Abstract
A bioenhancement strategy for improving the anaerobic degradation efficiency of ship domestic sewage under microaerobic conditions was proposed in this study. Strains Stenotrophomonas sp. MSPP05 and Prevotella sp. MSPP07 with high organic-degrading efficiency and extracellular hydrolase yield were used for the bioenhancement of activated sludge. In batch experiments, the removal rates of chemical oxygen demand and total nitrogen reached 94.5% and 66.9% after 72 h of degradation. The activities of dehydrogenase, extracellular amylase, and protease in the treatment group were 1.2, 1.4, and 2.0 times higher than those in the control group. Microbial community analysis showed that exogenous enhanced strains competed with original microorganisms and became dominant. One-stage continuous stirred tank reactor with bioenhanced activated sludge ran steadily for 90 days with average effluent COD and TN concentrations of 87.5 and 14.6 mg/L. The feasibility of improving organic-degrading efficiency through bioenhancement by using exogenous hydrolase-producing strains was confirmed under microaerobic conditions. This work provided a theoretical basis for improving treatment effects and developing a new technique for ship domestic sewage treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the emphasis on marine environmental protection, the research and application of efficient treatment technology of ship sewage, especially domestic sewage, has been a hot issue in the field of ship pollution prevention. Our early investigation of ships in the Yangtze River found that the inefficient use of processing devices and the direct discharging of untreated domestic sewage are prominent problems. The core reason for these problems is that power outage during anchoring prevents continuous aeration and inactivates aerobic microorganisms (Hu 2020). As a consequence, ship sewage cannot be processed regularly after the system was restarted. Therefore, an effective treatment technique for domestic sewage in inland ships in China without additional aeration is worthwhile to explore.
In the absence of additional oxygen supply, sewage treatment systems are microaerobic environments with low contents of dissolved oxygen (DO). This unique microaerobic condition provides niches for anaerobes and microaerobes. The presence of oxygen leads to high populations of facultative acidogens and results in the high yield of excreted enzymes. Microaeration-based anaerobic digestion (AD), which has the advantages of accelerating hydrolysis, scavenging hydrogen sulfide, and affecting microbial diversity, has been used in the treatment of organic waste and domestic sewage to improve the stability of anaerobic systems (Chen et al. 2020; Lim et al. 2014; Nguyen and Khanal 2018). Lim and Wang (2013) reported that methane yield had been increased by 21.0% after pretreating the liquid phase with 37.5 mL of O2/LR-d for 4 days. This increase can be attributed to the maintenance of the reducing environment for organic degradation created by facultative microorganisms through oxygen consumption. Díaz et al. (2011) also indicated that an appropriate microaeration rate can significantly enhance the hydrolysis of substrates and the yield of products. Liu et al. (2020) reported that microaeration was beneficial for enhancing NH4+-N removal performance.
The degradation of organic compounds under microaerobic conditions involves a series of complex reactions through the action of microorganisms and enzymes. The major contributors to chemical oxygen demand (COD) in ship sewage are carbohydrates, proteins, and lipids. The extracellular hydrolysis of organic matter is a rate-limiting step in the overall degradation process. The growth and metabolic activity of microorganisms and yield of extracellular hydrolase determine degradation efficiency under microaerobic conditions (Nguyen et al. 2007). The addition of hydrolase-producing microorganisms is conducive to promoting hydrolysis and improving the overall degradation efficiency of organics. Dehydrogenase is an indicator of overall sludge microbial activity because it is present intracellularly in all living microbial cells and does not accumulate extracellularly. Organic matter is oxidized by dehydrogenases through the transfer of protons and electrons from substrates to acceptors as a part of respiratory pathways (Filipovic et al. 2020). Therefore, dehydrogenase is widely used in the detection and evaluation of activated sludge, total bacterial colony numbers, water toxicity, and soil pollution (Xie et al. 2008). In this work, the changes in hydrolase and dehydrogenase activity in bioenhanced activated sludge were investigated to estimate the variation in the activity of organic-degrading strains.
A strategy of bioenhancement of activated sludge systems with hydrolase-producing microorganism was put forward to solve the problems of slow growth rate of anaerobic microorganism. The extracellular hydrolase-producing bacteria Stenotrophomonas sp. MSPP05 and Prevotella sp. MSPP07, which were screened and identified in the laboratory, were used as bioenhancement strains to promote the activity of extracellular hydrolase. Thus, decomposition from macromolecules into small molecules that can easily enter cells is promoted for subsequent degradation. Genus Stenotrophomonas has been reported to be capable of synthesizing amylase, protease, and agarose (Kumar et al. 2014; Wang et al. 2015; Zhu et al. 2016), as well as fixing nitrogen, degrading alkanes and aromatic hydrocarbons, and alleviating the toxicity of oily sewage (Bashandy et al. 2020; Gargouri et al. 2017; Hassanshahian et al. 2013; Luo et al. 2009). Genus Prevotella has been reported to secrete proteases (Wang and Hsu 2005). In the treatment of organic solid waste by using an anaerobic membrane bioreactor, Prevotella increased rapidly and reached a relative abundance of 43.2% (Inaba et al. 2020).
This study aimed to investigate the performance of Stenotrophomonas sp. MSPP05 and Prevotella sp. MSPP07 in treating domestic sewage in batch and continuous experiments and in influence of microbial community structures. The activities of extracellular hydrolase and dehydrogenase and degradation rates of COD and total nitrogen (TN) in bioenhanced group were compared with those in control group. The results might confirm the feasibility of Stenotrophomonas sp. MSPP05 and Prevotella sp. MSPP07 in bioenhancing activated sludge to improve the domestic sewage treatment efficiency in inland river ships under microaerobic conditions. This study lays a foundation for the development of bioenhanced degradation and the investigation of the mechanism underlying ship sewage treatment under microaerobic conditions.
Materials and methods
Synthetic domestic sewage
The synthetic wastewater was prepared according to the reference (Jiang et al. 2019), with the composition of 800 mg/L glucose, 500 mg/L sodium acetate, 550 mg/L NH4Cl, 250 mg/L peptone, and 124 mg/L KH2PO4·3H2O, but 130 m/L sodium glycerophosphate was replaced with 10 mg/L MgSO4, 10 mg/L FeSO4·7H2O, and 10 mg/L CaCl2 to supply trace elements. During operation process, the influent was synthetic wastewater with average concentration of 1240 mg/L CODcr, 125 mg/L NH4+-N, and 140 mg/L TN.
Bacterial strains, seed, and bioenhanced sludge
Two bacteria with high amylase and protease yields were screened and isolated by using the degradation object as the sole carbon source. These two strains had the highest similarity with genera Stenotrophomonas and Prevotella as revealed through 16S rDNA sequencing and were designated as Stenotrophomonas sp. MSPP05 and Prevotella sp. MSPP07. These two strains were used to bioenhance activated sludge in strengthening the activity of extracellular hydrolase and accelerate decomposition from macromolecules into micromolecules. The strains were cultivated separately in Luria–Bertani medium at 30 °C for 24 h at 120 rpm in a rotary shaker (Zhicheng, Shanghai, China). After culture, the cells were collected via centrifugation (6000×g, 4 °C) for the bioenhancement of activated sludge.
The seed sludge was collected from the anoxic zone in Dalian Lingshui Wastewater Treatment Plant. The mixed liquor suspended solids (MLSS), mixed-liquor volatile suspended solids (MLVSS), and settling volume (SV30%) were determined based on the reference (Mesquita et al. 2011). Bioenhanced activated sludge was constructed as follows: after natural sedimentation, the supernatant was removed, and sediment was added to the enriched medium with an equal volume of supernatant. The cells of Stenotrophomonas sp. MSPP05 and Prevotella sp. MSPP07 were obtained via centrifugation and added to the activated sludge at a ratio of 1 wt%. The mixture was cultured at 30 °C for 24 h at 120 rpm in a rotary shaker under microaerobic conditions. Sedimentation, supernatant removal, enriched medium addition, and cultivation were repeated thrice. After the last cultivation, bioenhanced activated sludge was obtained through settlement. The steps performed to control activated sludge were the same as those conducted to control bioenhanced sludge except that no functional strains added.
Batch and continuous experiments
Batch culture experiments were carried out in a series of 1 L shake flasks with 500 mL of simulated sewage and 100 g of activated sludge. The shake flasks were sealed and incubated with static culture for 72 h at a constant temperature of 25 °C. The sludge sample was collected for the determination of dehydrogenase activity, and the supernatant was sampled for measuring the contents of COD and TN and the activity of extracellular amylase and protease. Three series of experiments were operated in parallel.
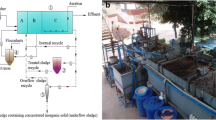
Continuous experiments were operated in continuous stirred tank reactor (CSTR) for simultaneous COD and nitrogen removal without additional aeration. The CSTR was a plastic cylindrical reactor (200 mm diameter, 300 mm height) with the tank volume of 9.4 L. The liquid was mixed continuously at 80 rpm by an overhead mechanical stirrer. The reactor was maintained at 25±1°C in a temperature-controlled room and continuously ran for 120 days. The CSTR was batch fed with the synthetic wastewater by a peristaltic pump, and hydraulic retention time was 24 h. Bioenhanced activated sludge was applied at a ratio of 10 wt%. There is no additional oxygen supply except for the overhead stirring device. Water samples of effluent were taken out every 2 days to determine COD, TN, and other indicators.
Analytical methods
DO was determined by using a portable DO meter (F4-Field, Mettler Toledo, Switzerland). COD and TN were determined by using Hach preloading tube reagents and a chromometer. Dehydrogenase activity was measured via the 2,3,5-triphenyltetrazolium chloride (TTC) reduction method (Hou et al. 2020). Under the action of a reducing agent, TTC is reduced into deep red triphenyl formazan (TF), which can be quantitatively analyzed through ultraviolet spectroscopy. Enzyme activity unit (μmol/(g·min)) was defined as the amount of dehydrogenase required to catalyze the substrate and produce 1 μmol of TF from 1g of dry sludge weight within 1 min. Amylase activity was determined through 3,5-dinitrosalicylic acid colorimetry. Enzyme activity unit (μmol/(mL·min)) was defined as the amount of amylase required to hydrolyze starch and produce 1 μmol of reducing sugar from 1 mL of measured liquid within 1 min (Van Staden and Mulaudzi 2000). Protease activity was determined through ninhydrin colorimetry. Enzyme activity unit (μmol/(mL·min)) was defined as the amount of protease required to hydrolyze protein and produce 1 μmol of amino acid from 1 mL of measured liquid within 1 min (Cupp-Enyard 2008).
Microbial community analysis
The activated sludge samples of the control and bioenhanced group were filtered and enriched by using a 0.22-μm sterile filtration membrane for bacterial diversity analysis. Total genomic DNA was extracted by using a bacterial genomic DNA extraction kit (TaKaRa, Japan) in accordance with a reference method (Green and Sambrook 2012). The V3 and V4 regions of 16S rDNA genes were amplified by using the aforementioned genomic DNA as a template and specific primer (341F: 5′-CCTACGGGAGGCAGCAG-3′; 806R: 5′-GCCAATGGACTACHVGGGTWTCTAAT-3′). PCR products were purified and sent out to the Personal Biotechnology Co., Ltd (Shanghai, China) for sequencing library construction and high-throughput sequencing on the MiSeq PE300 platform (Illumina, USA). The obtained high-quality sequences were analyzed by the QIIME2 DADA2 procedure. The amplification sequence variations (ASVs) were acquired as clustering results. Silva database was used for taxa annotation, and QIIME2 software was utilized to determine microbial community composition at different taxonomic levels. The indexes of α-diversity (Ace, Chao1, Shannon, and Simpson) was calculated for each sample. For function prediction, the sequenced 16S rRNA gene was BLASTed with a database of known metabolic function genes by using PICRUSt.
Results and discussion
Biological enhancement of activated sludge
The major components causing high COD were polysaccharides and proteins. Since construction of microbial community for bioenhancement focused on the specific physico-chemical properties of the bioprocess, the strains Stenotrophomonas sp. MSPP05 and Prevotella sp. MSPP07 that produced extracellular protease and amylase were selected for the enhancement of activated sludge.
The basic performance indexes of activated sludge before and after bioenhancement are presented in Table 1. After bioenhancement, the color of activated sludge changed from ash black to tawny due to the increase in the number of active microorganisms. Particle diameter increased, and the pH value tended toward neutral. The increase in SV30% from 21.0 to 25.0% was presumably related to the increase in particle diameter or extracellular polysaccharide production after the addition of functional strains. The ratio of SV30% and MLSS represents the settling ability, which lower than 120 mg/L is considered acceptable and over 150 mg/L is considered bulking (Jenkins et al. 2003). The settling performance of the control group (CG) and the bioenhanced group (BEG) were all satisfactory and conducive to reducing suspended solid content and improving effluent water quality. When the sludge was inoculated into simulated domestic sewage at a proportion of 10%, the MLVSS/MLSS values of the CG and BEG reached 68.0% and 74.0%, respectively. The concentration of organic solid components in the BEG increased, indicating improved potential in domestic sewage treatment.
COD and TN removal performance in batch experiments
Batch experiments were conducted for 72 h to explore the COD and TN removal performance of the bioenhanced activated sludge. The DO content in the shake flasks decreased from the initial value of 0.18 to 0.09 mg/L. This decrement indicated microaerobic conditions in biological treatment. The COD and TN removal rates are shown in Fig. 1. At each time point, the removal rate in the BEG was higher than that in the CG. In the initial 24 h of treatment, the COD concentrations in the two groups both decreased significantly. In the BEG, COD concentration decreased from the initial value of 1380.0 to 301.0 mg/L, while that in the CG decreased from 1395.0 to 451.0 mg/L. COD concentration further reduced during 24–72 h of degradation at a rate slower than the initial rate. After 72 h of degradation, COD concentration and removal rate in the BEG reached 76.0 mg/L and 94.5%, respectively, whereas those in the CG were 193.0 mg/L and 86.2%, respectively. The trend shown of TN degradation was similar to that presented of COD removal. At 72 h of degradation, the TN concentrations in the BEG and CG were 41.0 and 59.0 mg/L, respectively, with corresponding removal rates of 66.9% and 52.4%, respectively.
The above results demonstrated that the BEG showed accelerated and improved nutrient removal after the addition of functional strains. In addition, the microaerobic conditions promoted the degradation of organic matter. Xu et al. (2014) found that adequate microaeration intensity noticeably enhanced the hydrolysis of carbohydrate and protein by 21–27% and 38–64%, respectively. On account of the accelerated acidogenesis, more than threefold of intermediate metabolites were produced. Zhang et al. (2019) reported the autotrophic denitrification process under microaerobic condition; sulfide and nitrate removal rates of 100% and 53.8% were obtained with air aeration rate of 20 mL/min.
The operation of adding functional strains into activated sludge to improve the catabolism of organics under microaerobic conditions is a promising technique to solve practical problems in domestic sewage treatment on ships. The activities of microorganisms and extracellular hydrolase in the BEG are worthy of further study.
Analysis of dehydrogenase and hydrolases
Dehydrogenase activity
As an indicator of overall microbial activity of the activated sludge, dehydrogenase activities in the CG and BEG were compared and depicted in Fig. 2. The dehydrogenase activity in the BEG was higher than that in the CG at each sampling point. The common trend shown by the two groups was that the enzyme activity increased gradually during 0–24 h of treatment and increased dramatically during 24–48 h of treatment. This behavior could be due to the toxic effect of high concentrations of organics on microbial cells during the early treatment stage such that the total microbial population showed a slow growth trend. The biomass of microorganisms increased rapidly after adaptation and metabolism with pollutants as nutrient sources. After 48 h, the dehydrogenase activity and active microorganism numbers in the CG decreased, whereas those in the BEG increased continuously to 0.69 μmol/(g·min), which was 1.2 times higher than that in the CG.
In activated sludge treatment systems, organic pollutants in wastewater are degraded through absorption, metabolism, and utilization by microorganisms. In a sense, the activity of microorganisms determines the degradation efficiency of organics. Dehydrogenase is an indicator of overall microbial activity because it is present only in living microbial cells, but not accumulated extracellularly. Dehydrogenase can also catalyze the oxidation of organic pollutants by transferring a hydrogen atom from an oxidized matrix to a hydrogen accepter (Xie et al. 2008). The activity of dehydrogenase indicates not only microbial activity but also metabolic capacity of activated sludge.
Activity of extracellular hydrolases
The detection results of actual domestic sewage in the early stage showed that the major components causing high COD were polysaccharides and protein. Amylase and protease were selected as representatives to investigate the differences in the performances of extracellular hydrolase secreted by microorganisms in the CG and BEG. Enzyme activity is shown in Fig. 3. During the experimental period, the amounts of extracellular amylase and protease in the CG slowly increased with the maximum values of 3.2 and 5.2 μmol/(mL·min), respectively. Enzyme activity in the BEG was significantly higher than that in the CG. Enzyme activity showed rapid growth after slow growth during 0–24 h. At 72 h of treatment, amylase and protease activities in the BEG were 7.6 and 15.8 μmol/(mL·min), respectively, which were 1.4 and 2.0 times higher than those in the CG.
Extracellular hydrolysis, the first and rate-limiting step of organic degradation, plays an important role and directly affects the overall degradation efficiency of organic matter (Nguyen and Khanal 2018). The addition of bioenhanced strains with a high yield of extracellular hydrolases increased amylase and protease activities and promoted the degradation of starchy material into simple sugars and protein into amino acids. It accelerated the cellular entry and thorough degradation of small-molecule organics, thus improving the overall degradation efficiency of organic matter. It also confirmed that microaeration enhanced secretion of hydrolase and accelerated energetic conversions of intermediates to sustain overall stability in AD processes (Nguyen and Khanal 2018).
Bacterial community composition
Samples were collected from the CG and BEG at 24 and 72 h of degradation and named CG-24, CG-72, BEG-24, and BEG-72 to study the shift in microbial community structures after bioenhancement. The effective sequence quantities of the four samples after fragment sequencing, low-quality sequence filtering, and denoising were 105231, 100151, 133067, and 137695. The obtained data, as reflected by the coverage indices and rarefaction curves, met the requirements of subsequent sequencing and analysis.
The ɑ-diversity indexes of the four samples are shown in Table 2. The Chao1 index represents the richness of microbial communities, the Shannon and Simpson index reflects the homogeneity of the samples, and Faith’s PD represents diversity based on evolution. The data showed that the community richness and diversity in the control groups (CG-24 and CG-72) were higher than that in the bioenhanced groups (BEG-24 and BEG-72) likely because after the addition of the functional strains, the growth of indigenous strains in the activated sludge was inhibited, and the abundance of the bacterial communities decreased.
To study the common and unique species between the samples of the CG and BEG, Venn diagrams based on ASVs was used for community analysis. The ASV abundance data were used to make Venn diagram (Fig. 4) based on the number of unique and common ASVs among each sample, but not the abundance value. The results indicated that the added enhanced strains gradually decreased in terms of microbial species but increased in prominence in the bacterial community in the CG.
The representative sequences of ASVs in four samples were compared and identified to obtain the bacterial taxa information at different levels. The top 20 taxa at each level of phylum, class, order, and genus were selected to display relative abundance in Fig. 5. Proteobacteria and Bacteroidetes were the common dominant phylum (Fig. 5a). Consistent with the report that many functional strains belonging to Proteobacteria are the most common microbes encountered in wastewater treatment (Jiang et al. 2019), Proteobacteria had the highest proportion in four samples. Bacteroidetes is one of the most abundant heterotrophic bacteria in the marine environment, which can degrade granular organic substances, especially high-molecular-weight compounds (Dorador et al. 2009).
The microbial compositions in the CG and BEG were significantly different at the genus level (Fig. 5d). A total of 391 genera were detected in the four samples. Thermomonas, which was the largest dominant genus in the CG (25.6% of CG-24 and 26.3% of CG-72) and Terrimonas (8.2 % of CG-24 and 7.4% of CG-72), accounted for less than 0.13% in BEG-24 and BEG-72. In the BEG, genus Stenotrophomonas (12.7% of BEG-24 and 22.5% in BEG-72) and Prevotella (19.2% of BEG-24, 4.4% in BEG-72) were the dominant genera. Soonwooa accounted for 0.5% of the microbial community in BEG-24 and 15.3% in BEG-72. The dominant genera have the advantages of rapid growth, strong adaptability to the environment, and high yields of extracellular hydrolases, which means they are promising candidates for sewage treatment. The horizontal microbial composition and proportion in CG-24 and CG-72 had negligible changes, and the microbial community structure of the original activated sludge system was relatively stable. The exogenous enhanced strains Stenotrophomonas sp. MSPP05 and Prevotella sp. MSPP07 competed with the original microorganisms in the sludge and became the dominant bacteria in the BEG. The microbial community structure of BEG-24 was significantly different from that of BEG-72, and the dominant bacterial group in the activated sludge system underwent succession.
To predict the functional potential, the databases of the Kyoto Encyclopedia of Genes and Genomes (KEGG) was used to analyze core biological metabolic pathways. In this database, metabolic pathways are classified into six categories, including metabolism, genetic information processing, environmental information processing, cellular processes, organismal systems, and human diseases. Each metabolic pathway is further classified into several grades. Figure 6 shows the relative metabolic abundance analyzed through KEGG. The results showed that all metabolic pathways were abundant in the four samples and that bioenhancement did not increase or decrease the varieties of metabolic pathways in the system. The abundances of nucleotide, cofactor and vitamin, energy, carbohydrate, and amino acid metabolic pathways were higher than those of others. The relative abundance of the three major pathways of carbohydrate, amino acid, and lipid metabolism in the BEG was higher than that in the CG. These results revealed that bioenhancement improved the properties of organic metabolism at the molecular level.
The predicted relative abundance of metabolism. A, Xenobiotics biodegradation and metabolism; B, nucleotide metabolism; C, metabolism of terpenoids and polyketides; D, metabolism of other amino acids; E, metabolism of cofactors and vitamins; F, lipid metabolism; G, glycan biosynthesis and metabolism; H, energy metabolism; I, carbohydrate metabolism; J, biosynthesis of other secondary metabolites; K, amino acid metabolism
Performance of continuous experiments
The performance of continuous experiment on COD and TN removals by bioenhanced sludge in CSTR was investigated in this study. The process of organic matter removal is shown in Fig. 7. According to the distinguishing degradation characteristics, the process was divided into two stages, including start-up period (stage I) and stable circulation period (stage II). During the start-up period (0–20 days), COD and TN removal efficiencies increased gradually. When effluent COD and TN decreased to 125 and 20 mg/L, the system was successfully started up. It was inferred that both facultative aerobes and anaerobes in the system grew and metabolized well. In stage II, the system ran steadily for 90 days with effluent COD and TN lower than the emission standard for inland river domestic sewage in China. The lowest effluent COD concentration (54 mg/L) was achieved on 112 days with the corresponding degradation efficiency of 95.6%. The average concentration and removal efficiencies of COD were 87.5 mg/L and 92.9%, while those of TN were 14.6 mg/L and 87.8%, respectively. During the complete process, the average oxidation-reduction potential (ORP) was −250.6 mV, which agreed with the ORP range under microaerobic conditions in the literature that is optimal for growth and products yield of facultative microorganism (Nguyen and Khanal 2018). The control experiment using non-enhanced sludge was conducted subsequently. During operation for over 80 days, the average removal efficiencies of COD and TN were 74.2% and 46.1%, respectively. The performance revealed that bioenhanced activated sludge is of good COD and TN removing effects in continuous experiments under non-aerated conditions.
Conclusions
Strains Stenotrophomonas sp. MSPP05 and Prevotella sp. MSPP07, which have the properties of efficient substrate degradation and high extracellular hydrolase yield, were used to construct bioenhanced activated sludge. The batch and continuous experiments demonstrated that bioenhancement had positive effects on COD and TN removal by promoting activities of dehydrogenase, extracellular amylase, and protease. A longer-term operation of bioenhanced activated sludge to treat actual ship sewage needs further study. One-stage CSTR with bioenhanced activated sludge started up after 20 days and ran steadily for 90 days under microaerobic conditions; the average effluent removal rates for COD and TN were 92.9% and 87.8%. This study provided theoretical foundations for improving treatment effects without additional aeration and produced good economic benefits and social significance in promoting efficient treatment of domestic sewage from inland river ships.
References
Bashandy SR, Abd-Alla MH, Dawood MFA (2020) Alleviation of the toxicity of oily wastewater to canola plants by the N2-fixing, aromatic hydrocarbon biodegrading bacterium Stenotrophomonas maltophilia-SR1. Appl Soil Ecol 154:103654
Chen Q, Wu WQ, Qi DC, Ding YH, Zhao ZH (2020) Review on microaeration-based anaerobic digestion: State of the art, challenges, and prospectives. Sci Total Environ 710:136388
Cupp-Enyard C (2008) Sigma’s non-specific protease activity assay-casein as a substrate. J Vis Exp 19:899
Díaz I, Lopes AC, Pérez SI, Fdz-Polanco M (2011) Determination of the optimal rate for the microaerobic treatment of several H2S concentrations in biogas from sludge digesters. Water Sci Technol 64:233–238
Dorador C, Meneses D, Urtuvia V, Demergasso C, Vila I, Witzel KP, Imhoff JF (2009) Diversity of Bacteroidetes in high-altitude saline evaporitic basins in northern Chile. J Geophys Res Biogeosci 114:G00D05
Filipovic L, Romic M, Sikora S, Babic KH, Filipovic L, Gerke HH, Romic D (2020) Response of soil dehydrogenase activity to salinity and cadmium species. J Soil Sci Plant Nutr 20:530–536
Gargouri B, Contreras MM, Ammar S, Segura-Carretero A, Bouaziz M (2017) Biosurfactant production by the crude oil degrading Stenotrophomonas sp. B-2: chemical characterization, biological activities and environmental applications. Environ Sci Pollut Res 24:3769–3779
Green MR, Sambrook J (2012) Molecular cloning: a laboratory manual (Fourth Edition). Cold Spring Harbor Laboratory Press, New York.
Hassanshahian M, Ahmadinejad M, Tebyanian H, Kariminik A (2013) Isolation and characterization of alkane degrading bacteria from petroleum reservoir waste water in Iran (Kerman and Tehran provenances). Mar Pollut Bull 73:300–305
Hou F, Liu JN, Zhang YH, Zhao C, Xiao XD, Zou JL, Li Q, Hu S, Wang H, Jiang BJ (2020) Synthesis of metallic copper modified g-C3N4 by molecular self-assembly structure and its combined catalytic performance with activated sludge. J Hazard Mater 388:121754
Hu RH (2020) “Zero discharge and total degradation” Marine Domestic Sewage Treatment Equipment and Its Application Prospects. China Ship Survey 12:68–71
Inaba T, Su T, Aoyagi T, Aizawa H, Sato Y, Suh C, Lee JH, Hori T, Ogata A, Habe H (2020) Microbial community in an anaerobic membrane bioreactor and its performance in treating organic solid waste under controlled and deteriorated conditions. J Environ Manag 269:110786
Jenkins, D., Richard, M.G., Daigger, G., 2003. Manual on the causes and control of activated sludge bulking, foaming and other solids separation problems. Lewis Publishing, Boca Raton, FL.
Jiang L, Chen X, Qin M, Cheng SH, Wang YX, Zhou WZ (2019) On-board saline black water treatment by bioaugmentation original marine bacteria with Pseudoalteromonas sp. SCSE709-6 and the associated microbial community. Bioresour Technol 273:496–505
Kumar SS, Sangeeta R, Soumya S, Ranjan RP, Bidyut B, Kumar DMP (2014) Characterizing novel thermophilic amylase producing bacteria from Taptapani hot spring, Odisha, India. Jundishapur J Microb 7(12):e11800
Lim JW, Wang JY (2013) Enhanced hydrolysis and methane yield by applying microaeration pretreatment to the anaerobic co-digestion of brown water and food waste. Waste Manag 33:813–819
Lim JW, Chiam JA, Wang JW (2014) Microbial community structure reveals how microaeration improves fermentation during anaerobic co-digestion of brown water and food waste. Bioresour Technol 171:132–138
Liu L, Li N, Tao C, Zhao Y, Gao J, Huang Z, Zhang J, Gao J, Zhang J, Cai M (2020) Nitrogen removal performance and bacterial communities in zeolite trickling filter under different influent C/N ratios. Environ Sci Pollut Res, on publish.
Luo YR, Tian Y, Huang X, Yan CL, Hong HS, Lin GH, Zheng TL (2009) Analysis of community structure of a microbial consortium capable of degrading benzo(a)pyrene by DGGE. Mar Pollut Bull 58:1159–1116
Mesquita DP, Amaral AL, Ferreira EC (2011) Identifying different types of bulking in an activated sludge system through quantitative image analysis. Chemosphere 85:643–652
Nguyen D, Khanal SK (2018) A little breath of fresh air into an anaerobic system: how microaeration facilitates anaerobic digestion process. Bioresour Technol 36:1971–1983
Nguyen PHL, Kuruparan P, Visvanathan C (2007) Anaerobic digestion of municipal solid waste as a treatment prior to landfill. Bioresour Technol 98:380–387
Van Staden JF, Mulaudzi LV (2000) Flow injection spectrophotometric assay of α-amylase activity. Anal Chim Acta 421:19–25
Wang HT, Hsu JT (2005) Optimal protease production condition for Prevotella ruminicola 23 and characterization of its extracellular crude protease. Anaerobe 11:155–162
Wang ZG, Sun LH, Cheng J, Liu CL, Tang XF, Zhang HF, Liu Y (2015) The optimization of fermentation conditions and enzyme properties of Stenotrophomonas maltophilia for protease production. Biotechnol Appl Biochem 63(2):292–299
Xie J, Hu WR, Pei HY, Dun MN, Qi F (2008) Detection of amount and activity of living algae in fresh water by dehydrogenase activity (DHA). Environ Monit Assess 146:473–478
Xu S, Selvam A, Wong JW (2014) Optimization of micro-aeration intensity in acidogenic reactor of a two-phase anaerobic digester treating food waste. Waste Manag 34:363–369
Zhang RC, Xu XJ, Chen C, Shao B, Zhou X, Yuan Y, Lee DJ, Ren NQ (2019) Bioreactor performance and microbial community analysis of autotrophic denitrification under micro-aerobic condition. Sci Total Environ 647:914–922
Zhu YB, Zhao R, Xiao AF, Li LJ, Jiang ZD, Chen F, Ni H (2016) Characterization of an alkaline β-agarase from Stenotrophomonas sp. NTa and the enzymatic hydrolysates. Int J Biol Macromol 86:525–534
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Funding
This work was supported by the China MSA project (grant number 80817006), which is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
Qing Chen integrated the data and was a major contributor in writing the manuscript. Wanqing Wu and Linghua Zhang provided the research idea and instruments. Fang Wei performed the determination of chemical indexes. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible editor: Robert Duran
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PNG 41 kb)
Rights and permissions
About this article
Cite this article
Chen, Q., Wu, W., Zhang, L. et al. Shifts in enzymatic activities and microbial community structures in the bioenhanced treatment of ship domestic sewage under microaerobic conditions. Environ Sci Pollut Res 28, 51242–51250 (2021). https://doi.org/10.1007/s11356-021-14232-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-14232-7