Abstract
Selenium is an essential mineral naturally found in soil, water, and some of the food and is required as essential elements in human and animal body. Se supplementation is required especially for those having Se deficiency. Food supplement of selenium has several forms such as selenocysteine, selenite, selenomethionine, and selenate. Recently, Se supplement as selenium nanoparticles (SeNPs) has gained worldwide attention due to its bioactivities and properties. In the present study, we determined the potential hepatotoxicity of nano and bulk selenium using low and high doses in mice. Twenty-five Swiss albino mice (n=5) were randomly divided into 5 groups and treated orally for 28 days: Group 1: sterile saline (0.9%) as a control; Group 2: sodium selenite (1mg/kg); Group 3: sodium selenite (4mg/kg); Group 4: selenium nanoparticles (1mg/kg); and Group 5: selenium nanoparticles (4mg/kg). Administration of nano-selenium (70–90 nm) led to an increase in the activities of serum transaminases (ALT and AST), while no significant effects were noted on biochemical variables indicative of changes in heme synthesis pathway and oxidative stress like blood δ-aminolevulinic acid dehydratase (δ-ALAD), hepatic reactive oxygen species (ROS), catalase activity, superoxide dismutase (SOD), malondialdehyde assay (MDA), reduced glutathione (GSH) and oxidized glutathione (GSSG), glutathione peroxidase (GPx) compared to controls, and a high dose of sodium selenite. Our results suggest that nano-selenium at low dose (1mg/kg) exhibited antioxidant effects in the liver compared to the high dose (4mg/kg) of SeNPs and sodium selenite (1 and 4 mg/kg). The data from the present study might be useful for pharmacologists and toxicologists in providing future directions while designing selenium-based therapeutic strategies.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Selenium (Se) is a micronutrient that is essential for proper physiological function. It is an important nutritional trace element, and plays a significant role in the catalytic activity of glutathione peroxidase (GPx) and glutathione reductase (GR). It also helps in improving the immune system and maintains redox balance of the body (Amin et al. 2017). Selenium uptake occurs from food or water in the form of selenite, selenate, selenocysteine, and selenomethionine and has been associated with different selenoproteins in the body (Amini and Pirhajati Mahabadi 2018).
The total amount of selenium in an adult human varies from 10–20 mg and depends on various factors including geographical location or health status of the person (Kieliszek 2019). The Recommended Dietary Allowance (RDA) for Se is 55 μg for adults/day (Zwolak and Zaporowska 2012). It has been reported that gastrointestinal absorption in animals and humans for various Se compounds ranges from 45 to 95% of the ingested dose (Benko et al. 2012). The deficiency of selenium in the diet causes muscular dystrophy, cardiomyopathy, and chronic degenerative diseases (Amin et al. 2017). The upper tolerable intake level is 300 μg/day. Excessive Se causes toxicity and symptoms like gastrointestinal disorders, irritability, fatigue, hair loss, nail brittleness, dizziness, and nausea. The acute toxicity of selenium in humans has been reported to cause nausea, vomiting, and gastrointestinal disorder. In animals, selenium toxicity is associated with thickening of hair and degenerative changes in hooves and blind stagger (Hadrup et al. 2019). Oral Se exposure has been reported to cause hepatic and renal damage in rodents. The liver and kidney are known to be the major target organs of Se (Chen and Tappel 1995), the two major target organs of Se absorption. It is thus clear that Se at the lower level is beneficial, while at moderately high level, it may lead to toxic effects. Selenium at the sub nutritional dose acts also as an antioxidant while it is prooxidant at the supra-nutritional dose (Majeed et al. 2018). It is widely known that Se at the low dose is involved in the synthesis of glutathione peroxidase and thioredoxins (Venardos et al. 2004), few important antioxidants for preventing oxidative stress (Khurana et al. 2019). On the other hand, the higher dose of selenium is known to cause toxicity to the body (Maiyo and Singh 2017). At super nutrient dose, the ingestion of selenite is reduced by GSH leading to the formation of CH3Se− which reacts with oxygen to produce CH3Se− and ROS radicals.
Selenium nanoparticles have recently been reported to be preferred in various therapeutic applications in place of bulk selenium which has a wide therapeutic window. LD50 of sodium selenite and selenium nanoparticles was found to be 15.37mg and 113.87 mg, respectively (Bhattacharjee et al. 2014, Zhang et al. 2001). Selenium nanoparticles are widely used in the treatment of arthritis, cancer, diabetics, etc. (Guan et al. 2018; Khurana et al. 2019). Zhao et al. (2018) reported that SeNPs are more efficacious than selenite in the production of ROS. Multiple steps are needed in the reduction of selenite to selenide anion, while for SeNPs, only one step is required for the reduction of elemental selenium atom to selenide anion to trigger redox cycling with oxygen to produce ROS (Zhao et al. 2018). Nanomedicine thus might play an important role in the treatment of liver diseases (Sharma et al. 2019). However, it is equally important to evaluate the potential toxicity of the nanoform of potential therapeutics or preventive agent(s) as they are capable of crossing various cellular barriers leading to possible damage to tissues.
In the current study, we thus determined the dose-dependent effects of nano and bulk selenium on mouse liver along with its antioxidant and prooxidant effects following 28 days exposure.
Materials and method
Chemicals
Selenium nanoparticles were purchased from Nanoshel, Pvt. Ltd, while sodium selenite was procured from Sigma–Aldrich Chemicals Co., St. Louis (USA). All other chemicals used in the study were of analytical grade.
Characterization of Se nanoparticles
Characterization of selenium nanoparticles was done using transmission electron microscopy (TEM), dynamic light scattering (DLS) measurements, and zeta potential. The average particle size and size distribution of SeNPs were determined using a Nanosizer 90 ZS system (Malvern Instruments, Southborough, MA, USA). The measurements were carried out using a disposable cuvette at 25°C. Prepared nanoparticles were diluted with triple distilled water and measured using a Zetasizer. One drop of SeNPs was put on the carbon-coated copper grid, and an excess amount of sample was removed carefully followed by negative staining using 2% phosphotungstic acid, air-dried the samples in vacuum desiccator, and observed under the transmission electron microscope (Naqvi et al. 2010).
Animals
Adult (5–6 weeks old), Swiss albino male mice (body weight of 22–25g) were procured from the animal facility of CSIR-Indian Institute of Toxicology Research, Lucknow, India. Before the start of the experiment, the mice were acclimatized for 1 week in the institute’s animal house at the room temperature of (25 ± 2°C) with 12 h light/dark cycles. The mice were provided free access to the standard chow pellet diet (Altromin, Germany) and drinking water. The feed contained diet components such as metabolized energy, crude nutrients and moisture, carbohydrates, minerals, trace elements, added vitamins, amino acids, and fatty acid. The animals admitted humane care as per the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India, and prior permission was permitted from the Institutional Animal Ethics Committee of the National Institute of Pharmaceutical Education and Research, Raebareli (Approval # NIPER/RBL/IAEC/42/August 2019).
Experimental design
All the animals were randomly divided into 5 groups of 5 animals each and were orally administered for 28 days as below:
-
Group1: Sterile saline (0.9%) as a control (orally)
-
Group 2: Sodium selenite, 1mg/kg, as a low dose group
-
Group 3: Sodium selenite, 4mg/kg, as a high dose group
-
Group 4: Selenium nanoparticles, 1mg/kg as a low dose
-
Group 5: Selenium nanoparticles, 4mg/kg, as a high dose group
Blood and tissue sample collection
The mice were sacrificed after 28 days of exposure by cervical dislocation and blood was collected by retro-orbital puncture and transferred in heparinized tubes that were used for estimation of haematological parameters like (Hb, PCV, and ALAD activity). Heparinized blood was centrifuged for 15 min at 2500 rpm to collect sera that were used for the estimation of liver enzymes and other biochemical parameters. The liver was excised from the body and rinsed in ice-cold saline. Organ weight was recorded relative to body weight after wiping with blotting paper. The 10% (w/v) homogenate of the liver was prepared by homogenizing at 2000g and centrifugation at 10,000g for 15min, and the supernatant was collected for estimation of various biochemical parameters.
Haematological parameters estimation
Estimation of total haemoglobin content (Hb)
The total haemoglobin content was measured following Clegg and King (1942). The blood was taken with the help of a pipette up to the 20 μl mark and added to the haemoglobin meter tube. The 0.1N HCL was added up to the 20% mark into the tube. The water was added by dropper into the tube and mixed with the help of a dropper. The addition of water was stopped until the tube colour was matched with standard haemoglobin tubes in the stands (Ghai 2012).
Estimation of Packed cell volume (PCV)
0.5 ml of blood was taken in a Wintrobe tube using a pipette. Blood was drawn into the tube carefully assuring that the pipette reached the base of the tube and removing it gently. The tube was centrifuged for 30 min. at 15,000 g. The layers were separated and the percentage PCV was calculated by dividing the total volume of RBC by the whole blood (Ghai 2012).
Estimation of δ-aminolevulinic acid dehydratase (ALAD) activity
ALAD activity was determined using the method of Berlin and Schaller (1974). A total of 0.2 ml of heparinized blood was mixed with 1.3 ml of distilled water and incubated for 37°C for 10 min in an experimented tube and blank tube. The standard ALA (1 ml) was added to the experimented tube, while 1 ml of trichloroacetic acid (TCA) was added in the blank tube and incubated for 37°C for 1h. The reaction was stopped by adding 1 ml of TCA to the experimented tube and 1ml of ALA to the blank tube. The mixture was centrifuged and 1 ml of aliquot was taken in the test tube to the tube containing an equal volume of Ehrlich reagent. After 5 min, absorbance was measured at 555 nm.
Biochemical estimation
Liver function test
Serum alanine transaminase (ALT) and aspartate transaminase (AST) activities were determined following the method of Reitman and Frankel (1957). The assay system contained 1ml buffer/substrate solution, 0.1ml of serum, and was incubated for exactly 60min (for ALT) and 30min for AST at 37°C in a water-bath. One millilitre chromogen solution was added, mixed, and allowed to stand for 20 min at room temperature and 10 ml of 0.4 n NaOH was added subsequently. The extinction was read at 505 nm against blank. The controls were run in parallel, the substrate being added after deproteinization.
Determination of proteins
The protein concentration in tissue was determined following Folin’s Ciocalteau phenol reagent (Lowry et al. 1951). The reagent C which is the alkaline copper reagent [Reagent C = 50ml = 50ml of Reagent A (2% sodium carbonate in 0.1 N NaOH) + 1ml of Reagent B (0.5% copper sulphate in 1% sodium potassium tartrate)] of 1ml was added to the 200μl of tissue homogenates and incubated at the room temperature for 10 min. After adding reagent C, the Folin’s Ciocalteau phenol reagent (Folin’s Ciocalteau phenol reagent with water at the ratio of 1:1) of 100 μl was added to the mixture and incubated in dark at RT for 30 min. Bovine serum albumin (BSA) was used as the standard in the range of 10–640 μg which was also treated in the same manner as described above. The blue colour was developed and absorbance was determined at 660nm (Lowry et al. 1951).
Determination of reactive oxygen species (ROS)
The reactive oxygen species in the tissue sample was determined by the reported fluorometric method with some minor modifications. The reaction mixture was prepared by taking 5 μl of 5 μM 2,7-dichlorofluorescein (DCFDA) and added to the tissue supernatant of 10 μl and preceded with the addition of 885 μl of distilled water. The resultant mixture was incubated for 30 min which allows the DCFDA to get converted into DCF, and the fluorescence was recorded at 485/525 nm. The standard plot for DCF was prepared in the range of (10,000 to 0.01nm) and processed in the similar manner as described as above. The results were expressed as fluorescence units per mg of protein (Socci et al. 1999).
Determination of thiobarbituric acid reactive substances (TBARS)
The amount of MDA in the tissue sample was determined by the TBARS assay (Ohkawa et al. 1979). The lipid contents were determined by adding 750 μl of 0.8% thiobarbituric acid to the 100 μl of supernatant (10% homogenates), 100 μl of 8.1% sodium dodecyl sulphate (SDS) solution, and 750 μl of 20% acetic acid, and 300 μl of distilled water were added. Then the mixture was kept in a water bath at 95°C for 1h. A pink colour complex was observed in the samples and the absorbance was determined at 535nm. The standard was prepared by 100 μM tetraethoxypropane in the range of 5–80 μM and was treated similarly as samples (Ohkawa et al. 1979).
Determination of superoxide dismutase (SOD)
Superoxide dismutase activity was measured following the method of Kakkar et al. (1984). A total of 1.2 ml of sodium pyrophosphate (0.052 M, pH 8.3) was added in 0.2ml of supernatant, and then 0.3 mL phenazine methosulphate (186 μM), 0.3 ml of nitro blue tetrazolium (300 μM), 0.2ml nicotinamide adenine dinucleotide (780mM), and 0.2 ml of distilled water were added in a test tube. The mixture was incubated at 30°C for 90 s. The reaction was ceased by adding 1ml of glacial acetic acid within 90 s and the violet colour complex formed was determined at the absorbance of 560nm. For blank, 0.2ml of distilled water was used instead of supernatant and the above described process was followed (Kakkar et al. 1984).
Determination of Catalase activity
Catalase activity was determined by Sinha (1972). The assay was performed by adding 1ml of phosphate buffer (0.01M, pH 7.7) to the 100 μl of the supernatant; later, 500 μl distilled water and 500 μl of 0.2 M H2O2 were added. The resultant mixture was incubated at 37°C for 1min. The reaction was ceased by the addition of 2ml of % potassium dichromate and acetic acid in the ratio of 1:3 and kept in a boiling water bath at 95°C for 10 min. After cooling, the mixture absorbance was measured at 570nm. The standard was prepared from 0.2M hydrogen peroxide (H2O2) in the range of (2–64μm) (Sinha 1972).
Determination of reduced glutathione assay (GSH)
The reduced glutathione (GSH) was measured using the fluorometric method. The tissue homogenate was prepared by weighing 250 mg of tissue in 3.75ml of phosphate EDTA buffer and 1 mL of 25% HPO3 (for precipitating proteins). The mixture was centrifuged for 30min. at 10,000g at 4°C and the supernatant was separated. For GSH, 0.5ml of supernatant was dissolved in 4.5 ml of phosphate EDTA buffer, pH 8.0. The mixture was vortexed. Take 100 μl of the above mixture and mixed with 1.8ml phosphate EDTA buffer and 100μl of o-Phthalaldehyde solution (prepared in absolute methanol). The resultant mixture was incubated at room temperature for 15min. The standards were prepared from 10μm GSH solution in the range of (0.2–6.4μm) and performed in similar manners as the sample. The fluorescence was measured at the emission of 420nm and excitation of 350nm (Hissin and Hilf 1976).
Determination of oxidized glutathione assay (GSSG)
The oxidized glutathione was measured following the fluorometric method. The tissue homogenization process involved in this assay was similar to that of GSH. The resultant supernatant of 0.5ml was added to 200μl of N-ethylmaleimide (NEM) and incubated for 25 min. at room temperature. One hundred microlitres of the above solution was taken and mixed with 1.8ml phosphate EDTA buffer and 100 μl of o-Phthalaldehyde solution (prepared in absolute methanol). The resultant mixture was incubated at room temperature for 15 min. The standards were prepared from 10μm GSH solution in the range of (0.2–6.4μm) and performed in similar manners as the sample. The fluorescence was measured at the emission of 420nm and excitation of 350nm (Hissin and Hilf 1976).
Determination of glutathione peroxide (GPx) activity
The assay was performed by making slight modifications in the method of Flohe and Günzler (1984). Twenty per cent of tissue homogenate was prepared with phosphate buffer and 0.1 ml was taken and mixed with 0.1ml of 5mM GSH, 0.1ml of 25mM sodium azide, 0.1ml of 1.25 mM H2O2, and makeup to 2.5ml phosphate buffer (PB). The resultant mixture was incubated for 37°C for 10 min. The reaction was terminated by adding 2 ml of 1.65% metaphosphoric acid and centrifuged at 1500rpm for 10 min. The 2 ml supernatant was taken and mixed with 2ml of 0.4M disodium hydrogen phosphate and 1ml of 1mM DTNB. The resultant mixture was incubated for 10 min. at 37°C. The absorbance was recorded at 420 nm. The blank was prepared similarly as mentioned above; instead of 0.1 ml supernatant, we added 0.1PB (Flohe and Günzler 1984).
Determination of liver selenium concentration
Liver selenium concentration was determined using inductively coupled plasma mass spectrometry (ICP-MS). Liver samples were collected from mice and digested with nitric acid and perchloric acid (3: 1) ratio till we got white powder (Patwa et al. 2020). White powder was dissolved in double distilled water to make it up a final volume of 10 ml. The solution was read on ICP-MS and concentration was expressed as ng/ml (Takahashi et al. 2000).
Statistical analysis
All data in this study are presented as mean ± SEM for each group. Statistical differences between the groups were determined by one-way analysis of variance followed by multiple comparisons with Tukey’s test using GraphPad Prism statistical software (version 6). P < .05 was considered statistically significant.
Results
Physicochemical characterization of nanoparticles
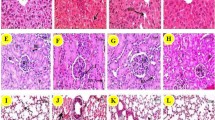
The size and morphology of SeNPs were determined by TEM. Figure 1A shows TEM for the selenium nanoparticles which reveal the average size of SeNPs around 70 nm with spherical morphology. The Zeta potential was determined as −27.2 (Fig. 1B), whereas using DLS, the size of nanoparticles was found to be around 90–100 nm (Fig. 1C). The figure clearly suggests that nano-Se is spherical, and the particles are monodispersed in solution and not agglomerated.
Effect of sodium selenite and selenium nanoparticles on body weight and organ weight
Reduction in body weight was noted in animals exposed to sodium selenite (low and high dose) compared to low- and high-dose group of SeNPs and also controls (Fig. 2A). Similar trend was also observed in the liver/body weight ratio, where no significant difference was observed between sodium selenite (low and high dose) and Se NPs exposed animals (low and high dose) (Fig. 2B).
Effect of sodium selenite and selenium nanoparticles on haematological parameters
The effects of sodium selenite and selenium nanoparticles on haematological variables suggest that exposure to selenite and selenium nanoparticles at the low and high dose did not significantly altered blood δ-aminolevulinic acid dehydratase (ALAD) activity, haematocrit (%), and haemoglobin (%) levels (Fig. 3).
Effect of sodium selenite and selenium nanoparticles on liver function markers
The effects of sodium selenite and selenium nanoparticles on liver function markers are depicted in Fig. 4. Sodium selenite and selenium nanoparticles did not statistically altered alanine transaminase (AST) activity, while selenium nanoparticles at the high dose significantly (p<0.05) increased ALT activity (Fig. 4). Sodium selenite (low and high dose) and selenium nanoparticles (low dose) did not significantly alter AST activity in serum compared with control.
Effect of sodium selenite and selenium nanoparticles on oxidative stress parameters in the liver
The effects of sodium selenite and selenium nanoparticles on biochemical variables indicative of oxidative stress in the liver suggest that the high dose (4mg/kg) of sodium selenite and selenium nanoparticles produced a significant decrease in liver GSH (*p<0.05, **p<0.01) (Fig. 5A) compared to the controls. Figure 5B also showed that GSSG contents in the liver increased significantly on sodium selenite (high dose) exposure compared to control (*p<0.05). On the other hand, selenium nanoparticles (high dose) did not affect GSSG compared to control. Figure 5C depicts that the liver ROS level was significantly increased by high-dose selenium nanoparticles compared to control. However, exposure to sodium selenite increased the liver ROS level, but no significant difference was found when compared to the control group. Figure 5D revealed that the liver glutathione peroxidase (GPx) activity was significantly decreased by the high dose of sodium selenite and selenium nanoparticles (*p<0.05). The high dose of both sodium selenite and selenium nanoparticles decreased the activities of liver catalase and superoxide dismutase (SOD), the two antioxidant enzymes (Fig. 5E, F), but no significant difference was found when compared with the control. Figure 5G suggests that the liver lipid peroxidation (MDA) increased on exposure to high dose of selenium nanoparticles, but no significant difference was found when compared with the control. On the other hand, low dose (1mg/kg) of both sodium selenite and selenium nanoparticles led to non-significant effects on liver GSH, GSSG, ROS, MDA, and antioxidant enzymes (Catalase, SOD, and GPx) compared to control.
Effects of sodium selenite and selenium nanoparticles on liver oxidative stress in mice. A GSSG (oxidized glutathione); B GSH (Reduced glutathione); C ROS (reactive oxygen species); D GPx (Glutathione peroxide); E Catalase (F) SOD (superoxide dismutase); G MDA (TBARS assay). All the data were represented as SEM. The statistically significant difference when compared with the control group was indicated as *p<0.05, **p<0.001
Determination of liver selenium concentration
Selenium concentrations in animals exposed to bulk selenium and Se NPs on liver selenium concentration is shown in Fig. 6. As expected, a higher dose led to a more pronounced accumulation of selenium in the liver compared to the low dose.
Discussion
Selenium is the co-factor for approximately 25 selenoproteins including glutathione peroxidase (GPx) which is a cytosolic enzyme that detoxifies and protects the cells from oxidative damage. It has been reported that dietary selenium deficiency redistributes the intracellular selenium among the selenoproteins in rats (Toppo et al. 2008). On the other hand, selenium supplementation has a role in the activity of GPx and mRNAs. Both may thus serve as the biomarkers of selenium status (Hesketh 2008). Variation in GPx genes is known to induce oxidative damage which is further responsible for inducing cancer by causing DNA damage (Peng et al. 2009). It is thus suggested that due to its narrow therapeutic margin and toxicity, the use of selenite is limited.
The popularity of selenium nanoparticles on the other hand, has gained increased attention in the medical field due to its reduced toxicity and increased bioavailability. It is well known that traditional selenium is “poorly absorbed” and highly toxic. Thus, it is important to increase the bioavailability and allow the control release of drugs using selenium-based carriers. Nano-selenium has also attracted attention as a food additive particularly in selenium-deficient individuals (Hosenedlova et al. 2018). In the present study, we assessed the effect of bulk and nano-selenium using two variable doses on hepatic organ based on specific biomarkers and also any possible relation with changes in oxidative stress (Shirsat et al. 2016).
We noted that there was a reduction in body weight gain in selenite as well as SeNPs exposed animals at both low and high doses compared with control. We noted that both selenite and selenium nanoparticles at the dose of 1mg/kg/bw and 4mg/kg/bw did not show any marked effects on the organ/body weight ratio in the mouse liver compared to the controls. The liver:body weight ratio remained unaltered by both selenite and selenium nanoparticles after 28 days of exposure. Growth retardation is the best indicator for toxicity as suggested by US National Research Council concluded in 1976. The growth inhibition is mainly due to the reduction in growth hormone and insulin-like growth factor-1 (Grønbaek et al. 1995). The selenium nanoparticles (nano-Se) demonstrated protective effects against cyclophosphamide-induced hepato- and genotoxicity at the dose of 2 mg/kg per body weight (Bhattacharjee et al. 2019). Selenite was more prominent in exhibiting toxic effects than others in rats (Zhang et al. 2005). It was reported that 3 ppm selenium caused a significant reduction in the body weight gain post 13 weeks of sub-chronic exposure (Jia et al. 2005). The 20 mg/kg selenium nanoparticles significantly decreased the body weight gain in 14 days study (Shakibaie et al. 2013). The parameters involved in heme synthesis pathway (ALAD, Hb, and PCV) remained unaltered by both selenite and selenium nanoparticles but a moderate increase was noted in low-dose SeNPs exposed animals suggesting the more pronounced role of nano-selenium in heme synthesis.
We also studied the effects of bulk and nano-selenium on serum activities of transaminases (ALT and AST), which are considered a specific biomarkers for assessing the hepatocellular injury (Reichling and Kaplan 1988). Serum AST is a hepatocyte cytosolic enzyme, and an increase in its activities suggests increased plasma permeability in view of hepatocyte damage (Li et al. 2012), while ALT, on the other hand, is considered a more specific marker of hepatic injury in pre-clinical models. The magnitude of increase in ALT activity in our study, was more pronounced than AST, which can be attributed to the facts that i) AST is bound to mitochondria while, ii) ALT has a longer half-life (Boone et al. 2005). An increase in serum ALT activity might thus be with or without an increase in AST activity. Our results also suggest a significant increase in ALT activity but no change in AST activity. There are reports particularly the one by Shakibaie et al. (2013) which reported that the repeated administration of selenium nanoparticles at the doses of 2.5, 5, 8, 10, and 20 mg/kg for 14 days did not elevate the activities of these hepatic enzymes (ALT and AST), whereas both selenite and selenium nanoparticles at the dose of 2 mg and 4 mg/kg for 14 days also did not significantly change the ALT and AST activities. We also observed that selenium nanoparticles at the dose of 4 mg/kg/body weight alone significantly increased ALT activity, whereas neither low nor high dose of selenium nanoparticles had any effect on ALT and AST activities following 28 days exposure in mice. This might be due to the fact that selenium offers protection to the mammalian cells, and it is also the component of selenocysteine (in glutathione peroxidases), selenomethionine, and other selenoproteins like selenoprotein P and thioredoxin reductase (Burk and Hill 2010).
Glutathione (GSH; L-γ-glutamyl-Lcysteinyl-glycine) is a small (Mol weight 307) thiol-containing molecule that comprises a thiol redox status synthesized from L-glutamate, L-cysteine, and γ-glycine in γ-glutamyl-cysteine synthase and GSH synthase in two consecutive steps (Haddad and Harb 2005). The redox reactions of GSH involve GPx and GSSG reductases which act as catalysts. Zhang et al. (2005) reported that 2 and 4 mg/kg dose of orally administered selenite and selenium nanoparticles significantly decreased GSH and GPx levels in mice after 15 days of exposure (Zhang et al. 2005). We also noted that GSH level was significantly reduced by selenite and selenium nanoparticles at the high doses. It has been reported that SeNPs had 1/7th acute toxicity compared to inorganic selenium (Zhang et al. 2001). In the current study, we observed a significant decrease in GSH compared to control at the high dose of both selenite and selenium nanoparticles. However, the same effects were not noted at the low-dose selenium nanoparticles treated group. Hepatic GSSG increased significantly at the high dose (4mg/kg) of selenite but not SeNPs. We do not have possible hypothetical explanation for the mechanism involved behind this interesting observation. The GPx activity was significantly decreased by both selenite and SeNPs at 4 mg compared to controls. Glutathione peroxidase (GPx) is a selenocysteine-dependent enzyme. GPx in cells is the most important hydrogen peroxide (H2O2-)-scavenging enzyme which converts hydrogen peroxide to water. We also noted that the ROS level was significantly increased by SeNPs high dose (4 mg/kg) but not at the low dose. Malondialdehyde (MDA), an aldehyde product and a marker of lipid peroxidation, binds with the thiol group in proteins which ultimately alters biological functioning (Ghorbani Taherdehi et al. 2019). In our study, we noted increased MDA levels in both high doses of selenite and selenium nanoparticles. From the results of the present study, it can be concluded that both selenite and selenium nanoparticles were able to increase GSH, GPx, SOD, and catalase at a low dose (1 mg/kg), while selenium nanoparticles showed more pronounced effects.
A key finding of this study is that both bulk and nano-selenium potentiated ALAD, a sulfhydryl enzyme, of the heme-biosynthesis pathway. Selenium being part of the glutathione peroxidase (GPx) a selenocysteine-dependent enzyme might be facilitating ALAD activation, and subsequent depletion of ROS. Lower dose of selenium nanoparticle increased ALAD activity and haemoglobin levels, while elevated serum ALT activity in high-dose-treated group indicates involvement of oxidative stress parameters indicated by ROS and MDA in hepatic toxicity. Selenium concentration using ICP-MS also suggests that the bio-distribution of high dose of both bulk and selenium nanoparticles in liver is more pronounced compared to the low dose which could be the one of reasons for the observed higher toxicity at the high dose. The results also indicated that 28 days selenium exposure may significantly raise selenium concentration in the liver in comparison to control animals.
Conclusion
The selenium nanoparticles are well-known antioxidant at the lower dose but at the higher doses, it acts as a prooxidant (He et al. 2014). The low-dose selenium nanoparticles exert beneficial effects when compared to selenite. Furthermore, selenium nanoparticles exhibited toxicity at high dose which is indicated by the elevated level of prooxidant and hepatic markers such as AST and ALT. Selenium nanoparticles at the higher dose exert the same effect as selenite as indicated by oxidative stress and elevation of ALT activity. Thus, we may conclude that both sodium selenite and selenium nanoparticle at higher doses have similar toxicity, whereas compared to low doses of sodium selenite and SeNPs, we observed that low-dose SeNPs exhibit less toxicity compared to high dose, and thus may be used as a chemoprotective agent. In contrast, it is observed that the lower dose of selenium nanoparticle exhibits antioxidant effects and thus can be safely used in medicine and nutrition (such as food products, nutraceutical, etc. (Wang et al. 2007)). We recommend more detailed future studies particularly for determining the potency, efficacy, and safety profile using more dose variations.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Amin KA, Hashem KS, Alshehri FS, Awad ST, Hassan MS (2017) Antioxidant and hepatoprotective efficiency of selenium nanoparticles against acetaminophen-induced hepatic damage. Biol Trace Elem Res 175(1):136–145
Amini SM, Pirhajati Mahabadi V (2018) Selenium nanoparticles role in organ systems functionality and disorder. Nanomed Res J 3(3):117–124
Benko I, Nagy G, Tanczos B, Ungvari E, Sztrik A, Eszenyi P, Prokisch J, Banfalvi G (2012) Subacute toxicity of nano-selenium compared to other selenium species in mice. Environ Toxicol Chem 31(12):2812–2820
Berlin A, Schaller KH (1974) European standardized method for the determination of delta - aminolevulinic acid dehydratase activity in blood. Z Klin Chem Klin Biochem 12:389–390
Bhattacharjee A, Basu A, Ghosh P, Biswas J, Bhattacharya S (2014) Protective effect of Selenium nanoparticle against cyclophosphamide induced hepatotoxicity and genotoxicity in Swiss albino mice. J Biomater Appl 29(2):303–317
Bhattacharjee A, Basu A, Bhattacharya S (2019) Selenium nanoparticles are less toxic than inorganic and organic selenium to mice in vivo. Nucleus 62:259–268
Boone L, Meyer D, Cusick P, Ennulat D, Bolliger AP, Everds N, Meador V, Elliott G, Honor D, Bounous D, Jordan H, for the Regulatory Affairs Committee of the American Society for Veterinary Clinical Pathology (2005) Selection and interpretation of clinical pathology indicators of hepatic injury in preclinical studies. Vet Clin Pathol 34(3):182–188
Burk RF, Hill KE (2010) 4.13 - Glutathione Peroxidases. In: McQueen CA (ed) Comprehensive Toxicology, 2nd edn. Elsevier, Oxford, pp 229–242
Chen H, Tappel AL (1995) Vitamin E, selenium, trolox C, ascorbic acid palmitate, acetylcysteine, coenzyme Q, β-carotene, canthaxanthin, and (+)-catechin protect against oxidative damage to kidney, heart, lung and spleen. Free Radic Res 22(2):177–186
Clegg JW, King EJ (1942) Estimation of haemoglobin by the alkaline haematin method. Br Med J 2:329–333
Flohe L, Günzler WA (1984) [12] Assays of glutathione peroxidase. Methods Enzymol 105:114–120
Ghai C (2012) A textbook of practical physiology. JP Medical Ltd.
Ghorbani Taherdehi F, Nikravesh MR, Jalali M, Fazel A, Gorji Valokola M (2019) Evaluating the protective role of ascorbic acid in malathion-induced testis tissue toxicity of male rats. Int J Prev Med 10:45–45
Grønbaek H, Frystyk J, Ørskov H, Flyvbjerg A (1995) Effect of sodium selenite on growth, insulin-like growth factor-binding proteins and insulin-like growth factor-I in rats. J Endocrinol 145(1):105–112
Guan B, Yan R, Li R, Zhang X (2018) Selenium as a pleiotropic agent for medical discovery and drug delivery. Int J Nanomedicine 13:7473–7490
Haddad JJ, Harb HL (2005) L-gamma-Glutamyl-L-cysteinyl-glycine (glutathione; GSH) and GSH-related enzymes in the regulation of pro- and anti-inflammatory cytokines: a signaling transcriptional scenario for redox(y) immunologic sensor(s)? Mol Immunol 42(9):987–1014
Hadrup N, Loeschner K, Mandrup K, Ravn-Haren G, Frandsen HL, Larsen EH, Lam HR, Mortensen A (2019) Subacute oral toxicity investigation of selenium nanoparticles and selenite in rats. Drug Chem Toxicol 42(1):76–83
He Y, Chen S, Liu Z, Cheng C, Li H, Wang M (2014) Toxicity of selenium nanoparticles in male Sprague–Dawley rats at supranutritional and nonlethal levels. Life Sci 115(1-2):44–51
Hesketh J (2008) Nutrigenomics and selenium: gene expression patterns, physiological targets, and genetics. Annual Reviews in Nutrition 28:157–177
Hissin PJ, Hilf R (1976) A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal Biochem 74(1):214–226
Hosenedlova B, Kepinska M, Stalickova S, Ferandez C, Ruttkay-Nedecky B, Peng Q, Baron M, Melcova M, Opatrilova R, Zidkova J, Bjorklund G, Sochor J, Kizek R (2018) Nano-selenium Nanomedicine 13:2107–2128
Jia X, Li N, Chen J (2005) A subchronic toxicity study of elemental nano-Se in Sprague-Dawley rats. Life Sci 76(17):1989–2003
Kakkar P, Das B, Viswanathan P (1984) A modified spectrophotometric assay of superoxide dismutase
Khurana A, Tekula S, Saifi MA, Venkatesh P, Godugu C (2019) Therapeutic applications of selenium nanoparticles. Biomed Pharmacother 111:802–812
Kieliszek M (2019) Selenium-fascinating microelement, properties and sources in food. In: Molecules Vol 24
Li G, Ye Y, Kang J, Yao X, Zhang Y, Jiang W, Gao M, Dai Y, Xin Y, Wang Q, Yin Z, Luo L (2012) l-Theanine prevents alcoholic liver injury through enhancing the antioxidant capability of hepatocytes. Food Chem Toxicol 50(2):363–372
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Maiyo F, Singh M (2017) Selenium nanoparticles: potential in cancer gene and drug delivery. Nanomedicine 12(9):1075–1089
Majeed W, Zafar M, Bhatti A, John P (2018) Therapeutic potential of selenium nanoparticles. J Nanosci Nanotechnol 9(1):1–4
Naqvi S, Samim M, Abdin MZ, Ahmad FJ, Maita AN, Prashant CK, Dinda AK (2010) Concentration - dependent toxicity of iron oxide nanoparticles mediated by increased oxidative stress. International Journal of Nanomedicine 5:983-999
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2):351–358
Patwa J, Thakur A, Sharma A, Flora SJS (2020) Monoisoamyl DMSA reduced copper-induced neurotoxicity by lowering 8-OHdG level, amyloid beta and Tau protein expressions in Sprague-Dawley rats. Metallomics 12(9):1428–1448
Peng G, Yim EK, Dai H, Jackson AP, Burget I, Pan MR, Hu R, Li K, Lin SY (2009) BRIT1/MCPH1 links chromatin remodelling to DNA damage response. Nature Cell Biology 11:865–872
Reichling JJ, Kaplan MM (1988) Clinical use of serum enzymes in liver disease. Dig Dis Sci 33(12):1601–1614
Reitman S, Frankel S (1957) A calorimetric method for the determination of serum glutamic oxaloacetic and glutamic pyruvic transaminases. Am J Clin Pathol 28:56–63
Shakibaie M, Shahverdi AR, Faramarzi MA, Hassanzadeh GR, Rahimi HR, Sabzevari O (2013) Acute and subacute toxicity of novel biogenic selenium nanoparticles in mice. Pharm Biol 51(1):58–63
Sharma H, Flora SJS, Naqvi S (2019) Nanomedicine: A new therapeutic approach in liver diseases. Austin J Nanomedicine & Nanotechnology 7:1053
Shirsat S, Kadam A, Mane RS, Jadhav VV, Zate MK, Naushad M, Kim KH (2016) Protective role of biogenic selenium nanoparticles in immunological and oxidative stress generated by enrofloxacin in broiler chicken. Dalton Trans 45(21):8845–8853
Sinha AK (1972) Colorimetric assay of catalase. Anal Biochem 47(2):389–394
Socci D, Bjugstad K, Jones H, Pattisapu J, Arendash G (1999) Evidence that oxidative stress is associated with the pathophysiology of inherited hydrocephalus in the H-Tx rat model. Exp Neurol 155(1):109–117
Takahashi S, Takahashi I, Sato H, Kubota Y, Yoshida S, Muramatsu Y (2000) Determination of major and trace elements in the liver of Wistar rats by inductively coupled plasma-atomic emission spectrometry and mass spectrometry. Lab Anim 34(1):97–105
Toppo S, Vanin S, Bosello V, Tosatto SCE (2008) Evolutionary and structural insight into the multifaceted glutathione peroxidase superfamily. Antioxidant and Redox Signaling 10:1501–1513
Venardos K, Harrison G, Headrick J, Perkins A (2004) Effects of dietary selenium on glutathione peroxidase and thioredoxin reductase activity and recovery from cardiac ischemia-reperfusion. J Trace Elem Med Biol 18(1):81–88
Wang H, Zhang J, Yu H (2007) Elemental selenium at nano size possesses lower toxicity without compromising the fundamental effect on selenoenzymes: comparison with selenomethionine in mice. Free Radic Biol Med 42(10):1524–1533
Zhang J-S, Gao X-Y, Zhang L-D, Bao Y-P (2001) Biological effects of a nano red elemental selenium. Biofactors 15(1):27–38
Zhang J, Wang H, Yan X, Zhang L (2005) Comparison of short-term toxicity between Nano-Se and selenite in mice. Life Sci 76(10):1099–1109
Zhao G, Wu X, Chen P, Zhang L, Yang CS, Zhang J (2018) Selenium nanoparticles are more efficient than sodium selenite in producing reactive oxygen species and hyper-accumulation of selenium nanoparticles in cancer cells generates potent therapeutic effects. Free Radic Biol Med 126:55–66
Zwolak I, Zaporowska H (2012) Selenium interactions and toxicity: a review. Cell Biol Toxicol 28(1):31–46
Acknowledgements
KP and MSD acknowledge the award of Senior Research Fellowships by the Department of Pharmaceuticals, Ministry of Chemical and Fertilizer, Government of India. SN was further supported by the Women Scientist Scheme (WOS-A), Department of Science and Technology, Government of India (grant number SR/WOS-A/LS-1224/2015).
Author information
Authors and Affiliations
Contributions
PK: literature search, animal dosing, biochemical assays, metal estimation, and illustration of the Figs; MD: participation in experimental work, biochemical assay, draft manuscripts; SN: literature search, data calculation, statistical analysis, first draft of manuscript; SJSF: conceptualized, overall supervision, manuscript review and finalization.
Corresponding author
Ethics declarations
Ethical approval
The animals were given humane care as per the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India, and prior permission was taken from the Institutional Animal Ethics Committee of the National Institute of Pharmaceutical Education and Research, Raebareli (Approval # NIPER/RBL/IAEC/42/August 2019).
Consent to participate
Not applicable
Consent to publish
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Mohamed M. Abdel-Daim
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kondaparthi, P., Deore, M., Naqvi, S. et al. Dose-dependent hepatic toxicity and oxidative stress on exposure to nano and bulk selenium in mice. Environ Sci Pollut Res 28, 53034–53044 (2021). https://doi.org/10.1007/s11356-021-14400-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-14400-9