Abstract
Dimethoate ([O,O-dimethyl S-(N-methylcarbamoylmethyl) phosphorodithioate]) is an organophosphate insecticide and acaricide widely used for agricultural purposes. Genotoxicity refers to the ability of a chemical agent interact directly to DNA or act indirectly leading to DNA damage by affecting spindle apparatus or enzymes involved in DNA replication, thereby causing mutations. Taking into consideration the importance of genotoxicity induced by dimethoate, the purpose of this manuscript was to provide a mini review regarding genotoxicity induced by dimethoate as a result of oxidative stress. The present study was conducted on studies available in MEDLINE, PUBMED, EMBASE, and Google scholar for all kind of articles (all publications published until May, 2020) using the following key words: dimethoate, omethoate, DNA damage, genetic damage, oxidative stress, genotoxicity, mutation, and mutagenicity. The results showed that many studies were published in the scientific literature; the approach was clearly demonstrated in multiple tissues and organs, but few papers were designed in humans. In summary, new studies within the field are important for better understanding the pathobiological events of genotoxicity on human cells, particularly to explain what cells and/or tissues are more sensitive to genotoxic insult induced by dimethoate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dimethoate ([O,O-dimethyl S-(N-methylcarbamoylmethyl) phosphorodithioate]) is an organophosphorous compound widely employed in agriculture as insecticide (Van Scoy et al. 2016). It was registered in 1962 for use being destined to control a wide range of insects, as for example plant hoppers, mites, flies, and aphids (Dissanayake et al. 2021). To date, dimethoate has been applied to crops such as, grain, fruit, and vegetables (Badry et al. 2021; Van Scoy et al. 2016). Furthermore, the insecticide has been used for non-agricultural purposes, as for example landscape maintenance and pest control (Van Scoy et al. 2016).
Genotoxicity is the pathobiological phenomenon characterized by genetic damage (Ribeiro et al. 2017). This means that harmful agent is classified as genotoxic if it is able to injury the genetic material. To date, several compounds either from endogenous or exogenous sources are identified as genotoxic in the scientific literature (Ribeiro et al. 2017). For this reason, it is assumed that different agents present in the environment continuously damage DNA molecule either to humans or to other species. Herein, it is very important to investigate what agents are able to induce genetic damage under different end-points and paradigms, especially those in close contact with humans and other species for long time. This scientific knowledge is very important since it protects living organisms against potential harm.
It is well discussed in literature that oxidative stress can activate a variety of pathways that leads to an oxidative imbalance damaging mammalian cells and, after extended periods, promote carcinogenesis (Quezada-Maldonado 2021; Reuter et al. 2010). The increase of ROS may be due to endogenous oxidative stress, from hepatic metabolism and the action of the enzyme P450, mitochondria activities or from NADPH enzymes, or from exogenous origin, such as those generated by chemical substances (Klaunig 2018). ROS molecules are involved in genotoxicity as a result of gene mutations on injured cell or by its interference in transduction and/or transcription factors (Klaunig 2018).
It is well known that dimethoate is very soluble in water and, therefore, it possesses soil persistence as a result of good properties closely related to good efficacy and fast degradation (Lima do Rego et al. 2021; NPIC 2019). In this context, the environmental consequences of dimethoate are of great concern.
To the best of our knowledge, few review articles regarding dimethoate toxicity are being published with certain regularity. Even so, these papers do not discuss genotoxicity as a result of oxidative stress (Reuber 1984; Van Coy et al. 2016; EFSA 2018). Particularly, little information has been available on the impact of dimethoate as well as its relevant metabolites focusing the side effects on human health. For this reason, further understanding on the impact to the human health for mitigating the noxious activities induced by dimethoate is important to protect humans and other species against potential harm. Herein, a search of the scientific peer-reviewed literature on dimethoate toxicity will provide new insights into the pathobiological mechanisms induced by the insecticide in mammalian cells. These data are relevant not only for the regulators, but also the policy makers.
Taking into consideration the genotoxicity as a result of oxidative stress may be induced by dimethoate, the aim of this study was to provide a mini review taking into account four aspects: (i) the publications over the years, (ii) the test-system used, (iii) the main findings published, and (iv) the identification of gaps within the field, in order to purpose future perspectives, whose goal is to protect humans against potential harm.
Material and methods
The search of the scientific literature was conducted to MEDLINE, PUBMED, EMBASE, and Google scholar for all kind of articles (all publications to May, 2020) using the following key words: dimethoate, omethoate, DNA damage, mutagenicity, mutation, genetic damage, oxidative stress, and genotoxicity. No time limit was imposed to the search, whose goal was to identify the maximum number of papers published within the field. Case reports, papers not written in English language, and reviews were not included to the study.
Results
Genotoxicity
A total of 79 papers were achieved between 1983 and 2020, but only 55 fulfilled the requirements adopted in this setting (Fig. 1). In 1983, a total of three papers were published within the field. Among them, Woodruff et al. (1983) did not detect the presence of chromosome loss in Drosophila melanogaster. The insecticide did not induce a significant amount of ring chromosome loss. In the same year, Nehez et al. (1983) evidenced the presence of micronucleated cells in bone marrow cells from mice exposed to dimethoate. However, this finding was not confirmed by Degraeve and Moutschen (1983), since negative results were found in rat by means of dominant lethal assay. All published articles rescued in the scientific searching are shown in Table 1.
Taking into account the papers published using mammalian eukaryotic cells, the majority of studies were performed using experimental test system, in particular rodents (rat and mouse). It is important to highlight that only 7 papers were published in humans, whereas 18 papers were published to animal experimental models. Among them, human lymphocytes are the preferred cells to evaluate the genotoxicity induced by dimethoate. For example, an earlier study conducted by Kizilet et al. (2019) has detected an increase of micronucleated cells in human lymphocytes continuously exposed to dimethoate. These findings were confirmed by others (Kizilet et al. 2019; Undeger and Basaran 2005; Jamil et al. 2004).
On the other hand, it has revealed that dimethoate is able to increase telomere length (Wang et al. 2019; Duan et al. 2017) as well as to induce abnormal expression of some cell regulatory proteins, such as p53 and 21 (Duan et al. 2017). Using single cell gel comet assay as a very sensitive assay for detecting DNA strand breaks. Samarawickrema et al. (2008) showed that dimethoate induces genetic damage in cord blood cells (Samarawickrema et al. 2008). Conversely, a weak genotoxicity has been verified by Bianchi-Santamaria et al. (1997) in human lymphocytes. Taken as a whole, it seems that dimethoate is able to induce genetic injury in humans, as a result of DNA strand breaks.
When performing the use of experimental models in rodents for evaluating the genotoxic potential of the insecticide, several studies have been detected so far. Some studies have demonstrated that dimethoate is able to induce genetic damage being closely associated with oxidative stress in liver and brain cells of rats (Yahia and Ali 2018; Li et al. 2016; Astiz et al. 2009a,b). Other authors have also detected the presence of genetic damage in rat peripheral lymphocytes as well (Qi et al. 2017). In mice exposed to several doses of dimethoate, an increased number of micronucleated cells was noticed (Undeger et al. 2000; Nehez and Desi 1996; Geetanjali et al. 1993). Moreover, lipid peroxidation and subsequent DNA injury in liver and kidney cells were found in mice exposed to dimethoate (Ayed-Boussema et al. 2012b). Such findings are in agreement with others (Dedek et al. 1984).
When Oncorphydus muykiss were investigated, genotoxicity and lipid peroxidation were also detected in liver and brain following dimethoate exposure (Dogan et al. 2011). The same results were obtained to guinea pigs (Mehl et al. 1994). However, this was not confirmed when rats were exposed to dimethoate evaluating the same target organs (Cunningham et al. 1994).
Other species have also been studied over mammals for identifying the genotoxicity induced by dimethoate. For example, changes in polymorphisms of some genes were detected in zebrafish exposed to dimethoate (Rong and Yin 2004). Recently, Hayat et al. (2018) have detected DNA damage in hemolymph from bees environmentally exposed to the insecticide. Following the same approach, other authors have demonstrated genetic injury in hemolymph from Folsomia candida in soil crops (Cardoso et al. 2017); hemocytes from Insceta orthoptera (Karpeta-kaczmarek et al. 2016), midgut glands from Xerolycosa memoralis (Wilczek et al. 2016), and African Catfish, Clarias gariepinus (Amaeze et al. 2020).
Interestingly, there are many published papers confirming that dimethoate is able to induce DNA mutations resulting in the biological scenario of species resistance. These findings were confirmed by studies on changes in the DNA sequence from Acht (acethylcholine) gene polymorphisms in several organisms, such as Aphis gossyppi Glover (Lokeshwari et al. 2016; Shang et al. 2014; Sun et al. 2005); Tetranuchus urticae (Khajehali et al. 2010); Bactrocera oleae (Kakani et al. 2008); and Musca domestica (Kristensen et al. 2006). Nevertheless, this was not confirmed by others (Carletto et al. 2010).
In order to clarify if dimethoate is able to induce point mutations in close contact with genome, several studies have employed the AMES tested by means of Escherichia coli. In fact, the study conducted by Ansari and Malik (Ansari and Malik 2009a,b) have demonstrated positive mutagenicity and interference with DNA repair system in E. coli exposed to the insecticide. These results are in agreement with Aleem and Malik (2005) and Rehana et al. (1995, 1996).
In the Tradescantia plant bioassay, increased micronucleated cells were also detected after exposure to dimethoate (Fardic et al. 2017; Mohamed and Ma 1999). When the genotoxic potential of dimethoate was investigated to Drosophila meganogaster, conflicting results were presented. Osaba et al. (1999), Xamena et al. (1988), and Woodruff et al. (1983) failed to detect any genotoxicity in this experimental test system. It is important to stress that the studies evaluated low doses of dimethoate. Probably, this could explain the negative data found.
Oxidative stress
We found many published papers demonstrating that dimethoate is a powerful oxidant agent in mammalian cells (Fig. 1). Of particular importance, the data have revealed that the pesticide is a harmful agent in multiple tissues and organs. These findings are summarized in Table 2. For example, dimethoate produced free radicals and blocked the antioxidant defense system in erythrocytes. Rats exposed to a single low dose of dimethoate (0.01% LD[50]) caused lipid peroxidation associated with induction of superoxide dismutase and catalase activities (John et al. 2001). The authors have yet revealed inhibition of glutathione S-transferase and acetylcholinesterase activities in rats exposed to dimethoate (John et al. 2001). Analogous results were found by means of increase in superoxide dismutase, malondialdehyde, and catalase levels in the same cells (Ben Amara et al. 2012; Barski and Spodniewska, 2012; Abdallah et al. 2011; Gargouri et al. 2011). Membrane-bound enzymes such as Ca(2+)-ATPase and acetylcholinesterase (AChE), Na(+)-K(+)-ATPase were also inhibited after dimethoate exposure (Ben Amara et al. 2012; Pan et al. 2010; Singh et al. 2006).
When rats were exposed during 30 days to dimethoate at 0.2 g/L dose in drinking water, severe oxidative stress in lung was evidenced by increasing malondialdehyde, protein carbonyl groups, and advanced oxidation protein products (Wang et al. 2016). An increase in superoxide dismutase, glutathione peroxidase, catalase followed by decreased acetylcholinesterase and butyrylcholinesterase activities, glutathione, and non-protein thiols levels were observed as well (Wang et al. 2016).
Epididymis spermatozoa were treated for 3 h at 37 °C with increasing concentrations of dimethoate (50, 100, and 200 μm) (Ben Abdallah et al. 2012). The results showed that the insecticide caused strong oxidative damage in spermatozoa as depicted by increased malondialdehyde levels (Ben Abdallah et al. 2012) followed by increased lipid peroxidation (Astiz et al. 2019 a, b; Jallouli et al. 2016). However, a decrease in superoxide dismutase, glutathione, and catalase were detected in vivo (Jallouli et al. 2016; Ben Abdallah et al. 2012). In mice, the same results were found, because subchronic exposure to dimethoate at 20 mg/kg/day for 30 days increased lipid peroxidation and decreased the levels of antioxidant enzymes in testis (Jallouli et al. 2015).
Liver is also a potential target for dimethoate toxicity. Rats treated with dimethoate (i.p. 1/250 LD50) for three times a week during 5 weeks induced fatty acid peroxidation in hepatocytes (Astiz et al. 2009a,b). When dimethoate was administered at doses ranging from 45 to 90 mg/kg, the results revealed an increase in cytochrome P450, lipid peroxidation, superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase levels in hepatic cells at higher doses only (Sharma et al. 2005a).
In further consideration of liver, the effects of low doses of dimethoate demonstrated similar outcomes as those found to high doses. This is because dimethoate increased the levels of superoxide dismutase, cytochrome P450, lipid peroxidation, catalase, glutathione peroxidase, and reductase at doses 6, 20, and 30 mg/kg (Kwape et al. 2013; Saafi et al. 2011; Sharma et al. 2005b). Glutathione-S-transferase increased at 6 and 30 mg/kg doses (Sharma et al. 2005b). Others have yet demonstrated that exposure to dimethoate for 30 days at 2 g/L dose trigged oxidative stress increasing malondialdehyde levels followed by decreasing glutathione and non-protein thiol levels in rat liver. A low expression of superoxide dismutase, glutathione peroxidase, and catalase activities were also noticed in this cellular type (Ben Amara et al. 2011).
In mice treated with concentrations ranging from 1 to 30 mg/kg for 30 consecutive days, dimethoate was able to inhibit acetylcholinesterase activities in liver cells. The pesticide increased lipid peroxidation and protein carbonyl levels in a dose-dependent manner. Catalase activity increased at doses higher than 5 mg/kg (Yan et al. 2015; Ayed-Boussema et al. 2012b). It seems that results show that lipid peroxidation as well as antioxidative defense mechanisms in rodents display different responses, being dependent upon pesticide treatments and doses (Yang et al. 2012).
Other mammalian species such as guinea pig shows the same results found in rodents. Dimethoate induced a significant increase in lipid peroxidation, and decrease in the activities of catalase and glutathione-S-transferase in liver of guinea pigs exposed to dimethoate at 14 mg/kg during 21 days (Al-Awthan and Bahattab 2019). The insecticide also increased serum levels of hepatic marker enzymes (AST, ALT, and ALP) in guinea pig at high dose administrated (80 mg/kg) (Al-Awthan et al. 2019, 2014).
Daily administration of dimethoate (20 and 40 mg/kg b.w.) for 30 days induced elevated levels of specific markers in pancreas, as for example, amylase and lipase. Interestingly, these biochemical dysfunctions were associated with high ROS levels and lipid peroxidation in pancreas suggesting the presence of oxidative damage in this metabolic organ (Kamath and Rajini 2007). In particular, dimethoate was able to significant increase in pro-fibrotic cytokine (TGF-β1) and this is strongly associated with reduction of the antioxidant enzymes, such as reduced glutathione, catalase, and superoxide dismutase activities (Messallam et al. 2018).
In kidney, dimethoate was administered at doses of 1, 5, 10, 15, and 30 mg/kg for 30 consecutive days in BALB/c mice. The pesticide inhibited acetylcholinesterase activities in kidney of mice followed by increased lipid peroxidation and protein carbonyl levels in a dose-dependent manner (Li et al. 2016; Saafi-Ben Salah et al. 2012). A decrease in gluthatione and plasma urea levels and an increase in superoxide dismutase and catalase activities were observed (Ben Amara et al. 2013; Ayed-Boussema et al. 2012b).
In heart, female Wistar rats were exposed to dimethoate for consecutive 30 days (0.2 g L-1 of drinking water). The results demonstrated that the insecticide promoted oxidative stress with high levels of malondialdehyde, protein carbonyl levels, and advanced protein oxidation (Ben Amara et al. 2013). An increase of superoxide dismutase, catalase, and glutathione peroxidase activities was also detected in the heart cells of rats after dimethoate exposure (Amara et al. 2013).
Dimethoate was administered at doses 0.6, 6, and 30 mg/kg for 30 days in rats for investigating the harmful effects in brain cells. The results revealed an increase in levels of superoxide dismutase, lipid peroxidation, cytochrome P450, catalase, glutathione peroxidase, and reductase in brain cells at 6 and 30 mg/kg doses. A decrease in glutathione was observed at 30 and 6 mg/kg. Glutathione-S-transferase increased at 30 mg/kg dose (Yahia and Ali 2018; Sharma et al. 2005a,b).
Following the findings for brain cells, dimethoate also induced an increase in catalase, superoxide dismutase, cytochrome P450, glutathione peroxidase, lipid peroxidation, and glutathione reductase at higher doses administrated (45, 75, and 90 mg/kg) for 24 h. Likewise, there were no significant differences in glutathione and glutathione-S-transferase activities in these animals. Nevertheless, there was a significant increase in glutathione-S-transferase in brain cells at 90 mg/kg dose only (Sharma et al. 2005b). Particularly, dimethoate (i.p. 1/250 LD50) was administrated for 5 weeks causing fatty acid peroxidation (Astiz et al. 2009b). The administration of low doses of dimethoate to rats induced severe oxidative stress in some specific brain regions, such as cortex, substantia nigra, and hippocampus (Astiz et al. 2013).
In non-mammalian cells, the same results were found. Erythrocytes of Oncorhynchus mykiss exposed to subtheal doses of dimethoate for 5, 15, and 30 days induced an increase in glutathione peroxidase activity and high levels of superoxide dismutase in brain tissue. Lipid peroxidation increased after the exposure in both tissues and it was positively correlated with duration of exposure (Dogan et al. 2011).
In frogs, dimethoate at 10 and 20 ppm doses treated for 24, 48, 72, or 96 h, the results showed that malondialdehyde levels increased significantly in stomach and lung. Reduced glutathione was changed in muscle and lung, being increased in stomach and tongue. With respect to antioxidant enzymes (glutathione-S-transferase and reductase and catalase), their activities were decreased in tongue, and increased in lung (Isnas et al. 2012; Özkol et al. 2012).
The monogonont rotifer Brachionus koreanus transcript analysis after exposure to dimethoate indicated that the transcriptional level of Bk-Cu/Zn-SOD was increased in a dose-dependent fashion (Kim et al. 2015).
Terrestrial isopods from the species Porcellionides pruinosus were treated with the recommended dose application (0.4 mg/kg soil) and a sublethal concentration (10 mg/kg soil) of dimethoate. The results showed that dimethoate caused oxidative stress by inhibition of the acetylcholinesterase enzyme, associated with changes in the levels of glutathione-S-transferase, catalase, and lipid peroxidation. In addition, the study demonstrated that the two concentrations used of dimethoate promoted the activation of different general detoxification mechanisms (Ferreira et al. 2015). The soil organism Enchytraeus albidus exposed to dimethoate for 2, 4, 8, 14, and 21 days caused cholinesterase inhibition (Novais et al. 2014).
Wolf spiders Xerolycosa nemoralis exposed to dimethoate displayed high activity of catalase and glutathione-S-transferase. Moreover, exposure of individuals to dimethoate increased catalase activity, and improved reductase glutathione activity (Stalmach et al. 2015).
Acute toxicity value (LC50) in Gammarus pulex exposed to dimethoate demonstrated the biological competence of dimethoate for inducing oxidative stress. In particular, the results revealed that malondialdehyde, gluthationes, superoxide dismutase, and catalase were increased (Serdar 2019).
In combination with other non-heavy essential metals, such as cadmium, Galba truncatula exposed to 0–400 μg L−1 of dimethoate, and 0–1000 μg L−1 of cadmium chloride demonstrated that dimethoate induced oxidative stress as a result of changes in some biochemical parameters in freshwater snails such as increased levels of superoxide dismutase, glutathione-S-transferase, glutathione peroxidase, and catalase activities and malondialdehyde, and glutathione (GSH) levels (Bannaee et al. 2019). The association of the dimethoate and cadmium increased the effects on Galba truncatula. It is important to stress that dimethoate stimulated to the bioconcentration of cadmium in snails as a result of increasing oxidative stress (Bannaee et al. 2019).
The common carp, Cyprinus carpio, exposed to 16 and 32 μg L−1 of dimethoate increased aspartate aminotransferase level in gills, the activity of catalase (0.2 ml L−1) in kidney, but it decreased the activity of lactate dehydrogenase and glucose 6-phsphate dehydrogenase in liver cells. Dimethoate significantly increased catalase activities in gills. Fish exposure to dimethoate decreased total antioxidant activity and glycogen levels in liver. A significant increase was detected in malondialdehyde and catalase activities in liver and kidney of fish exposed to dimethoate alone (Shadegan et al. 2018).
Conclusion and directions for future research
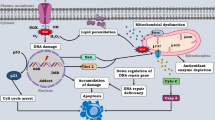
Overall, this study was able to present the current scientific knowledge on genotoxicity as a result of oxidative stress induced by dimethoate in multiple tissues and organs (Fig. 2). The approach has been documented in the literature so far, but few papers were conducted in humans. Therefore, further studies to address the risk to mammals for dimethoate are welcomed. Additionally, an analytical method for monitoring dimethoate in human body fluids is timely. This information is important to establish dose-response relationship of exposure and levels of oxidative stress and genotoxicity.
On the other hand, it is important to clarify what cells and/or tissues are more sensitive to genotoxicity induced by dimethoate as well. In particular, clarification of the gene mutation potential in vivo follow up studies to the positive mutagenic effects detected in mammalian and non-mammalian cells in vitro with dimethoate must be provided.
One of the most obvious limitations to any literature review is the quality of the available information. Since the mini-review has investigated the genotoxicity as a result of oxidative stress by dimethoate, to search other contexts and paradigms are also relevant in the context of chemical toxicity. For example, it would be interesting to know if and to what extent, dimethoate is able to interfere with cell cycle regulatory proteins in order to establish the role of apoptosis and cellular death after exposure to the insecticide. Curiously, the scientific literature was not able to conclude on the endocrine disruptor potential of dimethoate. Therefore, the interaction of dimethoate with the thyroid-signaling pathway in humans cannot be excluded. Certainly, these data play a crucial role for validating some end-points from published studies using microorganisms and rodents in so far as predict the real risk of dimethoate on carcinogenesis.
Data availability
Not applicable.
References
Abdallah FB, Gargouri B, Bejaoui H, Lassoued S, Ammar-Keskes L (2011) Dimethoate-induced oxidative stress in human erythrocytes and the protective effect of vitamins C and E in vitro. Environ Toxicol 26(3):287–291
Ahmad W, Shaikh S, Nazam N, Lone MI (2014) Protective effects of quercetin against dimethoate-induced cytotoxicity and genotoxicity in Allium sativum test. Int Sch Res Notices 2014:632672
Al-Awthan YS, Hezabr SM, Al-Zubairi AM, Al-Hemiri FA (2014) Effects of aqueous extract of Withania somnifera on some liver biochemical and histopathological parameters in male guinea pigs. Pak J Biol Sci 17(4):504–510
Al-Awthan YS, Salem Bahattab O (2019) Protective Role of Carissa edulis Ethanolic Extract against dimethoate-induced hepatotoxicity in guinea pigs. Pak J Biol Sci 22(6):299–308
Aleem A, Malik A (2005) Genotoxicity of the Yamuna River water at Okhla (Delhi), India. Ecotoxicol Environ Saf 61(3):404–412
Amaeze NH, Komolafe BO, Salako AF, Akagha KK, Briggs TD, Olatinwo OO, Femi MA (2020) Comparative assessment of the acute toxicity, haematological and genotoxic effects of ten commonly used pesticides on the African Catfish, Clarias gariepinus Burchell 1822. Heliyon. 6(8):e04768
Amara IB, Soudani N, Hakim A, Troudi A, Zeghal KM, Boudawara T, Zeghal N (2013) Protective effects of vitamin E and selenium against dimethoate-induced cardiotoxicity in vivo: biochemical and histological studies. Environ Toxicol. 28(11):630–643
Ansari MI, Malik A (2009a) Genotoxicity of agricultural soils in the vicinity of industrial area. Mutat Res 673(2):124–132
Ansari MI, Malik A (2009b) Genotoxicity of wastewaters used for irrigation of food crops. Environ Toxicol 24(2):103–115
Astiz M, de Alaniz MJ, Marra CA (2009a) Effect of pesticides on cell survival in liver and brain rat tissues. Ecotoxicol Environ Saf. 72(7):2025–2032
Astiz M, Diz-Chaves Y, Garcia-Segura LM (2013) Sub-chronic exposure to the insecticide dimethoate induces a proinflammatory status and enhances the neuroinflammatory response to bacterial lypopolysaccharide in the hippocampus and striatum of male mice. Toxicol Appl Pharmacol. 272(2):263–271
Astiz M, de Alaniz MJ, Marra CA (2009b) Antioxidant defense system in rats simultaneously intoxicated with agrochemicals. Environ Toxicol Pharmacol 28(3):465–473
Ayed-Boussema I, Rjiba K, Mnasri N, Moussa A, Bacha H (2012a) Genotoxicity evaluation of dimethoate to experimental mice by micronucleus, chromosome aberration tests, and comet assay. Int J Toxicol 31(1):78–85
Ayed-Boussema I, Rjiba K, Moussa A, Mnasri N, Bacha H (2012b) Genotoxicity associated with oxidative damage in the liver and kidney of mice exposed to dimethoate subchronic intoxication. Environ Sci Pollut Res Int. 19(2):458–466
Badry A, Schenke D, Treu G, Krone O (2021) Linking landscape composition and biological factors with exposure levels of rodenticides and agrochemicals in avian apex predators from Germany. Environ Res 193:110602
Banaee M, Sureda A, Taheri S, Hedayatzadeh F (2019) Sub-lethal effects of dimethoate alone and in combination with cadmium on biochemical parameters in freshwater snail, Galba truncatula. Comp Biochem Physiol C Toxicol Pharmacol 220:62–70
Barski D, Spodniewska A (2012) Activity of selected antioxidative enzymes in rats exposed to dimethoate and pyrantel tartrate. Pol J Vet Sci 15(2):239–245
Ben Abdallah F, Fetoui H, Zribi N, Fakfakh F, Ammar-Keskes L (2012) Antioxidant supplementations in vitro improve rat sperm parameters and enhance antioxidant enzyme activities against dimethoate-induced sperm damages. Andrologia Suppl 1:272–279
Ben Amara I, Soudani N, Hakim A, Bouaziz H, Troudi A, Zeghal KM, Zeghal N (2012) Dimethoate-induced oxidative damage in erythrocytes of female adult rats: possible protective effect of vitamin E and selenium supplemented to diet. Toxicol Ind Health. 28(3):222–237
Ben Amara I, Soudani N, Troudi A, Bouaziz H, Boudawara T, Zeghal N (2011) Antioxidant effect of vitamin E and selenium on hepatotoxicity induced by dimethoate in female adult rats. Ecotoxicol Environ Saf 74(4):811–819
Ben Amara I, Karray A, Hakim A, Ben Ali Y, Troudi A, Soudani N, Boudawara T, Zeghal KM, Zeghal N (2013) Dimethoate induces kidney dysfunction, disrupts membrane-bound ATPases and confers cytotoxicity through DNA damage. Protective effects of vitamin E and selenium. Biol Trace Elem Res 156(1-3):230–242
Benting J, Nauen R (2004) Biochemical evidence that an S431F mutation in acetylcholinesterase-1 of Aphis gossypii mediates resistance to pirimicarb and omethoate. Pest Manag Sci 60(11):1051–1055
Bianchi L, Zannoli A, Pizzala R, Stivala LA, Chiesara E (1994) Genotoxicity assay of five pesticides and their mixtures in Saccharomyces cerevisiae D7. Mutat Res 321(4):203–211
Bianchi-Santamaria A, Gobbi M, Cembran M, Arnaboldi A (1997) Human lymphocyte micronucleus genotoxicity test with mixtures of phytochemicals in environmental concentrations. Mutat Res 388(1):27–32
Cardoso DN, Silva ARR, Cruz A, Lourenço J, Neves J, Malheiro C, Mendo S, Soares AMVM, Loureiro S (2017) The comet assay in Folsomia candida: a suitable approach to assess genotoxicity in collembolans. Environ Toxicol Chem 36(9):2514–2520
Carletto J, Martin T, Vanlerberghe-Masutti F, Brévault T (2010) Insecticide resistance traits differ among and within host races in Aphis gossypii. Pest Manag Sci 66(3):301–307
Cunningham ML, Elwell MR, Matthews HB (1994) Relationship of carcinogenicity and cellular proliferation induced by mutagenic noncarcinogens vs carcinogens. III. Organophosphate pesticides vs tris(2,3-dibromopropyl)phosphate. Fundam Appl Toxicol 23(3):363–369
Dedek W, Grahl R, Schmidt R (1984) A comparative study of guanine N7-alkylation in mice in vivo by the organophosphorus insecticides trichlorphon, dimethoate, phosmet and bromophos. Acta Pharmacol Toxicol (Copenh) 55(2):104–109
Degraeve N, Moutschen J (1983) Genotoxicity of an organophosphorus insecticide, dimethoate, in the mouse. Mutat Res 119(3):331–337
Deshpande NM, Dhakephalkar PK, Kanekar PP (2001) Plasmid-mediated dimethoate degradation in Pseudomonas aeruginosa MCMB-427. Lett Appl Microbiol 33(4):275–279
Dissanayake KN, Chou RC, Thompson A, Margetiny F, Davie C, McKinnon S, Patel V, Sultatos L, McArdle JJ, Clutton RE, Eddleston M, Ribchester RR (2021) Impaired neuromuscular function by conjoint actions of organophosphorus insecticide metabolites omethoate and cyclohexanol with implications for treatment of respiratory failure. Clin Toxicol (Phila) 14:1–20
Dogan D, Can C, Kocyigit A, Dikilitas M, Taskin A, Bilinc H (2011) Dimethoate-induced oxidative stress and DNA damage in Oncorhynchus mykiss. Chemosphere 84(1):39–46
Du L, Wang H, Xu W, Zeng Y, Hou Y, Zhang Y, Zhao X, Sun C (2013) Application of ultraperformance liquid chromatography/mass spectrometry-based metabonomic techniques to analyze the joint toxic action of long-term low-level exposure to a mixture of organophosphate pesticides on rat urine profile. Toxicol Sci 134(1):195–206
Duan X, Yang Y, Wang S, Feng X, Wang T, Wang P, Liu S, Li L, Yao W, Cui L, Wang W (2017) Changes in the expression of genes involved in cell cycle regulation and the relative telomere length in the process of canceration induced by omethoate. Tumour Biol 39(7):1010428317719782
European Food Safety Authority (EFSA), Arena M, Auteri D, Barmaz S, Brancato A, Brocca D, Bura L, Carrasco Cabrera L, Chiusolo A, Civitella C, Court Marques D, Crivellente F, Ctverackova L, De Lentdecker C, Egsmose M, Erdos Z, Fait G, Ferreira L, Greco L, Ippolito A, Istace F, Jarrah S, Kardassi D, Leuschner R, Lostia A, Lythgo C, Magrans JO, Medina P, Mineo D, Miron I, Molnar T, Padovani L, Parra Morte JM, Pedersen R, Reich H, Sacchi A, Santos M, Serafimova R, Sharp R, Stanek A, Streissl F, Sturma J, Szentes C, Tarazona J, Terron A, Theobald A, Vagenende B, Van Dijk J, Villamar-Bouza L (2018) Peer review of the pesticide risk assessment of the active substance dimethoate. EFSA J 16(10):e05454
Ellingham TJ, Christensen EA, Maddock MB (1986) In vitro induction of sister chromatid exchanges and chromosomal aberrations in peripheral lymphocytes of the oyster toadfish and American eel. Environ Mutagen 8(4):555–569
Fadic X, Placencia F, Domínguez AM, Cereceda-Balic F (2017) Tradescantia as a biomonitor for pesticide genotoxicity evaluation of iprodione, carbaryl, dimethoate and 4,4'-DDE. Sci Total Environ 575:146–151
Ferreira NG, Morgado R, Santos MJ, Soares AM, Loureiro S (2015) Biomarkers and energy reserves in the isopod Porcellionides pruinosus: the effects of long-term exposure to dimethoate. Sci Total Environ 502:91–102
Gargouri B, Mansour RB, Abdallah FB, Elfekih A, Lassoued S, Khaled H (2011) Protective effect of quercetin against oxidative stress caused by dimethoate in human peripheral blood lymphocytes. Lipids Health Dis 10:149
Geetanjali D, Rita P, Reddy PP (1993) Effect of ascorbic acid in the detoxification of the insecticide dimethoate in the bone marrow erythrocytes of mice. Food Chem Toxicol 31(6):435–437
Hayat K, Afzal M, Aqueel MA, Ali S, Saeed MF, Khan QM, Ashfaq M, Damalas CA (2018) Insecticide exposure affects DNA and antioxidant enzymes activity in honey bee species Apis florea and A. dorsata: Evidence from Punjab, Pakistan. Sci Total Environ 635:1292–1301
Isnas M, Yegin E, Celik I (2012) Effects of omethoate on certain oxidative biomarkers in various tissues of frogs (Rana ridibunda) at acute exposure. Toxicol Ind Health 28(1):27–34
Jallouli M, Dhouib Iel B, Dhouib H, Gharbi N, El Fazaa S (2015) Effects of dimethoate in male mice reproductive parameters. Regul Toxicol Pharmacol. 73(3):853–858
Jallouli M, El Bini DI, Dhouib H, Lasram M, Gharbi N, El Fazaa S (2016) Disruption of steroidogenesis after dimethoate exposure and efficacy of. N-acetylcysteine in rats: an old drug with new approaches Environ Sci Pollut Res Int 23(8):7975–7984
Jamil K, Shaik AP, Mahboob M, Krishna D (2004) Effect of organophosphorus and organochlorine pesticides (monochrotophos, chlorpyriphos, dimethoate, and endosulfan) on human lymphocytes in-vitro. Drug Chem Toxicol 2:133–144
John S, Kale M, Rathore N, Bhatnagar D (2001) Protective effect of vitamin E in dimethoate and malathion induced oxidative stress in rat erythrocytes. J Nutr Biochem. 12(9):500–504
Kakani EG, Ioannides IM, Margaritopoulos JT, Seraphides NA, Skouras PJ, Tsitsipis JA, Mathiopoulos KD (2008) A small deletion in the olive fly acetylcholinesterase gene associated with high levels of organophosphate resistance. Insect Biochem Mol Biol 38(8):781–787
Kamath V, Rajini PS (2007) Altered glucose homeostasis and oxidative impairment in pancreas of rats subjected to dimethoate intoxication. Toxicology. 231(2-3):137–146
Karpeta-Kaczmarek J, Kubok M, Dziewięcka M, Sawczyn T, Augustyniak M (2016) The level of DNA damage in adult grasshoppers Chorthippus biguttulus (Orthoptera, Acrididae) following dimethoate exposure is dependent on the insects' habitat. Environ Pollut 215:266–272
Khajehali J, Van Leeuwen T, Grispou M, Morou E, Alout H, Weill M, Tirry L, Vontas J, Tsagkarakou A (2010) Acetylcholinesterase point mutations in European strains of Tetranychus urticae (Acari: Tetranychidae) resistant to organophosphates. Pest Manag Sci 66(2):220–228
Kim BM, Lee JW, Seo JS, Shin KH, Rhee JS, Lee JS (2015) Modulated expression and enzymatic activity of the monogonont rotifer Brachionus koreanus Cu/Zn- and Mn-superoxide dismutase (SOD) in response to environmental biocides. Chemosphere 120:470–478
Klaunig JE (2018) Oxidative Stress and Cancer. Curr Pharm Des. 24(40):4771–4778
Kızılet H, Yilmaz B, Uysal H (2019) Herbal medicine against genotoxicity of dimethoate, an insecticide, in mammalian somatic cells. Heliyon 5(3):e01337
Kristensen M, Huang J, Qiao CL, Jespersen JB (2006) Variation of Musca domestica L. acetylcholinesterase in Danish housefly populations. Pest Manag Sci 62(8):738–745
Kwape TE, Chaturvedi P, Kamau JM, George S (2013) Hepato-protective potential of methanol extract of leaf of Ziziphus mucronata (ZMLM) against dimethoate toxicity: biochemical and histological approach. Ghana Med J. 47(3):112–120
Li S, Cao C, Shi H, Yang S, Qi L, Zhao X, Sun C (2016) Effect of quercetin against mixture of four organophosphate pesticides induced nephrotoxicity in rats. Xenobiotica 46(3):225–233
Lima do Rêgo E, Santos da Silva JD, Costa Nakamura T, PHGD D, Oliveira UR, Souza JR (2021) Distribution of organochlorine, organophosphates, carbamate, thiocarbamate, pyrethroids, and strobilurins in surface sediments of the Rio de Ondas watershed by GC-MS. J Environ Sci Health B. 56(4):357–369
Lokeshwari D, Krishna Kumar NK, Manjunatha H (2016) Multiple mutations on the second acetylcholinesterase gene associated with dimethoateresistance in the melon aphid, Aphis gossypii (Hemiptera: Aphididae). J Econ Entomol 109(2):887–897
Mehl A, Schanke TM, Johnsen BA, Fonnum F (1994) The effect of trichlorfon and other organophosphates on prenatal brain development in the guinea pig. Neurochem Res 19(5):569–574
Mesallam DIA, Abdel Hamid OI, Ibrahem NE (2018) Ethanolic extract of fenugreek seeds moderates dimethoate-induced pancreatic damage in male rats. Environ Sci Pollut Res Int 25(4):3894–3904
Mohammed KB, Ma TH (1999) Tradescantia-micronucleus and -stamen hair mutation assays on genotoxicity of the gaseous and liquid forms of pesticides. Mutat Res 426(2):193–199
National Pesticide Information Center (NPIC). OSU extension pesticide properties database. http://npic.orst.edu/ingred/ppdmove.htm. 2014. Accessed 26 Jun 2019.
Nehéz M, Dési I (1996) The effect of dimethoate on bone marrow cell chromosomes of rats in subchronic four-generation experiments. Ecotoxicol Environ Saf 33(2):103–109
Nehéz M, Selypes A, Scheufler H, Fischer GW (1983) Effect of dimethoate and O-demethyldimethoate on bone marrow cells of CFLP mice. Regul Toxicol Pharmacol 3(4):349–354
Novais SC, Gomes NC, Soares AM, Amorim MJ (2014) Antioxidant and neurotoxicity markers in the model organism Enchytraeus albidus (Oligochaeta): mechanisms of response to atrazine, dimethoate and carbendazim. Ecotoxicology. 23(7):1220–1233
Osaba L, Aguirre A, Alonso A, Graf U (1999) Genotoxicity testing of six insecticides in two crosses of the Drosophila wing spot test. Mutat Res 439(1):49–61
Pan Y, Shang Q, Fang K, Zhang J, Xi J (2010) Down-regulated transcriptional level of Ace1 combined with mutations in Ace1 and Ace2 of Aphis gossypii are related with omethoate resistance. Chem Biol Interact 188(3):553–557
Qi L, Cao C, Hu L, Chen S, Zhao X, Sun C (2017) Metabonomic analysis of the protective effect of quercetin on the toxicity induced by mixture of organophosphate pesticides in rat urine. Hum Exp Toxicol 36(5):494–507
Quezada-Maldonado EM, Sánchez-Pérez Y, Chirino YI, García-Cuellar CM (2021) Airborne particulate matter induces oxidative damage, DNA adduct formation and alterations in DNA repair pathways. Environ Pollut 287:117313
Rehana Z, Malik A, Ahmad M (1995) Mutagenic activity of the Ganges water with special reference to the pesticide pollution in the river between Kachla to Kannauj (U.P.), India. Mutat Res 343(2-3):137–144
Rehana Z, Malik A, Ahmad M (1996) Genotoxicity of the Ganges water at Narora (U.P.), India. Mutat Res 367(4):187–193
Reuber MD (1984) Carcinogenicity of dimethoate. Environ Res 34(2):193–211
Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB (2010) Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med 49(11):1603–1616
Ribeiro DA, Yujra VQ, DE Moura CFG, Handan BA, DE Barros VM, Yamauchi LY, Castelo PM, Aguiar O Jr (2017) Genotoxicity induced by dental materials: a comprehensive review. Anticancer Res 37(8):4017–4024
Rong Z, Yin H (2004) A method for genotoxicity detection using random amplified polymorphism DNA with Danio rerio. Ecotoxicol Environ Saf 58(1):96–103
Saafi EB, Louedi M, Elfeki A, Zakhama A, Najjar MF, Hammami M, Achour L (2011) Protective effect of date palm fruit extract (Phoenix dactylifera L.) on dimethoate induced-oxidative stress in rat liver. Exp Toxicol Pathol 63(5):433–441
Saafi-Ben Salah EB, El Arem A, Louedi M, Saoudi M, Elfeki A, Zakhama A, Najjar MF, Hammami M, Achour L (2012) Antioxidant-rich date palm fruit extract inhibits oxidative stress and nephrotoxicity induced by dimethoate in rat. J Physiol Biochem 68(1):47–58
Samarawickrema N, Pathmeswaran A, Wickremasinghe R, Peiris-John R, Karunaratna M, Buckley N, Dawson A, de Silva J (2008) Fetal effects of environmental exposure of pregnant women to organophosphorus compounds in a rural farming community in Sri Lanka. Clin Toxicol (Phila) 46(6):489–495
Serdar O (2019) The effect of dimethoate pesticide on some biochemical biomarkers in Gammarus pulex. O. Environ Sci Pollut Res Int. 26(21):21905–21914
Shadegan MR, Banaee M (2018) Effects of dimethoate alone and in combination with Bacilar fertilizer on oxidative stress in common carp, Cyprinus carpio. Chemosphere 208:101–107
Shang Q, Pan Y, Fang K, Xi J, Wong A, Brennan JA, Cao C (2014) Extensive Ace2 duplication and multiple mutations on Ace1 and Ace2 are related with high level of organophosphates resistance in Aphis gossypii. Environ Toxicol 5:526–533
Sharma Y, Bashir S, Irshad M, Gupta SD, Dogra TD (2005a) Effects of acute dimethoate administration on antioxidant status of liver and brain of experimental rats. Toxicology. 206(1):49–57
Sharma Y, Bashir S, Irshad M, Nag TC, Dogra TD (2005b) Dimethoate-induced effects on antioxidant status of liver and brain of rats following subchronic exposure. Toxicology 215(3):173–181
Singh M, Sandhir R, Kiran R (2006) Erythrocyte antioxidant enzymes in toxicological evaluation of commonly used organophosphate pesticides. Indian J Exp Biol 44(7):580–583
Stalmach M, Wilczek G, Homa J, Szulinska E (2015) Antioxidative and immunological responses in the haemolymph of wolf spider Xerolycosa nemoralis (Lycosidae) exposed to starvation and dimethoate Environ Pollut 206:551-559.
Sun L, Zhou X, Zhang J, Gao X (2005) Polymorphisms in a carboxylesterase gene between organophosphate-resistant and -susceptible Aphis gossypii (Homoptera: Aphididae). J Econ Entomol 98(4):1325–1332
Undeğer U, Başaran N (2005) Effects of pesticides on human peripheral lymphocytes in vitro: induction of DNA damage. Arch Toxicol 79(3):169–176
Undeger U, Institóris L, Siroki O, Nehéz M, Dési I (2000) Simultaneous geno- and immunotoxicological investigations for early detection of organophosphate toxicity in rats. Ecotoxicol Environ Saf 45(1):43–48
Van Scoy A, Pennell A, Zhang X (2016) Environmental Fate and Toxicology of Dimethoate. Rev Environ Contam Toxicol 237:53–70
Vontas JG, Hejazi MJ, Hakes NJ, Cosmidis N, Loukas M, Janes RW, Hemingway J (2002) Resistance-associated point mutations of organophosphate insensitive acetylcholinesterase, in the olive fruit fly Bactrocera oleae. Insect Mol Biol 11(4):329–336
Wang J, Yu XF, Zhao JJ, Shi SM, Fu L, Sui DY (2016) Ginsenoside Rg3 attenuated omethoate-induced lung injury in rats. Hum Exp Toxicol. 35(6):677–684
Wang W, Zhang H, Duan X, Feng X, Wang T, Wang P, Ding M, Zhou X, Liu S, Li L, Liu J, Tang L, Niu X, Zhang Y, Li G, Yao W, Yang Y (2019) Association of genetic polymorphisms of miR-145 gene with telomere length in omethoate-exposed workers. Ecotoxicol Environ Saf 172:82–88
Wilczek G, Mędrzak M, Augustyniak M, Wilczek P, Stalmach M (2016) Genotoxic effects of starvation and dimethoate in haemocytes and midgut gland cells of wolf spider Xerolycosa nemoralis (Lycosidae). Environ Pollut 213:370–378
Woodruff RC, Phillips JP, Irwin D (1983) Pesticide-induced complete and partial chromosome loss in screens with repair-defective females of Drosophila melanogaster. Environ Mutagen 5(6):835–846
Xamena N, Velázquez A, Batiste-Alentorn M, Creus A, Marcos R (1988) Genotoxicity studies with four organophosphorus insecticides using the unstable white-zeste system of Drosophila melanogaster. Mutat Res 204(2):251–256
Yahia D, Ali MF (2018) Assessment of neurohepatic DNA damage in male Sprague-Dawley rats exposed to organophosphates and pyrethroid insecticides. Environ Sci Pollut Res Int 25(16):15616–15629
Yan X, Rong R, Zhu S, Guo M, Gao S, Wang S, Xu X (2015) Effects of ZnO nanoparticles on dimethoate-induced toxicity in mice. J Agric Food Chem 63(37):8292–8298
Yang J, Cao J, Sun X, Feng Z, Hao D, Zhao X, Sun C (2012) Effects of long-term exposure to low levels of organophosphorous pesticides and their mixture on altered antioxidative defense mechanisms and lipid peroxidation in rat liver. Cell Biochem Funct. 30(2):122–128
Funding
DAR and MBV are recipients from CNPq (Conselho Nacional de Desenvolvimento Cientifico e Tecnologico, grant number #001). DVS and ACMM are repicients from CAPES (Coordenação de Desenvolvimento de Pessoal de Nivel Superior).
Author information
Authors and Affiliations
Contributions
Conceptualization: ACMM, RCBS, MBV, CTFO and DAR; data search: MSS, DVS and MESA; formal analysis: ACMM, RCBS, MBV, CTFO and DAR; writing—review and editing: all authors.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Informed consent
For this type of study, formal consent is not required.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible editor: Mohamed M. Abdel-Daim
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Silva, M.S., De Souza, D.V., Alpire, M.E.S. et al. Dimethoate induces genotoxicity as a result of oxidative stress: in vivo and in vitro studies. Environ Sci Pollut Res 28, 43274–43286 (2021). https://doi.org/10.1007/s11356-021-15090-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-15090-z