Abstract
Acrylamide (ACR) has been previously associated with male sexual dysfunction and infertility. Eruca sativa (L.) (arugula or rocket) have been widely used in traditional remedies in Mediterranean region and western Asia and was known for its strong aphrodisiac effect since Roman times. The current study was designed to investigate LC/MS analysis of total ethanol extract Eruca sativa (L.) and the efficiency and mechanism of action of Eruca sativa seed extract (ESS) in reducing hypogonadism induced by acrylamide in male rats. Male Wistar rats were divided into 6 groups (n = 7): control group, Eruca sativa seed extract (ESS) at doses of 100 and 200 mg\kg, acrylamide (ACR), ACR + ESS 100 mg/kg, and ACR + ESS 200 mg/kg. The animals received ACR at a dose of 10 mg/kg b.wt for 60 days. Sperm indices, testicular oxidative stress, testosterone hormone, and testicular histopathology and immunohistochemistry of PCNA and caspase-3 were investigated. Moreover, the expression level of testicular B-cell lymphoma-2 (Bcl-2) and Bcl-2-associated X protein (Bax) genes was evaluated. In respect to the LC/MS of total ethanol extract Eruca sativa (L.) seed revealed tentative identification of 39 compounds, which belongs to different classes as sulphur-containing compounds, flavonoids, phenolic acid, and fatty acids. Administration of ESS extract (100, 200 mg/kg) improved semen quality, diminished lipid peroxidation, enhanced testicular antioxidant enzyme, restored serum testosterone level, and reduced testicular degeneration and Leydig cell death in the rats intoxicated with ACR. However, the effects of ESS at the dose of 200 mg/kg were similar to that of control group. Furthermore, ESS treatment significantly induced anti-apoptotic effect indicated by elevation of both Bcl-2 and Bax expressions. Nutriceutics of ESS extract protects testis against ACR-induced testicular toxicity via normalizing testicular steroidogenesis, keeping Leydig cells, and improving oxidative stress status.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acrylamide (ACR) is one of the major environmental public health problems and a food safety concern (Al-Serwi and Ghoneim 2015). ACR was firstly discovered in human food by the Swedish National Food Agency (2002). Potentially toxic acrylamide is largely derived from heat-induced reactions, at high-temperature (>120 °C) processes such as cooking, frying, toasting, roasting, or baking in carbohydrate-rich foods as in French fries and cereals, as well as coffee and almonds (Elhelaly et al. 2019; Sansano et al. 2017). ACR was found at concentrations that may exceed 2 mg/kg in a variety of popular foods (Raju et al. 2016). Furthermore, ACR is an industrial chemical used in production of polyacrylamide and acrylamide copolymers that is widely used in water treatment, papermaking, mining, textile, lotions, cosmetics, deodorants, and as electrophoresis gels (Aras et al. 2017; Kumar et al. 2018). However, polyacrylamides are non-toxic; the ACR monomer is highly toxic.
In the last decades, the interest against the environmental chemicals that affect human health especially those which caused testicular toxicity and infertility was increased. ACR is one of those environmental chemicals that have a great bad effect on human fertility (Yang et al. 2005). ACR is considered potent neurotoxin, class B carcinogen, and genotoxin as it induces DNA damage in the germ cells (Pourentezari et al. 2014; Abdel-Daim et al. 2020; Bin-Jumah et al. 2021; Farag et al. 2021a). In addition to that, it can increase rates of thyroid gland, central nervous system, and uterus tumours in experimental laboratory animals chronically exposed to acrylamide toxicity (Kumar et al. 2018). Glycidamide is a metabolite of ACR which could be responsible for the genotoxic and carcinogenic effects of ACR because it forms DNA adducts in different tissues (Kucukler et al. 2020). Many previous studies were done to investigate the effects of oral administration of ACR on the testicular functions. They mentioned that ACR caused severe testicular damage, significant reduction infertility due to formation of abnormal sperm, and a decrease in sperm count (Christina and Daniel 2015; Kalaivani et al. 2018; Kumar et al. 2018; Pourentezari et al. 2014; Yang et al. 2005). ACR reproductive toxicity could be owed to alkylation of SH groups in the sperm nucleus and tail, depletion of antioxidant, and/or DNA damage in the testis (Yang et al. 2005; Yassa et al. 2014). Therefore, unfavourable effects of ACR could be prevented by using biological activities of natural antioxidants that may be beneficial for clinical use (Farouk et al. 2021).
Nutriceutics got a lot of attention not only for their potential therapeutic effects, but also thanks to their safety. Eruca sativa L. is an annual herbaceous plant, which belongs to family of Brassicaceae and commonly known as Arugula, rocket, rugula, roquette, white pepper, or taramira (Garg and Sharma 2014). The high content of nutraceutical compounds in Eruca sativa has been associated to wide range of pharmacological activities such as anticancer activity on pancreatic adenocarcinoma cells (Martelli et al. 2019), anti-inflammatory, neuroprotective effects (Gugliandolo et al. 2018), antibacterial (Doulgeraki et al. 2017), antiulcer effect (Alqasoumi et al. 2009; S Jaafar and S Jaafar 2019), antidiabetic (Peter and EP 2016), hepatoprotective (Mashi 2017; Salman et al. 2010), antihyperlipidemic, antihypertensive (Seham et al. 2015), antioxidant activity (Maia et al. 2015), nephroprotective (Elgazar and AO 2013), and antiplatelet (Alarcón et al. 2014). In addition to aphrodisiac activity, ethanolic extract of E. sativa was reported to enhance androgen production and spermatogenesis (Ansari and Ganaie 2014). ESS oil showed a protective effect against nicotine-induced testicular damage regarding all morphometric and histological indices (Hussein 2013).
The current study was designed to evaluate the protective effect of ESS hydroethanolic extract administration on hypogonadism, Leydig cells apoptosis, and oxidative stress induced by acrylamide in rats’ testes and to identify some possible underlying pathways of its actions.
Materials and method
ESS seeds extract
The ESS seeds were purchased from a local market of medicinal plants (Haraz, Cairo, Egypt) and were authenticated at the herbarium of Botany Department, Faculty of science, Cairo University, Giza, Egypt. The extraction procedure was guided by Farag et al. (2021a) with few modifications. Five hundred grammes of powdered seeds was blended with 70% ethanol in a sealed glass vessel. Then the mixture was allowed to set for 72 h, in the dark at room temperature, and then was filtrated. After completion of maceration (3 times), the solvent was evaporated under vacuum using a rotary evaporator (Heidolph, 60 rpm Laborta 4000) at temperature below 50°C and RPM 60 to yield a viscous extract (Gulfraz et al. 2011). The obtained extract was stored at −20°C until used. The yielded extract weight was 73.5 g (extraction yield: 29.4% w/w). For preparation of doses, the extract was freshly dissolved in distilled water using 2% tween 80.
LC/MS of ESS extract
ESI-MS positive and negative ion acquisition mode was carried out on a XEVO TQD triple quadruple instrument: Waters Corporation, Milford, MA01757 USA, mass spectrometer; column : ACQUITY UPLC - BEH C18 1.7 μm - 2.1 × 50 mm column; flow rate: 0.2 mL\min; and solvent system: consisted of (A) water containing 0.1% formic acid and (B) methanol containing 0.1% formic acid. The sample (100 μg/mL) solution was prepared using high performance liquid chromatography (HPLC) analytical grade solvent of/MeOH, filtered using a membrane discfilter (0.2 μm), and then subjected to LC-ESI-MS analysis. Sample’s injection volumes (10 μL) were injected into the UPLC instrument equipped with reverse phase C-18 column (ACQUITY UPLC - BEH C18 1.7 μm particle size- 2.1 × 50 mm column). Sample mobile phase was prepared by filtering using 0.2 μm filter membrane disc and degassed by sonication before injection. Mobile phase elution was made with the flow rate of 0.2 mL/min using gradient mobile phase comprising two eluents: eluent A is H2O acidified with 0.1% formic acid, and eluent B is MeOH acidified with 0.1% formic acid. Elution was performed using the above gradient. The parameters for analysis were carried out using negative ion mode as follows: source temperature 150 °C, cone voltage 30 eV, capillary voltage 3 kV, desolvation temperature 440 °C, cone gas flow 50 L/h, and desolvation gas flow 900 L/h. Mass spectra were detected in the ESI between m/z 100 and 1000. The peaks and spectra were processed using the Maslynx 4.1 software and tentatively identified by comparing its retention time (Rt) and mass spectrum with reported data.
Chemicals and reagents
All chemicals used in the study were of analytical or HPLC grade. Acrylamide CAS number 79-06-1. Kits for measuring oxidative stress parameters, MDA (MD 25 29), GSH (GR 25 11), and SOD (SD 25 21), were purchased from Biodiagnostic Co. (Dokki, Giza, Egypt), in addition to testosterone ELISA kit (Abcam 108666, Cambridge, UK).
Animals
A total number of 42 male Wistar albino rats strain of Rattus norvegicus domestica weighing 190–220 g, 6 weeks of age, were obtained from the Animal House Colony at Vacsera, Egypt. This study protocol was revised and approved by Institutional Animal Care and Use Committee (IACUC), Cairo University (CU-II-F-83-18). Rats were kept at a constant temperature of 25 ± 1 °C, with a 12 h light/dark cycle with food and water ad libitum throughout the experimental period.
Experimental design
After 1 week of acclimatation, rats were randomly assigned to 6 groups. Seven rats were allocated per group. All treatments (ACR, ESS, and distilled water) were administrated orally by oral gavage. The first group was normal control (control): received 1 mL 2% tween 80 in distilled water. The second and third were groups that received Eruca sativa seed extract (ESS) at doses of 100 mg\kg and 200 mg\kg, respectively (Nazeam et al. 2018). The fourth group was acrylamide (ACR) that received 10 mg/kg b.wt (Zhu et al. 2010). The fifth and sixth groups received treatment of ESS at the former two doses concurrently with 10 mg/kg b.wt. ACR rats in all groups received the treatments daily as 1 mL/rat orally for 60 days. Twenty-four hour post last dose, all animals were anaesthetized under intraperitoneal injection with a mixture of 90 mg of ketamine/kg of b wt. and 10 mg of xylazine/kg b wt., and sperm analysis was conducted.
Sampling
Blood samples were collected retro-orbital sinuses into clean sterile serum-separating tubes, centrifuged at 3000g for 15 min, and 1 mL of serum was harvested and stored at −80°C until analysed within 1 month for testosterone concentrations. Rats were euthanized by decapitation between 09:00 AM and 11:00 AM to eliminate possible effects due to diurnal variation. Testes and epididymides were immediately removed; weighed and relative weights were calculated individually as testes or epididymides weight/body weight. Testes were allocated into duplicate, where one set was quickly stored at −80°C to be used for antioxidant assays and PCR, while the other set were kept in formalin to be used for histopathological examination and immune-histochemistry.
Sperm analysis
Sperm analysis was guided by method described by Oliveira et al. (2015). One cauda epididymis of each animal was minced with a scalpel, allowing sperm to be dispersed in 3 mmol/L of Hanks’ balanced salt solution (HBSS) at 37°C. The suspension was examined for evaluation of motility and viability followed by sperm concentration and morphology. Sperm motility was evaluated immediately by placing a drop of sperm suspension in a pre-warmed (37°C) microscope slide. The total motility was calculated as the average percentage of motile sperms (progressive plus non-progressive) in the 10 random fields under low power (×10). Sperm viability was estimated using dye exclusion staining technique (Eosin/Nigrosin) as described by Rato et al. (2013) where the red eosin stain penetrates spermatozoa with damaged cell membrane. Thus, stained spermatozoa were recognized as non-viable, whereas unstained spermatozoa were recognized as viable. Sperm count was assessed by diluting sperm in HBSS by a factor of 1:50. This solution was used to fill the two grids of a Neubauer counting chamber using a micro pipette. Sperms in the four large corner squares were counted under high power (×40) on an optical microscope. Sperm morphology was evaluated on eosin-nigrosin stained slides under oil immersion field (×10 magnification) (Kalaivani et al. 2018). Sperm cells with hook-shaped heads and no visible defects were considered normal, while those with head or tail defects were considered abnormal and were classified tail defects, deformed head, and detached head.
Assessment of testosterone hormone
ELISA procedure was used for quantitative determination of serum total testosterone concentration using competitive enzyme-linked immunosorbent assay kits following manufacturer’s instructions. The absorbance was measured at 450 nm using FilterMax F5 Multi-Mode Microplate Reader (Molecular Devices; San Jose, CA, USA), and testosterone concentrations were calculated from a calibration curve.
Assessment of testicular oxidative stress markers
Tissue homogenates were prepared from frozen testes samples in 10 volumes of 0.1 M Tris–EDTA buffer (pH 7.4) and centrifuged at 3200×g for 20 min at 4°C, and the supernatant fractions were utilized for the spectrophotometrical assessment of the levels of the following: reduced glutathione activity (GSH), with method based on the reduction of 5,5′-dithiobis (2-nitrobenzoic acid) (DTNB) with glutathione producing a yellow compound measured at 405 nm (Beutler 1963). Also, lipid peroxide (malondialdehyde MDA formation) was estimated spectrophotometrically using method based on thiobarbituric acid (TBA) reacted with malondialdehyde (MDA) in acidic medium at temperature of 95°C for 30 min lipid peroxidation (Ohkawa et al. 1979). SOD using SOD assay relies on the ability of the enzyme to inhibit the phenazine methosulphate-mediated reduction of nitroblue tetrazolium dye and estimated spectrophotometrically at 560 nm (Nishikimi et al. 1972).
Histopathological examination of testes
The testes were harvested from different groups then fixed in Bouin’s solution for 24–48 h. The specimens were processed for obtaining 4μm paraffin embedding sections and then stained with haematoxylin and eosin stain (H&E) following the methods that are described by Bancroft and Gamble (2008). The testicular scoring system was done following the criteria reported by Soliman et al. (2019). These criteria depend on scoring of the main spermatogenic cells (spermatogonial cells, primary spermatocytes, secondary spermatocytes, and spermatid cells) and Sertoli cells. The score ranged from 10 to 1 in thirty randomly chosen seminiferous tubules in each group under microscopic power field X 200.
Immune-histochemical analysis of proliferative cell nuclear antigen (PCNA) and caspase-3 expression in testicular tissue
The immune-histochemical analysis of PCNA and caspase-3 expressions in testicular tissues was performed according to Saeedan et al. (2021) and El-Marasy et al. 2018). The tissue sections were deparaffinized and rehydrated. The antigenic retrieval was done by pre-treating the tissue specimens with citrate buffer pH 6 for 20 min followed by the methods described by (Abu-Elala et al., 2015). All tissue sections were incubated with one of mouse monoclonal IgG2a (kappa light chain) PCNA antibody (Sc-56; Santa Cruz Biotechnology, Santa Cruz, CA, USA ) with dilution 1:50 and a mouse monoclonal IgG1 caspase-3 antibody (Sc-56053; Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 1:100 dilution in a humidified chamber for overnight. The primary antibodies were omitted with Tris-buffered saline (TBS) to obtain negative control slides to examine the specificity of the tested antibodies. The tissue sections were washed three times with Tris-buffered saline. The non-specific background was blocked by using blocking solution. The tissue specimens were incubated with secondary (HRP) antibody (ab205718; Abcam, Cambridge, UK). The tissue sections were incubated with DAB (Sigma) and then counterstained with Mayer haematoxylin and mounted. The image analysis was performed according to Farag et al. (2021b) by ImageJ Analyzer. In each group, five sections were evaluated, and in each section, the colour density of the immune-positive cells was analysed in five random fields.
mRNA expression analysis by quantitative real-time PCR (qRT-PCR)
The expression of the B-cell lymphoma-2 (Bcl-2) and the Bcl-2-associated X protein (Bax) genes in the testis was analysed by qRT-PCR. To isolate the total RNA, frozen testes samples were homogenized in liquid nitrogen and lysed in QIAzol according to the manufacturer’s instructions (Qiagen). RNA concentration and purity were checked with an UV-spectrophotometer at 260 nm and 280 nm. Ethidium bromide stained agarose gel electrophoresis (1.5%) was used to evaluate the integrity of isolated RNA. For cDNA synthesis, total RNA (1 μg) together with oligo dT primers (0.5 ng), dNTPs (10 mM), and reverse transcriptase and its buffer (Thermo Fisher Scientific, Inc.) were mixed in a total volume of 20 μl reaction and then incubated at 37°C for 1 h, followed by 70°C for 10 min in the thermal cycler. To determine the relative mRNA expression level of target gene, synthesized cDNA (1 μl), specific primers (1 μl each primer, 10 pM), and SYBR green PCR master mix (Qiagen) were mixed in a total volume of 25 μl. The PCR thermal conditions were set as follows: initial denaturation at 95°C for 4 min, followed by 40 cycle (94°C for 10 s, 56°C for 15 s, and 72°C for 20 s), and a final extension at 72°C for 7 min (Soliman et al. 2020). Specific primers were checked using primer Blast software and were synthesized by Invetrigen, Inc. (Table 1).
Statistical analysis
All experiments were performed in triplicates. The data were expressed as mean ± standard deviation (SD) except for scored data expressed as median ± standard error (SE) (n=7). Statistical analyses were carried out using SPSS software package (version 24.0.) and performed using one-way ANOVA followed by L.S.D test and Duncan post hoc test for normally distributed data, whilst the scored data that are not normally distributed was analysed by Kruskal-Wallis, the nonparametric test of ANOVA, to examine the significance among the studied groups (Youssef et al. 2020).
Results
LC/MS analysis of total ethanol extract Eruca sativa (L) seed revealed tentative identification of 39 compounds, which belongs to different classes as sulphur-containing compounds, flavonoids, phenolic acid, and fatty acids. Figure 1 A and B and Table 2 showed the identified compounds.
-
1.
The detected sulphated compounds were glucoerucin, glucoalyssin, desulphated-glucoraphanin, desulphated-sinidrin, and desulphated-(glucosyl-disulfonyl)-butyl glucosinolate. Peak no. 3 with molecular ion m/z 420.2190 and fragment ion at 258, 179, 147, 135, and 103 due to the loss of hexose unit [M-H-162-SO3,]- corresponds to glucoerucin C12H23NO9S3. Peak no. 5 with molecular ion m/z 450.3180 and fragment ion at 371due to the loss of [M-H-SO3]- corresponds to glucoalyssin C13H24NO10S3; peaks no, 24, 31, and 33 with molecular ion peak at m/z 359.0452, 280.9142, and 522.5403corespond to desulphated-glucoraphanin, desulphated-sinidrin, and desulphated-(glucosyl-disulfonyl)-butyl glucosinolate, respectively.
-
2.
The flavonoid glycosides which were detected belong to O-glycosides and their aglycones as derivatives of the following: isorhamnetin, quercetin, kaempferol, myricetin, naringenin, proanthocyanin, and procyanidin. The analysed extract shows intense molecular ion peaks with M-162, M-146, and M-132 fragments in MS spectrum which is indicative of the cleavage of a O-hexoside or O-rhamnoside (deoxyhexose) and O-pentoside. Previous results demonstrated on the family suggested that kaempferol, quercetin, and isorhamentin glycosides present in species of the family were of the O-glycoside type (Bell et al. 2015).
-
2.1
Peaks no. 8, 17, 20, and 31 belong to isorhamentin derivatives as peak no. 8 showed molecular ion at [M+H]+ at m/z 681.3137with a main fragment ion at m/z 315 corresponding to isorhamentin nucleus derived from loss of (M+H-hexose – CH2-hexose), so peak no. 8 is assigned for isorhamnetin-O-hexoside-O-acetyl hexoside. Peak no. 17 with molecular ion at [M+H]+ at m/z 447.2711 with a main fragment ion at m/z 315 derived from loss of ( M+H-pentose) is assigned for isorhamnetin-O-pentoside. Peak no. 20 showed the molecular ions [M+H]+ at m/z 479.2237 which undergo loss of hexose moiety and subsequent loss of a methyl from its methoxy group (−15 Da) and fragment ion m/z 300; peak 20 is assigned for isorhamentin-O-hexoside. Peak no. 31 showed the molecular ions [M+H]+at m/z 317.3259 which is assigned for isorhamentin aglycone.
-
2.2
Quercetin derivatives; peaks no. 7, 9, 10, 11, 12, 13, and 14 showed main fragments 303 which correspond to quercetin (aglycone + H)+. Peak no. 7 present as glycoside showed molecular ion [M+H]+ m/z at 951.6696 and fragment ion at 789, 627, 465, and 303 which correspond to M+H-hexose-hexose-hexose-caffeoyl), so it is assigned for quercetin -O-dihexosyl-O-caffeoylhexoside. Peak no. 9 showed a molecular ion [M+H]+m/z at 789.3619 and fragment ion at 627,465, and 303 which correspond to [M+H –hexose –hexose- hexose], so peak no. 9 is quercetin-O-trihexoside. Peak no. 10 with molecular ion peak at 627.3523 and fragment ion at 465 and 303 corresponds to [M+H –hexose –hexose), so peak 10 is assigned for quercetin-O-dihexoside. Peak 11 with molecular ion peak at 995.5999 and fragment ions at 833, 671, 369, and 303 corresponds to [M+H –hexose –hexose- sinapoylhexoside), so peak no. 11 is assigned for quercetin-O-dihexoside-O-sinapoylhexoside. Peak no. 12 with molecular ion peak at 817.4893 and fragment ions at 611, 465, and 303 corresponds to [M+H –sinapoyl-rhamnose –hexose), so peak no. 12 is assigned for quercetin-O-sinapoyl-O-rhamneside. Peak no. 13 with molecular ion peak at 979.5961 and fragment ions at 817, 611, 465, and 303 corresponds to [M+H –hexose –sinapoyl–rhamnose - hexose], so peak no. 13 is quercetin-O-rhammnosyl-hexoside-O-sinapoyl-hexoside. Peak no. 14 with molecular ion peak at 611.4440 and fragment ions at 465 and 303 corresponds to (M+H –hexose –rhamnose), so peak no. 14 is assigned for quercetin-O-rhamnosyl-hexoside.
-
2.3
Kaempferol derivatives: Peak no. 18 with molecular ion peak at 449.2255 and fragment peak at 287corresponds to aglycone-kaempferol (M+H-hexose), so peak no. 18 is kaempferol-O-hexoside. Peak no. 26 with molecular ion peak at 285.1703 corresponds to kaempferol-aglycone.
-
2.4
Myricetin derivatives: Peak 15 with molecular ion m/z 463.2641 and the base peak of aglycone appears at m/z 317, due to the loss of rhamnoside unit [M-146-H]-, and it corresponds to myricetin-O-rhamnoside.
-
2.5
Naringenin: Peak 19 showed a molecular ion peak at m/z 447.2711, [M-H]-, the base peak of aglycone at m/z 271 due to the loss of glucuronide [M-H -176]-, so peak 19 is assigned for naringenin-O-glucuronide.
-
2.6
Proanthocyanidin and Procyanidin: Peaks 21 and 23showed a molecular ion peak at m/z 833.5497[M-H]- and 869.4695 M-H]- and fragment ions at 815, 707, 289 and 309, 289, 275.
-
2.1
-
3.
Phenolic acids readily ionize in negative ESI mode and are mainly found as conjugates with sugars, organic acids, or bound to cell wall structures; they are characterized by their high polarity and thus eluted at an early retention time. In LC/MS analysis revealed the identification of caffeoyl-O-hexoside with molecular ion peak 343.9477 and main fragments 343 and 181 and caffeoyl quinic acid (chlorogenic acid) 353.4993 (peaks 22, 25); peak 4 corresponds to sinapic acid at 225.2157, 162.
-
4.
In the second half of the chromatographic run (18–28min), spectra show several unsaturated, hydroxylated fatty acids in addition to one sterol. Fatty acids; starting with 327.3518 oxo-dihydroxy-octadecenoic acid C18H32O5, 329.3802 tri-hydroxy-octadecenoic acid C18H34O5, 274.3674. octadecatetraenoic acid, 277.2927 octadecatetraenoic acid C18H30O2, 279.3026 linoleic C18H32O2, 313.3795, arachidic acid, 339 erucic acid (docosenoic acid C22H42O2), 255.3566 oleicC18H34O2, 327.3459 trihydroxy octadecadienoic acid, and 414.3915 β -sitosterol.
Effect of ESS on relative weights and sperm parameters
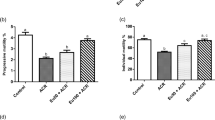
Though induced acrylamide toxicity using 10 mg/kg ACR for 60 days significantly diminished the relative epididymides weight, the decrease in relative testes weight was not significant. The concurrent administrations of ESS hydroethanolic extracts 200 mg/kg b.wt significantly prevented the later toxic effect (Fig. 2A). Sperm parameters including motility, viability, normal spermatozoa percentage, and sperm count showed significant reduction in ACR group compared to normal control and non-intoxicated ESS-treated groups. Interestingly, no differences were found between the control rats and ESS-treated intoxicated groups rats indicating a significant protective effect of ESS against ACR toxicity (Fig. 2B, D), where in ESS 200 mg/kg group, the viability and normal sperms percentage were induced insignificantly different than normal, ACR + ESS (100 mg/kg) and ACR + ESS (200 mg/kg) groups unlike the motility. The percentage of abnormal sperms was increased by ACR toxicity (10 mg/kg b.wt for 60 days). The consumption of ESS extracts (100, 200 mg/kg b.wt for 60 days) generically improved of sperm morphology and reduced percentage of abnormal sperms (Fig. 2C). ESS 200 mg/kg significantly restored sperm counts to almost normal level (Fig. 2D).
Effect ESS extract at doses of 100 and 200 mg/kg. b.wt on (A) relative testicular weight and sperm parameters, (B) motility, viability, and normal spermatozoa %, (C) abnormal spermatozoa% (bent tail, deformed head, and detached head), (D) sperm count (cells × 107/mL) in male rats with and without ACR-induced toxicity; 10 mg/kg for 60 days. (Mean ± SD, n=7), d superscript indicate statistical significance compared to ACR group at p<0.05
Effect of ESS on the level of testosterone hormones
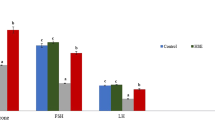
Induced acrylamide toxicity in rats (10 mg/kg b.wt for 60 days) decreased testosterone concentrations significantly (p < 0.05) compared with normal control group. Moreover, concurrent treatment with ESS extracts, ACR-intoxicated male rats restored testosterone concentrations as shown in Fig. 3.
Effect of ESS on testicular oxidative stress markers
Testicular SOD and reduced GSH antioxidant activities were significantly (p < 0.05) decreased in ACR group compared to normal control and ESS-treated groups. However, elevated lipid peroxidation activities exhibited by increased significant MDA (p < 0.05) were reported in ACR group indicated compared to normal and ESS-treated groups (Fig. 4). The regulation of ACR toxicity was in dose-dependent manner where the dose of 200 mg/kg induced better improvement than 100 mg/kg. Although the differences between both doses’ antioxidant effects were significant only for MDA, 200 mg/kg almost normalized the levels of SOD, GSH, and MDA. ESS administration in ACR non-treated groups showed insignificant increased antioxidant activity than normal.
Testicular antioxidant activity of ESS extract at doses of 100 and 200 mg/kg b.wt in male rats with and without acrylamide-induced toxicity (ACR) 10 mg/kg for 60 days. (A) SOD, (B) reduced GSH, and (C) MDA. (Mean ± SD, n=7), a, b, c, d superscripts indicate statistical significance compared to ACR group at p<0.05
Effect of ESS on histopathology of the testis and testicular lesion scoring
Figure 6 A summarized the effects of both ACR and ESS on the testicular lesion scoring in the different experimental groups. The testes of normal control group, ESS (100 mg/kg), and ESS (200 mg/kg) groups revealed mature normal active seminiferous tubules with normal main spermatogenic, Sertoli, and Leydig cells (Fig. 5A, B, C). The ACR group showed moderate to severe testicular degeneration in the form of reduction in the number of the spermatogonial cells, primary and secondary spermatocytes with marked increase in the number of multinucleate spermatid giant cells (Fig. 5D). Vacuolation of Sertoli cells and apoptosis of Leydig cells were also observed. Numerous seminiferous tubules appeared completely free from spermatogenic series with only vacuolated Sertoli cells (Fig. 5E). Leydig cells were apoptotic with inter-tubular oedema. The groups treated with ACR + ESS (100 mg/kg) showed a moderate improvement of testicular lesions with in complete spermatogenic series (Fig. 5F, G), whilst ACR + ESS (200 mg/kg) revealed marked enhancement in the testicular and epididymal lesions that was characterized by re-establishment of the normal spermatogenic series, Sertoli, and Leydig cells (Fig. 5H, I).
Histopathological pictures of the testes in different experimental groups (H&E X200). (A) Normal control group, (B) ESS (100 mg/kg), and (C) ESS (200 mg/kg) treated groups; showing normal histological architecture of mature active seminiferous tubules. (D) ACR-treated group showing testicular degeneration with reduction of spermatogenic series and appearance of spermatid giant cell (arrow). (E) ACR-treated group showing complete absence of spermatogenic series in some seminiferous tubules (arrows). (F–G) ACR + ESS (100 mg/kg) treated group showing normal seminiferous tubules. (H–I) ACR + ESS (200 mg/kg) treated group showing normal seminiferous tubules with hyperplasia of Leydig cells (arrows)
Effect of ESS on immune-histochemistry of PCNA and caspase-3
Both spermatogonial cells and primary spermatocytes showed intense PCNA expression, while secondary spermatocytes and spermatid showed less immunopositive reaction. The normal, ESS (100 mg/kg), and ESS (200 mg/kg) treated groups showed strong immunoreactivity against PCNA (Fig. 7A, B, C) and very weak immunopositive reaction against caspase-3 marker (Fig. 8A, B, C), whilst ACR group showed a significant reduction of the PCNA expression (Fig. 7D, E) and a significant elevation in caspase-3 expression in spermatogenic and Leydig cells (Fig. 8D) when compared with normal control group. The groups treated with ACR + ESS (100 mg/kg) and ACR + ESS (200 mg/kg) showed a significant elevation of PCNA (Fig. 7F, G, H, I) and a significant reduction in caspase-3 expression in both spermatogenic series and Leydig cells compared to ACR group (Fig. 8E, F). No significant difference was detected between the control, ACR + ESS (100 mg/kg), and ACR + ESS (200 mg/kg) groups in PCNA and caspase-3 protein expressions (Figs. 6B, 6C,7, 8).
PCNA expression in testes of different experimental groups (H&E ×200). (A) Control group, (B) ESS (100 mg/kg), and (C) ESS (200 mg/kg) treated groups; showing strong immunoreactivity in spermatogenic cells (arrow). (D–E) ACR-treated group showing absence of immune-positive reaction in some seminiferous tubules (arrows). (F–G) ACR + ESS (100 mg/kg) and (H–I) ACR + ESS (200mg/kg) treated groups showing intense immunoreactivity in spermatogenic series (arrow) and interstitial cells
Caspase-3 expression in testes of different experimental groups (H&E ×200). (A) Control group, (B) ESS (100 mg/kg), and (C) ESS (200 mg/kg) treated groups showing weak immunoreactivity in spermatogenic and Leydig cells. (D) ACR-treated group showing strong immunoreactivity in Leydig cells (arrows). (E) ACR + ESS (100 mg/kg) treated group showing weak immunoreactivity in Leydig cells (arrow). (F) ACR + ESS (200 mg/kg) treated groups showing weak immunoreactivity in spermatogenic (arrow) and Leydig cells
Effect of ESS on gene expression
To investigate the molecular mechanism underlying acrylamide-induced testicular damage, the transcriptional expression of some apoptosis-related genes was analysed. RT-qPCR analysis demonstrated that the mRNA expression of Bcl-2, an anti-apoptotic factor, was markedly reduced in the testis of ACR-intoxicated group, while the transcriptional level of Bax, a pro-apoptotic protein, was enhanced significantly compared to the normal control (Fig. 9). Administration of ESS 100 and ESS 200 along with ACR caused significant reductions in testicular Bax mRNA expression compared to ACR group, whereas no significant difference was observed in comparison with the control group. In the same context, Bcl-2 expression level in testes homogenates of rats treated with ESS 200 + ACR was restored near the normal control level and was significantly up-regulated as compared to ACR-intoxicated group (Fig. 9)
Effect of Eruca sativa seed (ESS) on (A) relative Bcl-2 and (B) Bax transcriptional expression in the testes of rats intoxicated with acrylamide (ACR). ß-actin was used for normalization; mRNA levels are expressed as fold changes relative to the normal control. All data are represented as mean ± SD, n = 7 rats/group. a, b, c, d superscripts indicate significant variation compared to ACR, respectively at p ≤ 0.05
Discussion
Testicular toxicity induced by ACR has been reported in different rodents’ models. Many previous studies reported major changes in gonadal and pituitary hormones as well as changes in testicular histopathological and gene expression (Yang et al. 2005; Wang et al. 2010; Ma et al. 2011). That could be contributed to ACR’s highly water solubility molecule which facilitates its absorption and distribution (Mannaa et al. 2006). Also, ACR could interact with nucleophilic groups (-SH, -NH2 or –OH) forming GSH S-conjugates and initiating biotransformation of electrophiles into mercapturic acids (Awad et al. 1998) and its metabolite glycidamide proved to induce DNA adducts in different tissues (Kucukler et al. 2020). Interestingly, the current investigation reveals that ESS (200 mg/kg) induced a high protective role against ACR toxic effects on the testicular cells through up-regulating Bcl-2 and Bax proteins, anti-apoptotic effect, and antioxidant capacity. Also, The ESS-treated groups alone showed features less or more similar to the normal control group, even the slight elevations in the relative testis weight and sperm count, which may reflect the possible enhancing properties of ESS on the male fertility and performance (Abd El-Aziz et al. 2016; Hussein 2013). Moreover, our results reported, for the first time, the protective effect of ESS hydroalcoholic extracts against acrylamide-induced injurious effects on male reproductive indices in rats.
Tentative identification of the chemical constituents of the total ethanol extract of Eruca sativa (L.) seed by LC/MS analysis revealed the presence of different bioactive compounds that belongs to various classes as sulphur-containing compounds including glucoerucin, glucoalyssin, desulphated-glucoraphanin, desulphated-sinidrin, and desulphated-(glucosyl-disulfonyl)-butyl glucosinolate and flavonoids that belongs to O-glycosides and their aglycones as derivatives of the following: isorhamnetin, quercetin, kaempferol, myricetin, naringenin, proanthocyanidin-proanthocyanin, and procyanidin, in addition to phenols as caffeoyl-O-hexoside and chlorogenic acid and fatty acids as oxo-dihydroxy-octadecenoic, tri-hydroxy-octadecenoic, octadecatetraenoic, linoleic, arachidic, and erucic acids. The sulphated and desulphated compounds detected were previously reported in the plant (Kim and Ishii 2006). Flavonoids have been frequently reported in family Brassicaceae to which Eruca sativa belongs (Bell et al. 2015). The former constituents are probably correlated to ESS antioxidant and anti-apoptotic effects.
In the present study, the ESS-treated groups alone showed features less or more similar to the normal control group, even the slight elevations in the relative testis weight and sperm count, which may reflect the possible enhancing properties of ESS on the male fertility and performance (Abd El-Aziz et al. 2016; Hussein 2013). However, our results reported, for the first time, the protective effect of ESS hydroalcoholic extracts against acrylamide-induced injurious effects on male reproductive indices in rats.
In the present study, daily acrylamide dosing of 10 mg/kg b.wt for 60 days induced significant reduction in relative epididymis weight, sperm cell count, sperm motility, viability, and normal sperm percentages. Similar results were previously reported in many studies using doses as low as 6.5 mg/kg b.wt for 21 days (Christina and Daniel 2015; Kalaivani et al. 2018; Ma et al. 2011; Pourentezari et al. 2014; Song et al. 2008; Wang et al. 2010; Yang et al. 2005). Tail and head deformities (Fig. 1 C) were the most reported morphological abnormalities with ACR-induced toxicity in rodents (Dobrzyn´ska and Gajewski 2000). The toxic effect of ACR on sperm motility, viability, and morphology was explained as ACR or its metabolite glycinamide induce mutation in germ and somatic cells through binding to dopamine receptors and spermatid protamines, thus inhibiting kinesin and dynein activity, resulting in interference of intracellular transport (Ghanayem et al. 2005; Pourentezari et al. 2014). The previously mentioned toxic effects of ACR on the sperm indices were inhibited by concurrent administration of ESS hydroethanolic extract (100 and 200 mg/kg) with ACR in dose-dependent manner. The improving activity of ES on sperm concentrations, morphology, viability, and motility was previously reported in healthy rodent by Hussein (2013) and Salma et al. (2018) and hydrogen peroxide intoxicated rats (Nowfel and Al-Okaily 2017). The protective effect of ESS against ACR toxicity can thus be explained by its antioxidant activity.
Our results indicated reduced superoxide dismutase (SOD) activities and glutathione (GSH) and significant elevation of lipid peroxidase (malondialdehyde) concentration in ACR-treated group, and these results come in harmony with Abdel-Daim et al. (2014), Alturfan et al. (2012), He et al. (2017), Lebda et al. (2014), and Yousef et al. (2007). ACR was proved to interact with GSH forming glutathione S-conjugates and initiating intracellular electrophiles metabolism (Awad et al. 1998). The two main antioxidants of semen are superoxide dismutase (SOD) and glutathione (GSH) (Zhang et al. 2010). However, depletion of GSH and SOD to certain critical levels may enhance lipid peroxidation (Aneja et al. 2004) causing high testicular concentrations of reactive oxygen species ROS which can damage proteins, lipids, and DNA of testicular cells inducing deleterious effects on quality of germ cell development and increase its apoptosis (Aziz et al. 2004; Sanocka and Kurpisz 2004). On the other hand, significant increase was observed in testicular antioxidant power in ESS extracts treated rats (100 and 200 mg/kg b.wt) with and without ACR toxicity. The increased SOD and GSH activities after oral administration of ESS extracts were supported by previous studies on testes of abamectin intoxicated male rats (Meligi and Hassan 2017) and on the kidney of mercuric chloride intoxicated rats. Lipid peroxidase (malondialdehyde) concentration increased significantly in ACR group supported by Alturfan et al. (2012) and Lebda et al. (2014). The reduction in MDA activities by ESS extract is supported by Alqasoumi et al. (2009) in hepatic tissue. The antioxidant property of Eruca sativa is attributed to its high content of carotenoids, fibres, minerals, glucosinolates, isothiocyanates, and flavonoids such as kaempferol, quercetin, and isorhamnetin, flavanols, and phenolic compounds (Garg and Sharma 2014; Nowfel and Al-Okaily 2017). Hence, ESS extracts decreased oxidative damage of acrylamide on testicular tissue that certainly contributed to the increased sperm count and quality.
Testosterone hormone concentrations significantly decreased in ACR group compared to control and ESS-treated groups (100, 200 mg/kg b.wt) supported by that obtained in rats using 15 and 30 mg/kg/day for 90 days (Yassa et al. 2014), 5–30 mg/kg/day ACR in weaning male rats (Ma et al. 2011), and 0.05% (w/v) ACR in drinking water for 21 days in rats (Lebda et al. 2014) and in mice using 5–60 mg/kg/day for 5 days ACR (Yang et al. 2005). ESS improving activity on testosterone concentrations was consistent with previously reported results in cadmium exposed rats (Al-okaily and Al-shammari 2016), nicotine intoxicated rats (Abd El-Aziz et al. 2016), and healthy rats (Al-Qudah 2017). The prolonged ACR administration results in increase of reactive oxygen species ROS reduce serum testosterone and impairment of sperm production function. This impairment may result from irregular hormonal synthesis and incomplete spermatogenesis.
The primary functions of testes and the male reproductive endocrine system are regulation of sex steroid hormones which are essential for normal reproductive function (O'Donnell et al. 2017). LH hormone and other steroidogenic stimuli create steroid hormones biosynthesis that initiated with cholesterol to form the first steroid, pregnenolone, in all steroidogenic tissues (Miller and Bose 2011). ACR may induce death of Leydig cells as further supported by our histopathological and immunohistochemical findings, and these results were agreed with Pourentezari et al. (2014) and Yang et al. (2005). Death of Leydig cells resulted in marked reduction of testosterone in serum and, subsequently, decreased the spermatogenesis in the testis. Many previous studies reported major changes in gonadal and pituitary hormones as a result of changes in testicular histopathological and gene expression (Camachoa et al. 2012).
In the testis, the dynamics of germ cells is precisely controlled by a balance between cell proliferation and apoptotic cell death Therefore, dysregulation of this fine-tuned balance results in reproductive impairment and infertility in male (Jeyaraj et al. 2003). In the current investigation, we unravelled the effects of ESS on the oxidant and antioxidant markers (lipid peroxidation, SOD, GSH) and apoptotic and anti-apoptotic markers (Bax, caspase-3, Bcl-2), along with testicular proliferation marker (PCNA) in ACR-intoxicated rats.
As it has been evidenced that ACR exposure is greatly associated with reproductive toxicity due to oxidative stress and apoptosis (Kaçar et al. 2018; Radad et al. 2020), the findings of the present study showed that ACR significantly increased the Bax mRNA expression in rats’ testis. Furthermore, ACR group showed a marked up-regulation of caspase-3 protein, which is required for both the intrinsic and extrinsic apoptotic pathways in the testis (Moreno et al. 2006). Cooperatively, activation of these proteins induces the testicular cells to undergo apoptosis rather than proliferation and survival. ACR exposure also significantly decreased the mRNA levels of the anti-apoptotic protein Bcl-2. These results indicated an accelerated rate of apoptosis in ACR-intoxicated testis, which might contribute to the observed decreased sperm mobility and viability. In accordance, previous studies reported activation of caspase-3 and Bax/Bcl-2 dysregulation in the germ cells upon acrylamide exposure both in vivo (Kaçar et al. 2018; Yilmaz et al. 2017) and in vitro (Liu et al. 2015; Yilmaz et al. 2017). Together, the data presented also support the previous suggestions that the mitochondrial dysfunction and redox-dependent apoptotic responses are greatly involved as critical events during ACR-induced testicular toxicity (Liu et al. 2015; Yildizbayrak and Erkan 2018).
Notably, administration of ESS to ACR-intoxicated rats effectively counteracted the ACR-induced testicular apoptosis as evident from down-regulation of Bax and caspase-3 expression in the testis compared to ACR group. Moreover, ESS potentially recovered Bcl-2 expression which clearly demonstrated that ESS could regulate testicular apoptosis. Our results are in line with other evidence showing the anti-apoptotic properties of ESS which may be linked to its bioactive constituents such as phenolic compounds and flavonoids with remarkable antioxidant capacity (Grami et al. 2020).
Conclusion
To sum up, Eruca sativa hydroethanolic extract especially at the dose of 200 mg/kg b.wt. induced a high protective role against the toxic effects ensued by acrylamide on the testicular cells through up-regulating Bcl-2 and Bax proteins, anti-apoptotic effect, and via the enhancement of the antioxidant capacity which may correlate to its identified bioactive constituents such as phenolic compounds and flavonoids.
Data Availability
Data available upon request.
Code availability
Not applicable
Abbreviations
- ESS:
-
Eruca sativa seed
- ACR:
-
Acrylamide
- Bcl-2:
-
Testicular B-cell lymphoma-2
- Bax:
-
Bcl-2-associated X protein
References
Abd El-Aziz G, El-Fark M, Hamdy R (2016) Protective effect of Eruca sativa seed oil against oral nicotine induced testicular damage in rats. Tissue Cell 48:340–348
Abdel-Daim MM, Abd Eldaim MA, Hassan AGA (2014) Trigonella foenum-graecum ameliorates acrylamide-induced toxicity in rats: roles of oxidative stress, proinflammatory cytokines, and DNA damage. Biochem Cell Biol 93(3):192–198. https://doi.org/10.1139/bcb-2014-0122
Abdel-Daim MM, Abo El-Ela FI, Alshahrani FK, Bin-Jumah M, Al-Zharani M, Almutairi B, Alyousif MS, Bungau S, Aleya L, Alkahtani S (2020) Protective effects of thymoquinone against acrylamide-induced liver, kidney and brain oxidative damage in rats. Environ Sci Pollut Res 27:37709–37717
Abu-Elala NM, Abd-Elsalam RM, Marzouk MS (2015) Molecular and immunohistochemical diagnosis of Photobacterium damselae subspecies piscicida during naturally occurring disease in Egypt. J World Aquacult Soc 46(6):583–595
Alam M, Kaur G, Jabbar Z et al (2007) Eruca sativa seeds possess antioxidant activity and exert a protective effect on mercuric chloride induced renal toxicity. Food Chem Toxicol 45:910–920
Alarcón M, Fuentes M, Carrasco G, Palomo I (2014) Novel role of Eruca sativa mill. (Rocket) extract: antiplatelet (NF-κB inhibition) and antithrombotic activities. Nutrients 6:5839–5852
Al-okaily B, Al-shammari Z (2016 ) The impact of Eruca sativa seeds on Leydig’s cells number and hormonal profile in cadmium exposed rats. Kufa J Vet Med Sci 7
Alqasoumi S, Al-Sohaibani M, Al-Howiriny T, Al-Yahya M, Rafatullah S (2009) Rocket "Eruca sativa": a salad herb with potential gastric anti-ulcer activity. World J Gastroenterol 15:1958–1965
Al-Qudah M (2017) Effects of Eruca sativa leaves extracts on testes, fertility potential and testosterone concentration in male rats. Annual Research & Review in Biology 16:1–7
Al-Serwi RH, Ghoneim FM (2015) The impact of vitamin E against acrylamide induced toxicity on skeletal muscles of adult male albino rat tongue: light and electron microscopic study. J Microsc Ultrastruct 3:137–147
Alturfan EI, Beceren A, Şehirli AÖ, Demiralp ZE, Şener G, Omurtag GZ (2012) Protective effect of N-acetyl-L-cysteine against acrylamide-induced oxidative stress in rats. Turk J Vet Anim Sci 36:438–445
Aneja R, Katyal A, Chandra R (2004) Stimulation of lipid peroxidation and impairment of glutathione-dependent defense system in Wistar rats treated with cryptopine, a rare non-narcotic opium alkaloid. Eur J Drug Metab Pharmacokinet 29:31–36
Ansari M, Ganaie M (2014) Ameliorative effect of rocket leaves on fertility in streptozotocin-induced diabetic rats. Internet Res 3:89–97
Aras D, Cakar Z, Ozkavukcu S, Can A, Cinar O (2017) In Vivo acrylamide exposure may cause severe toxicity to mouse oocytes through its metabolite glycidamide. PLoS One 12:e0172026
Awad M, Abdel-Rahman M, SA H (1998) Acrylamide toxicity in isolated rat hepatocytes. Toxicol in Vitro 12:699–704
Aziz N, Saleh R, Sharma R et al (2004) Novel association between sperm reactive oxygen species production, sperm morphological defects, and the sperm deformity index. Fertil Steril 81:349–354
Bancroft JD, Gamble, M. (Eds.) (2008) Theory and practice of histological techniques. Elsevier health sciences
Bell L, Oruna-Concha MJ, Wagstaff C (2015) Identification and quantification of glucosinolate and flavonol compounds in rocket salad (Eruca sativa, Eruca vesicaria and Diplotaxis tenuifolia) by LC-MS: highlighting the potential for improving nutritional value of rocket crops. Food Chem 172:852–861
Bennett R, Mellon F, Botting N et al (2002) Identification of the major glucosinolate (4-mercaptobutyl glucosinolate) in leaves of Eruca sativa L. (salad rocket). Phytochemistry 60:25–30
Bennett R, Rosa E, Mellon F, Kroon P (2006) Ontogenic profiling of glucosinalates, flavonoids and other secondary metabolite in Eruca sativa (salad rocket), Diplotaxis erucoides (wall rocket), Diplotaxis tenuifolia (wild rocket), and Bunias orientalis Pasini Pasini (Turkish rocket). J Agric Food Chem 54:4005–4015
Beutler E (1963) Improved method for the determination of blood glutathione. J Lab Clin Med 61:882–888
Bin-Jumah MN, AA AL-H, Abdelnaeim N, Kamel M, Fouda MMA, MMA A, Saadeldin IM, Abdel-Daim MM (2021) 12. Potential protective effects of Spirulina platensis on liver, kidney, and brain acrylamide toxicity in rats. Environ Sci Pollut Res 28(11):13031–13046. https://doi.org/10.1007/s11356-021-12422-x
Camachoa L, Latendresseb J, Muskhelishvilib L et al (2012) Effects of acrylamide exposure on serum hormones, gene expression, cell proliferation, and histopathology in male reproductive tissues of Fischer 344 rats. Toxicol Lett 211:135–143
Christina OU, Daniel UO (2015) Effects of acrylamide on the reproductive hormones and sperm quality in male rats. International Journal of Science and Research 6:5–8
Dobrzyn´ska M, Gajewski A (2000) Induction of micronuclei in bone marrow and sperm head abnormalities after combined exposure of mice to low doses of X-rays and acrylamide. Teratog Carcinog Mutagen 20:133–140
Doulgeraki A, Efthimiou G, Paramithiotis S et al (2017) Effect of rocket (Eruca sativa) extract on MRSA growth and proteome: metabolic adjustments in plant-based media. Front Microbiol 8-782
Elgazar AF, AO A (2013) Nephroprotective and diuretic effect of three medicinal herbs against gentamicin-induced nephrotoxicity in male rats. PJN 12(12):715–722
Elhelaly AE, AlBasher G, Alfarraj S, Almeer R, BahbahEI FMMA, Bungău SG, Aleya L, Abdel-Daim MM (2019) Protective effects of hesperidin and diosmin against acrylamide-induced liver, kidney, and brain oxidative damage in rats. Environ Sci Pollut Res Int 26(34):35151–35162. https://doi.org/10.1007/s11356-019-06660-3
El-Marasy SA, Abd-Elsalam RM, Ahmed-Farid OA (2018) Ameliorative effect of silymarin on scopolamine-induced dementia in rats. Open access Macedonian journal of medical sciences 6(7):1215–1224
Farag OM, Abd-Elsalam RM, Ogaly HA, Ali SE, El Badawy SA, Alsherbiny MA et al (2021a) Metabolomic profiling and neuroprotective effects of purslane seeds extract against acrylamide toxicity in rat’s brain. Neurochem Res 46(4):819–842
Farag OM, Abd-Elsalam RM, El Badawy SA, Ogaly HA, Alsherbiny MA, Ahmed KA (2021b) Portulaca oleracea seeds’ extract alleviates acrylamide-induced testicular dysfunction by promoting oxidative status and steroidogenic pathway in rats. BMC Complementary Medicine and Therapies 21:122. https://doi.org/10.1186/s12906-021-03286-2
Farouk SM, Gad FA, Almeer R, Abdel-Daim MM, Emam MA (2021) Exploring the possible neuroprotective and antioxidant potency of lycopene against acrylamide-induced neurotoxicity in rats' brain. Biomed Pharmacother 138:111458
Flanders A, Abdulkarim S (1985) The composition of seed and seed oils of taramira(Eruca sativa). J Am Oil Chem Soc 62:1134–1135
Garg G, Sharma V (2014) Eruca sativa(L.): Botanical description, crop improvement, and medicinal properties. J Herbs Spices Med Plants 20:171–182
Ghanayem BI, Witt KL, El-Hadri L, Hoffler U, Kissling GE, Shelby MD, Bishop JB (2005) Comparison of germ cell mutagenicity in male CYP2E1-null and wild-type mice treated with acrylamide: evidence supporting a glycidamide-mediated effect. Biol Reprod 72:157–163
Grami D, Rtibi K, Hammami I et al (2020) Protective action of eruca sativa leaves aqueous extracts against bisphenol a-caused in vivo testicular damages. J Med Food 23:600–610
Gugliandolo A, Giacoppo S, Ficicchia M, Aliquo A, Bramanti P, Mazzon E (2018) Eruca sativa seed extract: a novel natural product able to counteract neuroinflammation. Mol Med Rep 17:6235–6244
Gulfraz M, Sadiq A, Tariq H et al (2011) Phytochemical analysis and antibacterial activity of Eruca sativa seed. Pak J Bot 43:1351–1359
He Y, Tan D, Mi Y, Bai B, Jiang D, Zhou X, Ji S (2017) Effect of epigallocatechin-3-gallate on acrylamide-induced oxidative stress and apoptosis in PC12 cells. Hum Exp Toxicol 36:1087–1099
Hussein ZF (2013) Study the effect of Eruca sativa leaves extract on male fertility in albino mice. J Univ Nat Sci 16:143–146
Jeyaraj D, Grossman G, Petrusz P (2003) Dynamics of testicular germ cell apoptosis in normal mice and transgenic mice overexpressing rat androgen-binding protein. Reprod Biol Endocrinol 1:1–14
Jin J, Koroleva O, Gibson T et al (2009) Analysis of phytochemical composition and chemoprotective capacity of rocket (Eruca sativa and Diplotaxis tenuifolia) leafy salad following cultivation in different environments. J Agric Food Chem 57:5227–5234
Kaçar S, Şahintürk V, Can B, Musmul A (2018) L-cysteine partially protects against acrylamide-induced testicular toxicity. Balkan medical journal 35:311–319
Kalaivani M, Saleena UV, Katapadi KGK, Kumar YP, Nayak D (2018) Effect of acrylamide ingestion on reproductive organs of adult male wistar rats. J Clin Diagn Res 12
Kim S-J, Ishii G (2006) Glucosinolate profiles in the seeds, leaves and roots of rocket salad (Eruca sativaMill.) and anti-oxidative activities of intact plant powder and purified 4-methoxyglucobrassicin. Soil Sci Plant Nutr 52:394–400
Kucukler S, Caglayan C, Darendelioğlu E, Kandemir FM (2020) Morin attenuates acrylamide-induced testicular toxicity in rats by regulating the NF-κB, Bax/Bcl-2 and PI3K/Akt/mTOR signaling pathways. Life Sciences 261:118301
Kumar J, Das S, Teoh SL (2018) Dietary acrylamide and the risks of developing cancer: facts to ponder. Front Nutr 5:14
Lebda M, Gad S, Gaafar H (2014) Effects of lipoic acid on acrylamide induced testicular damage. Materia socio-medica 26:208–212
Lelario F, Bianco G, Bufo S, Cataldi T (2012) Establishing the occurrence of major and minor glucosinolates in Brassicaceae by LC-ESI-hybrid linear ion-trap and Fourier-transform ion cyclotron resonance mass spectrometry. Phytochemistry 73:74–83
Liu Z, Song G, Zou C et al (2015) Acrylamide induces mitochondrial dysfunction and apoptosis in BV-2 microglial cells. Free Radic Biol Med 84:42–53
Ma Y, Shi J, Zheng M, Liu J, Tian S, He X, Zhang D, Li G, Zhu J (2011) Toxicological effects of acrylamide on the reproductive system of weaning male rats. Toxicol Ind Health 27:617–627
Maia ML, C-S L, A C et al (2015) Eruca sativa: benefits as antioxidants source versus risks of already banned pesticides. J Environ Sci Health B 50:338–345
Mannaa F, Abdel-Wahhab MA, Ahmed HH, Park MH (2006) Protective role of Panax ginseng extract standardized with ginsenoside Rg3 against acrylamide-induced neurotoxicity in rats. J Appl Toxicol 26(3):198–206. https://doi.org/10.1002/jat.1128
Martelli A, Citi V, Piragine E et al. (2019) Anticancer activities of erucin a H2S-donor isothiocyanate from Eruca sativa Mill.: Is H2S the real player? Ther Appl Nitric Oxide. Cancer Inflamm Disord 327–328
Martínez-Sanchez A, Llorach R, Gil M, Ferreres F (2007) Identification of new flavonoid glycosides and flavonoid profiles to characterize rocket leafy salads (Eruca vesicaria and Diplotaxis tenuifolia). J Agric Food Chem 55:1356–1363
Mashi S (2017) Effect of Eruca sativa leaves extract on liver enzymes and lipid profile in phosphoric acid induced liver damage in male rabbits. J Entomol Zool Stud 5:1011–1015
Meligi NM, Hassan HF (2017) Protective effects of Eruca sativa (rocket) on abamectin insecticide toxicity in male albino rats. Environ Sci Pollut Res 24:9702–9712
Miller WL, Bose HS (2011) Early steps in steroidogenesis: intracellular cholesterol trafficking. J Lipid Res 52:2111–2135
Moreno R, Lizama C, Urzúa N et al (2006) Caspase activation throughout the first wave of spermatogenesis in the rat. Cell Tissue Res 325:533–540
Nazeam J, El-Hefnawy H, Omran G, A-N S (2018) Chemical profile and antihyperlipidemic effect of Portulaca oleracea L. seeds in streptozotocin-induced diabetic rats. J Nat Prod Res Former Nat Prod Lett 32:1484–1488
Nishikimi M, Rao NA, Yagi K (1972) The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun 46:849–854
Nowfel AJ, Al-Okaily BN (2017) Oxidative stress: role of Eruca sativa extract on male reproduction in rats. Advances in Animal and Veterinary Sciences 5:39–46
O'Donnell L, Stanton P, de Kretser DM (2017) Endocrinology of the male reproductive system and spermatogenesis, Endotext. MDText. com, Inc
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Oliveira PF, Tomás GD, Dias TR, Martins AD, Rato L, Alves MG, Silva BM (2015) White tea consumption restores sperm quality in prediabetic rats preventing testicular oxidative damage. Reprod BioMed Online 31:544–556
Pasini F, V V, Caboni M, D’Antuono L (2012) Determination of glucosinolates and phenolic compounds in rocket salad by HPLC-DAD–MS: evaluation of Eruca sativa mill. and Diplotaxis tenuifolia L. genetic resources. Food Chem 133:1025–1033
Peter SJ, EP S (2016) Global current trends in natural product for diabetes managements Int. J Pharm Pharm Scii 8:21–28
Pourentezari M, Talebi A, Abbasi A, Khalili MA, Mangoli E, Anvari M (2014) Effects of acrylamide on sperm parameters, chromatin quality, and the level of blood testosterone in mice. Iranian journal of reproductive medicine 12:335
Radad K, Amir YE, Al-Emam A, Al-Shraim M, Bin-Jaliah I, Krewenka C, Moldzio R (2020) Minocycline protects against acrylamide-induced neurotoxicity and testicular damage in Sprague-Dawley rats. J Toxicol Pathol 33:87–95
Raju J, Kocmarek A, Roberts J, Taylor M, Patry D, Chomyshyn E, Caldwell D, Cooke G, Mehta R (2016) Lack of adverse health effects following 30-weeks of dietary exposure to acrylamide at low doses in male F344 rats. Toxicol Rep 3:673–678
Rato L, Alves M, Dias T, Lopes G, Cavaco J, Socorro S, Oliveira P (2013) High-energy diets may induce a pre-diabetic state altering testicular glycolytic metabolic profile and male reproductive parameters. Andrology 1:495–504
Rochfort S, Trenerry V, Imsic M et al (2008) Class targeted metabolomics: ESI ion trap screening methods for glucosinolates based on MSn fragmentation. Phytochemistry 69:1671–1679
S Jaafar N, S Jaafar I (2019) Eruca Sativa Linn.: pharmacognostical and pharmacological properties and pharmaceutical preparations. Asian Journal of Pharmaceutical and Clinical Research, 39-45
Saeedan AS, Soliman GA, Abdel-Rahman RF, Abd-Elsalam RM, Ogaly HA, Foudah AI, Abdel-Kader MS (2021) Artemisia judaica L. diminishes diabetes-induced reproductive dysfunction in male rats via activation of Nrf2/HO-1-mediated antioxidant responses. Saudi Journal of Biological Sciences 28(3):1713–1722
Salma U, Khan T, Shah A (2018) Antihypertensive effect of the methanolic extract from Eruca sativa Mill. (Brassicaceae) in rats: muscarinic receptor-linked vasorelaxant and cardiotonic effects. J Ethnopharmacol 224:409–420
Salman S, Rogerson SJ, Kose K, Griffin S, Gomorai S, Baiwog F, Winmai J, Kandai J, Karunajeewa HA, O'Halloran SJ, Siba P, Ilett KF, Mueller I, Davis TM (2010) Pharmacokinetic properties of azithromycin in pregnancy. Antimicrob Agents Chemother 54:360–366
Sanocka D, Kurpisz M (2004) Reactive oxygen species and sperm cells. Reprod Biol Endocrinol 2:1–7
Sansano M, Heredia A, Peinado I, Andres A (2017) Dietary acrylamide: what happens during digestion. Food Chem 237:58–64
Seham S, Magda S, Madiha M (2015) Effect of some plant oils and garlic on lipids of rats fed on high cholesterol diet. J Int Food Res 22:1307–1314
Soliman GA, Saeedan AS, Abdel-Rahman RF, Ogaly HA, Abd-Elsalam RM, Abdel-Kader MS (2019) Olive leaves extract attenuates type II diabetes mellitus-induced testicular damage in rats: Molecular and biochemical study. Saudi Pharmaceutical Journal 27:326–340
Soliman GA, Abdel-Rahman RF, Ogaly HA, Althurwi HN, Abd-Elsalam RM, Albaqami FF, Abdel-Kader MS (2020) Momordica charantia extract protects against diabetes-related spermatogenic dysfunction in male rats: molecular and biochemical study. Molecules 25(22):5255
Song H, Wang R, Geng Z, Cao S, Liu T (2008) Subchronic exposure to acrylamide affects reproduction and testis endocrine function of rats. Zhonghua Nan Ke Xue 14:406–410
Tao H, Wang L, Cui Z et al (2008) Dimeric proanthocyanidins from the roots of Ephedra sinica. Planta Med 74:1823–1825
Wang H, Huang P, Lie T, Li J, Hutz RJ, Li K, Shi F (2010) Reproductive toxicity of acrylamide-treated male rats. Reprod Toxicol 29:225–230
Weckerle B, Michel K, Balázs B et al (2001) Quercetin 3, 3’, 4’-tri-O-β-D-glucopyranoside from leaves of Eruca sativa (Mill.). Phytochem 57:547–551
Yang H-J, Lee S-H, Jin Y, Choi J-H, Han C-H, Lee M-H (2005) Genotoxicity and toxicological effects of acrylamide on reproductive system in male rats. J Vet Sci 6:103–109
Yassa HA, George SM, Refaiy Ael R, Moneim EM (2014) Camellia sinensis (green tea) extract attenuate acrylamide induced testicular damage in albino rats. Environ Toxicol 29:1155–1161
Yildizbayrak N, Erkan M (2018) Acrylamide disrupts the steroidogenic pathway in Leydig cells: possible mechanism of action. Toxicol Environ Chem 100:235–246
Yilmaz B, Yildizbayrak N, Aydin Y, Erkan M (2017) Evidence of acrylamide- and glycidamide-induced oxidative stress and apoptosis in Leydig and Sertoli cells. Hum Exp Toxicol 36:1225–1235
Yousef MI, Kamel KI, El-Guendi MI, El-Demerdash FM (2007) An in vitro study on reproductive toxicity of aluminium chloride on rabbit sperm: the protective role of some antioxidants. Toxicology 239:213–223
Youssef FS, Menze ET, Ashour ML (2020) A potent lignan from prunes alleviates inflammation and oxidative stress in lithium/pilocarpine-induced epileptic seizures in rats. Antioxidants 9(7):575. https://doi.org/10.3390/antiox9070575
Zhang J, Yue W, Ren Y, Zhang C (2010) Enhanced role of elaidic acid on acrylamide-induced oxidative stress in epididymis and epididymal sperm that contributed to the impairment of spermatogenesis in mice. Toxicol Ind Health 26:469–477
Zhu H, Wang Y, Liu Y, Xia Y, Tang T (2010) Analysis of flavonoids in Portulaca oleracea L. by UV–vis spectrophotometry with comparative study on different extraction technologies. Food Anal Methods 3:90–97
Acknowledgements
Authors are thankful to Plant Taxonomy, Department of Botany, Faculty of Science, Cairo University for confirming the taxonomic identity of the plant.
Author information
Authors and Affiliations
Contributions
Conceptualization: Kawkab A. Ahmed, Reham M. Abd-Elsalam, Shymaa A. El Badawy, and Hanan A. Ogaly
Methodology, validation, and formal analysis: Shymaa A. El Badawy, Ola M. Farag, Reham M. Abd-Elsalam, Hanan A. Ogaly, and Faten M. Ibrahim
Investigation, data curation, and writing the original draft preparation: all authors
Writing, review and editing: Shymaa A. El Badawy, Hanan A. Ogaly, Reham M. Abd-Elsalam, Faten M. Ibrahim, and Kawkab A. Ahmed
Corresponding author
Ethics declarations
Ethics approval
The applied experimental protocol was approved by the Institutional Animal Care and Use Committee (IACUC), Cairo University (# CU-II-F-84-18).
Consent to participate
Not applicable
Consent for publication
Not applicable
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Mohamed M. Abdel-Daim
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abd-Elsalam, R.M., El Badawy, S.A., Ogaly, H.A. et al. Eruca sativa seed extract modulates oxidative stress and apoptosis and up-regulates the expression of Bcl-2 and Bax genes in acrylamide-induced testicular dysfunction in rats. Environ Sci Pollut Res 28, 53249–53266 (2021). https://doi.org/10.1007/s11356-021-14532-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-14532-y