Abstract
Cadmium (Cd), a toxic heavy metal, produces various forms of environmental contaminations and health problems in human. In this study, we aimed to examine the localization of several apoptotic markers in human placentas from pregnant women who were environmentally exposed to Cd. Twelve pregnant women participated in this analysis and they were divided into 2 groups according to their living areas: high-Cd (H-Cd) and low-Cd (L-Cd) groups. After delivery, the placentas were immediately harvested, and the placental width, length, and weight were measured. The placental Cd concentration was determined by using ICP-MS. The expression of three apoptotic markers, cleaved caspase-3, cleaved lamin A/C, and TUNEL, was examined in immunohistochemistry. In results, the placental Cd concentration in the H-Cd group was higher than that in the L-Cd group. In contrast, a significant decrease in the BW (birth weight):PW (placenta weight) ratio representing the placental nutrient transport function was found in the H-Cd group, and an inverse correlation between placental Cd concentration and BW:PW ratio was demonstrated. Additionally, significant elevations in the expression of cleaved caspase-3, cleaved lamin A/C proteins, and TUNEL were shown in the H-Cd placenta. Moreover, positive correlations were found between the placental Cd concentration and the expression of cleaved caspase-3 and TUNEL. Collectively, our findings suggest that the exposure of pregnant women to environmental Cd might induce Cd to be transferred to the body and then accumulated in the placenta, resulting in disturbance of the placental function and eventual apoptosis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The placenta is a specialized organ composed of two portions that are developed from maternal and fetal tissues. Its maternal portion is called the basal plate composed of extra-villous trophoblasts, uterine deciduae, and other immune cells, whereas the fetal portion, the chorionic plate, is formed by chorionic mesenchymal cells covered by a single-layered tissue called the amnion. The surface of the chorionic plate is attached by the umbilical cord occupied by umbilical vessels that give rise to chorionic vessels distributing into the chorionic plate. The chorionic villi projecting from the chorionic plate into the intervillous space are composed of four cell-layers which include syncytiotrophoblast, cytotrophoblast, connective tissue core, and fetal capillary, and they form the placental blood barrier (Huppertz 2008). This specialized feto-maternal organ plays important roles in nutrient transport, gas exchange, hormonal production, and prevention of xenobiotics (Myllynen et al. 2005). On the other hand, it has been reported that heavy metals can be accumulated in the placenta and transferred to the fetus, leading to a low birth-weighted neonate (Kippler et al. 2012). Therefore, the placenta is frequently used as a human organ biomarker for developmental toxicology and monitoring (Lyengar and Rapp 2001; Myllynen et al. 2005).
Cadmium (Cd) is a heavy toxic metal that naturally occurs in the earth’s crust at the concentration of approximately 0.1 mg/kg (Tchounwou et al. 2012). The anthropogenic sources such as industrialization, mining, and agriculture can produce the shift of Cd distribution into the environment, leading to the Cd contamination in soil, air, and water (Yu et al. 2017). In Thailand, the Cd contamination is found in paddy fields in Mae Ku, Mae Tao, and Phrathat Phadaeng subdistricts, Mae Sot district, Tak province, that are utilized as agricultural fields receiving irrigation water from Mae Tao creek (Simmons et al. 2005). Consequently, the agricultural food products such as rice growing on these areas can uptake Cd, and the consumption of those products can transfer Cd into the human body by the food chain (Suwatvitayakorn et al. 2019). The body burden of Cd produces the structural and functional damages in several organs such as brain, kidney, and placenta (Mukherjee et al. 2010; Phuapittayalert et al. 2013; Satarug et al. 2019).
For protection of the Cd toxicity, metallothionein (MT), a low-molecular-weight and cysteine-rich protein, is useful by binding and trapping Cd in the cell, resulting in reduction of the concentration and toxicity in the body (Klaassen et al. 2009). The correlation between MT expression and placental Cd concentration has been reported (Kippler et al. 2012; Phuapittayalert et al. 2016). Despite the protecting effect of MT against the Cd toxicity, the overloading of Cd in placenta can induce pathological features including syncytial knots, aggregations of syncytiotrophoblast nuclei after apoptosis (Phuapittayalert et al. 2013). A critical executioner of apoptosis is cysteine-dependent aspartate-specific proteases 3 (caspase-3) that is activated (cleaved) through either intrinsic or extrinsic pathway (El-Baz et al. 2015; Elmore 2007). The elevation in expression of cleaved caspase-3 protein was reported to induce the cell death in mouse placentas and JEG-3 cells after Cd treatment (Zhu et al. 2019). The cleavage of caspase-3 was shown to trigger such alterations as DNA fragmentation and degradation of cytoskeletal and nuclear proteins (Elmore 2007; Erboga and Kanter 2016). One method to detect the DNA fragmentation is terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) (Elmore 2007). After Cd treatment, the increment of TUNEL-positive reaction was concomitant with the rise of cleaved caspase-3 protein in mouse placenta and human trophoblast cells (Zhu et al. 2019). In contrast with elevation of apoptotic cells, the attenuation of PCNA (proliferating cell nuclear antigen) was shown to occur in placenta of Cd-injected rat (Erboga and Kanter 2016). Additionally, lamin A/C, an intermediate filament, plays a role in the nuclear envelope integrity, and it is suggested to involve in the placental apoptotic process (Botta et al. 2019). However, the association of lamin A/C expression and the placenta Cd accumulation by environmental exposure remains unelucidated.
As stated above, these apoptotic markers can be used for clarification of the mechanism behind the placental toxicity and dysfunction by Cd; therefore, we aimed to examine the expression and localization of these three apoptotic markers in human placentas of pregnant women exposed to environmental Cd in their living areas.
Materials and methods
Participants and sample collection
This study was approved by the University of Phayao Human Ethnic Committee (No. 2/073/59). The 12 of among 51 placental samples were obtained from our previous study during 2013–2016 (Phuapittayalert et al. 2016). These healthy pregnant women were residents in Mae Sot district and participants in the Antenatal Care and Labor Units of Mae Sot General Hospital, Mae Sot district, Tak province. Pregnant women who drink alcohol or smoke are not allowed to participate in this study. Then, participants read written information about this study and expressed consents with signatures on it before engaging in the experiment. At 36-week gestational age, the maternal blood and urinary samples were collected for Cd measurement using a spectroscopy (Merck, Germany) and renal function tests with an enzymatic method. Then, the pregnant women were divided into two groups according to their living areas, blood, and urinary Cd concentrations. The six participants who lived in Mae Ku, Mae Tao, and Phrathat Phadaeng subdistricts which have been reported as high Cd-contaminated areas (Simmons et al. 2005), and whose blood and urinary Cd concentrations were more than 0.5 μg/L and 2 μg/g for creatinine (Nordberg et al. 2015), were classified as a high Cd-exposed (H-Cd) group. The remaining six participants who resided in other low Cd-contaminated areas, and whose blood and urinary Cd concentrations were less than 0.5 μg/L and 2 μg/g for creatinine, were classified as a low Cd-exposed (L-Cd) group.
Postnatally, the general characteristics of newborns including the body weight (BW), the gestational age, the birth length, and the head and chest circumferences were recorded. Then, the harvested placentas were weighed and measured. To unifying placental samples for analysis of Cd contamination, the central part of each placenta was cut by carbon knife blades and then subdivided into two halves: one half was stored in metal-free cryo-tubes at −20 °C for metal analysis and the other half was fixed in 4% paraformaldehyde for immunohistochemical analyses.
Trace element analysis
The measurement of Cd concentration was performed in the same way as reported in our previous study (Phuapittayalert et al. 2016). Briefly, placental tissues of 1 g were digested with 5 mL nitric acid at 200 °C for 20 min. After cooling down, deionized water was added to adjust the volume. Finally, the placental Cd concentration was measured by the inductively coupled plasma mass spectrometer (ICP-MS) (Agilent Technologies, Japan).
Immunohistochemistry
The localizations and expressions of cleaved caspase-3 and lamin A/C proteins in the paraffin-embedded placental sections were examined using the immunohistochemical technique. Briefly, the placental embedded paraffin sections were cut at 5 μm and mounted on glass slides. The sections were then performed the antigen retrieval in citrate buffer before individually incubated at 4 °C overnight with polyclonal rabbit anti-cleaved caspase-3 or anti-cleaved lamin A/C IgG at 1:100 dilutions (Cell Signaling Technology, Massachusetts, USA) in 1% blocking reagent. The placental sections were then incubated for 1 h at room temperature with goat anti-rabbit antibody at 1:100 dilutions (Cell Signaling Technology, Massachusetts, USA) in 1% blocking reagent followed with avidin-biotin complex (ABC) kit (Vectorstain, Burlingame, UK). The positive reactions were visualized using DAB prior to counterstained with hematoxylin. The stained section with primary antibody omission was used as a negative control. For the positive control, the small intestine and liver were harvested from 6 μg TNF-α injected mice. The immunoreactivities were observed under the light microscope (Olympus, DP21, Japan), and the 20 microscopic fields per placental sections were randomly taken at 20X magnification for further measurement of the immunoreaction intensity using ImageJ software (NIH). The color detection was performed under the IHC toolbox by selection and measurement of DAB intensity area. Then, the whole area of placental section was measured and recorded. Finally, the intensity of immunoreaction per area of placental villi was calculated and further statistically analyzed.
TUNEL assay
Some sections of the placentas were stained with DeadEnd™ Colorimetric TUNEL System (Promega, Wisconsin, USA) to examine DNA fragmentation. They were then incubated with solutions of a rTdT reaction mixture, whereas, as the negative control, omission of rTdT enzyme in the reaction mixture was applied to some other sections. Additionally, sections of the placentas treated with DNase I was used as the positive control. After incubation with biotinylated nucleotides and streptavidin HRP, sites of the immuno-positive reactions were visualized by DAB. Finally, all the sections were observed under light microscope (Olympus, DP21, Japan), and 20 rectangle fields in individual sections were taken under microscope at 20X magnification for further quantification of the immunoreaction analysis by ImageJ software (NIH) as describe above.
Statistical analysis
All data were shown in mean ± standard error of the mean (SEM). The statistical analysis between the two groups was determined by non-parametric (Mann-Whitney U test) and parametric statistical (independent t-test) analyses. The correlations between two variables were then tested by Pearson or Spearman correlations. To evaluation of statistical signification, p values were set at 0.05.
Results
The general features of pregnant women and newborns
Between the L-Cd and the H-Cd groups, no significant differences were found in the mean age (L-Cd: 23.83±2.17 and H-Cd: 28.33±2.61 years, p>0.05), the body weight (L-Cd: 63.67±4.31 and H-Cd: 58.42±0.89 kg, p>0.05), the body height (L-Cd: 1.57±0.02 and H-Cd: 1.55±0.02 m2, p>0.05), the body mass index (BMI) (L-Cd: 25.79±1.80 and H-Cd: 24.40±0.80 kg/m2, p>0.05), and the number of childbearing of pregnant women (L-Cd: 1.33±0.20 and H-Cd: 1.83±0.38, p>0.05), as shown in our previous study (Phuapittayalert et al. 2016).
No significant differences were also found in the gestational age (L-Cd: 39.50±0.59 and H-Cd: 39.33±0.68 weeks, p>0.05), the body weight at birth (L-Cd: 2975±130.12 and H-Cd: 2925±81.42 g, p>0.05, the body length at birth (L-Cd: 50.83±0.52 and H-Cd: 51.83±0.76 cm, p>0.05), and the circumference of the head (L-Cd: 33.08±0.94 and H-Cd: 32.17±0.52 cm, p>0.05) and the circumference of the chest (L-Cd: 39.50±0.59 and H-Cd: 39.33±0.68 cm, p>0.05) of newborns between the two groups, as previously reported (Phuapittayalert et al. 2016).
Maternal blood and urinary Cd concentrations and their renal functions
After 36-week gestational age, the Cd concentration in blood and urine samples was measured. The blood and urinary Cd concentration in the H-Cd group was significantly higher than that in the L-Cd group (p≤0.05) (Table 1), and both values were in a positive correlation (p≤0.01, rs=0.886).
To determine the renal function of pregnant women, the creatinine values in serum and urine were measured, and the estimated glomerular filtration rate (eGFR) and estimated creatinine clearance (CCr) were calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation (Levey et al. 2009) and the Cockcroft and Gault equation (Cockcroft and Gault 1976), respectively. The mean serum creatinine value was not statistically different between the H-Cd and the L-Cd groups (p>0.05). In contrast, both urine creatinine and eGFR values were found to significantly decrease in the H-Cd group (p≤0.05) (Table 1). In addition, the decrease in the eGFR value was positively correlated with that in the urine creatinine value (p≤0.05, rs=0.683), but it was inversely related to the values of serum creatinine (p≤0.01, rs=−0.884), blood Cd (p≤0.01, rs=−0.826), and urinary Cd concentrations (p≤0.05, rs=−0.683). Finally, we found a lower value of CCr in the H-Cd (p≤0.05) (Table 1), a positive correlation between CCr and eGFR values (p≤0.01, rs=0.830), and a negative association between CCr value and serum creatinine value (p≤0.01, rs=−0.864), blood Cd (p≤0.05, rs=−0.668), and urinary Cd concentrations (p≤0.01, rs=−0.891).
Placental Cd concentrations and its characteristics
At postpartum, the mean placental weights were 491.67±58.65 and 566.67±48.72 g in the L-Cd and H-Cd groups, respectively (p>0.05). There were no differences in mean placental width, length, and thickness between the two groups (p>0.05). The ratios of birth weight to placenta weight (BW:PW ratios), which represent the sufficiency of placental nutrient transfer to the fetus, were significantly lower in the H-Cd group than those in the L-Cd group (p≤0.05) (Table 2). On the other hand, the placental Cd concentration was approximately 3.5-fold (p≤0.05) higher in the H-Cd group than those in the L-Cd group. Interestingly, the placental Cd concentrations negatively associated with the BW:PW ratios (p≤0.05, rs=−0.718).
Immunohistochemical localization findings
Cleaved caspase-3 protein
The immunoreactivity for cleaved caspase-3 protein was found in both maternal and fetal portions of the placenta.
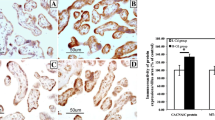
Note that the immunoreaction intensity for cleaved caspase-3 protein in chorionic villi was highest in the cytoplasm and nucleus of fetal endothelial cells, followed by the cytoplasm of syncytiotrophoblast, syncytial knot, Hofbauer cell, and cytotrophoblast in both L-Cd (Fig. 1a) and H-Cd groups (Fig. 1 b and c). In a quantitative measurement of the immunoreactivity in chorionic villi by using IHC toolbox, ImageJ software (NIH), the immunoreactivity in the H-Cd group (31.67 ± 3.90) was approximately 2-fold (p≤0.05) higher than that in the L-Cd group (16.12 ± 2.57) (Fig. 2a).
Immuno-light micrographs of cleaved caspase-3 protein expression in chorionic plate, chorionic villus, and basal plate of human placenta. Note in chorionic villi the immunoreaction in cytoplasm and nucleus of fetal endothelial cells (FEC), cytoplasm of cytotrophoblasts (CTB), syncytiotrophoblasts (STB), syncytial knots (STK), Hofbauer (H) cells, and the vasculosyncytial membrane (VSM) in L-Cd (a) and H-Cd (b and c) groups. In chorionic plate, immunoreactivity was shown in cytoplasm of chorionic mesenchymal cells (CM) and endothelial cells of chorionic vessels (ECV) in both L-Cd (d) and H-Cd (e) groups. In basal plate, the reaction was found in cytoplasm of decidual cells (DC) in L-Cd (f) whereas the immunoreactivity was shown in both cytoplasm and nucleus of decidual cells in H-Cd (g) groups. Sections of the liver harvested from mice preinjected with 6 μg TNF-α were used as a positive control; the reaction sites were seen in hepatocyte (HT) surrounding the central vein (CV) (h). Placental section processed by omitting anti-cleaved caspase-3 antibody was used as a negative control (i). Bars represent 50 μm in a and b, and 20 μm in c–i
The immunoreactivity for cleaved caspase-3 protein was quantitatively measured in both L-Cd and H-Cd groups. Note that the expression of cleaved caspase-3 protein in H-Cd group was significantly higher than in the L-Cd group (**p≤0.01) (a). In addition, the expression of cleaved caspase-3 and the placental Cd concentration were positively correlated (p≤0.05, rs=0.594) (b). In contrast, an inverse correlation between cleaved caspase-3 protein and BW:PW ratio was statistically significant (p≤0.05, rs= −0.671) (c)
Additionally, the immunoreactivity was found in the vasculo-syncytial membrane which is an interface between maternal and fetal blood circulations and formed by the syncytiotrophoblast, fetal endothelial cells, and their basal lamina (Fig. 1 b and c). In chorionic plate, the immunoreactivity for cleaved caspase-3 protein was localized predominantly in the cytoplasm and nucleus of endothelial cells of chorionic vessels, whereas it was slightly found in the cytoplasm of chorionic mesenchymal cells in both L-Cd (Fig. 1d) and H-Cd (Fig. 1e) groups.
In maternal portion of the placenta, the immunoreactivity for cleaved caspase-3 protein was mainly localized in large-round cell in the uterine decidua termed decidual cells in both L-Cd (Fig. 1f) and H-Cd (Fig. 1g) groups. However, it was localized in the cytoplasm of decidual cells in the L-Cd group, whereas it was in both cytoplasm and nucleus of decidual cells in the H-Cd group. In addition, the immunoreactivity intensity was positively correlated to the placental Cd concentration (p≤0.05, rs=0.594) (Fig. 2b). In contrast, it was inversely correlated with the BW:PW ratio (p≤0.05, rs= −0.671) (Fig. 2c).
As a positive control for the immunoreaction, sections of the liver harvested from adult mice pre-injected with 6 μg TNF-α were immunostained with the same antibody, resulting in distinct immunopositivity (Fig. 1h). In negative control experiments, omission of the primary antibody resulted in the abolition of all immunostaining (Fig. 1i).
Cleaved lamin A/C protein
The immunoreactivity for cleaved lamin A/C, representing the damage levels of the nuclear envelope, was found in almost all portions of the placenta.
In the chorionic villi, the immunoreactivity for cleaved lamin A/C of the nuclear envelope was most intense in fetal endothelial cells, followed by syncytiotrophoblasts, syncytial knots, Hofbauer cells, and cytotrophoblast in both L-Cd (Fig. 3a) and H-Cd (Fig. 3 b and c) groups.
Immuno-light micrographs of the cleaved lamin A/C protein expressed in 3 different areas: chorionic plate, chorionic villus, and basal plate. Intensely immuno-positive cells were found in nuclear envelope and cytoplasm of fetal endothelial cells (FEC), cytotrophoblasts (CTB), syncytiotrophoblasts, syncytial knots (SKT), and Hofbauer cells (H) of chorionic villus in L-Cd group (a) and H-Cd group (b and c). Weak immunoreactivities were shown in nuclear envelope and cytoplasm of chorionic mesenchymal cells of chorionic plate in both L-Cd (d) and H-Cd (e) groups. A few decidual cells were immunoreactive for cleaved lamin A/C protein in L-Cd (f) and H-Cd (g) groups. Sections of small intestine collected from mice pre-injected with 6μg TNF-α were used as the positive control; the positive cells are indicated by head arrow (h). The staining omitting anti-cleaved lamin A/C antibody was used as the negative control (i). Bars represent 50 μm in a and b, and 20 μm in c–i
In the chorionic plate, the immunoreactivity for cleaved lamin A/C was seen in the nuclear envelope and cytoplasm of chorionic mesenchymal cells in both L-Cd (Fig. 3d) and H-Cd (Fig. 3e) groups.
In basal plate, the immunoreactivity for cleaved lamin A/C protein was small in the nuclear envelope and cytoplasm of decidual cells in both L-Cd (Fig. 3f) and H-Cd (Fig. 3g) groups.
As a positive control, sections of the small intestine collecting from mice pre-injected with 6μg TNF-α were immunostained with the same antibody, resulting in distinct immunopositivity (Fig. 3h). In negative control experiments, omission of the primary antibody resulted in the abolition of all immunostaining (Fig. 3i).
In a quantitative measurement, the immunoreactivity for cleaved lamin A/C in unit areas of the chorionic villi from the L-Cd group (15.54±2.58) was significantly weaker than that from the H-Cd group (32.31±5.66) (p≤0.01) (Fig. 4a). The immunoreactivity for cleaved lamin A/C were positively correlated with that for cleaved caspase-3 protein (p≤0.05, rs=0.650) (Fig. 4b). Although the immunoreactivity for cleaved lamin A/C showed a tendency of correlation to the concentration of placental Cd, it was not statistically significant. (p=0.07, rs=0.585). In contrast, the immunoreactivity for cleaved lamin A/C was negatively correlated to the BW:PW ratio (p≤0.05, rs= −0.745) (Fig. 4c).
The lamin A/C protein immunoreactivities were quantitatively measured in L-Cd and H-Cd groups. The percentage of cleaved lamin A/C protein expression in H-Cd group were statistically increased when comparing to those in L-Cd groups (a) (p≤0.05, rs=0.650). In addition, the cleaved lamin A/C expressions were positively correlated with cleaved caspase-3 (Casp3) expressions (p≤0.05, rs=0.585) but not placental Cd (PCd) concentration (p=0.07, rs=0.585) (b). In contrast, cleaved lamin A/C immunoreactivities were inversely related to BW:PW ratios (p≤0.05, rs= −0.745) (c)
TUNEL findings
The TUNEL-positive reaction, representing DNA fragmentation, was found in chorionic villi, basal, and chorionic plates.
In chorionic villus, the reaction was predominantly found in the nucleus of fetal endothelial cells, syncytiotrophoblasts, syncytial knots, cytotrophoblasts, and Hofbauer cells of both L-Cd (Fig. 5a) and H-Cd (Fig. 5 b and c) groups. In chorionic plate, the reaction was found in the nucleus of chorionic mesenchymal cells and endothelial cell of chorionic vessels in both L-Cd (Fig. 5d) and H-Cd (Fig. 5e) groups. In the basal plate, the reaction was found in the nucleus of decidual cells in both L-Cd (Fig. 5f) and H-Cd (Fig. 5g) groups.
The TUNEL immune-positive reaction was expressed in 3 different areas of placenta including chorionic plate, chorionic villus, and basal plate. In chorionic villus, it was expressed in nucleus of fetal endothelial cells (FEC), Hofbauer cells (H), cytotrophoblasts (CTB), and syncytiotrophoblasts (STB) lining the chorionic villus and syncytial knots (STK) in both L-Cd (a) and H-Cd (b and c) groups. In chorionic plate, the reaction was found in nucleus of chorionic mesenchymal cells (CM) and endothelial cells of chorionic vessels (ECV) in both L-Cd (d) and H-Cd (e) groups. Finally, the immuno-positive reaction was in nucleus of decidual cells of basal plate in both L-Cd (f) and H-Cd (g) groups. Positive and negative control sections were shown in h and i, respectively. Bars represent 50 μm in a and b, and 20 μm in c–i
As a positive control for the TUNEL, placental sections were treated with DNase I, resulting in distinct reaction (Fig. 5h). As a negative control, rTdT Enzyme was omitted in the reaction mixture, resulting in absence of the reaction (Fig. 5i).
The mean percentage of TUNEL-positive cells in chorionic villi were significantly higher by about 2-folds in the H-Cd group (6.00± 0.99) than that in the L-Cd group (2.80±0.32) (p≤0.01) (Fig. 6a). The TUNEL reaction intensities were positively correlated to the placental Cd concentration (p≤0.01, rs=0.869), the immunoreactivities for cleaved caspase-3 (p≤0.01, rs=0.758), and for cleaved lamin A/C (p≤0.05, rs=0.705) (Fig. 6b), but negatively correlated with the BW:PW ratios (p≤0.05, rs= −0.658) (Fig. 6c).
The TUNEL reaction was quantitatively measured in both L-Cd and H-Cd groups. The increment of TUNEL reaction was shown in H-Cd group by comparing to L-Cd group (p≤0.01) (a). Additionally, the TUNEL immunoreactivities were positive correlated with placental Cd (PCd) concentrations (p≤0.01, rs=0.869), cleaved caspase-3 (Casp3) immunoreactivities (p≤0.01, rs=0.758), and cleaved lamin A/C (LMAC) immunoreactivities (p≤0.05, rs=0.705) (b) but inversely correlated with BW:PW ratios (p≤0.05, rs= −0.658) (c)
Discussion and conclusion
Major findings of the present study are a significantly higher placental Cd concentration in pregnant women in the H-Cd group than that in the L-Cd group, a positive correlation of the expression levels of such apoptotic markers as cleave caspase-3, cleaved lamin A/C, and the TUNEL reaction in chorionic villi with the placental Cd concentration, and an inverse correlation of their expression levels and the placental Cd concentration levels with the BW:PW ratio.
A previous study by others has reported the Cd concentration at high levels in blood and urine of pregnant women who lived in such high Cd contaminated areas as Mae Ku, Mae Tao, and Prathat Phadaeng subdistricts, Mae Sot district, Tak province where a high Cd level was detected in paddy soil and rice grain (Simmons et al. 2005). It was thus suggested that environmental Cd might be transferred to human body via the food chain, especially rice intake (Swaddiwudhipong et al. 2007). A previous study reported that a long-term environmental or occupational exposure to Cd can induce kidney damages (Järup et al. 2000). Serum creatinine, eGFR, and CCr are usually used as biomarkers for the kidney function (Levey et al. 2009; Cockcroft and Gault 1976). In our study, even though the serum creatinine values were not statistically different between the two groups, the values of the eGFR and CCr decreased in the H-Cd group and both values were inversely related to the blood Cd concentrations. After Cd exposure, the decrease of eGFR indicates the lower glomerular function, and a decreased value of eGFR less than 60 mL/min/1.73m2 was related to an increased risk for nephrotoxicity (Akesson et al. 2005). Although the value of eGFR in our participants was greater than 90 mL/min/1.73m2, which is defined as the normal condition, the high blood Cd level in the H-Cd group was regarded as a sign of a lower glomerular function as already demonstrated an early sign of adverse effects on kidney function (Akesson et al. 2005). A more reduced value of eGFR has been reported in a population who were co-exposed to Cd and lead and associated with a progression of chronic kidney diseases in the United States (Navas-Acien et al. 2009). The association of decreased eGFR value with a high blood Cd concentration has been shown in Korean women but not in men; therefore, Cd-induced renal damages might be different between genders (Hwangbo et al. 2011). Therefore, it is reasonable to infer that pregnant women who had high blood Cd and lower eGFR values in our study might be more susceptible to Cd-induced lower function of the kidney. It was further suggested that, after Cd uptake into the body, maternal blood Cd can be transferred across placenta to the fetus via due metal transporters in a competitive manner with other metals, resulting in an essential metal deficiency (Kippler et al. 2012; Phuapittayalert et al. 2016; Somsuan et al. 2019) and low birth weight neonates (Kippler et al. 2012).
In normal condition, the BW:PW ratio represents a placental function to maintain a sufficient nutrient supply to the fetus (Salavati et al. 2018), and its mean value in newborns is 6:1 (Cunningham et al. 2010). In contrast, the BW:PW in H-Cd group was 5.15:1, significantly lower than the normal value, in the present study. Alterations in the BW:PW ratio have previously been reported under various conditions. A higher BW:PW ratio has been shown in newborns delivered from diabetic pregnant women, suggesting that the placental and fetal growths were disturbed by an increasing maternal glycemic level (Gloria-Bottini et al. 2016) and the maternal insulin resistance (Taricco et al. 2003). On the contrary, a lower BW:PW ratio has been demonstrated in diabetic and smoking pregnant women, suggesting that the hypoxia and oxidative stress represent a proposed mechanism behind this inappropriate ratio (Ganer Herman et al. 2017). Moreover, our study have shown an inverse correlation of placental Cd concentrations and the BW:PW ratios. Therefore, we suggest that Cd might induce the oxidative stress and hypoxia in the placenta and then disturb the placental function of nutrient transfer to the fetus.
After Cd uptake into the cell, MT protein increases and binds with Cd to form Cd-MT complex, by which the toxicity of Cd is prevented, and this trapping into the cell reduces the distribution of Cd (Klaassen et al. 2009). Therefore, MT has frequently been used as a biomarker of Cd accumulation in several organs including placenta (Kippler et al. 2012). Our previous study has shown upregulations of MT mRNA and protein in high Cd-accumulated placentas and a positive correlation between the MT expression and the placental Cd concentration (Phuapittayalert et al. 2016). In our previous study (Phuapittayalert et al. 2016), MT protein was shown to be predominantly localized in syncytiotrophoblasts and endothelial cells in the placenta of the H-Cd group. In the present study, the same cell species of the placenta as the previous study was positive for apoptosis markers including cleaved caspase-3, cleaved lamin A/C, and TUNEL. Moreover, we found the significant correlations between quantitative MT expression and quantitative positivity for apoptotic markers (data not shown). It is suggested that placental Cd induced the elevation of MT expression to prevent Cd toxicity, and that the overloading of Cd to MT triggers the placental apoptosis. The Cd-induced placental morphological changes have been reported such as a reduction of trophoblast cells (Lee et al. 2009), decreases in villous/placental volumes and fetal capillary volume (Bush et al. 2000), and increases of syncytial knots, nucleus aggregations of syncytiotrophoblasts undergoing apoptosis (Phuapittayalert et al. 2013).
The expression of cleaved caspase-3, a key executioner caspase, has frequently been used as an apoptotic marker in several organs including the placenta (El-Baz et al. 2015; Selvaratnam et al. 2013). In our present study, cleaved caspase-3 protein, which was detected more highly in the H-Cd group, was localized in different areas of the placenta, fetal endothelial cells, and syncytiotrophoblasts of chorionic villi with the former more dominant. The latter two cells form the vasculo-syncytial membrane (VSM) that plays an important role in regulation of the gas and nutrients transport between the mother and the fetus (Huppertz 2008). Additionally, our present study showed a positive correlation between cleaved caspase-3 protein expression and placental Cd concentration. Thus, we suggest that the environmental Cd exposure might interfere the structure and function of VSM, resulting in Cd transport across the placenta to the fetus. In support of this suggestion, the upregulation of caspase-3 protein has been reported in the placenta of CdCl2-administrated mice by others (Zhu et al. 2019), and a positive correlation has been reported between the caspase-3 protein expression and the heavy metal concentration in intrauterine growth retardation (IUGR) placenta, suggesting that the Cd-activated caspase-3 protein might be one of the proposed mechanisms behind the IUGR condition (El-Baz et al. 2015).
In view of the dominant expression of cleaved lamin A/C protein in fetal endothelial cells and syncytiotrophoblasts and the positive correlation of lamin A/C protein expression with cleaved caspase-3 expression in the present study, it might be shown that the Cd in placenta activated the caspase-3 protein and then it induced the breakdown of nuclear envelope in placental cells. In this regard, several previous studies by others should be noted: caspase-3 has been shown to be involved in expression of cleaved lamin A/C and B during breast cancer cell apoptosis (Kivinen et al. 2005), and the expression of caspase-3 expressions has been shown to increase in atrioventricular nodal myocyte from lamin A/C mutant mice when compared with wild-type mice. These findings suggest that diminished lamin A/C level regulates the apoptotic process in the conduction system of heart (Wolf et al. 2008). Additionally, the promotion of cellular aging in fibroblasts has been shown to be induced by oxidative damage or loss of C-terminal cysteine residues of lamin A (Pekovic et al. 2011). In another study using SW480 cells, the cleavage of lamin A, B1, and B2 has been shown after CdCl2 treatment but not sodium arsenite, suggesting that CdCl2 is involved in promotion of the protease function (Hashimoto et al. 2017). The other study has shown that lamin B1-GFP (green fluorescent protein) molecule is localized on the nuclear membrane in normal cells, while lamin A-GFP molecule is localized in the nucleoplasm and cytoplasm during apoptosis, suggesting that a partial cleavage of lamin A is involved in complete disintegration of the nuclear membrane (Broers et al. 2002).
Not only the disintegration of the nuclear envelope but also the breakdown of DNA occurs during apoptosis (Elmore 2007). In our present study, TUNEL reaction, one of DNA fragmentation, was predominantly found in the nucleus of fetal endothelial cells, syncytiotrophoblasts, and decidual cells. In addition, the reaction density of TUNEL-positive cells was positively correlated with placental Cd concentration and the intensity of immunoreactivities for cleaved lamin A/C and cleaved caspase-3. The increased immunoreactions for TUNEL and cleaved caspase-3 protein have been reported to occur in the testis of mouse offspring, suggesting that the parental Cd exposure could affect the testicular development of male offspring (Nan et al. 2020). In addition, the elevation of TUNEL-positive number has been reported to occur in mouse placentas and JEG-3 cells after Cd treatment, but apoptotic cells have been shown to decrease after activation of autophagy via p62-mediated caspase-9 degradation, suggesting that Cd induces cell apoptosis via caspase-9-dependent mitochondrial signaling pathway in placental trophoblasts (Zhu et al. 2019). During increment of apoptotic cells, the PCNA, a proliferating cell nuclear antigen, has been reported to diminish in mouse placenta after Cd treatment, suggesting that Cd exposure throughout pregnancy could induce the placental damage by suppressing the trophoblast proliferation (Erboga and Kanter 2016). Therefore, we suggested that environmental Cd exposure could activate caspase-3 protein-mediated DNA fragmentation, resulting in placental damages by suppression of the trophoblast proliferation. It was further suggested that the effect of environmental Cd exposure on PCNA expression in human placenta should be evaluated.
Collectively, we conclude that the exposure to environmental Cd throughout pregnancy induces uptake of Cd into the body, and its effects on maternal kidney functions, and then its accumulation in the placenta, resulting in induction of apoptosis and functional disturbance in the placenta.
Data availability
Not applicable.
References
Akesson A et al (2005) Tubular and glomerular kidney effects in Swedish women with low environmental cadmium exposure. Environ Health Perspect 113:1627–1631. https://doi.org/10.1289/ehp.8033
Botta C, Pellegrini G, Hässig M, Pesch T, Prähauser B, Wunderlin S, Guscetti F, Schneeberger M, Schmitt S, Basso W, Hilbe M, Schuler G, Borel N (2019) Bovine fetal placenta during pregnancy and the postpartum period. Vet Pathol 56:248–258. https://doi.org/10.1177/0300985818806453
Broers JL, Bronnenberg NM, Kuijpers HJ, Schutte B, Hutchison CJ, Ramaekers FC (2002) Partial cleavage of A-type lamins concurs with their total disintegration from the nuclear lamina during apoptosis. Eur J Cell Biol 81:677–691. https://doi.org/10.1078/0171-9335-00282
Bush PG, Mayhew TM, Abramovich DR, Aggett PJ, Burke MD, Page KR (2000) A quantitative study on the effects of maternal smoking on placental morphology and cadmium concentration. Placenta 21:247–256. https://doi.org/10.1053/plac.1999.0470
Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41. https://doi.org/10.1159/000180580
Cunningham F, Leveno K, Bloom S, Hauth J, Rouse D, Spong C (2010) Chapter 3: implantation, embryogenesis, and placental development. In: Cunningham F, Leveno K, Bloom S, Hauth J, Rouse D, Spong C (eds) Williams obstetrics, 23rd edn. McGraw-Hill, New York, pp 36–77
El-Baz MA, El-Deeb TS, El-Noweihi AM, Mohany KM, Shaaban OM, Abbas AM (2015) Environmental factors and apoptotic indices in patients with intrauterine growth retardation: a nested case-control study. Environ Toxicol Pharmacol 39:589–596. https://doi.org/10.1016/j.etap.2015.01.009
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35:495–516. https://doi.org/10.1080/01926230701320337
Erboga M, Kanter M (2016) Effect of cadmium on trophoblast cell proliferation and apoptosis in different gestation periods of rat placenta. Biol Trace Elem Res 169:285–293. https://doi.org/10.1007/s12011-015-0439-8
Ganer Herman H, Miremberg H, Schreiber L, Bar J, Kovo M (2017) The association between disproportionate birth weight to placental weight ratio, clinical outcome, and placental histopathological lesions. Fetal Diagn Ther 41:300–306. https://doi.org/10.1159/000448949
Gloria-Bottini F, Neri A, Coppeta L, Magrini A, Bottini E (2016) Correlation between birth weight and placental weight in healthy and diabetic puerperae Taiwanese. J Obstet Gynecol 55:697–699. https://doi.org/10.1016/j.tjog.2015.03.013
Hashimoto K, Majumdar R, Tsuji Y (2017) Nuclear lamins and progerin are dispensable for antioxidant Nrf2 response to arsenic and cadmium. Cell Signal 33:69–78. https://doi.org/10.1016/j.cellsig.2017.02.012
Huppertz B (2008) The anatomy of the normal placenta. J Clin Pathol 61:1296–1302. https://doi.org/10.1136/jcp.2008.055277
Hwangbo Y, Weaver VM, Tellez-Plaza M, Guallar E, Lee BK, Navas-Acien A (2011) Blood cadmium and estimated glomerular filtration rate in Korean adults. Environ Health Perspect 119:1800–1805. https://doi.org/10.1289/ehp.1003054
Järup L, Hellström L, Alfvén T, Carlsson MD, Grubb A, Persson B, Pettersson C, Spång G, Schütz A, Elinder CG (2000) Low level exposure to cadmium and early kidney damage: the OSCAR study. Occup Environ Med 57:668–672. https://doi.org/10.1136/oem.57.10.668
Kippler M, Tofail F, Gardner R, Rahman A, Hamadani JD, Bottai M, Vahter M (2012) Maternal cadmium exposure during pregnancy and size at birth: a prospective cohort study. Environ Health Perspect 120:284–289. https://doi.org/10.1289/ehp.1103711
Kivinen K, Kallajoki M, Taimen P (2005) Caspase-3 is required in the apoptotic disintegration of the nuclear matrix. Exp Cell Res 311:62–73. https://doi.org/10.1016/j.yexcr.2005.08.006
Klaassen CD, Liu J, Diwan BA (2009) Metallothionein protection of cadmium toxicity. Toxicol Appl Pharmacol 238:215–220. https://doi.org/10.1016/j.taap.2009.03.026
Lee CK, Lee JT, Yu SJ, Kang SG, Moon CS, Choi YH, Kim JH, Kim DH, Son BC, Lee CH, Kim HD, Ahn JH (2009) Effects of cadmium on the expression of placental lactogens and Pit-1 genes in the rat placental trophoblast cells. Mol Cell Endocrinol 298:11–18. https://doi.org/10.1016/j.mce.2008.09.028
Levey AS, Stevens LA, Schmid CH, Zhang Y(L), Castro AF III, Feldman HI, Kusek JW, Eggers P, van Lente F, Greene T, Coresh J, for the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150:604–612. https://doi.org/10.7326/0003-4819-150-9-200905050-00006
Lyengar GV, Rapp A (2001) Human placenta as a “dual” biomarker for monitoring fetal and maternal environment with special reference to potentially toxic trace elements. Part 3: toxic trace elements in placenta and placenta as a biomarker for these elements. Sci Total Environ 280:221–238. https://doi.org/10.1016/s0048-9697(01)00827-0
Mukherjee R et al (2010) Melatonin protects against alterations in hippocampal cholinergic system, trace metals and oxidative stress induced by gestational and lactational exposure to cadmium. EXCLI J 9:119–132
Myllynen P, Pasanen M, Pelkonen O (2005) Human placenta: a human organ for developmental toxicology research and biomonitoring. Placenta 26:361–371. https://doi.org/10.1016/j.placenta.2004.09.006
Nan Y, Yi SJ, Zhu HL, Xiong YW, Shi XT, Cao XL, Zhang C, Gao L, Zhao LL, Zhang J, Xu DX, Wang H (2020) Paternal cadmium exposure increases the susceptibility to diet-induced testicular injury and spermatogenic disorders in mouse offspring. Chemosphere 246:125776. https://doi.org/10.1016/j.chemosphere.2019.125776
Navas-Acien A, Tellez-Plaza M, Guallar E, Muntner P, Silbergeld E, Jaar B, Weaver V (2009) Blood cadmium and lead and chronic kidney disease in US adults: a joint analysis. Am J Epidemiol 170:1156–1164. https://doi.org/10.1093/aje/kwp248
Nordberg GF, Nogawa K, Nordberg M (2015) Chapter 32—cadmium. In: Nordberg GF, Fowler BA, Nordberg M (eds) Handbook on the Toxicology of Metals, Fourth edn. Academic Press, San Diego, pp 667–716. https://doi.org/10.1016/B978-0-444-59453-2.00032-9
Pekovic V, Gibbs-Seymour I, Markiewicz E, Alzoghaibi F, Benham AM, Edwards R, Wenhert M, von Zglinicki T, Hutchison CJ (2011) Conserved cysteine residues in the mammalian lamin A tail are essential for cellular responses to ROS generation. Aging Cell 10:1067–1079. https://doi.org/10.1111/j.1474-9726.2011.00750.x
Phuapittayalert L, Norkaew T, Supanpaiboon W, Chuenchoojit S, Hipkeao W, Swaddiwudhipong W, Sakulsak N (2013) Increasing of syncytial knot and fibrinoid deposit in high-Cd accumulated human placentas. Int J Morphol 31:1210–1215
Phuapittayalert L, Saenganantakarn P, Supanpaiboon W, Cheunchoojit S, Hipkaeo W, Sakulsak N (2016) Increasing CACNA1C expression in placenta containing high Cd level: an implication of Cd toxicity. Environ Sci Pollut Res Int 23:24592–24600. https://doi.org/10.1007/s11356-016-7841-4
Salavati N, Gordijn SJ, Sovio U, Zill-E-Huma R, Gebril A, Charnock-Jones DS, Scherjon SA, Smith GCS (2018) Birth weight to placenta weight ratio and its relationship to ultrasonic measurements, maternal and neonatal morbidity: a prospective cohort study of nulliparous women. Placenta 63:45–52. https://doi.org/10.1016/j.placenta.2017.11.008
Satarug S, Boonprasert K, Gobe GC, Ruenweerayut R, Johnson DW, Na-Bangchang K, Vesey DA (2019) Chronic exposure to cadmium is associated with a marked reduction in glomerular filtration rate. Clin Kidney J 12:468–475. https://doi.org/10.1093/ckj/sfy113
Selvaratnam J, Guan H, Koropatnick J, Yang K (2013) Metallothionein-I- and -II-deficient mice display increased susceptibility to cadmium-induced fetal growth restriction. Am J Physiol Endocrinol Metab 305:E727–E735. https://doi.org/10.1152/ajpendo.00157.2013
Simmons RW, Pongsakul P, Saiyasitpanich D, Klinphoklap S (2005) Elevated levels of cadmium and zinc in paddy soils and elevated levels of cadmium in rice grain downstream of a zinc mineralized area in Thailand: implications for public health. Environ Geochem Health 27:501–511. https://doi.org/10.1007/s10653-005-7857-z
Somsuan K, Phuapittayalert L, Srithongchai Y, Sonthi P, Supanpaiboon W, Hipkaeo W, Sakulsak N (2019) Increased DMT-1 expression in placentas of women living in high-Cd-contaminated areas of Thailand. Environ Sci Pollut Res Int 26:141–151. https://doi.org/10.1007/s11356-018-3598-2
Suwatvitayakorn P, Ko MS, Kim KW, Chanpiwat P (2019) Human health risk assessment of cadmium exposure through rice consumption in cadmium-contaminated areas of the Mae Tao sub-district, Tak, Thailand. Environ Geochem Health 42:2331–2344. https://doi.org/10.1007/s10653-019-00410-7
Swaddiwudhipong W, Limpatanachote P, Mahasakpan P, Krintratun S, Padungtod C (2007) Cadmium-exposed population in Mae Sot District, Tak Province: 1. Prevalence of high urinary cadmium levels in the adults. J Med Assoc Thail 90:143–148
Taricco E, Radaelli T, Nobile de Santis MS, Cetin I (2003) Foetal and placental weights in relation to maternal characteristics in gestational diabetes. Placenta 24:343–347. https://doi.org/10.1053/plac.2002.0913
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Heavy metal toxicity and the environment. Exp Suppl 101:133–164. https://doi.org/10.1007/978-3-7643-8340-4_6
Wolf CM, Wang L, Alcalai R, Pizard A, Burgon PG, Ahmad F, Sherwood M, Branco DM, Wakimoto H, Fishman GI, See V, Stewart CL, Conner DA, Berul CI, Seidman CE, Seidman JG (2008) Lamin A/C haploinsufficiency causes dilated cardiomyopathy and apoptosis-triggered cardiac conduction system disease. J Mol Cell Cardiol 44:293–303. https://doi.org/10.1016/j.yjmcc.2007.11.008
Yu R, He L, Cai R, Li B, Li Z, Yang K (2017) Heavy metal pollution and health risk in China. Glob Health J 1:47–55. https://doi.org/10.1016/S2414-6447(19)30059-4
Zhu HL et al (2019) Activation of autophagy inhibits cadmium-triggered apoptosis in human placental trophoblasts and mouse placenta. Environ Pollut (Barking, Essex: 1987 254:112991. https://doi.org/10.1016/j.envpol.2019.11299
Acknowledgements
We would like to thank Dr. Witaya Swaddiwudhipong and staffs of Mae Sot General Hospital for the collaboration and sample collection. Additionally, we are grateful to Professor Dr. Thomas Brunner, Department of Biology, University of Konstanz in supply of sections of the liver and small intestine from mice pre-injected with 6μg TNF-α used as specimens for positive control in this study. Finally, we appreciate the School of Medical Science, University of Phayao for the generous support. We acknowledge the Overseas resident Professor Hisatake Kondo in Khon Kaen University for editing the manuscript.
Funding
This study was granted by the School of Medical Science, University of Phayao (Project Nos. MS612001 and MS612002).
Author information
Authors and Affiliations
Contributions
Nathamon Tanasrivaroottanun performed the placental tissue processing and sectioning. Wisa Supanpaiboon conducted the metal analysis. Wiphawi Hipkaeo and Natthiya Sakulsak designed the methodology. Laorrat Phuapittayalert investigated the immunohistochemistry technique, TUNEL assay, data analysis, and was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the University of Phayao Human Ethnic Committee, University of Phayao, Thailand (No. 2/073/59). All participants have read the information sheet and signed a consent form before engaging in the experiment.
Consent for publication
Not applicable.
Conflict of interests
The authors declare no competing interests.
Additional information
Responsible Editor: Lotfi Aleya
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Phuapittayalert, L., Tanasrivaroottanun, N., Hipkaeo, W. et al. Increased expression of apoptotic markers in human full-term placenta after exposure to elevated environmental cadmium levels during pregnancy. Environ Sci Pollut Res 28, 51795–51807 (2021). https://doi.org/10.1007/s11356-021-14431-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-14431-2