Abstract
This study investigated the combined effects of seawater acidification and hypoxia on the antioxidant response in gonads of the thick shell mussel Mytilus coruscus. Mussels were collected along the Shengsi Island, East China Sea, where oxygen and pH fluctuations frequently occur in summer. Mussels were exposed to three pH (8.1, 7.7, and 7.3) and two dissolved oxygen (DO) levels (6 and 2 mg L−1) for 21 days followed by a 10-day recovery period (pH 8.1 and DO 6 mg L-1). Gonad surface area (GSA) and activity of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX), glutathione (GSH), glutathione S-transferase (GST), and malondialdehyde (MDA) in gonad were measured at days 21 and 31. Complex and enzyme-specific responses were observed after the 21-day exposure period. Overall, PCA analysis revealed a stronger effect of pH than DO. Integrated biomarker response (IBR) analysis demonstrated that low pH and DO decreased mussel’s antioxidant system and increased oxidative damage with potential consequences for gonad development. Mussels exposed to low pH and DO were only partly able to recover a normal enzymatic activity after 10-day recovery period. This suggests that mussels exposed to short-term pH and DO fluctuations event in the field may suffer lasting negative impacts.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ocean acidification (OA) refers to the perturbation of the seawater carbonate chemistry when excess carbon dioxide from the atmosphere is absorbed by the ocean (Birchenough et al. 2015; Orr et al. 2005; Feely et al. 2004). The atmospheric CO2 concentration has increased from 280 to 400 ppm since the beginning of the industrial revolution. Projections suggest that it may reach up to 1200 ppm by the end of this century leading to an average 0.4 pH units decrease in surface ocean (Caldeira and Wickett 2003). A large body of evidence is showing that these changes have the potential to cause dramatic consequences on marine organisms and ecosystem (Noor and Das 2019; Jin et al. 2020). Among marine animals, bivalves are particularly sensitive to OA (Tan and Zheng 2020) with impacts on survival, calcification, growth, and reproduction. Exposure to OA leads to increased energy costs to maintain acid-base regulation and compensate for calcium carbonate dissolution (Anthony et al. 2008; Doney et al. 2009; Nakamura et al. 2011; Orr et al. 2005).
Hypoxia, a decrease in oxygen concentration, is another global driver in coastal waters (Deutsch et al. 2011; Zhang et al. 2010; Schmidtko et al. 2017; Breitburg et al. 2018), which is mainly caused by global warming and eutrophication (Breitburg et al. 2018). Over 400 hypoxic zones were documented in the coastal zone since 2008 for a total of 245000 km2 area. For example, hypoxic zones have been observed in the East China Sea since the 1950s (Diaz and Rosenberg 2008; Li et al. 2011; Chen et al. 2020). Large amount of nutrients released from the Yangtze River increased the primary productivity in the East China Sea, which consequently leads to eutrophication and hypoxia. These hypoxic zones could be greater than 15000 km2 (Chen et al. 2020).
Previous studies on mussels have showed that expression of oxidative stress-related genes and activity of antioxidant enzyme activities were affected by hypoxia (e.g. Woo et al. 2013; Sui et al. 2017; Nogueira et al. 2017, Huang et al. 2018a, 2018b). These are linked to increased production of cellular reactive oxygen species (ROS) (e.g. Ekau et al. 2010; Levin et al. 2009), and a potential risk of oxidative damage (Hermes-Lima et al. 2015).
Exposure to OA and hypoxia can inhibit gonadal development in marine invertebrates (Parker et al. 2018; Mos et al. 2016; Kurihara et al. 2013; Uthicke et al. 2014; Aguirre-Velarde et al. 2019), as a consequence of shifts in energy budget (Sui et al. 2016), subsequently affecting reproduction and population dynamics. However, the combined effects of hypoxia and acidification are still poorly understood. Marine mussels M. coruscus were collected from the Shengsi Island, East China Sea, where they regularly experience low levels of dissolved oxygen and pH during the wet season (Sui et al. 2017). To better understand the combined effect of OA and hypoxia, they were exposed to decreased pH and DO using a full factorial design. We hypothesized that OA and hypoxia will negatively impact their gonad antioxidant responses and their reproduction.
Materials and methods
Animals
Thick shell mussels M. coruscus (80 ± 10 mm shell length, 160 ± 20 mg dry tissue weight) were sampled from the Shengsi island, East China Sea (30° 33′ 00.945″ N, 121° 49′ 59.757″ E) and immediately transferred to the laboratory. Undamaged mussels were selected and acclimated for 2 weeks in 500L aquaria containing aerated seawater with salinity (25 psu), temperature (25 °C), oxygen concentration (6 mg L−1), and pH value (8.1) The light regime was 12 h light/12h dark and mussels were fed with the microalgae Chlorella spp. every 12 h (25,000 cells mL−1).
Experimental design
Mussels were exposed to pH (three levels: 8.1, 7.7, and 7.3) and DO (two levels: 6 and 2 mg L−1). Each treatment had three replicates (tanks) containing 30 mussels. Target pH 8.1 was selected as the present average at the sampling site; pH 7.7 corresponds to the extreme present variability and the extreme pH projected for the year 2100 and the minimum of present natural variability at the sampling site (Li et al. 2014), and pH 7.3 is the expected pH by 2300 (Caldeira and Wickett 2005). DO 6 mg L−1 is within the present range of variability at the sampling site while 2 mg L−1 corresponds to extreme minimum of documented DO in the East China Sea (2–3 mg L−1 in summer; Chen et al. 2007a, 2007b). pH was maintained by bubbling pure CO2. The flow rate was controlled by pH feedback STAT systems (DAQ-M) operated by the CapCTRL software (Loligo Systems Inc, Tjele, Denmark). DO was maintained by injecting N2 and air via an O2 regulator (Loligo Systems Aps, Tjele, Denmark). Daily, salinity was measured daily using a multiparameter instrument (model 5200A, YSI, USA) and total alkalinity (AT) was determined by titration with methyl red-methylene blue. pCO2, saturation states of calcite (Ωca), aragonite (Ωar), and dissolved inorganic carbon (DIC) were calculated using the CO2SYS software. A summary of the carbonate chemistry is presented in Table 1. Mussels were maintained for 21 days under the treatment conditions and then transferred back to the same condition than during acclimation (salinity 25 psu, temperature 25 °C, DO 6 mg L−1, and pH 8.1) for a 10-day recovery period.
Tissue collection and preparation
Gonads were collected at days 21 and 31. As female accounted for more than 80% of the mussel population used for the experiment, only females were used for analyses. For each treatment, six females were obtained and then dissected. Gonads were carefully excised, surface dried with tissue paper, thoroughly washed with phosphate buffer (50 mM; pH 7.4), and kept on ice. The gonads were photographed and the surface area (GSA in mm2) was measured using the software ImageJ. Aliquots from each tissue pool were immediately frozen in liquid nitrogen and stored at −80 °C for further analysis. The gonads were thawed on ice and homogenized (1:4, w-v) in 0.1 M Tris-HCl buffer (pH 7.5) containing 0.15 M KCl, 0.5 M sucrose, 1 mM EDTA, 1 mM Dithiothreitol (DTT, Sigma), and 40 μg mL-1 Aprotinin (Sigma). Homogenization was performed at 4 °C using 12–15 strokes of a motor driven Teflon Potter-Elvehjem homogenizer. Homogenized samples were sonicated for 2 min at 0 °C with a Braun Labsonic U sonifier at 50% duty cycles and then centrifuged at 12000g for 45 min at 4 °C. Supernatants were collected for biochemical analysis.
Antioxidant assays
Commercial kits from the Nanjing Jiancheng Bioengineering Institute (Nanjing, China) were used for antioxidant assays. Optical density values were measured using a microplate reader (Flexstation® 3, Molecular Devices, California, USA). The protein content of enzyme crude extract was determined using Coomassie Brilliant Blue (G-250) method (Bradford 1976). The protein content was used to normalize enzyme activities.
Superoxide dismutase assay
The nitro blue tetrazolium (NBT) method was used to measure superoxide dismutase (SOD) activity according to Sun et al. (1988). NBT was reduced to blue forms by O2- generated by the xanthine/xanthine oxidase system, which has a strong absorbance at 560 nm. One unit (U) of SOD is defined as the amount that inhibits the rate of NBT reduction by 50%.
Catalase assay
Catalase (CAT) activity was measured using a spectrophotometric assay of hydrogen peroxide based on the formation of its stable complex with ammonium molybdate at 405 nm (Góth 1991). In brief, a H2O2 degradation reaction catalyzed by CAT was terminated by adding ammonium molybdate, and the intensity of a yellow complex formed by molybdate and H2O2 at 405 nm was measured. One U of CAT activity is defined as the degradation of 1 μmol H2O2 per second per mg of protein.
Glutathione assay
GSH level was measured according to the method of Ringwood et al. (1999) by reading the optical density of the yellow substance formed when 5,5′-dithio-2-nitrobenzoic acid was reduced by glutathione (GSH) at 412 nm. GSH content in the extract was determined as nmol mg-1 protein by a standard curve generated with GSH at diverse concentrations.
Glutathione peroxidase assay
Activity of glutathione peroxidase (GPX) was measured by quantifying the rate of oxidating reduced glutathione (GSH) to oxidized glutathione (GSSG) induced by H2O2 (Xia and Zhou 1987). The method for measuring the amount of GSH is described above. One U of GPX is defined as the amount that reduces the level of GSH by 1μmol L-1 in 1 min per mg of protein.
Malondialdehyde assay
Malondialdehyde (MDA) was assessed by measuring the thiobarbituric acid reactive substances (TBARS). MDA in the lipid peroxide degradation product can be condensed with thiobarbituric acid (ZBA) to form a red product with a maximum absorption peak at 532nm. The TBARS formed was measured in a microplate reader at 532 nm and quantified as malondialdehyde equivalents using1,1,3,3-tetramethoxypropane as the standard (Ohkawa et al. 1979). MDA content in the extract is expressed as nmol TBARS mg-1 protein.
Glutathione S-transferase assay
The glutathione S-transferase (GST) activity was determined according to Habig et al. (1974), using 2 mM 1-chloro-2,4-dinitrobenzene (CDNB) and 2 mM reduced glutathione in 0.1 M potassium phosphate buffer, pH 7.0. The absorbance was monitored for 2 min at 30 °C at 340 nm for a better detection in the microplate reader. One U of GST activity is the amount which catalyzes the conjugation of 1mM of substrate per minute.
Integrative biomarker index
Six biomarkers (SOD, CAT, GPX, MDA, GST, GSH) were integrated in a IBR index according to Beliaef and Burgeot (2002) as: (1) calculation of mean and standard deviation for each biomarker; (2) standardization for each sample: Y = (X − m)/s, where Y = standardized biomarker response, X = response value of each biomarker, m = mean value of the biomarker, and s= standard deviation of the biomarker; (3) Z was then calculated as Z = Y or Z = −Y for biomarker induced or inhibited; (4) score for the biomarker was computed as S = Z + |min|, where S≥0 and |min|= absolute minimum value of Y for each biomarker; (5) scores were visualized in a star plot and correspond to radial coordinates. IBR was finally calculated as:
where α = angle formed by each two consecutive scores, and n = the number of biomarkers.
Statistical analysis
All statistical analyses were performed using SPSS 18.0. Shapiro-Wilk test was used to test the normality of the data, and Levene’s test was used to test the homogeneity of the variance prior to statistical analysis. Effects of pH, hypoxia, and their interactions on SOD, CAT, GPX, GSH, GST MDA, and GSA were analyzed by two-way analysis of variance (ANOVA). When relevant, one-way ANOVA, Tukey’s HSD post hoc test, and Student’s t test were used to resolve the effect of individual parameters. Principal component analysis (PCA) was conducted using Origin 2018. Results were expressed as mean ± SEM and a threshold of p <0.05 for significant difference.
Results
Gonad surface area
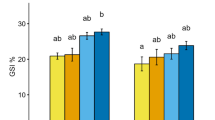
pH, DO, and their interactions had a significant effect on gonad surface area (GSA) after the 21-day exposure period (Table 2) while only pH and the interaction between pH and DO had significant effects after the 10-day recovery period (Table 3). Overall, exposure to low pH led to a decrease in GSA. Low DO significantly decreased GSA under pH 8.1 after exposure and under pH 7.3 after recovery (Fig. 1).
Gonad surface area (GSA) of M. coruscus exposed to different DO concentrations (6 and 2 mg L-1) and pH levels (8.1, 7.7, and 7.3) after a 21-day exposure period and after a 10-day recovery period. Letters indicate significant effects (p<0.05; post hoc test) between pH treatments within DO level and asterisks significant effects (p<0.05; post hoc test) between DO treatments within pH level
Antioxidant assays
After the exposure period, SOD activity was significantly impacted by DO (Table 2) leading to an increased activity under low DO (Fig. 2). No significant effect was observed for SOD activity after the recovery period (Table 3).
After the 21-day exposure period, pH and DO had a significant effect on CAT activity, but not their interaction (Table 2). Both low pH and low DO led to a decrease in CAT activity (Fig. 3). However, no significant effect was observed after the 10-day recovery period (Table 3).
GPX activity was significantly affected by pH, DO, and their interaction both after the exposure (Table 2) and recovery periods (Table 3). When significant, pH and DO led to an increased GPX activity. After the exposure period, at DO 6 mg L-1, GPX activity was significantly increased at pH 7.7 compared to the other two pHs. At DO 2 mg L-1, GPX activity increased with decreasing pH (Fig. 2.). Exposure to low DO significantly increased GPX activity at pH 8.1 and 7.3 but not at pH 7.7. After recovery, low DO significantly increased GPX activity under pH 7.7 and 7.3 (Fig. 4).
Glutathione peroxidase (GPX) activity in the gonad of M. coruscus exposed to different DO concentrations (6 and 2 mg L-1) and pH levels (8.1, 7.7, and 7.3) after a 21-day exposure period and after a 10-day recovery period. Letters indicate significant effects (p<0.05; post hoc test) between pH treatments within DO level and asterisks significant effects (p<0.05; post hoc test) between DO treatments within pH level
After the exposure period, both pH and DO had a significant effect on GSH activity but not their interaction (Table 2). Exposure to low pH and DO led to an increased GSH activity (Fig. 5). The tested parameters had no significant effect on GSH activity after a ten-day recovery (Table 3).
After the exposure period, pH, DO, and their interaction had a significant effect on GST activity (Table 2). When exposed to DO 6 mg L-1, GST activity decreased with decreasing pH. Under DO 2 mg L-1; GST activity was significantly lower at pH 7.3 compared to the other two tested pHs. Exposure to low DO only significantly impacted by GST activity at pH 8.1 (Fig. 6). After the recovery period, only pH had a significant effect on GST activity (Table 3) leading to a reduced activity at pH 7.3 compared to other two pHs (Fig. 6). The interaction between DO and pH was found significant only after exposure (Table 2).
Glutathione S-transferase (GST) activity in the gonad of M. coruscus exposed to different DO concentrations (6 and 2 mg L-1) and pH levels (8.1, 7.7, and 7.3) after a 21-day exposure period and after a 10-day recovery period. Letters indicate significant effects (p<0.05; post hoc test) between pH treatments within DO level and asterisks significant effects (p<0.05; post hoc test) between DO treatments within pH level
Only pH had a significant effect on MDA level after the 21-day exposure period (Table 2). The MDA level was significantly lower at pH 8.1 compared to the two other pHs (Fig. 7). After the recovery period, no significant differences in MDA levels were observed (Table 3).
Integrated biomarker response
Standardized biomarkers (SOD, CAT, GPX, MDA, GST, GSH) were plotted in IBR star plots (Fig. 8). Overall, the IBR values tend to decrease with decreasing pH and DO with the exception of the pH 7.3 and DO 2 mg L-1 treatment (Fig. 8).
Principal component analysis
Principal component analysis (PCA) showed that PC1 expressed 64.94% and PC2 13.08% of total variance after the 21-day exposure (Fig. 9). PC1 allowed to discriminate between pH 8.1 and decreased pHs and was mostly influenced by CAT, GST, GPX, GSH, MDA, and GSA. PC2 was mostly influenced by SOD activity (Fig. 10).
Discussion
The purpose of this study was to clarify the antioxidant response in the gonad of the marine mussel M. coruscus under ocean acidification and hypoxia and its connection with gonad development.
Exposure to environmental stressors modulates the dynamic balance between the generation and removal of reactive oxygen species (ROS). ROS can be removed by antioxidant enzymes mostly on the polyunsaturated fatty acids in the plasma membrane phospholipids. As a consequence, modification in the level of MDA, the product of lipid peroxidation, is an indicator of changes in ROS dynamics (Diguiseppi et al. 1984).
The relative contribution of each antioxidant enzyme to protect against oxidative stress is not well known. In this study, the effect of pH, DO, and their interaction was enzyme-specific after a 21-day exposure. When significant, low pH and DO led to a decrease in SOD, CAT, and GST activity and an increase in GPX and GSH activity and MDA level.
As a vital scavenger of H2O2, CAT is considered a second line of antioxidant defense (Chelikani et al. 2004). Although CAT activity often increases as a result of the increased H2O2 under oxidative stress (Hermes-Lima 2004), multiple stressors may change this trend (Matozzo et al. 2013). In our study, CAT activity was significantly reduced by exposure to low pH and DO. Woo et al. (2013) also found that CAT activity decreased when mussels Mytilus galloprovincialis were exposed to hypoxia.
GPX not only participates in the conversion of H2O2 to water and molecular oxygen but also plays a vital role in the use of GSH as a reducing agent to convert other lipids to non-toxic products (Sies et al. 1997). In our study, GPX activity was significantly increased under certain combinations of low pH and low DO. Lima et al. (2019) showed that the GPX activity in oyster Crassostrea gasar was not impacted by an exposure to a combination of low pH and phenanthrene for 96 h. Johannsson et al. (2018) found GPX activity increased in the brain and gills of Characid fish Cyphocharax abramoides during hypoxia. Although both GPX and CAT could catalyze the decomposition of H2O2, their different changes suggested that GPX is more capable of scavenging free radicals than CAT, which is consistent with previous research (Dorval et al. 2003). Besides acting as a substrate for GPX and glutathione reductase, GSH is also a major thiol compound that acts as a protective agent for a variety of toxic substances through thiol groups (Habig et al. 1974; Moreno et al. 2005). In our experiment, the GSH activity was increased by low pH and DO, showing a similar trend with GPX. Some studies have shown that OA and hypoxia or the combination with other stressors either decrease or increase GPX activity, depending on the species and specific tissue (Huang et al. 2018a, 2018b; Khan and Ringwood 2016). In addition to being an important regulator of detoxifying oxygen free radicals, glutathione has also been reported to be associated with reproductive success in oysters. Ringwood and Conners (2000) found that parental depletion of GSH may increase the susceptibility of embryos of the oyster Crassostrea virginica to metal toxicity. Hence, another explanation for the increase in GSH is to maintain the health state of the gonads.
GST plays different functions in the process of chemical detoxification, through which it greatly increases the basis of sulfhydryl reaction metabolites and reduces oxygen species (Aniya et al. 1993; Sheehan et al. 2001). In our experiment, the GST activity was significantly decreased under low pH and DO, indicating a disruption in the balance of GST, supporting similar observations by Lima et al. (2019).
MDA level is often measured in combination with antioxidant enzymes. The activity of antioxidant enzymes indirectly reflects the ability of the organism to scavenge oxygen free radicals, while the level of MDA indirectly reflects the severity of the attack of the organism cells by free radicals. In our results, MDA level was significantly increased under low pHs, suggesting oxidative damage.
At the end of the recovery period, enzyme activities were partially restored with no significant effects of pH, DO, or their interaction for SOD, CAT, GSH, and MDA. GST activity was only partially restored but was still significantly impacted by the exposure to low pH during the first 21 days. Exposure to low pH and DO had still significant effects on GPX after the recovery period. The consequences of these longer lasting impacts are undetermined.
Different enzymes have different sensitivity to environmental stressors. To better capture the overall stress response, the analysis of several enzymes and even other biomarkers can be combined. The IBR index is such a powerful tool. It has been widely used in many studies to assess stress responses and ecological risks (Cao et al. 2019; Damiens et al. 2007; Xie et al. 2016). In this study, exposure to low pH and DO led to a decrease in IBR/n with DO 2 mg L-1 and pH 7.3 showing the highest IBR/n value, reflecting this group was the most highly impacted one. Similarly, the lowest values of GSA were observed at lowest pH and DO. When exposed to stressing conditions, mussels exhibit physiological trade-offs. Under extreme environmental pressures, they may divert energy from reproduction to costly physiological defenses (Petes et al. 2008; Béguel et al. 2013). Our results suggest that the combined stress of ocean acidification and hypoxia negatively impact GSA as a consequence of such shift in energy allocation with potential long-lasting consequences on their fitness.
Conclusion
Our results suggest that exposure to acidification and hypoxia modulates mussel’s gonadal antioxidant system. The effect is enzyme-dependent and when significant effects are observed, acidification and/or hypoxia decreased the activity of CAT and GST, but increased GPX, GSH activity and MDA level. pH had a stronger effect on the antioxidant response than DO. These changes in the antioxidant system are associated with a decrease of GSA. A more specific link between the antioxidant system and gonad development is worth exploring in the future. A partial recovery of the antioxidant system of mussels exposed to low pH and DO was observed after a 10-day recovery but without positive consequences for GSA. A full recovery may take longer time and the short and long-term consequences of these changes need to be further investigated.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Aguirre-Velarde A, Thouzeau G, Jean F, Mendo J, Cueto-Vega R, Kawazo-Delgado M, Vásquez-Spencer J, Herrera-Sanchez D, Vega-Espinoza A, Flye-Sainte-Marie J (2019) Chronic and severe hypoxic conditions in Paracas Bay, Pisco, Peru: consequences on scallop growth, reproduction, and survival. Aquaculture 512:734259
Aniya Y, Shimoji M, Naito A (1993) Increase in liver microsomal glutathione s-transferase activity by phenobarbital treatment of rats: possible involvement of oxidative activation via cytochrome p450. Biochem Pharmacol 46(10):1741–1747
Anthony KR, Kline DI, Diaz-Pulido G, Dove S, Hoegh-Guldberg O (2008) Ocean acidification causes bleaching and productivity loss in coral reef builders. Proc Natl Acad Sci U S A 105(45):17442–17446
Béguel JP, Huvet A, Quillien V, Lambert C, Fabioux C (2013) Study of the antioxidant capacity in gills of the pacific oyster Crassostrea gigas in link with its reproductive investment. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 157(1):63–71
Beliaef B, Burgeot T (2002) Integrated biomarker response: a useful tool for ecological risk assessment. Environ Toxicol Chem 21(6):1316–1322
Birchenough SN, Reiss H, Degraer S, Mieszkowska N, Borja Á, Buhl-Mortensen L, Braeckman U, Craeymeersch J, Mesel ID, Kerckhof F, Kröncke I, Parra S, Rabaut M, Schröder A, Colen CV, Hoey GV, Vincx M, Wätjen K (2015) Climate change and marine benthos: a review of existing research and future directions in the North Atlantic. Wiley Interdiscip Rev Clim Chang 6(2):203–223
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1-2):248–254
Breitburg D, Levin LA, Oschlies A, Grégoire M, Chavez FP, Conley DJ, Jacinto GS (2018) Declining oxygen in the global ocean and coastal waters. Science 359(6371):eaam7240
Caldeira K, Wickett ME (2003) Anthropogenic carbon and ocean pH. Nature 425(6956):365–365
Caldeira K, Wickett ME (2005) Ocean model predictions of chemistry changes from carbon dioxide emissions to the atmosphere and ocean. J Geophys Res Oceans 110(C9):C09S04
Cao R, Zhang T, Li X, Zhao Y, Wang Q, Yang DL, Qu Y, Liu H, Dong ZJ, Zhao JM (2019) Seawater acidification increases copper toxicity: a multi-biomarker approach with a key marine invertebrate, the Pacific Oyster Crassostrea gigas. Aquat Toxicol 210:167–178
Chelikani P, Fita I, Loewen PC (2004) Diversity of structures and properties among catalases. Cell Mol Life Sci 61(2):192–208
Chen CC, Gong GC, Shiah FK (2007a) Hypoxia in the east china sea: one of the largest coastal low-oxygen areas in the world. Mar Environ Res 64(4):399–408
Chen JH, Mai KS, Ma HM, Wang XJ, Deng D, Liu XW, Xu W, Liufu ZG, Zhang WB, Tan BP, Ai QH (2007b) Effects of dissolved oxygen on survival and immune responses of scallop (Chlamys farreri jones et preston). Fish & Shellfish Immunology 22(3):272–281
Chen CC, Gong GC, Chou WC, Shiah FK (2020) Hypoxia in autumn of the East China Sea. Mar Pollut Bull 152:110875
Damiens G, Gnassia-Barelli M, Loquès F, Roméo M, Salbert V (2007) Integrated biomarker response index as a useful tool for environmental assessment evaluated using transplanted mussels. Chemosphere 66(3):574–583
Deutsch C, Brix H, Ito T, Frenzel H, Thompson L (2011) Climate-forced variability of ocean hypoxia. Science 333(6040):336–339
Diaz RJ, Rosenberg R (2008) Spreading dead zones and consequences for marine ecosystems. Science 321(5891):926–929
Diguiseppi J, Fridovich I, McCord JM (1984) The toxicology of molecular oxygen. CRC Crit Rev Toxicol 12(4):315–342
Doney SC, Fabry VJ, Feely RA, Kleypas JA (2009) Ocean acidification: the other CO2 problem. Annu Rev Mar Sci 1:169–192
Dorval J, Leblond VS, Hontela A (2003) Oxidative stress and loss of cortisol secretion in adrenocortical cells of rainbow trout (Oncorhynchus mykiss) exposed in vitro to endosulfan, an organochlorine pesticide. Aquat Toxicol 63(3):229–241
Ekau W, Auel H, Pörtner HO, Gilbert D (2010) Impacts of hypoxia on the structure and processes in pelagic communities (zooplankton, macro-invertebrates and fish). Biogeosciences 7(5):1669–1699
Feely RA, Sabine CL, Lee K, Berelson W, Kleypas J, Fabry VJ, Millero FJ (2004) Impact of anthropogenic CO2 on the CaCO3 system in the oceans. Science 305(5682):362–366
Góth L (1991) A simple method for determination of serum catalase activity and revision of reference range. Clin Chim Acta 196(2-3):143–151
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferase, the first enzymatic step in mercapturic acid formation. J Biol Chem 249(22):7130–7139
Hermes-Lima M (2004) Oxygen in biology and biochemistry: role of free radicals. Functional Metabolism, Regulation and Adaptation, pp 319–366
Hermes-Lima M, Moreira DC, Rivera-Ingraham GA, Giraud-Billoud M, Genaro-Mattos TC, Campos ÉG (2015) Preparation for oxidative stress under hypoxia and metabolic depression: revisiting the proposal two decades later. Free Radic Biol Med 89:1122–1143
Huang XZ, Liu YM, Liu Z, Zhao ZK, Dupont S, Wu FL, Huang W, Chen JF, Hu MH, Lu WQ, Wang YJ (2018a) Impact of zinc oxide nanoparticles and ocean acidification on antioxidant responses of Mytilus coruscus. Chemosphere 196:182–195
Huang XZ, Liu ZK, Xie Z, Dupont S, Huang W, Wu FL, Kong H, Liu LP, Sui YM, Lin DH, Lu WQ, Hu MH, Wang YJ (2018b) Oxidative stress induced by titanium dioxide nanoparticles increases under seawater acidification in the thick shell mussel Mytilus coruscus. Mar Environ Res 137:49–59
Jin P, Hutchins DA, Gao K (2020) The impacts of ocean acidification on marine food quality and its potential food chain consequences. Front Mar Sci 7:543979
Johannsson OE, Giacomin M, Sadauskas-Henrique H, Campos DF, Braz-Mota S, Heinrichs-Caldas WD, Baptista R, Wood CM, Almeida-Val VMF, Val AL (2018) Does hypoxia or different rates of re-oxygenation after hypoxia induce an oxidative stress response in Cyphocharax abramoides (Kner 1858), a Characid fish of the Rio Negro? Comp Biochem Physiol A Mol Integr Physiol 224:53–67
Khan B, Ringwood AH (2016) Cellular biomarker responses to hypoxia in eastern oysters and Atlantic ribbed marsh mussels. Mar Ecol Prog Ser 546:123–133
Kurihara H, Yin R, Nishihara GN, Soyano K, Ishimatsu A (2013) Effect of ocean acidification on growth, gonad development and physiology of the sea urchin Hemicentrotus pulcherrimus. Aquat Biol 18(3):281–292
Levin LA, Ekau W, Gooday AJ, Jorissen F, Middelburg JJ, Naqvi SWA, Neira C, Rabalais NN, Zhang J (2009) Effects of natural and human-induced hypoxia on coastal benthos. Biogeosciences 6(10):2063–2098
Li X, Bianchi TS, Yang Z, Osterman LE, Allison MA, DiMarco SF, Yang G (2011) Historical trends of hypoxia in Changjiang River estuary: applications of chemical biomarkers and microfossils. J Mar Syst 86(3-4):57–68
Li HM, Shi XY, Chen P, Zhang CS (2014) Effects of pH and DO on the migration and transformation of phosphate in the process of mixing in the Changjiang Estuary. Mar Environ Sci 33:497–502
Lima D, Mattos JJ, Piazza RS, Righetti BPH, Monteiro JS, Grott SC, Bebianno MJ (2019) Stress responses in Crassostrea gasar exposed to combined effects of acute pH changes and phenanthrene. Sci Total Environ 678:585–593
Matozzo V, Chinellato A, Munari M, Bressan M, Marin MG (2013) Can the combination of decreased pH and increased temperature values induce oxidative stress in the clam Chamelea gallina and the mussel Mytilus galloprovincialis? Mar Pollut Bull 72(1):34–40
Moreno I, Pichardo S, Jos A, Gómez-Amores L, Mate A, Vazquez CM, Camean AM (2005) Antioxidant enzyme activity and lipid peroxidation in liver and kidney of rats exposed to microcystin-LR administered intraperitoneally. Toxicon 45(4):395–402
Mos B, Byrne M, Dworjanyn SA (2016) Biogenic acidification reduces sea urchin gonad growth and increases susceptibility of aquaculture to ocean acidification. Mar Environ Res 113:39–48
Nakamura M, Ohki S, Suzuki A, Sakai K (2011) Coral larvae under ocean acidification: survival, metabolism, and metamorphosis. PLoS One 6(1):e14521
Nogueira L, Mello DF, Trevisan R, Garcia D, da Silva AD, Dafre AL, de Almeida EA (2017) Hypoxia effects on oxidative stress and immunocompetence biomarkers in the mussel Perna perna (Mytilidae, Bivalvia). Mar Environ Res 126:109–115
Noor NM, Das SK (2019) Effects of elevated carbon dioxide on marine ecosystem and associated fishes. Thalassas: An International Journal of Marine Sciences 35(2):421–429
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Orr JC, Fabry VJ, Aumont O, Bopp L, Doney SC, Feely RA, Gnanadesikan A, Gruber N, Ishida A, Joos F, Key RM, Lindsay K, Maier-Reimer E, Matear R, Monfray P, Mouchet A, Najjar RG, Plattner GK, Rodgers KB, Sabine CL, Sarmiento JL, Schlitzer R, Slater RD, Totterdell IJ, Weirig MF, Yamanaka Y, Yool A (2005) Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 437(7059):681–686
Parker LM, O'Connor WA, Byrne M, Dove M, Coleman RA, Pörtner HO, Scanes E, Virtue P, Gibbs M, Ross PM (2018) Ocean acidification but not warming alters sex determination in the Sydney rock oyster, Saccostrea glomerata. Proc R Soc B Biol Sci 285(1872):20172869
Petes LE, Menge BA, Harris AL (2008) Intertidal mussels exhibit energetic trade-offs between reproduction and stress resistance. Ecol Monogr 78(3):387–402
Ringwood AH, Conners DE (2000) The effects of glutathione depletion on reproductive success in oysters, Crassostrea virginica. Mar Environ Res 50(1-5):207–211
Ringwood AH, Conners DE, Keppler CJ, DiNovo AA (1999) Biomarker studies with juvenile oysters (Crassostrea virginica) deployed in situ. Biomarkers 4:400–414
Schmidtko S, Stramma L, Visbeck M (2017) Decline in global oceanic oxygen content during the past five decades. Nature 542(7641):335–339
Sheehan D, Meade G, Foley VM, Dowd CA (2001) Structure, function and evolution of glutathione transferases: implications for classification of non-mammalian members of an ancient enzyme superfamily. Biochem J 360(1):1–16
Sies H, Sharov VS, Klotz LO, Briviba K (1997) Glutathione peroxidase protects against peroxynitrite-mediated oxidations: a new function for selenoproteins as peroxynitrite reductase. J Biol Chem 272(44):27812–27817
Sui YM, Kong H, Huang XZ, Dupont S, Hu MH, Storch D, Portner HO, Lu WQ, Wang YJ (2016) Combined effects of short-term exposure to elevated CO2 and decreased O2 on the physiology and energy budget of the thick shell mussel Mytilus coruscus. Chemosphere 155:207–216
Sui YM, Hu MH, Shang YY, Wu FL, Huang XZ, Dupont S, Storch D, Portner HO, Li JL, Lu WQ, Wang YJ (2017) Antioxidant response of the hard shelled mussel Mytilus coruscus exposed to reduced pH and oxygen concentration. Ecotoxicol Environ Saf 137:94–102
Sun Y, Oberley LW, Li Y (1988) A simple method for clinical assay of superoxide dismutase. Clin Chem 34(3):497–500
Tan K, Zheng H (2020) Ocean acidification and adaptive bivalve farming. Sci Total Environ 701:134794
Uthicke S, Liddy M, Nguyen HD, Byrne M (2014) Interactive effects of near-future temperature increase and ocean acidification on physiology and gonad development in adult Pacific sea urchin, Echinometra sp. A. Coral Reefs 33(3):831–845
Woo S, Denis V, Won H, Shin K, Lee G, Lee TK, Yum S (2013) Expressions of oxidative stress-related genes and antioxidant enzyme activities in Mytilus galloprovincialis (Bivalvia, Mollusca) exposed to hypoxia. Zool Stud 52:15
Xia YM, Zhou LZ (1987) Detection methods of glutathione peroxidase in blood and tissue. The American Journal of Chinese Medicine 16:29–32
Xie J, Zhao Y, Wang Q, Wu HF, Teng J, Yang DL, Cao I, Chen LZ, Li F, Ji CL, Cong M, Zhao JM (2016) An integrative biomarker approach to assess the environmental stress in the north coast of Shandong Peninsula using native oysters, Crassostrea gigas. Mar Pollut Bull 112(1-2):318–326
Zhang J, Gilbert D, Gooday AJ, Levin L, Naqvi SWA, Middelburg JJ, Scranton M, Ekau W, Pena A, Dewitte B, Oguz T, Monteiro PMS, Urban E, Rabalais NN, Ittekkot V, Kemp WM, Ulloa O, Elmgren R, Escobar-Briones E, Van der Plas AK (2010) Natural and human-induced hypoxia and consequences for coastal areas: synthesis and future development. Biogeosciences 7(5):1443–1467
Acknowledgments
We are very grateful to all who participated in this study and to the reviewers.
Funding
This research was supported by funding from the research Grant (31872587) from the Natural Science Foundation of China and the Guangdong South China Sea Key Laboratory of Aquaculture for Aquatic Economic Animals, Guangdong Ocean University (KFKT2019ZD04). This work was also supported by the Open Fund of Guangxi Key Laboratoryof Aquatic Genetic Breeding and Healthy Aquaculture.
Author information
Authors and Affiliations
Contributions
JY, MH, HK, and YD conceived the study. GX, HK, XC, and HC carried out the experiment. HK, GX, and SD analyzed the data and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Animals were used following international, national, and/or institutional guidelines.
Consent for publication
The authors consent to publish.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Cinta Porte
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xu, G., Kong, H., Chang, X. et al. Gonadal antioxidant responses to seawater acidification and hypoxia in the marine mussel Mytilus coruscus. Environ Sci Pollut Res 28, 53847–53856 (2021). https://doi.org/10.1007/s11356-021-14584-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-14584-0