Abstract
The submitted work observed Cu, Ni, and Zn effects on selected physiological and stress parameters of the alga Raphidocelis (Pseudokirchneriella) subcapitata. In 96-h experiments, EC50 values for algal specific growth rates (SGR) inhibition in Cu, Ni, and Zn presence were estimated as 0.15, 0.50, and 0.20 mg l−1. In addition to growth inhibition, the effect of metals at various concentrations on algal SGR was also monitored. While these experiments confirmed approximately the same toxicity of Zn and Cu on SGR, Ni toxicity on this parameter was observed as the lowest. In terms of the effect of metals on the level of selected photosynthetic pigments, chlorophyll a, chlorophyll b, and carotenoids, the following inhibition orders can be established: Zn > Cu > Ni, Ni > Cu > Zn, and Ni > Cu ≥ Zn, respectively. As a novelty of our research, we included monitoring and evaluation of the intensity of stress, which was the response of algal cells to the presence of Cu, Ni, and Zn, and its correlation with respect to production factors and metal accumulation in algal cells. As stress factors, thiol (-SH) group and TBARS (thiobarbituric acid reactive substances) as significant indicators of lipid level peroxidation were determined. The content of -SH groups depended on the concentration of metal, and its level was the most stimulated by Zn, less by Cu and Ni. The TBARS content was 2 to 5 times higher in Cu than in Zn or Ni presence. In the presence of Zn and Ni, TBARS content reached approximately the same levels. For this parameter, the following rank order can be arranged: Cu >> Ni ≥ Zn. While Cu and Ni accumulation in R. subcapitata was confirmed, Zn accumulation was not determined or was below the detectable limit. Regression analyses revealed significant positive correlation between Cu accumulation and TBARS while carotenoids as possible antioxidants confirmed with TBARS mostly negative correlations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Metal pollution of an aquatic environment is a persistent problem mainly in some rivers in undeveloped countries. Among metals that frequently occur in freshwaters are Cu, Ni, and Zn. While their concentrations in European rivers are considerably variable with the maximum around 0.080, 0.010, and 0.005 mg l−1 for Zn, Cu, and Ni, respectively, in the rivers of undeveloped countries, the metals are usually present at several times higher concentrations (Paul 2017). Their increasing levels may be harmful to all living organisms, including water phytoplankton situated at the beginning of the water food chain, and they create ecological, evolutionary, nutritional, and environmental problems. Many metals, such as Co, Cu, Cr, Fe, Mg, Mn, Mo, Ni, Se, and Zn, are introduced as trace elements required for various biochemical and physiological functions (Tchounwou et al. 2012); however, they are also associated with toxicity when their level in the environment increased and overreached essential concentration.
Many sources have presented that Cu and Zn are essential for all species of phytoplankton (Liu et al. 2014); however, Ni is required only for prokaryotic phytoplankton such as cyanobacteria (Muyssen et al. 2004). Although Cu, Ni, and Zn are essential metals, they can also affect toxically at higher concentrations. Ecotoxicological tests of metal ions on green freshwater algae provide not only an estimation of the risk associated with metal toxicity to wild freshwater biota (Torres et al. 2008) but also the determination of phytoplankton’s ability to survive and produce biomass under stressful conditions. This can be helpful in the use of algae in bioreactors for the decontamination of municipal wastewaters (Malik 2004).
Determination of the half-maximal effective concentration of metal (EC50) is very useful for the estimation of metal toxicity (OECD 201 2002). Based on the EC50 values of Cu, Ni, and Zn determined for growth inhibition of some green algae species in 96-h tests, several authors have suggested that Cu is more toxic than Ni or Zn (Geis et al. 2000; Pereira et al. 2005; Magdaleno et al. 2014). The order of Ni or Zn in their toxicity on algal growth is not quite clear. While the first group of researchers (Geis et al. 2000; Magdaleno et al. 2014) have asserted that Zn is more toxic than Ni, the second group (Mehta and Gaur 1999; Pereira et al. 2005) have conversely maintained that Ni reduces algal growth more than Zn. Algal growth and biomass production are extraordinarily dependent on the efficiency of photosynthesis, which is regulated by the level of photosynthetic pigments presented in an algal cell. As Soto et al. (2011) have previously mentioned, metal ions can significantly inhibit the effectivity of photosynthesis. Some authors have reported that intracellularly presented metal ions cause the deformation of chloroplasts, including changes in the arrangement of thylakoids of algae such as Chlorella vulgaris or Raphidocelis subcapitata (Carfagna et al. 2013). Other authors have noticed that zinc ions (Zn) can inhibit the activity of enzymes that use divalent cations (Fe and Mg) as a cofactor for chlorophyll biosynthesis, and this can result in reduced production of photosynthetic pigments (Ikegami et al. 2005). Moreover, Küpper et al. (1996) presented that divalent metal ions such as Cu, Ni, or Zn can limit photosynthesis efficiency by substituting and replacing Mg in the porphyrin structure of chlorophylls. Consequently, the activity of metal ions reduces the function of chlorophylls as well as chlorophyll production. Piovár et al. (2011) mentioned that chlorophyll b is less sensitive to the presence of toxic metal concentrations than chlorophyll a; therefore, the production of chlorophyll b can be increased to ensure relatively effective photosynthesis under stressful conditions. However, chlorophylls are important for photosynthesis since autotrophic organisms synthesize other pigments as well, e.g., carotenoids. Carotenoids are supplementary pigments and belong to non-enzymatic antioxidants (McElroy and Kopsell 2009; Tan et al. 2020). They are essential for cell detoxification and participate in the termination of the lipid peroxidation chain (Karuppanapandian et al. 2011). The Chlorophyceae are generally believed to have the same major pigments as the higher plants and a list of observed carotenoids among species of this class are published by Rowan (1989). Although the reduction of the carotenoids content is the standard answer of green algae to the toxicity of metals in short-time experiments, phytoplankton can also increase their content in the reactive oxygen species (ROS) detoxification process (Bossuyt and Janssen 2004; Takaichi 2011; Miazek et al. 2015). Moreover, organisms such as algae also defend themselves against metal toxicity and ROS stress by producing specific substances associated with increased thiol group formation. While glutathione is preferentially included in ROS detoxification, phytochelatin neutralizes the charge of the metal (Le Faucheur et al. 2006). Stimulation of cell defensive mechanisms depends on the level of metal in cells or can be limited by the adaptability of organisms to metal and ROS stress. Insufficiently activated defense against ROS stress can result in lipid peroxidation and other intracellular damages.
While the research of metals in plants is focused on more parameters (Adamczyk-Szabela et al. 2017, 2020), in the algae, research is mostly focused on the specific growth rate (SGR) inhibition, biomass, and uptake of metals (e.g., Bossuyt and Janssen 2004; Chakraborty et al. 2010; Al-Hasawi et al. 2020). The main goals of this research were the study of heavy metal effects (Cu, Ni, and Zn) on the basic parameters of R. subcapitata like SGR and photosynthesis level (chlorophyll a, chlorophyll b, and carotenoids) that are parameters known from the standardized protocols like EN ISO 8692 (2012), OECD 201 (2002), or U.S. EPA712-C-96-164 (1996). Because metals can directly (like Cu) or indirectly (like Cd or Zn that are not typical redox active elements) increase oxidative stress levels, we focus to study two basic parameters that are mostly used in the experiments: thiobarbituric acid reactive substances as a parameter of lipoperoxidation (TBARS) and thiol protein groups (-SH) as parameter of protein oxidation. Thiol groups are changing as one of the most relevant parameters mostly in animals and plants, and proteins after exposure to metals and these factors are very rarely studied in algae (e.g., Karuppanapandian et al. 2011). Therefore, our research was focused on monitoring of such stress reactions and the correlation evaluation of the relationships between production and stress factors. Focusing on these parameters of oxidative stress is a novel and spread the complexity of our research.
Material and methods
Algal culture and composition of test medium
Green freshwater alga Raphidocelis subcapitata (Korshikov) Hindák, 1990 (synonym Pseudokirchneriella subcapitata, CCALA 433) from the collection at the Institute of Botany of the Czech Academy of Science in Třeboň (Czech Republic) was used in the tests. The test medium was prepared according to the STN EN ISO 8692 (2012) standard that is comparable to the OECD 201 (2002) guideline. Composition of the test medium was as follows: macronutrient, NaHCO3: 595.17 μmol l−1 (50 mg l−1); NH4Cl: 280.37 μmol l−1 (15 mg l−1); MgCl2.6 H2O: 59.02 μmol l−1 (12 mg l−1); CaCl2.2 H2O: 122.38 μmol l−1 (18 mg l−1); MgSO4.7 H2O: 60.85 μmol l−1 (15 mg l−1); K2HPO4: 9.16 μmol l−1 (1.6 mg l−1); micronutrient, FeCl3.6 H2O: 0.30 μmol l−1 (0.08 mg l−1); Na2EDTA.2 H2O: 0.27 μmol l−1 (0.1 mg l−1); H3BO3: 2.99 μmol l−1 (0.185 mg l−1); MnCl2.4 H2O: 2.10 μmol l−1 (0.415 mg l−1); ZnCl2: 0.02 μmol l−1 (3 × 10−3 mg l−1); CoCl2.6 H2O: 0.01 μmol l−1 (1.5 × 10−3 mg l−1); CuCl2.2 H2O: 6 × 10−5 μmol l−1 (1 × 10−5 mg l−1); Na2MoO4.2 H2O: 0.03 μmol l−1 (7 × 10−3 mg l−1). In these tests, the pH of medium was determined as 7.78 ± 0.28, and this value was at the end of experiments shifted to neutral or slightly acidic level. The changes in pH value did not exceed the limit specified in standardized norms like OECD 201 (2002) and STN EN ISO 8692 (2012).
Chemical and test design
The tested metals were added before the application of algal suspensions and used as chlorides (NiCl2.6 H2O (Lachema, Czech Republic, p.a.), ZnCl2 (Merck, Germany, p.a.), CuCl2.2 H2O (Merck, Germany, p.a.)), which were dissolved in distilled water. Fourteen Cu concentrations in the range 0.03–1.86 mg l−1 (0.53–29.33 μmol l−1), sixteen Ni concentrations in the range 0.05–1.98 mg l−1 (0.84–33.66 μmol l−1), and twelve Zn concentrations in the range 0.05–1.58 mg l−1 (0.73–24.20 μmol 1−1) were used in the tests for estimation of ECx values for algal specific growth rate inhibition. From the concentrations used to determine the ECx values, 7 concentrations of each metal in the range 1.5–24.0 μmol l−1 (0.093–1.525 mg l−1 for Cu; 0.100–1.480 mg l−1 for Ni; 0.100–1.580 mg l−1 for Zn) were selected and used for the determination of a specific growth rate, photosynthetic pigments, -SH groups, and TBARS content.
Cultivation management
Algal cells were added to 100-ml Erlenmeyer flasks with 50 ml of medium, and the initial algal density was 1.105 ± 6000 cells ml−1. During the tests, algal culture was kept for 96 h under constant conditions—illumination 1700 lux (22.1 μmol s−1 m−2), temperature 24 ± 1°C, and light/dark period of 18/6 h (STN EN ISO 8692 2012). During cultivation, the cultures were mixed in a shaker (Biosan Multi-Shaker PSU 20, Riga, Latvia).
Measurement of physiological and stress parameters
The standard STN EN ISO 8692 (2012) was used for the determination of a specific growth rate (SGR), which was measured once a day. Other parameters were determined after 96 h of the tests. Based on the previous determinations, the metal concentrations were selected to assess of algal specific growth rate (SGR) (OECD 201 2002; STN EN ISO 8692 2012). The specific growth rate was determined for each concentration once a day.
Specific growth rate
The algal cell number was determined before the calculation of the specific growth rate (SGR). There was a regression analysis applied for obtaining a regression functions for the calibration curve that expressed the dependence between the absorbance (at wavelengths 750 nm, where distilled water was used as reference) of the algal suspension and algal cell count. The microscopic method of counting of cells in the Bürker chamber was used for the detection of algal cell numbers for each diluted and spectrophotometric measured sample at wavelength 750 nm. The algal cell count in the ecotoxicological tests was expressed as the number of cell per ml (cells ml−1) and calculated by a modified regression function:
where N is the number of cells per ml and A750 is the absorbance of algal suspension determined at wavelengths 750 nm.
When the number of algal cells in the tests was established, the specific growth rate of algal suspensions was calculated according to the equation:
where μ = SGR is the specific growth rate (d−1), N the number of algal cells at time j of the test, Ni the number of algal cells at time i, tj the moment time at the end of the period, and ti the moment time at the start of the period.
Then the inhibition of SGR was calculated as follows:
where I is the inhibition of SGR in percent, SGR is the specific growth rate, and Ti is the tested concentration of the metal.
Photosynthetic pigments
Content of photosynthetic pigments (chlorophyll a, chlorophyll b, and carotenoids) was measured spectrophotometrically where 95% ethanol was used as a reference. For these determinations, 4.5 ml of algal suspension was centrifuged at 4000 RPM (2000g) for 15 min, the supernatant was removed, and 3 ml of 95% ethanol and glass balls were added to the pellet. The suspension was vortexed and then centrifuged at 4000 RPM (2000g) for 3 min. The absorbance of the supernatant was immediately measured at the wavelengths 665, 649, and 470 nm. Pigment concentrations were calculated according to the equations presented by Lichtenthaler and Wellburn (1983):
and calculated for algal suspension density.
Thiol (-SH) groups
The thiol (-SH) group concentration was determined spectrophotometrically in 0.5 ml ethanol algal extract, to which 0.1 ml DTNB solution (20 mmol dm−3 in ethanol) was added and vortexed for 15 s (Vortex mixer Gemmy UM-300, Twain). Before the assay, the suspension was stored in the dark at room temperature for 45 min, and then the absorbance at 412 nm was measured. Ethanol was used as the referenced substance (Viner et al. 1997).
TBARS
TBARS (thiobarbituric acid reactive substance) content was determined according to Heath and Packer (1968). This method measures the level of lipoperoxidation which at the end most relevant product is malondialdehyde (MDA). In this procedure, 1.0 ml of 20% (w/v) TCA + 0.5% (w/v) TBA solution (trichloroacetic acid + thiobarbituric acid) and 0.1 ml of 4% BHT (butylated hydroxytoluene) were added to 3 ml of ethanol algal extract. This mixture was stirred and incubated in a 90 °C water bath (VWR Unstirred Water Baths, VWB 2) for 30 min. Subsequently, the mixture was cooled in ice water for 10 min and then centrifuged at 4000 RPM (2900g) for 10 min. Absorbance was determined at 532 nm, and distilled water was used as a reference.
Thiol and TBARS concentrations were calculated according to Lambert-Beer law at extinct coefficient 13600 l mol−1 cm−1 and 0.155 dm3 mol−1 cm−1, respectively, and converted to algal suspension.
Heavy metal accumulation determination
Minimum of 0.69 mg of algae dry mass was mineralized in the mixture of HNO3:H2O2 (5 ml; 4:1) during the night and then for 60 min at 180°C in ZA-1 autoclave (Czech Republic). After cooling, the mineralized samples were diluted up to 25 ml with distilled water and measured by galvanostatic-dissolved chronopotentiometry on EcaFlow 150 GLP (Istran, Slovak Republic). This method is comparable with the method of AAS in the precision, accuracy, and sensitivity of measured results because reproducibility of this method is 2.5% at 50 μg l−1 for Ni(II) and, for method, where Cu Zn is measured with Cd and Pb, is 1.5% at 50 μg l−1 for Pb (Application list No. 65 n.d.-a, and 67 n.d.-b from https://www.istran.sk; Piršelová et al. 2010; Tkáčová et al. 2012; Manová et al. 2017).
Statistical analysis
ECx values for inhibition of algal suspension were estimated by Probit analysis and a confidence interval (95% CI) was determined for EC50 as a mean of a normal distribution of Cu, Ni, or Zn ECx concentrations. The obtained results were compared with the control and statistically evaluated by Student’s t-test. Dependence between the metal concentrations and examined parameters was assessed by correlation analysis according to Pearson. Statistical analyses and graphs are made in software MS Excel (Microsoft, USA).
Results
Estimated ECx values, specific growth rate, and photosynthetic pigment content of R. subcapitata under metal stress
Estimation of the effective concentration (ECx) of toxic substances is essential for ecotoxicological testing (OECD 201 2002). ECx values were estimated for algal specific growth rate inhibition as EC25, EC50, and EC75 values. These EC values expressed concentrations that caused 25, 50, and 75% SGR inhibition after 96-h exposure to Cu, Ni, or Zn (Table 1). For the SGR of algal suspension, Cu was determined as the most toxic among the tested metals. Therefore, metal effectivity to induce SGR inhibition increases in this order: Ni < Zn < Cu.
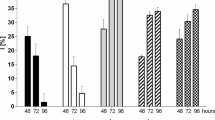
Although Cu concentration in the range 0.093–0.373 mg l−1 (except of 0.224 mg l−1) stimulated the algal specific growth rate during the first exposure hours (Fig. 1a), in the following hours, the SGR was already reduced. Finally, the Cu concentrations 0.149, 0.224, 0.261, and 0.373 mg l−1 caused after 96 h a decreasing SGR by 45, 74, 67, and 72% of the control, respectively (Fig. 1a). Other Cu concentrations such as 0.745 and 1.491 mg l−1 reduced algal SGR already at the time of 24 h after exposure to the metal (Fig. 1a). Subsequently, these Cu concentrations reduced SGR by 74–80% of the control after 96 h of exposure (Fig. 1a).
Specific growth rate (SGR) and photosynthetic (PS) pigments content after 96-h exposure of R. subcapitata to Cu, Ni, and Zn. The error bars indicate mean ± SD (n = 6). The symbols such as the asterisk, circle, and number sign define the statistical significance for each determined parameter in comparison to control. Statistical significance is at *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001 level.
Stimulation of SGR was also shown in the tests with Ni at all applied concentrations after 24-h exposure, and a statistically significant inhibitory effect of all tested concentrations on SGR occurred only after 96 h of exposure (Fig. 1c). When Ni was used in a concentration of 0.494, 0.741, and 1.482 mg l−1, the first inhibitory effect was observed after 48 and 72 h, respectively. In the highest tested concentration (1.482 mg l−1), SGR achieved approximately 57 and 34% of the control after 72 and 96 h of exposure, respectively.
After 24-h exposure of algae to selected concentrations of Zn, no SGR stimulation in comparison to control was determined (Fig. 1e). The effect of higher Zn concentrations was already inhibitory (Fig. 1e). With prolonged exposure, all used Zn concentrations reduced SGR statistically significantly. When Zn was used at the highest concentration (1.583 mg l−1), inhibition of SGR was obtained after 96-h exposure up to 85% of the control (Fig. 1e). This indicates for this concentration a very strong stressful effect of Zn on algal growth.
Photosynthetic pigments (chlorophyll a, b, and carotenoids) are relatively sensitive to metal stress. Production and intracellular concentrations of pigments usually decrease in toxic levels of metal ions.
Chlorophyll a (Chl a) is one of the most important pigments involved in photosynthesis. The intracellular concentration of Chl a was significantly decreased in the presence of all tested metals (Fig. 1b, d, f). A very strong inhibitory effect of all metals appeared at their concentration of 0.22, respectively, 0.24 mg l−1, when Chl a content reached at maximum, 70% of the control. Zn was confirmed to be the most toxic. The weakest toxic effect of Chl a was observed in the test with all used Ni concentrations. The inhibitory rank order for Chl a production could be arranged as Zn > Cu > Ni.
The Cu, Zn, and Ni in all applied concentrations significantly reduced (P < 0.001) chlorophyll b (Chl b) content (Fig. 1b, d, f). However, at low concentrations, the inhibition of Chl b production was stronger than that of Chl a mainly for Cu and Ni; the differences in the effect of the individual concentrations for all tested metals were smaller than those for Chl a.
Cells usually enhance the carotenoid (Car) level in the defense process against ROS; however, under extremely stressful metal conditions or in short-term exposure to metals, carotenoids production may either stagnate or decline. The Cu, Ni, and Zn used in our tests fully confirmed this tendency (Fig. 1b, d, f). While most of the metal concentrations from 0.149 mg l−1 significantly inhibited carotenoid production, the effect of lower concentrations was stimulatory or insignificant (Fig. 1b, d, f). The strongest inhibitory effect on Car production was confirmed for Zn at concentrations of 0.408 mg l−1 and greater (Fig. 1f).
Substances involved in stress reactions and heavy metal bioaccumulation
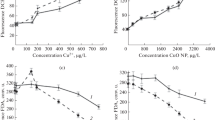
Glutathione and phytochelatin are also involved in the detoxification processes in cells. Their intracellular production can arise when cells are exposed to metals, causing oxidative stress. These substances are included among thiols, and their production was detected by determination of the level of reduced -SH groups in our experiments. As is presented in Fig. 2a, c, and e, the level of reduced -SH groups progressively increased with increasing metal concentration. Since the increased production of -SH groups in the presence of Cu and Zn appeared already at lower concentrations (0.149 and 0.192 mg l−1), it can be concluded that these metals caused stronger oxidative stress than Ni (Fig. 2a, c, e).
Lipid peroxidation of cells results mainly from oxidative stress, and the level of stress in the presence of metals was determined through TBARS level, which reached in the control 0.060 mol.10−6 cells (Fig. 2b, d, f). For Cu in the lowest used concentration (0.093 mg l−1), the TBARS level doubled (Fig. 2b). Moreover, TBARS production increased exponentially with increasing Cu concentration, and at the three highest Cu concentrations, the TBARS level was 10-fold higher than that in the control (Fig. 2b). Statistically significant differences in TBARS levels were also detected in Ni and Zn presence in all tested concentrations; however, these metals increased the TBARS level only slightly compared to Cu (Fig. 2d, f). The results confirmed that Cu induced very strong oxidative stress.
In Table 2 the concentrations are introduced corresponding to EC25, EC50, and EC75 values and the amounts in which they accumulated in algal cells. While Cu and Ni presence in R. subcapitata cells was confirmed, zinc content in the cells was below the detection limit of used method. The same situation was observed for Ni at the concentration 0.490 mg l−1.
Correlation analysis
Correlation analysis and correlation matrix for the studied parameters for each metal are introduced in Table 3. For metal accumulation were used concentrations and data from Table 2. Because for Zn we were not able to confirm its presence in the algal cells, the correlation coefficients for Zn accumulation and other observed parameters could not be calculated (Table 3). While Cu presence has a significant negative impact on SGR, Ni did not prove effect to growth of R. subcapitata in regression analysis. For all studied metals, the statistically significant positive correlation between Chl a and Car content was confirmed (Table 3). While for all metals a negative correlation among SGR and TBARS or Car was observed, highly positive statistically significant (P ≤ 0.01) correlation was confirmed between thiol (-SH) group and TBARS.
Discussion
Estimated ECx values and specific growth rate (SGR) of R. subcapitata under metal stress caused by accumulated metal
Based on the EC50 values estimated during our study, the order Cu > Zn > Ni was provided for the ability of the metal to induce algal SGR inhibition (Table 1). This order corresponds to the results presented by Geis et al. (2000) or Magdaleno et al. (2014) for R. subcapitata in 96-h tests (Table 4). Other authors have concluded that Ni is for algae more toxic than Zn; therefore, they have established the order as Cu > Ni > Zn (Pereira et al. 2005; Al-Hasawi et al. 2020). Lin et al. (2020) used Scenedesmus obliquus in which they tested the inhibition of SGR and observed the following rank: Ni > Cu > Zn. When the estimated EC25 values (for Cu, Ni, and Zn as 0.09, 0.18, and 0.12 mg l−1, respectively) were compared with Cu, Ni, and Zn concentrations detected in wastewaters by Üstün (2009) (0.012–0.179 mg l−1 of Cu, 0.059–0.202 mg l−1 of Ni, and 0.303–0.982 mg l−1 of Zn), it can be concluded that R. subcapitata could survive relatively well in wastewaters contaminated with Cu and Ni. Zn is especially toxic in these waters. Pereira et al. (2005) reported that concentrations corresponding to our estimated EC75 values strongly inhibit the growth of algae and increase the formation of defense agents, inducing stress. Toxicity of Cu, Zn, and Ni on specific growth rate of 8 freshwater algal species also introduced Fettweis et al. (2021) who, in addition to the effect of individual metals, also monitored the effects of their binary mixtures depending on the concentrations in which the metals entered into interactions and on algal species.
The weakest toxic effect of Ni (Fig. 1a, c, e) was also confirmed in the assessment of the SGR, which coincides with the results presented in the work of Lukavský et al. (2003). Different results were obtained by Mehta and Gaur (1999) in 96-h test for Chlorella vulgaris, for which Zn was less toxic than Cu and Ni. The structural composition of a cell wall, metal transporters equipment, metal potential of ionic mimicry, or metal essentiality are crucial for metal uptake by algae and their accumulation into cells (Domozych et al. 2012). While Ni has never been presented as an essential metal for alga R. subcapitata, the importance of Cu and Zn in the nutrition of green freshwater algae has been published several times (Boer et al. 2014). Cu and Zn are therefore required for the growth and development of algae, and thus the mechanisms of their intake are more developed than that for Ni. Because Cu and Zn are received more effectively, these metals can accumulate in the algal cells and manifest as being more toxic than Ni. These affirmations are entirely or partly supported by some papers (Ardestani et al. 2014; Zeraatkar et al. 2016); however, other authors (Mehta and Gaur 1999) have pointed out that the algal intracellular metal concentration decreased in the rank order Cu > Ni > Zn (Al-Hasawi et al. 2020). As a consequence, we did not exclude the opportunity that the accumulation of Ni can be preferred by algae more than that of Zn. In this case, Ni toxicity should be reduced by greater activity of algal defense mechanisms when compared to Zn. Although the content of carotenoids during our study was in most cases in Ni presence higher than that of Zn (Fig. 1d, f), -SH group, concentrations did not follow this trend, and their concentration was higher in tests with Zn (Fig. 2c, e). Based on these results, R. subcapitata probably accumulates Ni less than Cu, what is shown in Table 2. However, we cannot conclude the same for Zn that was not confirmed by the measure of electrochemical method with EcaFlow. Due to our knowledge, Zn accumulation in R. subcapitata was not measured, yet. For this reason, it is hard to compare our observation with other authors. Gao et al. (2016) also observed decrease of cell density of R. subcapitata in the presence of Zn, and that was observed in our experiments, too. Their EC50 value for cells with zinc and no phosphorous supply was calculated to 0.121 mg l−1, and that is in the range of confidence interval for our observation (Table 1).
The toxicity of metals to R. subcapitata can be influenced through more parameters, e.g., the presence of essential elements or pH value. Heijerick et al. (2002) observed Ca, Mg, Na, and K effects on Zn toxicity to this alga in the range of 5.5–8.0 pH values (Table 4). These authors confirmed that while K presence did not influence Zn toxicity to R. subcapitata competition between H+ and Zn2+ reduced zinc toxicity. While at pH 5.6, the authors observed 72-h EbC50 Zn2+ activity with the value 2.45×10−6 mol l−1, at pH 7.8, this value decreased up to 8.93 ×10−8 mol l−1 (Heijerick et al. 2002). In our experiments, we started at slightly basic pH (7.78±0.28). At the end of our experiments was the pH value slightly shifted to neutral or slightly acidic conditions. While in the standardized norms like OECD 201 (2002) and STN EN ISO 8692 (2012), norm is the condition that the control pH value does not change more than 1.5 units from the start to finish of experiment. This condition was kept. The pH changes were below 1.5 units. This requirement is essential because acidic conditions can change the toxicity and bioavailability of many metals. They are more bioavailable in the environment and more toxic to biota. Toxicity of Ni on R. subcapitata was decreased when Deleebeeck et al. (2009) increased Mg concentrations up to 5 mmol l−1 (72-h ErC50 = 1120 μg Nidiss l−1) or decreased pH value from 8.0 (72-h ErC50 = 145 μg Nidiss l−1) to 6.4 where 72-h ErC50 = 145 μg Nidiss l−1 (Table 4).
Photosynthetic pigments content
Metal ions can interact with substructures in the intracellular space, and this usually results in the inhibition or inactivation of important biological processes including photosynthetic pigment production (Table 4; Soto et al. 2011; Carfagna et al. 2013). The relationship between the content of pigments and photosynthesis activity, as well as the development of algal biomass, is very close (Miazek et al. 2015). Thus, biomass production should predictably decline with the decrease of photosynthetic pigment production or concentration. Nevertheless, Čypaitė et al. (2014) presented that photosynthetic pigment production is more sensitive to the toxicity of metal ions than algal growth. Different influence of metals on the growth and photosynthesis activity in metal presence also confirmed for C. vulgaris Ouyang et al. (2012) and for Spirulina platensis Akbarnezhad et al. (2020). While algal SGR can still be stimulated by lower metal concentrations, chlorophyll production can be reduced already at low concentrations. This statement was confirmed only for Chl b in our tests (Fig. 1b, d, f). For example, the lowest used metal concentration (approximately 0.09 mg l−1) reduced SGR by at most 36% (at 24 h in the presence of 0.096 mg Zn l−1 in Fig. 1e); Chl b content was reduced by 43 up to 66% of the control (Fig. 1a–f). Among the tested metals, Zn had the strongest toxic effect on Chl a production, but this was not confirmed in the correlation matrix in Table 3 due to the missing/not detectable presence of Zn in the cells. A negative correlation was calculated for Cu and Ni for both chlorophylls (Table 3). When Chakraborty et al. (2010) applied Cu, Ni, and Zn individually at a concentration of 0.60 mg l−1 (approximately 9.6 μmol l−1) on phytoplankton, the descending rank order for Chl a was established as Cu > Zn > Ni. This is slightly different from the order determined for this parameter in our tests, where Zn was observed to be the most toxic. For the alga R. subcapitata, a strong adverse effect of Zn on Chl a production was also confirmed by Atay and Özkoç (2010). Fargašová et al. (1999) presented an adverse effect of Ni on Chl a production in the alga Scenedesmus quadricauda, which was lower than that of Cu, and these results correspond to ours. When Danilov and Ekelund (2001) observed the photosynthetic efficiency of Chlamydomonas reinhardtii in short-term exposure experiments, they confirmed that Cu in concentration 0.10 mg l−1 (1.6 μmol l−1) reduced this parameter by 10% of the control, but the same concentration of Ni or Zn had a stimulatory effect and efficiency that surpassed the control by 60%. Stimulation of photosynthetic pigment production by 47% of the control in the test at low Ni concentrations (0.1–10.0 μmol l−1) on phytoplankton was also mentioned by Miazek et al. (2015). The stimulation of both chlorophyll pigments by Ni was not confirmed in our test (Fig. 1d). Already the lowest applied Cu concentration 0.093 mg l−1 (1.47 μmol l−1) inhibited Chl a content, which decreased to the control by 21%. Therefore, these results correspond to those of Danilov and Ekelund (2001) or Miazek et al. (2015).
Piovár et al. (2011) presented that chlorophyll b (Chl b) is less sensitive to toxic metals than Chl a, and photosynthetic organisms can increase the Chl b level to provide relatively effective photosynthesis in the protection process. Slight increase in Chl b content in C. vulgaris is also introduced by Kondzior and Butarewicz (2018). These authors observed that in the presence of Cu, carotenoids content was reduced, while the ratio of overall chlorophyll to carotenoids was increased. The stimulatory effect of metals on Chl b production was also observed by Wong and Chang (1991). These authors mentioned that Ni concentration from 0.10 to 1.00 mg l−1 (1.7 to 17.0 μmol l−1) significantly enhanced Chl b production in Chlorella pyrenoidosa in its log phase. On the contrary to these statements, Chl b content in our tests was in some cases reduced more than that of Chl a (Fig. 1b, d, f). However, Aggarwal et al. (2011) suggested that metals usually affect the decrease of Chl b content and this corresponds to our results. Fargašová et al. (1999) concluded that the Cu adverse effect on Chl b production in alga Scenedesmus quadricauda is stronger than that of Ni, and this result is completely contrary to ours. When the adverse effect of Cu on Chl b content was compared with that of Zn (Fig. 1b, f), the Zn inhibitory effect was higher only in concentrations of 0.186 and 1.583 mg l−1, and therefore, Cu was more toxic. This does not correspond; however, it is with the conclusions of Mosleh and Mofeed (2014). Nguyen-Deroche et al. (2012) suggested that the effect of metal on chlorophyll production is strongly dependent on phytoplankton species and that the mechanism of metal impact on chlorophyll amount is not completely clear at this time (Fettweis et al. 2021).
Some papers have presented that carotenoid concentration increases under unfavorable conditions, whereas other sources have pointed out that carotenoid levels can also decrease in the presence of extremely high metal concentrations or short-time tests, in which algae do not have enough time to adapt to stress conditions (Bossuyt and Janssen 2004; Karuppanapandian et al. 2011; Miazek et al. 2015; Kondzior and Butarewicz 2018). This type of stimulation or depression of carotenoid content was also observed in our experiments. While lower metal concentrations (0.093 mg Cu l−1) stimulated the content of carotenoids (Car), higher concentrations had a toxic effect on algae for the renewal of Car production in a relatively short time (Fig. 1b). Similarly, Chakilam (2012) observed that Cu and Zn at a concentration of 1 mg l−1 (16 μmol l−1) after 96 h did not reduce the carotenoid content of cyanobacteria Anabaena oryzae and Tolypothrix tenuis; however, their higher concentrations (10 and 100 mg l−1) already had a significant adverse effect. Bossuyt and Janssen (2004) observed that Car production in R. subcapitata after a week of exposure to Cu at 0.10 mg l−1 (1.6 μmol l−1) concentration was enhanced up to six times, and this in part corresponds to the results obtained in our experiments, where the same Cu concentration enhanced the Car content only by 5% of that in the control.
Substances involved in stress reactions
In the presence of toxic compounds, algae tend to protect against their toxic effect that is often connected with increased oxidation stress level in the cells. One of the first changes that can be observed at the thiol (protein) levels include glutathione (Guo et al. 2020). Metal ions including as well as bivalent Cu, Ni, and Zn have a high binding affinity to -SH groups, and so metals usually interact with glutathione (GSH), which can lead to disruption of the antioxidant system (Perales-Vela et al. 2006). However, cells can increase this GSH level in a defense regime to preclusion cellular damage by ROS (Hayat et al. 2012). Intracellular enhancement of GSH levels and the presence of metal ions in toxic levels already stimulate the production of phytochelatin (PC), which is primarily included in a scavenge of metals and their charge neutralization (Tsuji et al. 2003). Consequently, -SH group production commonly increases under metal stress conditions, and this was also observed in our experiments (Fig. 2a, c, e). Some authors (Le Faucheur et al. 2006) have mentioned that the ability of metals to induce the elevation of GSH and PC levels in the green alga Scenedesmus vacuolatus or diatom Thalassiosira sp. can grow in the following order: Zn < Ni < Cu. This does not correspond to our results, where this order was designated as Ni < Cu < Zn (Fig. 2a, b, c). Copper is known that produces highly reactive hydroxyl radicals in Haber-Weiss reaction similarly as Fe (Gunther et al. 1995), so there is a very high probability that changes in thiol content can be detected very soon because it is one of the first parameters that changed during increasing of the level of oxidative stress. However, due to our knowledge, there are no publications that measured this parameter at the presence of Cu, Ni, or Zn in R. subcapitata, and for this reason, we do not introduce these data in Table 4.
A hydroxyl radical is the most dangerous form of ROS. Because of its high reactivity, it can immediately oxidize the closest biomolecules such as pigments, proteins, lipids, and DNA (Karuppanapandian et al. 2011). For the ability of metals to enhance ROS production in Chlamydomonas sp. cells, metals were arranged in the following descending rank order: Pb2+ > Fe3+ > Cd2+ > Ag+ > Cu2+ > As5+ > Cr6+ > Zn2+ (Szivák et al. 2009). Copper (Cu) is more effective in the ROS level increasing, and its intracellular activity can result in more effective protein and lipid oxidation in algal cells than that of Ni or Zn (Table 4; Mehta and Gaur 1999; Soto et al. 2011). For the ability of Cu, Ni, and Zn to induce lipid peroxidation in Chlorella vulgaris, Mehta and Gaur (1999) arranged these metals in the order: Cu > Ni > Zn. Similarly, in our tests, Cu was confirmed to be the most toxic metal for this parameter (Fig. 2b, d, f). Production of TBARS in the lowest concentration (0.093 mg l−1) surpassed that of the control nearly two times and rapidly increased with increasing concentration. This was also statistically confirmed by a highly positive correlation in Table 3 (r = 0.9442, P < 0.001). The ability of metals to activate lipid peroxidation decreased in the following order: Cu >> Ni ≥ Zn. Despite the fact that we could only compare the agreement of our results with the work of Devasagayam et al. (2003), we assume that TBARS assay can interfere with some other cellular substances. In our TBARS assay, butylated hydroxytoluene was used for the prevention of autoxidation of unsaturated organic compounds, but that does not quite limit the concentration of interfering substances. It would be useful to modify this assay to allow more accurate results.
Conclusion
Pollution of the environment, including waters, is constantly increasing due to the intensification of agriculture and industrial activity. One of the serious environmental problems for the aquatic environment is also metal. They can act as stress factors and significantly affect all trophic levels of the aquatic ecosystem. Because algae represent a basic trophic level in water and are a source of food for many aquatic organisms, it is important to know and describe all risks that may be associated with changes in their environment and be reflected in changes in their production and vital abilities. Research aimed at a more thorough understanding of the relationships that occur between living organisms and their environment, in this case in water, provides, in any case, important knowledge about the functioning of the aquatic ecosystem and its ability or inability to cope with stress. Except stress factors determination, the novelty of the results presented here also consists in expressing the correlations between the production and stress factors to which algae are permanently exposed in the current state of the aquatic environment. Knowing the correlations will make it possible to predict how the aquatic ecosystem will behave in the event of increased adverse pressure. The results obtained can also serve as a useful source of information in environmental risk assessment.
Here present study focused on the effects of Cu, Ni, and Zn on selected physiological and stress parameters of alga Raphidocelis subcapitata. ECx values determined for growth inhibition of this alga in 96-h tests provided evidence that Cu is more toxic than Ni or Zn. A similar result was obtained by the observation of specific growth rate (SGR). Besides growth parameters, the content of photosynthetic pigments (Chl a, Chl b, and Car) depending on the concentration of the tested metals was also evaluated as one of the production parameters. The level of intensity of defensive mechanisms indicated that algal suspension of R. subcapitata responds to the presence of Cu or Zn by a greater production of reduced -SH groups than in the presence of Ni. Cu or Zn induced higher production of ROS than Ni. Although TBARS (thiobarbituric acid reactive substances) production was statistically significant in the presence of Zn or Ni, the ability of these metals to induce lipid peroxidation was negligible as compared with that of Cu. Copper induced 6 times higher lipid peroxidation in R. subcapitata cells than Zn or Ni. Pollution of aquatic ecosystems or wastewaters, however, is usually not due to only one pollutant. On the contrary, varied substances input into the environment and their reciprocal interactions and impact on biota, including algae, are relatively unknown. Tests, in which the effects of individual metals on organisms are followed, are basic for the study of their toxicity on organisms. From the perspective of more complex information about the effects of metals, it is necessary to focus attention primarily on the monitoring of the paired combination effects and interaction relations and include the research of oxidative stress level, too. In the presence, there are some pioneer publications focused on the monitoring of pollution in the aquatic environment. Development of new tools for fast assessment of ecotoxicological risk was rarely published (e.g., Moore et al. 2004; Rodrigues et al. 2021). For example, using of urban wet weather discharge (UWWD) management that focuses on UWWD, which often represents a significant source of pollution in all aquatic bodies (Gosset et al. 2019). In these bioassays, R. subcapitata together with Chlorella vulgaris, Daphnia magna, and Heterocypris incongruens are included. Moreover, microalgae growth inhibition assays are candidates for referent ecotoxicological assays that reduce the use of fish and other animal models in aquatic toxicology (Expósito et al. 2017).
References
Adamczyk-Szabela D, Lisowska K, Romanowska-Duda Z, Wolf W (2020) Combined cadmium – zinc interactions alter manganese, lead, copper uptake by Melissa officinalis. Sci Rep 10:1675. https://doi.org/10.1038/s41598-020-58491-9
Adamczyk-Szabela D, Romanowska-Duda Z, Lisowska K, Wolf WM (2017) Heavy metals uptake by herbs. V. Metal accumulation and physiological effects induced by thiuram in Ocimum basilicum L. Water Air Soil Pollut 228:334. https://doi.org/10.1007/s11270-017-3508-0
Aggarwal A, Sharma I, Tripathi BN, Munjal AK, Baunthiyal M, Sharma V (2011) Metal toxicity and photosynthesis. Chap. 16. In: Itoh S, Mohanty P, Guruprasad KN (eds) Photosynthesis: Overviews on Recent Progress and Future Perspectives Hardcover, 1st edn. IK International Publishing House, New Delhi, pp 229–236
Akbarnezhad M, Shamsaie Mehrgan M, Kamali A, Javaheriu Baboli M (2020) Effects of microelements (Fe, Cu, Zn) on growth and pigment contents of Arthrospira (Spirulina) platensis. Iran J Fih Sci 19:653–668 https://jifro.ir/article-1-2186-fa.pdf
Al-Hasawi ZM, Abdel-Hamid MI, Almutairi AW, Touliabah HE (2020) Response of Pseudokirchneriella subcapitata in free and alginate immobilized cells to heavy metals toxicity. Molecules 25(12):2847–2862. https://doi.org/10.3390/molecules25122847
Alves CM, Ferreira CMH, Soares EV, Soares HMVM (2017) A multi-metal risk assessment strategy for natural freshwater ecosystems based on the additive inhibitory free metal ion concentration index. Environ Pollut 223:517–523. https://doi.org/10.1016/j.envpol.2017.01.053
Application list No. 65 (n.d.-a). Determination of Ni in clean as well as and turbid water samples. [online] Available at https://www.istran.sk, cit. 6.5.2016.
Application list No. 67 (n.d.-b). Determination of Zn, Cd, Pb and Cu. [online] Available at https://www.istran.sk, cit. 6.5.2016.
Ardestani MM, van Straalen NM, van Gestel CAM (2014) The relationship between metal toxicity and biotic ligand binding affinities in aquatic and soil organisms: a review. Environ Pollut 195:133–147. https://doi.org/10.1016/j.envpol.2014.08.020
Atay Ş, Özkoç HB (2010) Effect of sediment on the bioavailability and toxicity of copper and zinc to a green alga. Fresenius Environ Bull 19:3027–3036 https://www.prt-parlar.de/download_feb_2010/
Boer JL, Mulrooney SB, Hausinger RP (2014) Nickel-dependent metalloenzymes. Arch Biochem Biophys 544:142–152. https://doi.org/10.1016/j.abb.2013.09.002
Bossuyt BTA, Janssen CR (2004) Long-term acclimation of Pseudokirchneriella subcapitata (Korshikov) Hindak to different copper concentrations: changes in tolerance and physiology. Aquat Toxicol 68:61–74. https://doi.org/10.1016/j.aquatox.2004.02.005
Carfagna S, Lanza N, Salbitani G, Basile A, Sorbo S, Vona V (2013) Physiological and morphological responses of lead or cadmium exposed Chlorella sorokiniana 211-8K (Chlorophyceae). SpringerPlus 2(147):1–7. https://doi.org/10.1186/2193-1801-2-147
Chakilam SR (2012) Metal effects on carotenoid content of Cyanobacteria. Int J Bot 8:192–197. https://doi.org/10.3923/ijb.2012.192.197
Chakraborty P, Babu PVR, Acharyya T, Bandyopadhyay D (2010) Stress and toxicity of biologically important transition metals (Co, Ni, Cu and Zn) on phytoplankton in a tropical freshwater system: an investigation with pigment analysis by HPLC. Chemosphere 80:548–553. https://doi.org/10.1016/j.chemosphere.2010.04.039
Čypaitė A, Žaltauskaitė J, Venclovienė J (2014) Assessment of chlorophyll-a, chlorophyll-b and growth rate in freshwater green algae Pseudokirchneriella subcapitata exposed to cadmium and copper. In: 9th Int Conf “ENVIRONMENTAL ENGINEERING” 22–23 May 2014, Vilnius, Lithuania, pp 1–7. https://doi.org/10.3846/enviro.2014.009
Danilov RA, Ekelund NGA (2001) Effects of Cu2+, Ni2+, Pb2+, Zn2+ and pentachlorophenol on photosynthesis and motility in Chlamydomonas reinhardtii in short-term exposure experiments. BMC Ecol 1:1. https://doi.org/10.1186/1472-6785-1-1
Deleebeeck NME, De Shamphelaere KAC, Janseen CR (2009) Effects of Mg2+ and H+ on the toxicity of Ni2+ to the unicellular green alga Pseudokirchneriella subcapitata: model development and validation with surface waters. 407:1901–1914. https://doi.org/10.1016/j.scitotenv.2008.11.052
Devasagayam TPA, Boloor KK, Ramasarma T (2003) Methods for estimating lipid peroxidation: an analysis of merits and demerits. Indian J Biochem Biophys 40:300–308 https://www.researchgate.net/publication/230695531_Methods_for_estimating_lipid_peroxidation_An_analysis_of_merits_and_demerits
Domozych DS, Ciancia M, Fangel JU, Mikkelsen MD, Ulvskov P, Willats WGT (2012) The cell walls of green algae: a journey through evolution and diversity. Front Plant Sci 3:1–7. https://doi.org/10.3389/fpls.2012.00082
Expósito N, Kumar V, Sierra J, Schuhmacher M, Papiol GG (2017) Performance of Raphidocelis subcapitata exposed to heavy metal mixtures. Sci Total Environ 601-602:865–873. https://doi.org/10.1016/j.scitotenv.2017.05.177
Fargašová A, Bumbálová A, Havránek E (1999) Ecotoxicological effects and uptake of metals (Cu2+, Cu+, Mn2+, Mo6+, Ni2+, V5+) in freshwater alga Scenedesmus quadricauda. Chemosphere 38:1165–1173. https://doi.org/10.1016/S0045-6535(98)00346-4
Fettweis A, Bergen B, Hansul S, De Schamphelaere K, Smolders E (2021) Correlated Ni, Cu, and Zn sensitivity of 8 freshwater algal species and consequences for low-level metal mixture effects. Environ Toxicol Chem 00:1–11. https://doi.org/10.1002/etc.5034
Gao C, De Schamphelaere KAC, Smolders E (2016) Zinc toxicity to the alga Pseudokirchneriella subcapitata decreases under phosphate limiting growth conditions. Aquat Toxicol 173:74–82. https://doi.org/10.1016/j.aquatox.2016.01.010
Geis SW, Fleming KL, Korthals ET, Searle G, Reynolds L, Karner DA (2000) Modifications to the algal growth inhibition test for use as a regulatory assay. Environ Toxicol Chem 19:36–41. https://doi.org/10.1002/etc.5620190105
Gosset A, Durrieu C, Barbe P, Bazin C, Bayard R (2019) Microalgal whole-cell biomarkers as sensitive tools for fast toxicity and pollution monitoring of urban wet weather discharges. Chemosphere 217:522–533. https://doi.org/10.1016/j.chemosphere.2018.11.03
Gunther MR, Hanna PM, Mason RP, Cohe MS (1995) Hydroxyl radical formation from cuprous ion and hydrogen peroxide: a spin-trapping study. Arch Biochem Biophys 316(1):515–522
Guo J, Peng J, Lei Y, Kanerva M, Li Q, Song J, Guo J, Sun H (2020) Comparison of oxidative stress induced by clarithromycin in two freshwater microalgae Raphidocelis subcapitata and Chlorella vulgaris. Aquat Toxicol 219:105376. https://doi.org/10.1016/j.aquatox.2019.105376
Hayat S, Hayat Q, Alyemen MN, Wani AS, Pichtel J, Ahmad A (2012) Role of proline under changing environments. Plant Signal Behav 7:1456–1466. https://doi.org/10.4161/psb.21949
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198. https://doi.org/10.1016/0003-9861(68)90654-1
Heijerick DG, De Schamphelaere KAC, Janssen CR (2002) Biotic ligand model development predicting Zn toxicity to the alga Pseudokirchneriella subcapitata: possibilities and limitations. Comp Biochem Physiol C 133:207–218
Ikegami I, Nemoto A, Sakashita K (2005) The formation of Zn-Chl a in Chlorella heterotrophically grown in the dark with an excessive amount of Zn2+. Plant Cell Physiol 46:729–735. https://doi.org/10.1093/pcp/pci079
Karuppanapandian T, Moon J-C, Kim C, Manoharan K, Kim W (2011) Reactive oxygen species in plants: their generation, signal transduction, and scavenging mechanisms. Aust J Crop Sci 5:709–725 http://www.cropj.com/kim_5_6_2011_709_725.pdf
Kondzior P, Butarewicz A (2018) Effect of heavy metals (Cu and Zn) on the content of photosynthetic pigments in the cells of algae Chlorella vulgaris. J Ecol Eng 19(3):18–28. https://doi.org/10.12911/22998993/85375
Küpper H, Küpper F, Spiller M (1996) Environmental relevance of heavy metal-substituted chlorophylls using the example of water plants. J Exp Bot 47:259–266. https://doi.org/10.1093/jxb/47.2.259
Le Faucheur S, Schildknecht F, Behra R, Sigg L (2006) Thiols in Scenedesmus vacuolatus upon exposure to metals and metalloids. Aquat Toxicol 80:355–361. https://doi.org/10.1016/j.aquatox.2006.10.002
Lichtenthaler HK, Wellburn AR (1983) Determination of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans 603:591–592. https://doi.org/10.1042/bst0110591
Lin Y, Abraham J, RoyChowdhury A, Su T-L, Braida W, Christodoulatos C (2020) Ecotoxicological response of Scenedesmus obliquus to pure energetic compounds and metal ions found in wastewater streams from munitions manufacturing. Algal Res 48:101927. https://doi.org/10.1016/j.algal.2020.101927
Liu J, Chakraborty S, Hosseinzadeh P, Yu Y, Tian S, Petrik I, Bhagi A, Lu Y (2014) Metalloproteins containing cytochrome, iron−sulfur, or copper redox centers. Chem Rev 114:4366–4469. https://doi.org/10.1021/cr400479b
Lukavský J, Furnadjieva S, Cepák V (2003) Toxicity of metals, Al, Cd, Co, Cr, Cu, Fe, Ni, Pb and Zn on microalgae, using microplate bioassay 1: Chlorella kessleri, Scenedesmus quadricauda, Scenedesmus subspicatus and Raphidocelis subcapitata (Selenastrum capricornutum). Int J Psychol Res 110:127–141. https://doi.org/10.1021/cr400479b
Magdaleno A, De Cabo L, Arreghini S, Salinas S (2014) Assessment of heavy metal contamination and water quality in an urban river from Argentina. Braz J Aquat Sci Technol 18:113–120 https://siaiap32.univali.br/seer/index.php/bjast/article/viewFile/4131/3441
Malik A (2004) Metal bioremediation through growing cells. Environ Int 30:261–278. https://doi.org/10.1016/j.envint.2003.08.001
Manová A, Beinrohr E, Čacho F (2017) Atomic absorption spectrometry with electrothermal atomization to determine trace amounts of arsenic. Acta Chim Slov 10(2):175–179. https://doi.org/10.1515/acs-2017-0029
McElroy JS, Kopsell DA (2009) Physiological role of carotenoids and other antioxidants in plants and application to turfgrass stress management. N Z J Crop Hortic Sci 37(4):327–333. https://doi.org/10.1080/01140671.2009.9687587
Mehta SK, Gaur JP (1999) Heavy-metal-induced proline accumulation and its role in ameliorating metal toxicity in Chlorella vulgaris. New Phytol 143:253–259. https://doi.org/10.1046/j.1469-8137.1999.00447.x
Miazek K, Iwanek W, Remacle C, Richel A, Goffin D (2015) Effect of metals, metalloids and metallic nanoparticles on microalgae growth and industrial product biosynthesis: a review. Int J Mol Sci 16:23929–23969. https://doi.org/10.3390/ijms161023929
Moore MN, Depledge MH, Readman JW, Leonard DRP (2004) An integrated biomarker-based strategy for ecotoxicological evaluation of risk in environmental management. Mutat Res 552:247–268. https://doi.org/10.1016/j.mrfmmm.2004.06.028
Mosleh YYI, Mofeed J (2014) Bio-chemical biomarkers in algae Scenedesmus obliquus exposed to heavy metals Cd, Cu and Zn. Life Sci J 11:994–1004 http://www.lifesciencesite.com/lsj/life1110/153_26973life111014_994_1004.pdf
Muyssen BTA, Brix KV, DeForest DK, Janssen CR (2004) Nickel essentiality and homeostasis in aquatic organisms. Environ Rev 12:113–131. https://doi.org/10.1139/a04-004
Nam S-H, Kwak JI, An Y-J (2018) Assessing applicability of the paper-disc method used in combination with flow cytometry to evaluate algal toxicity. Environ Pollut 234:979–987. https://doi.org/10.1016/j.envpol.2017.12.010
Nguyen-Deroche TLN, Caruso A, Le TT, Bui TV, Schoefs B, Tremblin G, Morant-Manceau A (2012) Zinc affects differently growth, photosynthesis, antioxidant enzyme activities and phytochelatin synthase expression of four marine diatoms. Sci World J 2012:1–15. https://doi.org/10.1100/2012/982957
OECD 201 (2002) OECD Guidelines for the Testing of Chemicals. Proposal for Updating Guideline 201 Freshwater Alga and Cyanobacteria, Growth Inhibition Test. 25 p. http://www.oecd.org/chemicalsafety/testing/1946914.pdf Accessed 8 April 2020
Ouyang H, Kong X-Z, He W, Qin N, He QS, Wang Y, Wang R, Xu FL (2012) Effects of five heavy metals at sub-lethal concentrations on the growth and photosynthesis of Chlorella vulgaris. Chin Sci Bull 57:3363–3370. https://doi.org/10.1007/s11434-012-5366-x
Paul D (2017) Research on heavy metal pollution of river Ganga: a review. Ann Agrar Sci 15:278–286. https://doi.org/10.1016/j.aasci.2017.04.001
Perales-Vela HV, Peña-Castro JM, Cañizares-Villanueva RO (2006) Heavy metal detoxification in eukaryotic microalgae. Chemosphere 64:1–10. https://doi.org/10.1016/j.chemosphere.2005.11.024
Pereira MJ, Resende P, Azeiteiro UM, Oliveira J, de Figueiredo DR (2005) Differences in the effects of metals on growth of two freshwater green algae (Pseudokirchneriella subcapitata (Korshikov) Hindak and Gonium pectorale Müller). Bull Environ Contam Toxicol 75:515–522. https://doi.org/10.1007/s00128-005-0782-0
Piovár J, Stavrou E, Kaduková J, Kimáková T, Bačkor M (2011) Influence of long-term exposure to copper on the lichen photobiont Trebouxia erici and the free-living algae Scenedesmus quadricauda. Plant Growth Regul 63:81–88. https://doi.org/10.1007/s10725-010-9515-4
Piršelová B, Jakabová S, Boleček P, Hegedűs O (2010) Determination of lead content in shoots of pea by method of flow-through chronopotenciometry and atomic absorption spectrometry. Zborník vedeckých prác doktorandov a mladých vedeckých pracovníkov „Mladí vedci 2010“ [Proceedings of doctoral students and young researchers “Young Scientists 2010”], 650-655. Available at: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwjL06fIvdPwAhWxzYUKHUR3C6kQFjABegQIAxAD&url=https%3A%2F%2Fconferences.ukf.sk%2Findex.php%2Fphdconf%2Fphdconf2010%2Fpaper%2Fdownload%2F121%2F81&usg=AOvVaw37C5JC5FdUl-9DMvSDAVLY.
Rodrigues S, Pinto I, Martins F, Formigo N, Antunes SC (2021) Can biochemical endpoints improve the sensitivity of the biomonitoring strategy using bioassays with standard species, for water quality evaluation? Ecotox Environ Safety 215:112151. https://doi.org/10.1016/j.ecoenv.2021.112151
Rowan KS (1989) Photosynthetic Pigments of Algae. Cambridge University Press, Cambridge, 334 p
Soto P, Gaete H, Hidalgo ME (2011) Assessment of catalase activity, lipid peroxidation, chlorophyll-a, and growth rate in the freshwater green algae Pseudokirchneriella subcapitata exposed to copper and zinc. Lat Am J Aquat Res 39:280–285 https://scielo.conicyt.cl/pdf/lajar/v39n2/art09.pdf
STN EN ISO 8692 (2012) Water quality. Fresh water algal growth inhibition test with unicellular green algae (ISO 8692:2012). https://www.iso.org/standard/54150.html
Szivák I, Behra R, Sigg L (2009) Metal-induced reactive oxygen species production in Chlamydomonas reinhardtii (Chlorophyceae). J Phycol 45:427–435. https://doi.org/10.1111/j.1529-8817.2009.00663.x
Takaichi S (2011) Carotenoids in Algae: Distributions, Biosyntheses and Functions. Mar Drugs 9:1101–1118. https://doi.org/10.3390/md9061101
Tan K, Zhang B, Zhang H, Ma H, Li S (2020) Enzymes and non-enzymatic antioxidants responses to sequential cold stress in polymorphic noble scallop Chlamys nobilis with different total carotenoids content. Fish Shellfish Immunol 97:617–623. https://doi.org/10.1016/j.fsi.2019.12.063
Tkáčová J, Horváth M, Kassai A, Horáková S (2012) Stanovenie kadmia a olova metódou prietokovej rozpúšťacej chronopotenciometrie vo vodách určených na ľudskú spotrebu. [Determination of cadmium and lead by flow-soluble chronopotentiometry method in waters intended for human]. Proceedings of the conference. Hydrochémia 2012:145–156 (in Slovak language). Available at: http://www.vuvh.sk/download/kniznica/zborniky/zb_hydrochemia12/17.pdf
Torres MA, Barros MP, Campos SCG, Pinto E, Rajamani S, Sayre RT, Colepicolo P (2008) Biochemical biomarkers in algae and marine pollution: A review. Ecotoxicol Environ Saf 71:1–15. https://doi.org/10.1016/j.ecoenv.2008.05.009
Tsuji N, Hirayanagi N, Iwabe O, Namba T, Tagawa M, Miyamoto S, Miyasaka H, Takagi M, Hirata K, Miyamoto K (2003) Regulation of phytochelatin synthesis by zinc and cadmium in marine green alga, Dunaliella tertiolecta. Phytochemistry 62:453–459. https://doi.org/10.1016/S0031-9422(02)00559-9
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Heavy metal toxicity and the environment. In: Luch A (ed) Molecular, Clinical and Environmental Toxicology. Experientia Supplementum, vol 101. Springer, Basel, pp 133–164. https://doi.org/10.1007/978-3-7643-8340-4_6
U.S. EPA (1996) Ecological Effects Test Guidelines OPPTS 850.5400 Algal Toxicity, Tiers I and II. United States Environmental Protection Agency. Prevention, Pesticides and Toxic Substances (7101) EPA712-C-96-164. April 1996.
Üstün GE (2009) Occurrence and removal of metals in urban wastewater treatment plants. J Hazard Mater 172:833–838. https://doi.org/10.1016/j.jhazmat.2009.07.073
Viner RI, Krainev AG, Williams TD, Schoneich C, Bigelow DJ (1997) Identification of oxidation sensitive peptides within the cytoplasmic domain of the sarcoplasmic reticulum Ca2+-ATPase. Biochemistry 36:7706–7716. https://doi.org/10.1021/bi970058z
Wong PK, Chang L (1991) Effects pf copper, chromium and nickel on growth, photosynthesis and chlorophyll a synthesis of Chlorella pyrenoidosa 251. Environ Pollut 72:127–139. https://doi.org/10.1016/0269-7491(91)90063-3
Zeraatkar AK, Ahmadzadeh H, Talebi AF, Moheimani NR, McHenry MP (2016) Potential use of algae for heavy metal bioremediation: a critical review. J Environ Manag 181:817–831. https://doi.org/10.1016/j.jenvman.2016.06.059
Acknowledgements
We thank Canadian lector Dr. Mike Sabo from the Department of Languages at the Faculty of Natural Sciences, Comenius University in Bratislava for the English language correction.
Availability of data and materials
All data are available at the workplace where the work was performed, and the material is commercially available.
Code availability
Commercially available software or software for the respective devices was used to process the results.
Funding
This study was supported by the Scientific Grant Agency of Ministry of Education, Science, Research and Sport of the Slovak Republic (VEGA) 1/0332/18.
Author information
Authors and Affiliations
Contributions
Alexandra Filová: investigation and writing–original draft. Agáta Fargašová: supervision, review and editing of the original draft, funding acquisition, and conceptualization. Marianna Molnárová: metal determination-partially review and editing of the original draft, funding acquisition, and conceptualization.
Corresponding author
Ethics declarations
Ethics approval
No animals or parts of animals were used in the tests.
Consent to participate
All authors agree to participate in the article.
Consent for publication
The institution in which the work was produced agrees with its publication.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Ludek Blaha
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Filová, A., Fargašová, A. & Molnárová, M. Cu, Ni, and Zn effects on basic physiological and stress parameters of Raphidocelis subcapitata algae. Environ Sci Pollut Res 28, 58426–58441 (2021). https://doi.org/10.1007/s11356-021-14778-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-14778-6