. After the autoclave cooled down to room temperature, the product was washed three times with water and ethanol. Finally, the brown MMOF particles were obtained by drying at 80°C for 12 h.
Modification of MMOF with dendrimer (MMOF@PPI)
The MMOF particles (200 mg) were added to the dendrimer solution (200 μL) and then it was stirred for 2 h at room temperature. The modified MMOF particles (MMOF@PPI) were separated using an external magnet, washed with methanol and ethanol, and then dried at 40°C overnight. Scheme 1 shows the schematic representation of the MMOF@PPI synthesis procedure.
Characterizations
Fourier transform infrared (FTIR) spectra of MMOF adsorbents were measured on Avatar FTIR spectrophotometer (Thermo Nicolet, USA). The crystalline structure of synthesized MMOF adsorbents was obtained using powder X-ray diffraction (PXRD) (Philips, Expert Pro, Netherlands) with Cu Kα radiation. The vibrating sample magnetometer (Meghnatis Daghigh Kavir Co., Iran) was also used to measure the magnetic properties of the MMOF adsorbents. The morphology and size of synthesized MMOF adsorbents were studied through field emission scanning electron microscopy (FESEM, TESCAN, Mira 3, Czech Republic). The N2 adsorption/desorption isotherms of synthesized adsorbents were determined at 77 K using the BELSORP-MINI II instrument (BEL Japan, Inc.). The zeta potential of the dendrimer-modified MMOF in deionized water was measured by the WALLIS zeta potential analyzer (Cordouan Technologies). The concentration of Pd ion before and after adsorption was determined using ICP optical emission spectrometer (Shimadzu, ICP-7000 ver. II).
Adsorption experiments
The absorption experiments were performed at 25°C using palladium solutions with MMOF and MMOF@PPI adsorbents. The effect of processing conditions (MMOF content, Pd ion concentration, contact time, and pH) on the adsorption efficiency was investigated. Aqueous solutions of Pd ion with different concentrations (20–100 ppm) were prepared in deionized (DI) water. A specific amount (10–20 mg) of MMOF adsorbent was added into 50 mL of Pd aqueous solution and shaken using an orbital shaker (Heidolph Unimax 1010, Germany) at 400 rpm. The pH of each solution was adjusted on different values in the range of 2–7 using HCl (0.01 M) and NaOH (0.01 M). At certain time intervals (5–150 min), the MMOF adsorbent was separated magnetically using an external strong magnet. Finally, the Pd ion concentration was determined with the ICP-OES instrument. The Pd uptake (qt) on the MMOFs at a given time (t) is calculated based on the following equation (Iqbal et al. 2016):
where V (L) is the volume of the Pd solution, m (g) is the mass of MMOF adsorbent, and C0 and Ct (mg/L) are the Pd ion concentration at t = 0 and t, respectively.
Regeneration study
The reusability of MMOFs as an important characteristic of adsorbents was investigated according to the procedure reported in the literature (Lin et al. 2019). The Pd ion was completely eluted from Pd-loaded MMOF and MMOF@PPI adsorbents at pH 1.0 for 24 h, followed by rinsing three times with 10 mL of acidified thiourea. Then it was rinsed twice with 20 mL of HCl (0.1 M) and distilled water. Such adsorption–desorption cycles were repeated twice.
Experimental design
The effect of adsorption process conditions on metal uptakes, a statistical approach based on response surface methodology (RSM), was carried out. Affecting parameters, including MMOF@PPI content (mg), Pd ion concentration (ppm), and contact time (min), were considered as independent variables, and Pd uptake (mg/g) was a dependent variable (response). The central composite design (CCD) consisting five levels (coded as −alpha, −1, 0, +1, and +alpha) was utilized (Table S1). The small CCD design matrix with corresponding results of Pd uptakes on MMOF@PPI adsorbent is tabulated in Table S2. The predicted response (Pd uptakes, Y) was expressed by the following second-order polynomial equation:
Where Xi and Xj were coded values of independent variables. α0, αi, αii, and αij were also regression coefficients for the constant, linear, quadratic, and interaction effects, respectively (Myers et al. 2009).
Results and discussion
Structural characterization
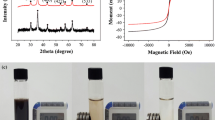
PXRD patterns of MOF and MMOF were obtained to study their crystalline structure (Fig. 1a). It can be seen that the MMOF particles exhibit the main diffraction peaks of MOF (2θ = 7.4, 8.6, and 25.7°) and Fe3O4. It indicated that the crystalline MOF was successfully grown onto the Fe3O4 surface. The successful modification of MMOF with PPI dendrimer was followed by FTIR spectroscopy of MMOF and MMOF@PPI (Fig. 1b). The characteristic peak of Zr-based MOF is visible in the FTIR spectrum of MMOF (Hasanzadeh et al. 2019). The peaks at 746 and 660 cm−1 illustrated the Zr-O vibration of Zr-based MOF. The adsorptions of carboxyl groups on the terephthalic acid ligands appeared at 1395, 1585, and 1658 cm−1 (Huo et al. 2019). In the spectrum of MMOF@PPI, the extra adsorption (around 1022 cm−1) belonged to the CN stretching vibration of the PPI dendrimer. Moreover, the increased adsorption band around 3440 cm−1 is attributed to both O-H stretching vibration of adsorbed water and the N-H stretching of PPI dendrimer primary amine groups (Kayal and Chakraborty 2018). Therefore, the MMOF particles were successfully modified with PPI dendrimer.
The nitrogen adsorption/desorption isotherm of MMOF and MMOF@PPI adsorbents was obtained to analyze their specific surface area and pore volume (Fig. 1c). They showed a hybrid I/II mixed type isotherm at 77 K with H4 hysteresis loop, consistent with previous literature (Chen et al. 2019a, b; Huo et al. 2019). The obtained results exhibit the high BET surface area of 507 m2/g for MMOF particles rather than Fe3O4 particles (about 11 m2/g), which is beneficial for the adsorption of metal ions. The pore volume of MMOF adsorbent was also increased to 0.58 cm3/g, suggesting successful synthesis of high specific surface area MOFs on magnetic particles. The BET surface area of MMOF@PPI (120 m2/g) was rationally expected to slightly decrease due to the surface modification of MMOF with dendrimer and surface coverage of the particles. Although the modification of MMOF with dendrimer leads to a reduction in the surface area, the provided large number of functional ending groups could contribute to efficient adsorption. Pore size distribution analysis of MMOF and MMOF@PPI (Fig. 1d) indicated the micromesoporous characteristics of magnetic adsorbents, which facilitate the diffusion of metal ions to accessible active sites within the pores.
Magnetic properties of the MMOF adsorbents were further studied. The magnetic hysteresis loops of MMOF and MMOF@PPI particles (Figure S1) revealed the relatively soft ferromagnetic behavior of magnetic adsorbents. Compared to that of MMOFs, higher saturation magnetization and coercivity were obtained for MMOF@PPI. The morphology of MMOF adsorbents was observed by FESEM. Figure 2 has confirmed the regular morphology with the semispherical shape of MMOF particles. The particle size of MMOF is in the range of 20–50 nm. Modification of MMOF with dendrimer leads to the formation of aggregated irregular microspheres.
Statistical analysis and model validation
The second-order polynomial equation was developed to correlate the Pd uptakes (Y) as the function of MMOF@PPI content (X1), Pd ion concentration (X2), and contact time (X3) by using the analysis of variance (ANOVA). The confidence level was selected to be 95%, which means the model terms will be significant if their probability value is smaller than 0.05. The results of ANOVA (Table S3) confirmed that the model is highly significant. Furthermore, it was concluded that the terms X1X3, X2X3, X22, and X32 have no significant effect on Pd uptake at the studied confidence level. Therefore, the final fitted regression equation in a coded unit is obtained as follows:
where X1, X2, and X3 represent the MMOF@PPI content, Pd ion concentration, and contact time, respectively. The coefficient of determination (R2) and adjusted R2 (\( {R}_{adj}^2 \)) are 0.984 and 0.975, respectively, advocating a close agreement between the model predicted data and the experiments (Figure S2). The high adequate precision of 36.32 (the value above 4) confirms adequate model discrimination. The obtained results suggested that the model was satisfactorily capable of predicting the Pd uptakes on dendrimer-modified MMOF adsorbents.
Figure 3 represents the 3D response surface and contour plot of the Pd uptakes as the function of MMOF@PPI content (mg) and Pd ion concentration (ppm) in the constant contact time of 50 min. It was found that as the amount of MMOF@PPI increased, the Pd uptake decreased. This reduction intensified at a higher level of MMOF@PPI content. This could be ascribed to the splitting effect of flux between the Pd ion concentrations and the dendrimer-modified MMOF surface. Increasing the MMOF@PPI content causes a decrease in the amount of Pd metal ions adsorbed onto the unit weight of MMOF@PPI. This could also be responsible for increasing metal uptakes observed with increasing initial Pd concentration.
The optimum conditions for efficient Pd uptakes were obtained based on the RSM model. The variables were set in their investigated range. The maximum Pd uptakes were predicted to be 235.6 mg/g at MMOF@PPI content of 12.5 mg, Pd ion concentration of 80 ppm, and contact time of 40 min. Further experiment at the optimal conditions was carried out to confirm the predictivity of the regression model. An uptake of 234 mg/g was obtained, demonstrating close agreement with predicted Pd uptake.
Adsorption kinetics
The effect of contact time on Pd uptake on MMOF and MMOF@PPI materials was assessed (Fig. 4). Figure 4a shows that the adsorption capacity of Pd ions increased rapidly in 80 min and gradually reached equilibrium at 150 min. A fast and efficient Pd ion uptake on both magnetic adsorbents was carried out in the first minutes. The Pd adsorption rate of dendrimer-modified MMOF was found to be faster than the unmodified one. The Pd uptake on both magnetic adsorbents exhibits a fast initial Pd uptake followed by slow adsorption until the equilibrium is reached. The equilibrium Pd uptake (qe) on MMOF and MMOF@PPI was found to be about 204 and 291 mg/g, respectively.
Three kinetic models including intraparticle diffusion (IPD), pseudo-first-order (PFO), and pseudo-second-order (PSO) models were utilized to describe the kinetics of Pd uptake on magnetic adsorbents by considering the following equations (Oladipo et al. 2014; Embaby et al. 2018):
where KP (mg/g·min0.5) is the rate constant of interparticle diffusion and I is the intercept representing the thickness of the boundary layer. qe and qt are the Pd uptakes at equilibrium and at a time t, respectively. k1 (1/min) and k2 (g/mg.min) are also the rate constants of PFO and PSO models, respectively (Ayub et al. 2019; Sharifi et al. 2019). The linear plots of kinetic models are shown in Fig. 4. Table 1 summarizes the calculated adsorption kinetic parameters for all models. The obtained results indicate that the Pd uptake on both magnetic adsorbents best abides the pseudo-first-order model. This result confirms that the physisorption could be considered as the main driving force of Pd(II) uptake on magnetic adsorbents (Dragan and Loghin 2013), consisting of the results obtained from adsorption isotherm. The value of the rate constant of MMOF@PPI was found to be higher than MMOF adsorbent, revealing the faster kinetics of Pd uptake on dendrimer functionalized materials. This may be ascribed to the synergistic effect of the nanoporous structure of MMOF particles and a large number of functional groups on the dendrimer surface.
Effect of pH
In this study, the effect of pH has been evaluated in the range of 2–7 to optimize the pH value for maximum Pd(II) uptake. Figure 5a shows the effect of pH on Pd(II) uptake on MMOF and MMOF@PPI adsorbents. It is obvious that the Pd(II) uptake on both magnetic adsorbents is strongly affected by pH value. It indicates that the maximum uptake (204 and 291 mg/g for MMOF and MMOF@PPI, respectively) has occurred at pH near 4. Dendrimer-modified MMOF exhibits higher Pd(II) uptake throughout all pH values rather than MMOF, which could be ascribed to the high binding affinity of PPI dendrimers toward metal ions (Yen et al. 2017). Moreover, the cationic zirconium nodes in MOF (UiO-66) are considered as further binding sites for Pd(II) ions (Lin et al. 2018). The zeta potential measurement indicated that the MMOF@PPI is positively charged at neutral pH value (~6.5) and low pH and thus capable of forming electrostatic adsorption. It is well-known that at low pH, the surface of the adsorbent would be protonated and gives positive zeta potential.
Adsorption isotherms
The effect of initial Pd ion concentration, as one of the most important data for understanding the mechanism of adsorption, was investigated in the range of 60 to 110 ppm. As shown in Fig. 5b, the dendrimer-modified MMOF has a higher Pd uptake capacity than the unmodified one with maximum uptake of 330 mg/g at an initial Pd concentration of 110 ppm. A large number of functional end groups on the surface of dendrimer-modified MMOF particles could be responsible for this increase in Pd uptake. Further investigations on the mechanism of Pd uptake were carried out using Langmuir, Freundlich, and Tempkin isotherm models. The Langmuir isotherm model assumes monolayer sorption onto specific homogeneous sites, while the Freundlich isotherm model describes the heterogeneous adsorption process (Mohammad et al. 2011; Liu et al. 2016). The linear forms of Langmuir, Freundlich, and Tempkin isotherm models are expressed by the following equations (Liu et al. 2016):
where Ce (mg/L) is the Pd ion concentration at equilibrium. KL (L/mg) and KF ((mg/g)(L/mg)1/nF) are the Langmuir and Freundlich constants, respectively. The term 1/n is related to the adsorption intensity (Heydari Moghaddam et al. 2019; Baziar et al. 2021). B (J/mol) and KT (L/mg) are also the Tempkin constants.
Figure S3 shows the linear plots of Langmuir, Freundlich, and Tempkin isotherms for Pd uptake on MMOF and MMOF@PPI adsorbents. Considering the linear isotherm constants (Table 2) revealed that the uptake of Pd ions on both magnetic adsorbents was well matched by the Langmuir isotherm model. It implies that the Pd ions were adsorbed homogeneously on a monolayer surface of magnetic adsorbents.
Adsorption thermodynamics
The adsorption thermodynamic was investigated at different temperatures in the range of 298–318 K under the optimum conditions. The thermodynamic parameters of Pd uptake on MMOF and MMOF@PPI materials are given in Table 3. Thermodynamic parameters including Gibbs free energy (ΔG0), enthalpy (ΔH0), and entropy (ΔS0) were calculated by the following equations (Lima et al. 2019; Y. Liu and Liu 2008; W. Zhang et al. 2019):
where R (8.314 J/mol.K) and T (K) are the universal gas constant and the temperature of the solution, respectively. The ΔG0 value decreased with increasing temperature from 298 to 318 K for both MMOF and MMOF@PPI materials. The negative value of ΔG0 indicated that the adsorption of Pd on both magnetic adsorbents is spontaneous. Moreover, the positive values of ΔH0 confirm the endothermic nature of the adsorption.
Regeneration and reusability of adsorbents
The regeneration and the potential reusability of an adsorbent are additional features for the commercialization of the product. Cycles of the adsorption–desorption process were conducted, and the result was shown in Figure S4. The results showed that the adsorption capacity of both magnetic adsorbents was slightly decreased, as the number of adsorption–desorption cycles was increased. However, this reduction was lower for the dendrimer-modified adsorbent. The MMOF@PPI retained 92% of its capacity after two cycles of adsorption–desorption, while this value was found about 85% for unmodified MMOF. The results confirmed the reversibility of adsorption sites on dendrimer-modified adsorbent. Further investigation on the structural stability of magnetic adsorbent in water and acidic environments (HCl 0.1 M) for 7 days at 25°C (Figure S5) has shown their good stability and potential practical applicability in harsh environments.
Comparison with other adsorbents
Table 4 summarizes the comparison between the Pd(II) uptake capacity of MMOF@PPI and other reported adsorbents. The data indicate that the dendrimer-modified MMOF exhibits fast and efficient adsorption of Pd(II) compared to other materials. MMOF@PPI also provides easy separation of Pd, thanks to its good magnetic properties. The high adsorption capacity of MMOF@PPI, which is benefited from a high specific surface area and a large number of functional groups, makes it an outstanding material for the recovery of precious metals and wastewater treatment.
Conclusions
A novel magnetic adsorbent (MMOF@PPI) was successfully synthesized and utilized for adsorption and recovery of Pd(II) from an aqueous solution. The most important findings are summarized as follows:
-
The prepared dendrimer-modified adsorbent possessed a superior figure of merits as compared to the unmodified one, such as high magnetic properties and a large number of active sites for Pd(II) uptake.
-
The Pd(II) uptake on dendrimer-modified magnetic adsorbent was described by the pseudo-first-order kinetic model.
-
The adsorption isotherm of dendrimer-modified magnetic adsorbent was fitted to the Langmuir isotherm model, suggesting homogeneous adsorption on a monolayer surface of adsorbents.
-
The Pd adsorption capacity of dendrimer-modified MMOF was 43% higher than the MMOF.
-
Investigating the impact of adsorption parameters through RSM revealed that the maximum metal uptake (291 mg/g) will be achieved at optimal conditions of 12.5 mg adsorbent content, 80 ppm Pd concentration, pH = 4, and contact time of 40 min.
-
The results of this research demonstrate that the MMOF@PPI adsorbent is not only suitable for metal ion adsorption, but also can be used for other applications such as wastewater treatment.
References
Abdi J, Mahmoodi NM, Vossoughi M, Alemzadeh I (2019) Synthesis of magnetic metal-organic framework nanocomposite (ZIF-8@SiO2@MnFe2O4) as a novel adsorbent for selective dye removal from multicomponent systems. Microporous Mesoporous Mater 273:177–188. https://doi.org/10.1016/j.micromeso.2018.06.040
Ayub S, Mohammadi AA, Yousefi M, Changani F (2019) Performance evaluation of agro-based adsorbents for the removal of cadmium from wastewater. Desalin Water Treat 142:293–299. https://doi.org/10.5004/dwt.2019.23455
Bagheri A, Taghizadeh M, Behbahani M, Asgharinezhad AA, Salarian M, Dehghani A, Ebrahimzadeh H, Amini MM (2012) Synthesis and characterization of magnetic metal-organic framework (MOF) as a novel sorbent, and its optimization by experimental design methodology for determination of palladium in environmental samples. Talanta 99:132–139. https://doi.org/10.1016/j.talanta.2012.05.030
Baziar M, Zakeri HR, Ghalehaskar S, Nejad ZD, Shams M, Anastopoulos I, Giannakoudakis DA, Lima EC (2021) Metal-organic and zeolitic imidazole frameworks as cationic dye adsorbents: physicochemical optimizations by parametric modeling and kinetic studies. J Mol Liq 332(June):115832. https://doi.org/10.1016/j.molliq.2021.115832
Chen G, Wang Y, Weng H, Wu Z, He K, Zhang P, Guo Z, Lin M (2019a) Selective separation of Pd(II) on pyridine-functionalized graphene oxide prepared by radiation-induced simultaneous grafting polymerization and reduction. ACS Appl Mater Interfaces 11(27):24560–24570. https://doi.org/10.1021/acsami.9b06162
Chen R, Tao CA, Zhang Z, Chen X, Liu Z, Wang J (2019b) Layer-by-layer fabrication of core-shell Fe3O4@UiO-66-NH2 with high catalytic reactivity toward the hydrolysis of chemical warfare agent simulants. ACS Appl Mater Interfaces 11(46):43156–43165. https://doi.org/10.1021/acsami.9b14099
Daliran S, Ghazagh-Miri M, Oveisi AR, Khajeh M, Navalón S, Âlvaro M, Ghaffari-Moghaddam M, Delarami HS, García H (2020) A pyridyltriazol functionalized zirconium metal-organic framework for selective and highly efficient adsorption of palladium. ACS Appl Mater Interfaces 12(22):25221–25232. https://doi.org/10.1021/acsami.0c06672
Donia AM, Atia AA, Elwakeel KZ (2007) Recovery of gold(III) and silver(I) on a chemically modified chitosan with magnetic properties. Hydrometallurgy 87(3–4):197–206. https://doi.org/10.1016/j.hydromet.2007.03.007
Dragan ES, Loghin DFA (2013) Enhanced sorption of methylene blue from aqueous solutions by semi-IPN composite cryogels with anionically modified potato starch entrapped in PAAm matrix. Chem Eng J 234:211–222. https://doi.org/10.1016/j.cej.2013.08.081
El Salam HMA, Zaki T (2018) Removal of hazardous cationic organic dyes from water using nickel-based metal-organic frameworks. Inorg Chim Acta 471:203–210. https://doi.org/10.1016/j.ica.2017.10.040
El-Shorbagy HG, El-Kousy SM, Elwakeel KZ, Abd El-Ghaffar MA (2021) Eco-friendly chitosan condensation adduct resins for removal of toxic silver ions from aqueous medium. Journal of Industrial and Engineering Chemistry, April 100:410–421. https://doi.org/10.1016/j.jiec.2021.04.029
Elwakeel KZ, El-Sayed GO, Darweesh RS (2013) Fast and selective removal of silver(I) from aqueous media by modified chitosan resins. Int J Miner Process 120:26–34. https://doi.org/10.1016/j.minpro.2013.02.007
Elwakeel KZ, Al-Bogami AS, Guibal E (2021) 2-Mercaptobenzimidazole derivative of chitosan for silver sorption – contribution of magnetite incorporation and sonication effects on enhanced metal recovery. Chem Eng J 403(May 2020):126265. https://doi.org/10.1016/j.cej.2020.126265
Embaby MS, Elwany SD, Setyaningsih W, Saber MR (2018) The adsorptive properties of UiO-66 towards organic dyes: a record adsorption capacity for the anionic dye alizarin red S. Chin J Chem Eng 26(4):731–739. https://doi.org/10.1016/j.cjche.2017.07.014
Far HS, Hasanzadeh M, Nashtaei MS, Rabbani M, Haji A, Moghadam BH (2020) PPI-dendrimer-functionalized magnetic metal-organic framework (Fe3O4@MOF@PPI) with high adsorption capacity for sustainable wastewater treatment. ACS Appl Mater Interfaces 12(22):25294–25303. https://doi.org/10.1021/acsami.0c04953
Guibal E, Von Offenberg Sweeney N, Vincent T, Tobin JM (2002) Sulfur derivatives of chitosan for palladium sorption. React Funct Polym 50(2):149–163. https://doi.org/10.1016/S1381-5148(01)00110-9
Hamedi A, Zarandi MB, Nateghi MR (2019) Highly efficient removal of dye pollutants by MIL-101(Fe) metal-organic framework loaded magnetic particles mediated by poly L-dopa. Journal of Environmental Chemical Engineering 7(1):102882. https://doi.org/10.1016/j.jece.2019.102882
Hasanzadeh M, Simchi A, Far HS (2019) Kinetics and adsorptive study of organic dye removal using water-stable nanoscale metal organic frameworks. Mater Chem Phys 233(May):267–275. https://doi.org/10.1016/j.matchemphys.2019.05.050
Hasanzadeh M, Simchi A, Far HS (2020) Nanoporous composites of activated carbon-metal organic frameworks for organic dye adsorption: synthesis, adsorption mechanism and kinetics studies. J Ind Eng Chem 81:405–414
Hayati B, Arami M, Maleki A, Pajootan E (2015) Thermodynamic properties of dye removal from colored textile wastewater by poly (propylene imine) dendrimer. Desalin Water Treat 56:97–106. https://doi.org/10.1080/19443994.2014.931529
Huang L, He M, Chen B, Bin H (2015) A designable magnetic MOF composite and facile coordination-based post-synthetic strategy for the enhanced removal of Hg2+ from water. J Mater Chem A 3(21):11587–11595. https://doi.org/10.1039/c5ta01484k
Huang L, He M, Chen B, Bin H (2016) A mercapto functionalized magnetic Zr-MOF by solvent-assisted ligand exchange for Hg2+ removal from water. J Mater Chem A 4(14):5159–5166. https://doi.org/10.1039/c6ta00343e
Huo J-B, Xu L, Chen X, Zhang Y, Yang J-CE, Yuan B, Ming-Lai F (2019) Direct epitaxial synthesis of magnetic Fe3O4@UiO-66 composite for efficient removal of arsenate from water. Microporous Mesoporous Mater 276(March):68–75. https://doi.org/10.1016/J.MICROMESO.2018.09.017
Iqbal M, Iqbal N, Bhatti IA, Ahmad N, Zahid M (2016) Response surface methodology application in optimization of cadmium adsorption by shoe waste: a good option of waste mitigation by waste. Ecol Eng 88(March):265–275. https://doi.org/10.1016/j.ecoleng.2015.12.041
Kanani-Jazi MH, Akbari S, Kish MH (2020) Efficient removal of Cr (VI) from aqueous solution by halloysite/poly(amidoamine) dendritic nano-hybrid materials: kinetic, isotherm and thermodynamic studies. Adv Powder Technol 31(9):4018–4030. https://doi.org/10.1016/j.apt.2020.08.004
Kayal S, Chakraborty A (2018) Activated carbon (type Maxsorb-III) and MIL-101(Cr) metal organic framework based composite adsorbent for higher CH4 storage and CO2 capture. Chem Eng J 334:780–788. https://doi.org/10.1016/j.cej.2017.10.080
Khan NA, Khan SU, Ahmed S, Farooqi IH, Dhingra A, Hussain A, Changani F (2019) Applications of nanotechnology in water and wastewater treatment: a review. Asian Journal of Water, Environment and Pollution 16(4):81–86. https://doi.org/10.3233/AJW190051
Khiarak BN, Hasanzadeh M, Mojaddami M, Far HS, Simchi A (2020) In situ synthesis of quasi-needle-like bimetallic organic frameworks on highly porous graphene scaffolds for efficient electrocatalytic water oxidation. Chem Commun 56(21):3135–3138. https://doi.org/10.1039/c9cc09908e
Kousha M, Daneshvar E, Esmaeli AR, Jokar M, Khataee AR (2012) Optimization of acid blue 25 removal from aqueous solutions by raw , esterified and protonated Jania adhaerens biomass. Int Biodeterior Biodegradation 69:97–105. https://doi.org/10.1016/j.ibiod.2012.01.007
Krawczyk M, Akbari S, Jeszka-Skowron M, Pajootan E, Fard FS (2016) Application of dendrimer modified halloysite nanotubes as a new sorbent for ultrasound-assisted dispersive micro-solid phase extraction and sequential determination of cadmium and lead in water Samples. J Anal At Spectrom 31(7):1505–1514. https://doi.org/10.1039/c6ja00096g
Li J, Wang S, Wang F, Xuran W (2019) Environmental separation and enrichment of gold and palladium ions by amino-modified three-dimensional graphene. RSC Adv 9(5):2816–2821. https://doi.org/10.1039/c8ra10506e
Li L, Xu Y, Zhong D, Zhong N (2020a) CTAB-surface-functionalized magnetic MOF@MOF composite adsorbent for Cr(VI) efficient removal from aqueous solution. Colloids Surf A Physicochem Eng Asp 586(Vi):124255. https://doi.org/10.1016/j.colsurfa.2019.124255
Li Y, Wang Y, He L, Meng L, Lu H, Li X (2020b) Preparation of poly(4-vinylpyridine)-functionalized magnetic Al-MOF for the removal of naproxen from aqueous solution. J Hazard Mater 383(August 2019):121144. https://doi.org/10.1016/j.jhazmat.2019.121144
Lim CR, Lin S, Yun YS (2020) Highly efficient and acid-resistant metal-organic frameworks of MIL-101(Cr)-NH2 for Pd(II) and Pt(IV) recovery from acidic solutions: adsorption experiments, spectroscopic analyses, and theoretical computations. J Hazard Mater 387(Ii):121689. https://doi.org/10.1016/j.jhazmat.2019.121689
Lima EC, Hosseini-bandegharaei A, Moreno-piraján JC, Anastopoulos I (2019) A critical review of the estimation of the thermodynamic parameters on adsorption equilibria. Wrong use of equilibrium constant in the Van â€TM T Hoof equation for calculation of thermodynamic parameters of adsorption. J Mol Liq 273:425–434. https://doi.org/10.1016/j.molliq.2018.10.048
Lin S, Bediako JK, Cho CW, Song MH, Zhao Y, Kim JA, Choi JW, Yun YS (2018) Selective adsorption of Pd(II) over interfering metal ions (Co(II), Ni(II), Pt(IV)) from acidic aqueous phase by metal-organic frameworks. Chem Eng J 345(March):337–344. https://doi.org/10.1016/j.cej.2018.03.173
Lin S, Zhao Y, Bediako JK, Cho CW, Sarkar AK, Lim CR, Yun YS (2019) Structure-controlled recovery of palladium(II) from acidic aqueous solution using metal-organic frameworks of MOF-802, UiO-66 and MOF-808. Chem Eng J 362(October 2018):280–286. https://doi.org/10.1016/j.cej.2019.01.044
Liu Y, Liu YJ (2008) Biosorption isotherms, kinetics and thermodynamics. Sep Purif Technol 61(3):229–242. https://doi.org/10.1016/j.seppur.2007.10.002
Liu L, Li C, Bao C, Jia Q, Xiao P, Liu X, Zhang Q (2012) Preparation and characterization of chitosan/graphene oxide composites for the adsorption of Au(III) and Pd(II). Talanta 93:350–357. https://doi.org/10.1016/j.talanta.2012.02.051
Liu J, Zeng M, Ronghai Y (2016) Surfactant-free synthesis of octahedral ZnO / ZnFe 2 O 4 heterostructure with ultrahigh and selective adsorption capacity of malachite green. Nat Publ Group 2015:1–10. https://doi.org/10.1038/srep25074
Liu Q, Deng CH, Sun N (2018) Hydrophilic tripeptide-functionalized magnetic metal-organic frameworks for the highly efficient enrichment of N-linked glycopeptides. Nanoscale 10(25):12149–12155. https://doi.org/10.1039/c8nr03174f
Mergola L, Stomeo T, Del Sole R (2020) Synthesis of photoswitchable submicroparticles and their evaluation as ion-imprinted polymers for Pd(II) uptake. Polym J 52(7):743–754. https://doi.org/10.1038/s41428-020-0319-8
Mincke S, Asere TG, Verheye I, Folens K, Vanden Bussche F, Lapeire L, Verbeken K, Van Der Voort P (2019) Functionalized chitosan adsorbents allow recovery of palladium and platinum from acidic aqueous solutions. Green Chem 21(9):2295–2306. https://doi.org/10.1039/C9GC00166B
Moawed EA, El-Hagrasy MA, Kamal M, El-Shahat MF (2016) Recovery and determination of palladium from its alloys using iminodiacetic polyurethane/carbon nanofibers sorbent. J Liq Chromatogr Relat Technol 39(8):415–421. https://doi.org/10.1080/10826076.2016.1169428
Moghadam BH, Hasanzadeh M, Simchi A (2020) Self-powered wearable piezoelectric sensors based on polymer nanofiber-metal-organic framework nanoparticle composites for arterial pulse monitoring. ACS Applied Nano Materials 3(9):8742–8752. https://doi.org/10.1021/acsanm.0c01551
Moghaddam H, Masoumeh RN, Dehghani MH, Akbarpour B, Azari A, Yousefi M (2019) Performance investigation of zeolitic imidazolate framework – 8 (ZIF-8) in the removal of trichloroethylene from aqueous solutions. Microchem J 150(August):104185. https://doi.org/10.1016/j.microc.2019.104185
Mohammad N, Hayati B, Arami M, Lan C (2011) Adsorption of textile dyes on pine cone from colored wastewater : kinetic , equilibrium and thermodynamic studies. Desalination 268(1–3):117–125. https://doi.org/10.1016/j.desal.2010.10.007
Molavi H, Shojaei A, Pourghaderi A (2018) Rapid and Tunable Selective adsorption of dyes using thermally oxidized nanodiamond. J Colloid Interface Sci 524:52–64. https://doi.org/10.1016/j.jcis.2018.03.088
Myers RH, Montgomery DC, Anderson-cook CM (2009) Response surface methodology: process and product optimization using designed experiments. John Wiley and Sons, USA
Nagarjuna R, Shivani S, Rajesh N, Ganesan R (2017) Effective adsorption of precious metal palladium over polyethyleneimine-functionalized alumina nanopowder and its reusability as a catalyst for energy and environmental applications. ACS Omega 2(8):4494–4504. https://doi.org/10.1021/acsomega.7b00431
Natale F, Di M, Orefice FLM, Erto A, Lancia A (2017) Unveiling the potentialities of activated carbon in recovering palladium from model leaching solutions. Sep Purif Technol 174:183–193. https://doi.org/10.1016/j.seppur.2016.10.022
Oladipo AA, Gazi M, Saber-samandari S (2014) Adsorption of anthraquinone dye onto eco-friendly semi-IPN biocomposite hydrogel : equilibrium isotherms, kinetic studies and optimization. J Taiwan Inst Chem Eng 45(2):653–664. https://doi.org/10.1016/j.jtice.2013.07.013
Parajuli D, Kawakita H, Inoue K, Funaoka M (2006) Recovery of gold(III), palladium(II), and platinum(IV) by aminated lignin derivatives. Ind Eng Chem Res 45(19):6405–6412. https://doi.org/10.1021/ie0603518
Qiu J, Feng Y, Zhang X, Jia M, Yao J (2017) Acid-promoted synthesis of UiO-66 for highly selective adsorption of anionic dyes: adsorption performance and mechanisms. J Colloid Interface Sci 499(March):151–158. https://doi.org/10.1016/j.jcis.2017.03.101
Ramesh A, Hasegawa H, Sugimoto W, Maki T, Ueda K (2008) Adsorption of gold(III), platinum(IV) and palladium(II) onto glycine modified crosslinked chitosan resin. Bioresour Technol 99(9):3801–3809. https://doi.org/10.1016/j.biortech.2007.07.008
Senosy IA, Lu ZH, Abdelrahman TM, Yang MNO, Guo HM, Yang ZH, Li JH (2020) The post-modification of magnetic metal-organic frameworks with β-cyclodextrin for the efficient removal of fungicides from environmental water. Environmental Science: Nano 7(7):2087–2101. https://doi.org/10.1039/c9en01372e
Sharifi S, Nabizadeh R, Akbarpour B, Azari A, Ghaffari HR, Nazmara S, Mahmoudi B, Shiri L, Yousefi M (2019) Modeling and optimizing parameters affecting hexavalent chromium adsorption from aqueous solutions using Ti-XAD7 nanocomposite: RSM-CCD approach, kinetic, and isotherm studies. J Environ Health Sci Eng 17(2):873–888. https://doi.org/10.1007/s40201-019-00405-7
Sharma S, Wu CM, Koodali RT, Rajesh N (2016) An ionic liquid-mesoporous silica blend as a novel adsorbent for the adsorption and recovery of palladium ions, and its applications in continuous flow study and as an industrial catalyst. RSC Adv 6(32):26668–26678. https://doi.org/10.1039/c5ra26673d
Wu MX, Gao J, Wang F, Yang J, Song N, Jin X, Mi P, Tian J, Luo J, Liang F, Yang Y-W (2018) Multistimuli responsive core–shell nanoplatform constructed from Fe3O4@MOF equipped with Pillar[6]arene nanovalves. Small 14(17):1–6. https://doi.org/10.1002/smll.201704440
Yang J, Zhang Z, Pang W, Chen H, Yan G (2019) Polyamidoamine dendrimers functionalized magnetic carbon nanotubes as an efficient adsorbent for the separation of flavonoids from plant extraction. Sep Purif Technol 227(March):115710. https://doi.org/10.1016/j.seppur.2019.115710
Yen CH, Lien HL, Chung JS, Der Yeh H (2017) Adsorption of precious metals in water by dendrimer modified magnetic nanoparticles. J Hazard Mater 322(February):215–222. https://doi.org/10.1016/j.jhazmat.2016.02.029
Yi Q, Fan R, Xie F, Zhang Q, Luo Z (2016) Recovery of palladium(II) from nitric acid medium using a natural resin prepared from persimmon dropped fruits residues. J Taiwan Inst Chem Eng 61:299–305. https://doi.org/10.1016/j.jtice.2016.01.009
Zhang B, Likang F, Wang S, Zhang L (2018) Adsorption of palladium(II) from aqueous solution using nanosilica modified with imidazoline groups. Mater Chem Phys 214(Ii):533–539. https://doi.org/10.1016/j.matchemphys.2018.04.120
Zhang W, Hu L, Hu S, Yang L (2019) Optimized synthesis of novel hydrogel for the adsorption of copper and cobalt ions in wastewater. RSC Adv 9(28):16058–16068. https://doi.org/10.1039/c9ra00227h
Zhang X, Wang J, Dong XX, Lv YK (2020) Functionalized metal-organic frameworks for photocatalytic degradation of organic pollutants in environment. Chemosphere 242. https://doi.org/10.1016/j.chemosphere.2019.125144
Zhao X, Liu S, Tang Z, Niu H, Cai Y, Meng W, Wu F, Giesy JP (2015) Synthesis of magnetic metal-organic framework (MOF) for efficient removal of organic dyes from water. Sci Rep 5(July):1–10. https://doi.org/10.1038/srep11849
Zhou L, Liu J, Liu Z (2009) Adsorption of platinum(IV) and palladium(II) from aqueous solution by thiourea-modified chitosan microspheres. J Hazard Mater 172(1):439–446. https://doi.org/10.1016/j.jhazmat.2009.07.030
Zhou L, Xu J, Liang X, Liu Z (2010) Adsorption of platinum(IV) and palladium(II) from aqueous solution by magnetic cross-linking chitosan nanoparticles modified with ethylenediamine. J Hazard Mater 182(1–3):518–524. https://doi.org/10.1016/j.jhazmat.2010.06.062
Availability of data and materials
Not applicable.
Funding
MH thanks the Iran National Elites Foundation (INEF, Grant No. 15-89661) for the financial support.
Author information
Authors and Affiliations
Contributions
Hossein Shahriyari Far: investigation, methodology, visualization, and writing: original draft. Mahdi Hasanzadeh: conceptualization, supervision, resources, formal analysis, funding acquisition, project administration, and writing — review and editing. Mohammad Shabani Nashtaei: investigation, methodology, and visualization. Mahboubeh Rabbani: conceptualization, supervision, resources, formal analysis, and validation.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent forpublication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Magnetic hysteresis loops, comparison of experimental and predicted Pd(II) uptake, Langmuir, Freundlich, and Tempkin isotherm plots, regeneration cycles of adsorbents, structural stability of magnetic adsorbent, experimental results of Pd (II) uptakes, and ANOVA table.
ESM 1
(DOC 587 kb)
Rights and permissions
About this article
Cite this article
Far, H.S., Hasanzadeh, M., Nashtaei, M.S. et al. Fast and efficient adsorption of palladium from aqueous solution by magnetic metal–organic framework nanocomposite modified with poly(propylene imine) dendrimer. Environ Sci Pollut Res 28, 62474–62486 (2021). https://doi.org/10.1007/s11356-021-15144-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-15144-2