Abstract
Cancer is a universal health issue, and many anticancer therapeutic drugs have been isolated from natural products. This study analyzed the cytotoxic and apoptotic activity of Plectranthus amboinicus leaf hexane (PALH) extract in MDA-MB-231 (median inhibitory concentration [IC50] = 39.26 μg/mL) and MCF7 (IC50 = 89.05 μg/mL) breast cancer cell lines. Cells appeared rounded and shrunken, indicating morphological changes due to apoptosis induction. The primary constituent of PALH was phenol, 5-methyl-2-(1-methylethyl) (44%). PALH extract treatment increased the percentage of late apoptotic cells in the MDA-MB231 cell line (58% ± 1.5% at 200 μg/mL) compared to the control group, as evidenced by the activated caspase-3 and caspase-7 identified and captured by fluorescence microscopy. The relative migration rate in MDA-MB-231 cells treated with 10 μg/mL of PALH extract for 48 h was significantly lower compared to the control group. Analysis of acute (2000 mg/kg/BW) and subacute (250 and 500 mg/kg/BW) toxicity of PALH extract in mice showed no mortality or adverse effects in the kidney and liver histology compared to the control group. PALH extract can be considered nontoxic as it does not cause any adverse changes and so can be proposed as a potential breast anticancer agent.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cancer is the uncontrolled growth of cells with the ability to invade different body parts. It is a health problem in many high- and low-income countries (Bray et al. 2012), with high morbidity and mortality. In 2018, there were an estimated 18.1 million new cases of cancer and 9.6 million deaths due to cancer (Bray et al. 2018). Breast cancer in women is one of the most common and primary causes of death due to cancer globally (Zhou et al. 2017). Although remarkable developments in diagnosis and treatment have been made, the prognosis and 5-year survival rate for patients are still unsatisfactory (Bao et al. 2016). Surgery, radiation therapy, chemotherapy, immunotherapy, adjuvant therapy, and targeted therapy are the modern approaches to treating cancer (Baskar et al. 2012; Sudhakar 2009). However, these approaches have failed to show expected results, in addition to having adverse effects, such as nausea, vomiting, fever, and tumor site pain (Kerns et al. 2014; Widakowich et al. 2007). Therefore, new phytochemicals with anticancer potential and lower toxicity levels and side effects are in demand and require exploration.
The National Cancer Institute (NCI) has tested about 35,000 plant species, of which 3000 have shown reproducible anticancer activity (Desai et al. 2008). This ability of plants to treat cancer is due to the bioactive secondary metabolites synthesized in them (Issa et al. 2006). Numerous studies have reported the use of plant extracts and isolated compounds for their anticancer activity. For example, crude extracts (Khan et al. 2020; Meeran et al. 2010; Shu et al. 2010) or specific compounds such as curcumin from Curcuma longa, genistein from Lupinus plyphyllus, tea polyphenols from Camellia sinensis, resveratrol from Vitis vinifera, sulforaphane from Brassica oleracea, silymarin from Silybum marianum, diallyl sulfide from Allium sativum, lycopene from Solanum lycopersicum, rosmarinic acid from Salvia rosmarinus, apigenin from Petroselinum crispum, and gingerol from Zingiber officinale (Khan et al. 2020; Wang et al. 2012) are used for their bioactivity, while others are more specific for some cancers, such as head and neck (Matovina et al. 2017), breast (Levitsky and Dembitsky 2015), pancreatic (Yue et al. 2017), prostate (Thomas-Charles and Fennell 2019), and colorectal cancer (Benarba and Pandiella 2018).
Plectranthus amboinicus (Lour.) Spreng (family Lamiaceae) is an aromatic succulent herb with distinctive-smelling leaves (Pillai et al. 2011). This herb occurs naturally throughout the warm regions of Asia, Africa (Arumugam et al. 2016), and the southern region of Saudi Arabia. It is a traditional medicinal herb used to treat several diseases, including otalgia, cephalalgia, dyspepsia, anorexia, intestinal disturbance, cholera, halitosis, nervous system disorders, bronchitis, chronic asthma, hiccoughs, strangury, malarial fever, hepatopathy, constipation, and muscle spasms (Asiimwe et al. 2014; Pillai et al. 2011; Warrier et al. 1996). It has numerous pharmacological properties, including antioxidant, antiepileptic, antitumor, neuropharmacological, antimutagenic, and antimicrobial properties (Patel et al. 2010a, b; Gurgel et al. 2009; Buznego and Perez-Saad 1999; Annapurani and Priya 1999). Few studies have reported possible toxic effects of P. amboinicus on vital organs (Asiimwe et al. 2014). However, although P. amboinicus shows anticancer activity, the acute and subacute toxicity of P. amboinicus leaf hexane (PALH) extract has not been investigated. This study assessed the cytotoxic, apoptotic activity of PALH extract on breast cancer cell lines and the acute and subacute toxicity on albino mice.
Materials and methods
Plant material
P. amboinicus was collected from Bani Malik (120 km east of the Jizan region; 17°19′14.4″N, 43°10′21.1″E), Saudi Arabia. The herb was taxonomically identified by Dr. Mohammed el Sheikh, and the voucher specimen (KSU/BRC-055) was deposited in our laboratory for future reference.
Soxhlet extraction
We extracted about 50 g of air-dried powdered leaves of P. amboinicus with 450 mL of hexane, chloroform, ethyl acetate, and methanol for 24 h in a Soxhlet apparatus. The extracts were evaporated using a vacuum rotary evaporator (Heidolph, Germany) at 45 °C until a constant weight was obtained. Then, we dissolved 10 mg of each extract in 1 mL of dimethylsulfoxide (DMSO) for activity tests.
Cell culture
We obtained two human breast cancer cell lines MCF7 (ACC 115) and MDA-MB-231 (ACC 732) from the German Collection of Microorganisms and Cell Cultures and maintained them in Dulbecco’s modified Eagle’s medium (DMEM). The medium was supplemented with 1 mM sodium pyruvate, 2 mM L-glutamine, 10% fetal bovine serum (FBS), and 1% penicillin/streptomycin. The cells were kept in a humidified incubator at 37 °C in 5% CO2.
Cytotoxicity assay
Cells (5 × 104 cells/well) were seeded in 24-well plates and exposed to PALH extract at concentrations of up to 200 μg/mL for 48 h. Next, we conducted a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, as described previously (Abutaha et al. 2020). Color changes were measured at a wavelength of 540 nm. Cell viability and the median inhibitory concentration (IC50) were determined using OriginPro 8.5. Finally, morphological changes were observed under a phase contrast microscope (Leica Microsystems, Germany).
Lactate dehydrogenase activity assay
We determined the lactate dehydrogenase (LDH) activity of the supernatants of cells (5 × 104 cells/well) incubated with the IC50 (μg/mL) of PALH extract by measuring the resultant increase in optical density (Nasr et al. 2018). DMSO (0.01%) was used as positive control, and Triton X-100 was used as negative control. We mixed 100 μL of the supernatant with 100 μL of the LDH assay mixture prepared according to the manufacturer’s instructions (Sigma-Aldrich, St. Louis, MO, USA). Absorbance was measured at a wavelength of 490 nm using a spectrophotometer plate reader (Biochrom, England).
Apoptosis induction assay
We investigated the induction of apoptosis by PALH extract using the Annexin-V and Dead Cell Kit (7-AAD) protocol following the user guide and the manufacturer’s instructions. Briefly, MDA-MB-231 cells were seeded in 6-well plates until 90% confluence and then incubated with 100 and 200 μg/mL of PALH extract or control (0.01% DMSO). After treatment, both the supernatant and the cells were combined, centrifuged, washed with phosphate-buffered saline (PBS), suspended in 1% FBS, and mixed with 100 μL of Muse™ Annexin-V and Dead Cell Reagent at 25 °C in the dark. After 20 min, non-apoptotic dead cells, non-apoptotic live cells, late apoptotic cells, and early apoptotic cells were analyzed using the Muse™ Cell Analyzer (Millipore, Billerica, MA, USA).
Caspase-3 and caspase-7 activity assay
Caspase activity was investigated using a caspase-3/7 assay kit (Life Technologies, Waltham, MA, USA). Briefly, cells were treated with PALH extract, as in the cytotoxicity assay, and incubated with 2 μM caspase-3/7 assay reagent following the manual’s instructions. The wells were imaged using a fluorescent microscope (EVOS, USA).
Migration assay
Cells were plated in a 12-well plate until 90% confluence, and then a scratch was made in each well using an autoclaved 10 μL pipette tip. Next, 10 μg/mL of (IC50) PALH extract was pipetted, and images were captured at 0, 24, and 48 h following treatment. Data represented the mean ± standard deviation (SD) (P < 0.05) in treated versus control groups (Student’s two-tailed t-test). The relative migration ratio was measured and quantified, as described previously (Abutaha et al. 2020).
Gas chromatography–mass spectrometry analyses and compound identification
PALH extract was diluted in high-performance liquid chromatography–grade chloroform and then injected into a gas chromatography–mass spectrometry (GC-MS) system (TurboMass, PerkinElmer, Inc., Waltham, MA, USA) equipped with an Elite-5MS column (30 m × 0.25 mm with 0.25 μm coating thickness). Analytical conditions were the same as previously reported (Abutaha et al. 2020). Finally, different compounds were identified by comparing the spectra with those in the WILEY (2006) and National Institute of Standard and Technology (2005) libraries.
Experimental animals
Healthy male and female albino mice with 25–35 g body weight (BW) were obtained from the Department of Zoology, King Saud University, Riyadh, Saudi Arabia. The mice were kept in cages for 7 days before dosing to acclimatize them to laboratory conditions. Three mice per group were housed in aerated plastic cages under standard conditions of a 12/12 h light/dark cycle and provided food and water ad libitum. All procedures were performed according to the Animal Ethics Committee, Biology Department, Al Imam Mohammad Ibn Saud Islamic University, Saudi Arabia.
Acute toxicity study
Acute toxicity study was carried out according to Organisation for Economic Co-operation and Development (OECD; No. 2008) guidelines. The mice were divided into two groups (n = 3) of both sexes. All animals were fasted overnight. After the fasting period, both groups were weighed, and PALH extract doses were calculated according to the mean BW. Each group was administered a single oral dose of 2000 mg/kg/BW of PALH extract dissolved in sterile distilled water. The control group received 200 μL of sterile distilled water only. Next, clinical observations, including mortality, behavior, neurological abnormalities, and signs of toxicity, were made initially for the first 4 h and thereafter once every 24 h for the next 14 days. The mice were euthanized on day 14. Liver and kidney samples were taken for tissue processing (Abutaha et al. 2020) and examined under a light microscope equipped with a digital camera to investigate any histological change (Jarrar et al. 2020).
Subacute toxicity study
We divided 18 mice (25–35 g BW) into three groups (1, 2, and 3; n = 10 each; 3 females/cage and 3 males/cage). Groups 1 and 2 were orally administered 250 and 500 mg/kg/BW, respectively, of PALH extract for 21 days. Group 3 (control) received distilled water in the same volume. The mice were euthanized on day 22. Liver and kidney samples were excised, sectioned, and further processed for histopathological examination (Jarrar et al. 2020).
Statistical analysis
Statistical calculations (Student’s t-test) were conducted using Microsoft Excel. The means of measurements were recorded with standard deviations (± SD). P ≤ 0.05 was considered statistically significant.
Results
The yields of P. amboinicus hexane, chloroform, ethyl acetate, and methanol crude extracts were 2.83%, 1.57%, 1.92%, and 3.82%, respectively. PALH extract was cytotoxic against human breast cancer cells. The IC50 value of PALH extract against MDA-MB-231 and MCF-7 cells was 39.26 and 89.05 μg/mL, respectively (Fig. 1a). Cell death after incubation with PALH extract at IC50 was also assessed by LDH release into the medium. We found a significant release of LDH in treated cells 48 h posttreatment (Fig. 1b).
Effect of PALH extract on MDA-MB-231 and MCF-7 cells using MTT and LDH assays. a MTT assay was used to assess the cytotoxicity at a wavelength of 540 nm using a microplate reader. b MDA-MB-231 (39.26 μg/mL) and MCF7 (89.05 μg/mL) cells were incubated with PALH extract for 48 h. LDH release was investigated at a wavelength of 490 nm using a microplate reader. Data represent the mean ± SD. Experiments were carried out in triplicate. P < 0.05 was considered statistically significant. DMSO (0.01%) and Triton X-100 were used as positive and negative controls, respectively. PALH, Plectranthus amboinicus leaf hexane; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; LDH, lactate dehydrogenase; SD, standard deviation; DMSO, dimethylsulfoxide
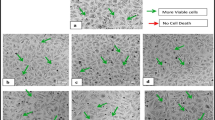
PALH extract treatment altered the morphology of MDA-MB-231 and MCF-7 cells, while control cells showed a healthy morphology. We also observed cytoplasmic shrinkage, detachment, and complete loss of cellular integrity in all PALH extract–treated cells (Fig. 2). Statistical analyses showed a significant difference between PALH extract–treated cells compared to control cells (P < 0.05). In addition, PALH extract treatment significantly induced apoptosis in MDA-MB-231 cells in a concentration-dependent manner.
Morphological changes in MDA-MB-231 (d) and MCF-7 (b) cells treated with 40 and 90 μg/mL of PALH extract, respectively, at 48 h. Morphological changes were observed under a phase contrast microscope. Cells were rounded and shrunken, indicating apoptosis induction. a and c Control MDA-MB-231 and MCF-7 cells. Arrows indicate cells undergoing morphological changes. PALH, Plectranthus amboinicus leaf hexane
Treatment of MDA-MB-231 cells with 100 and 200 μg/mL of PALH extract significantly inhibited cell viability to 40.0% ± 1.5% and 10.8% ± 1.1%, respectively, compared to corresponding vehicle controls (DMSO, 0.01%), which had a cell viability of 93.2% ± 0.4%; increased the percentage of late apoptotic cells to 47% ± 5.6% and 58% ± 1.5%, respectively, compared to vehicle control cells (2.6% ± 1.3%); and significantly increased early apoptotic cells to 0.2% ± 1.2% and 0.5% ± 0.5%, respectively, compared to control cells (0.4% ± 0.3%) (Fig. 3a, b). These results were also supported by the activated caspase-3 and caspase-7 identified and captured by fluorescence microscopy (Fig. 4).
Effect of PALH extract on MDA-MB-231 cell apoptosis. a Apoptotic effect of different concentrations of PALH extract were assessed using the Muse™ Annexin-V and Dead Cell Assay Kit. b Bar charts show an increase in the proportion of total apoptotic MDA-MB-231 cells after exposure to 100 and 200 μg/mL of PALH extract. Data represent the mean ± SD. Experiments were carried out in triplicate. P < 0.05 was considered statistically significant (Student’s two-tailed t-test). D, dead; EA, early apoptotic; LA, late apoptotic; L, live; PALH, Plectranthus amboinicus leaf hexane; SD, standard deviation
Activation of caspase-3 and caspase-7 in MDA-MB-231 cells. Cells treated with 40 μg/mL of PALH extract for 24 h showed an increase in the nuclear intensity (green) compared to untreated cells (0.01% DMSO). Activated caspase-3 and caspase-7 were identified and captured by fluorescence microscopy. Arrows indicate cells undergoing activation of caspase-3 and caspase-7. PALH, Plectranthus amboinicus leaf hexane; DMSO, dimethylsulfoxide
We used scratch assay to analyze the in vitro wound closure effect of PALH extract. We used 10 μg of PALH extract/mL to study the effect on MDA-MB-231 cell migration. The relative migration rates of PALH extract–treated cells (10 μg/mL) at 24 and 48 h were significantly lower compared to untreated control cells (Fig. 5), indicating that PALH extract treatment decreases the migration of MDA-MB-231 cells in a time-dependent manner. Micrographs in Fig. 5 show the coverage of scratched wounds in the presence or absence of PALH extract.
Images of in vitro migration analysis of MDA-MB-231 cells. a Effects of PALH extract on MDA-MB-231 cell migration. Cells were scratched and treated with 10 μg/mL of PALH extract and their images captured at 0, 24, and 48 h. b Data represent the mean ± SD. P < 0.05 from triplicate experiment versus the control (Student’s two-tailed t-test). PALH, Plectranthus amboinicus leaf hexane; SD, standard deviation
Acute toxicity
Gross pathological examination of the kidneys and liver of mice treated with 2000 mg/kg/BW of PALH extract (2000 mg/kg/BW) for 14 days showed no signs of toxicity and mortality. Histopathological examination of kidney (Fig. 6b) and liver (Fig. 6d) sections of mice treated with 2000 mg/kg/BW of PALH extract showed a normal renal and hepatic structure, with no histopathological changes compared to the control group (Fig. 6a, c, respectively). The liver of treated mice showed healthy central veins, portal area, blood sinusoids, and endothelial cells with normal radiating hepatic strands, and the kidneys showed healthy glomeruli, proximal convoluted tubules (PCTs), and distal convoluted tubules (DCTs).
Photomicrographs of kidney sections of a control mice and b female mice treated with 2000 mg/kg of PALH extract showing a normal renal architecture (H&E 400×). Photomicrographs of liver sections of c control mice and d female mice treated with 2000 mg/kg of PALH extract showing normal morphology of a hepatic lobule with a normal CV bounded by an intact endothelium (arrowhead). Parallel cords of hepatocytes (H) radiate from the CV toward the periphery of the hepatic lobule and are separated by blood sinusoids (BS) (H&E 400×). PCT, proximal convoluted tubule; DCT, distal convoluted tubule; G, glomerulus; BS, Bowman’s space; BV, blood vessels; H&E, hematoxylin and eosin; CV, central vein; PALH, Plectranthus amboinicus leaf hexane
Subacute toxicity
Daily oral administration of PALH extract to mice for 14 consecutive days did not affect their behavior compared to the control group. All the treatment groups appeared healthy, with no mortality and no signs of abnormal behavior observed throughout the study period.
Histopathological examination of kidney sections of groups 1 (Fig. 7c, d) and 2 (Fig. 7e, f) showed no significant microscopic changes compared to group 3 (control; Fig. 7a, b). The microscopic architecture of kidney sections of groups 1 and 2 had a similar appearance to that of group 3, with a healthy glomerulus, Bowman’s capsule, PCTs, and DCTs.
Photomicrographs of kidney sections of a female control mice, b male control mice, c female mice treated with 250 mg/kg of PALH extract, d male mice treated with 250 mg/kg of PALH extract, e female mice treated with 500 mg/kg of PALH extract, and f male mice treated with 500 mg/kg of PALH extract, all showing a normal renal architecture (H&E 400×). PCT, proximal convoluted tubule; DCT, distal convoluted tubule; G, glomerulus; BS, Bowman’s space; BV, blood vessels; H&E, hematoxylin and eosin; PALH, Plectranthus amboinicus leaf hexane
Histopathological examination of liver sections of group 3 (Fig. 8a, b) showed a healthy central vein, hepatic blood sinusoids, and portal triad, including the hepatic portal vein, hepatic artery, and interlobular bile duct. Groups 1 (Fig. 8c, d) and 2 (Fig. 8e, d) showed no microscopic alterations compared to group 3. Groups 1 and 2 had healthy central veins and hepatic sinusoids lined with endothelial cells with healthy radiating hepatocytes.
Photomicrographs of liver sections of a female control mice, b male control mice, c female mice treated with 250 mg/kg of PALH extract, d male mice treated with 250 mg/kg of PALH extract, e female mice treated with 500 mg/kg of PALH extract, and f male mice treated with 500 mg/kg of PALH extract, all showing a normal hepatic lobular architecture with well-brought-out hepatocytes and a prominent nucleus and nucleolus. Parallel cords of hepatocytes (H) radiate from the CV toward the periphery of the hepatic lobule and are separated by sinusoidal spaces (BS) (H&E 400×). PALH, Plectranthus amboinicus leaf hexane; CV, central vein; BS, Bowman’s space; H&E, hematoxylin and eosin
Histological findings showed that PALH extract was relatively nontoxic to mice. No signs of toxicity were observed in the liver and kidney sections of treated mice. However, more studies on the safety of PALH extract should be conducted before using it as a health product.
Gas chromatography–mass spectrometry analyses
GC-MS analyses of PALH extract showed 28 peaks on the chromatogram (Fig. 9). The extract primarily comprised phenol, 5-methyl-2-(1-methylethyl) (44%), in addition to 1-isopropyl-2,3-dimethoxybenzene, nonacosane, tetratetracontane, 2,6,10,14,18,22-tetracosahexae, and pentatriacontane (Fig. 9 and Table 1).
Discussion
Chemotherapy is considered one of the fundamental approaches to modern cancer therapy. Anticancer chemotherapeutic agents that result in apoptosis of cancer cells are the most promising strategy for cancer treatment (Lowe and Lin 2000). Therefore, candidate anticancer chemotherapeutic agents should induce apoptosis of cancer cells (Surh 2003). Apoptosis is known for its morphological alterations, such as membrane blebbing, cell shrinkage, rounding of cells, detachment, and chromatin condensation (Hengartner 2000). In this study, these alterations were detected in cells incubated with PALH extract. Apoptosis indicators also include phosphatidylserine translocation, which is identified using annexin-V staining (Boersma et al. 2005). In this study, these characteristics were similar to those reported in the past as evidence that cells undergo apoptosis (Khan et al. 2020; Shu et al. 2010; Wang et al. 2012).
Caspases are a family of protease enzymes that play an important role in apoptosis. Caspases are triggered by diverse death stimuli (Timmer and Salvesen 2007). The bulk of the proteolysis that occurs during apoptosis is performed by effector caspases. Among effector caspases, caspase-3 and caspase-7 play an important role in coordinating the events that occur during apoptosis, since the two are major executioner caspases (Slee et al. 1999). In this study, we observed an increase in caspase-3/7 green color intensity in breast cancer cells treated with PALH extract. Increased caspase-3 and caspase-7 activity has also been widely reported (Kumar et al. 2017; Motadi et al. 2020). However, it is dependent on the solvent used for extraction. Toxicity has been shown to be more commonly activated in nonpolar extracts (Ediriweera et al. 2016; Kim et al. 2020; Saleh et al. 2020).
The liver and kidneys of rats are vital organs used in many acute and subacute studies to assess the safety or toxicity of drugs or natural products (Loha et al. 2019; Satyapal et al. 2008). Our acute and subacute toxicity analysis did not show any abnormalities in the color, shape, size, and texture of the liver and kidneys of treated mice compared to the control group, which is consistent with findings of previous studies that reported no signs of toxicity and mortality after treatment with P. amboinicus extract at a dose of 2000 mg/kg (acute) or 200 and 400 mg/kg (subacute) (Pillai et al. 2011). Another study conducted on P. amboinicus extract by Asiimwe et al. (2014) showed no mortality at a 5000 mg/kg dose (acute) and at doses of 2500, 1250, and 625 mg/kg (subacute) of the aqueous extract for 28 days, although treatment-related toxicological abnormalities, such as necrosis and hemorrhages, increased with the dose. The toxicity reported could be attributed to overdosing. These results highlight the importance of adhering to the dose limit prescribed by herbalists on the basis of the patient’s health and physique.
Subacute oral toxicity analysis has been used for safety evaluation to obtain safety data before product commercialization (Arts et al. 2004; Bautista et al. 2004). In this study, we recorded no toxicity signs throughout the subacute and acute toxicity experiments, indicating that oral administration of PALH extract is nontoxic to mice.
GC-MS analyses showed the presence of many anticancer compounds in PALH extract. Most of these compounds show anticancer activity in vitro and/or in vivo. The primary constituent of PALH extract analyzed in this study was phenol, 5-methyl-2-(1-methylethyl) (> 44% of the total). This monoterpene is also a major constituent of other plants, such as Monarda bradburiana (57.7%), Trachyspermum copticum (72.3–37.2%), Lagoecia cuminoides (72.8–94.8%), Lippia gracilis (3.83–55.50%), and Monarda punctata (75.2%) (Salehi et al. 2018). Thymol (phenol, 5-methyl-2-(1-methylethyl)) has anticancer properties and inhibits several cancer cell lines, including glioblastoma, glioma, leukemia, breast cancer, mastocytoma, osteosarcoma, hepatocellular carcinoma, cervical cancer, laryngeal carcinoma, laryngeal carcinoma, and laryngeal carcinoma (Islam et al. 2019; Nagoor Meeran et al. 2017). Thymol induces apoptosis and necrosis, depolarizing the mitochondrial membrane potential, stimulating the cell cycle arrest in G0/G1, producing intracellular reactive oxygen species, and activating Bax and caspase cleavage. It also increases intracellular Ca2+ overload, phospholipase-C, and protein kinase-C–dependent Ca2+ release from the endoplasmic reticulum (Kang et al. 2016; Nagoor Meeran et al. 2017). The presence of terpinene in PALH extract can be due to its anticancer properties. In addition, it has cytotoxic and antioxidant properties against mouse leukemia (P388) (Jaafari et al. 2007) and B16-F10 (Ferraz et al. 2013) cells. Previous studies also reported that α-humulene is cytotoxic to UACC-257, A549, MCF-7, HT-29, L-929, DLD-1, and HeLa cancer cell lines (Legault and Pichette 2007; Salehi et al. 2018), and τ-cadinol and α-cadinol are cytotoxic to A-549, HT-29, and MCF-7 cancer cell lines (Chang et al. 2000; He et al. 1997).
Conclusions
PALH extract shows anticancer effects in MCF7 and MDA-MB-231 cells, with morphological changes that support the induction of apoptosis. In addition, the extract does not cause histological abnormalities in the liver and kidneys of treated animals. Its biological activities are due, in part, to thymol and other constituents. However, further investigation is required to confirm its effectiveness and safety in humans.
Data availability
All the data presented can be found online.
References
Abutaha N, Al-zharani M, Al-Doaiss AA, Baabbad A, Al-malki AM, Dekhil H (2020) Anticancer, antioxidant, and acute toxicity studies of a Saudi polyherbal formulation, PHF5. Open Chem 18:472–481
Annapurani S, Priya R (1999) Antimutagenic, antitumourogenic and antigenotoxic effects of polyphenol extracts of selected medicinal plants. Indian J Nutr Diet 36:431–435
Arts JH, Muijser H, Appel MJ, Kuper CF, Bessems JG, Woutersen RA (2004) Subacute (28-day) toxicity of furfural in Fischer 344 rats: a comparison of the oral and inhalation route. Food Chem Toxicol 42:1389–1399
Arumugam G, Swamy MK, Sinniah UR (2016) Plectranthus amboinicus (Lour.) Spreng: botanical, phytochemical, pharmacological and nutritional significance. Molecules 21:369
Asiimwe S, Borg-Karlsson A-K, Azeem M, Mugisha KM, Namutebi A, Gakunga NJ (2014) Chemical composition and toxicological evaluation of the aqueous leaf extracts of Plectranthus amboinicus (Lour.) Spreng. Int J Pharm Sci Invent 3:19–27
Bao J, Zhu L, Zhu Q, Su J, Liu M, Huang W (2016) SREBP-1 is an independent prognostic marker and promotes invasion and migration in breast cancer. Oncol Lett 12:2409–2416
Baskar R, Lee KA, Yeo R, Yeoh K-W (2012) Cancer and radiation therapy: current advances and future directions. Int J Med Sci 9:193–199
Bautista ARPL, Moreira E, Batista MS, Miranda M, Gomes I (2004) Subacute toxicity assessment of annatto in rat. Food Chem Toxicol 42:625–629
Benarba B, Pandiella A (2018) Colorectal cancer and medicinal plants: principle findings from recent studies. Biomed Pharmacother 107:408–423
Boersma HH, Kietselaer BL, Stolk LM, Bennaghmouch A, Hofstra L, Narula J, Heidendal GA, Reutelingsperger CP (2005) Past, present, and future of annexin A5: from protein discovery to clinical applications. J Nucl Med 46:2035–2050
Bray F, Jemal A, Grey N, Ferlay J, Forman D (2012) Global cancer transitions according to the Human Development Index (2008–2030): a population-based study. Lancet Oncol 13:790–801
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424
Buznego MT, Perez-Saad H (1999) Antiepileptic effect of Plectranthus amboinicus (Lour.) Spreng.(french marjoram). Rev Neurol 29:388–389
Chang S-T, Wang DS-Y, Wu C-L, Shiah S-G, Kuo Y-H, Chang C-J (2000) Cytotoxicity of extractives from Taiwania cryptomerioides heartwood. Phytochemistry 55:227–232
Desai AG, Qazi GN, Ganju RK, El-Tamer M, Singh J, Saxena AK, Bedi YS, Taneja SC, Bhat HK (2008) Medicinal plants and cancer chemoprevention. Curr Drug Metab 9:581–591
Ediriweera MK, Tennekoon KH, Samarakoon SR, Thabrew I, Dilip De Silva E (2016) A study of the potential anticancer activity of Mangifera zeylanica bark: evaluation of cytotoxic and apoptotic effects of the hexane extract and bioassay-guided fractionation to identify phytochemical constituents. Oncol Lett 11:1335–1344
Ferraz RP, Bomfim DS, Carvalho NC, Soares MB, da Silva TB, Machado WJ, Prata APN, Costa EV, Moraes VRS, Nogueira PCL (2013) Cytotoxic effect of leaf essential oil of Lippia gracilis Schauer (Verbenaceae). Phytomedicine 20:615–621
Gurgel A, da Silva J, Grangeiro A, Oliveira DC, Lima CM, da Silva A, Souza I (2009) In vivo study of the anti-inflammatory and antitumor activities of leaves from Plectranthus amboinicus (Lour.) Spreng (Lamiaceae). J Ethnopharmacol 2:361–363
He K, Zeng L, Shi G, Zhao G-X, Kozlowski JF, McLaughlin JL (1997) Bioactive compounds from Taiwania cryptomerioides. J Nat Prod 60:38–40
Hengartner MO (2000) The biochemistry of apoptosis. Nature 407:770–776
Islam MT, Khalipha AB, Bagchi R, Mondal M, Smrity SZ, Uddin SJ, Shilpi JA, Rouf R (2019) Anticancer activity of thymol: a literature-based review and docking study with emphasis on its anticancer mechanisms. IUBMB Life 71:9–19
Issa AY, Volate SR, Wargovich MJ (2006) The role of phytochemicals in inhibition of cancer and inflammation: new directions and perspectives. J Food Compos Anal 19:405–419
Jaafari A, Mouse HA, Rakib EM, Tilaoui M, Benbakhta C, Boulli A, Zyad A (2007) Chemical composition and antitumor activity of different wild varieties of Moroccan thyme. Rev Bras 17:477–491
Jarrar Y, Al-Doaiss A, Alfaifi M, Shati A, Al-Kahtani M, Jarrar B (2020) The influence of five metallic nanoparticles on the expression of major drug-metabolizing enzyme genes with correlation of inflammation in mouse livers. Environ Toxicol Pharmacol 80:103449
Kang S-H, Kim Y-S, Kim E-K, Hwang J-W, Jeong J-H, Dong X, Lee J-W, Moon S-H, Jeon B-T, Park P-J (2016) Anticancer effect of thymol on AGS human gastric carcinoma cells. J Microbiol Biotechnol 26:28–37
Kerns SL, Ostrer H, Rosenstein BS (2014) Radiogenomics: using genetics to identify cancer patients at risk for development of adverse effects following radiotherapy. Cancer Discov 4:155–165
Khan T, Ali M, Khan A, Nisar P, Jan SA, Afridi S, Shinwari ZK (2020) Anticancer plants: a review of the active phytochemicals, applications in animal models, and regulatory aspects. Biomolecules 10:47
Kim W, Park C, Park J, Cheong H, Kim S-J (2020) Pine needle hexane extract promote cell cycle arrest and premature senescence via p27 KIP1 upregulation gastric cancer cells. Food Sci Biotechnol 29:845–853
Kumar S, Sharma VK, Yadav S, Dey S (2017) Antiproliferative and apoptotic effects of black turtle bean extracts on human breast cancer cell line through extrinsic and intrinsic pathway. Chem Cent J 11:1–10
Legault J, Pichette A (2007) Potentiating effect of β-caryophyllene on anticancer activity of α-humulene, isocaryophyllene and paclitaxel. J Pharm Pharmacol 59:1643–1647
Levitsky DO, Dembitsky VM (2015) Anti-breast cancer agents derived from plants. Nat Prod Bioprospect 5:1–16
Loha M, Mulu A, Abay SM, Ergete W, Geleta B (2019) Acute and subacute toxicity of methanol extract of Syzygium guineense leaves on the histology of the liver and kidney and biochemical compositions of blood in rats. Evid Based Complement Alternat Med:2019 Mar 10;2019:5702159. https://doi.org/10.1155/2019/5702159. PMID: 30956682; PMCID: PMC6431459
Lowe S, Lin A (2000) Apoptosis in cancer. Carcinogenesis 21:485–495
Matovina C, Birkeland AC, Zick S, Shuman AG (2017) Integrative medicine in head and neck cancer. Otolaryngol Head Neck Surg 156:228–237
Meeran SM, Ahmed A, Tollefsbol TO (2010) Epigenetic targets of bioactive dietary components for cancer prevention and therapy. Clin Epigenetics 1:101–116
Motadi LR, Choene MS, Mthembu NN (2020) Anticancer properties of Tulbaghia violacea regulate the expression of p53-dependent mechanisms in cancer cell lines. Sci Rep 10:1–11
Nagoor Meeran MF, Javed H, Al Taee H, Azimullah S, Ojha SK (2017) Pharmacological properties and molecular mechanisms of thymol: prospects for its therapeutic potential and pharmaceutical development. Front Pharmacol 8:380
Nasr FA, Abutaha N, Al-Zahrani M, Farooq M, Wadaan MA (2018) Anticancer potential of plant extracts from Riyadh (saudi Arabia) on MDA-MB-231 breast cancer cells. Afr J Tradit Complement Altern Med 15:46–53
No OT (2008) 407: repeated Dose 28-day oral toxicity study in rodents. OECD guidelines for the testing of chemicals, Section 4
Patel RD, Mahobia NK, Singh MP, Singh A, Sheikh NW, Alam G, Singh SK (2010a) Antioxidant potential of leaves of Plectranthus amboinicus (Lour) Spreng. Pharm Lett 2:240–245
Patel R, Mahobia N, Waseem N, Upwar N, Singh S (2010b) Phyto-physicochemical investigation of leaves of Plectranthus amboinicus (Lour) Spreng. Pharm J 2:536–542
Pillai PG, Suresh P, Aggarwal G, Doshi G, Bhatia V (2011) Pharmacognostical standardization and toxicity profile of the methanolic leaf extract of Plectranthus amboinicus (Lour) Spreng. J Appl Pharm Sci 1:76
Saleh KA, Albinhassan TH, Al-Ghazzawi AM, Mohaya A, Shati AA, Ayoub HJ, Abdallah QM (2020) Anticancer property of hexane extract of Suaeda fruticose plant leaves against different cancer cell lines. Trop J Pharm Res 19:129–136
Salehi B, Mishra AP, Shukla I, Sharifi-Rad M, Contreras MM, Segura-Carretero A, Fathi H, Nasrabadi NN, Kobarfard F, Sharifi-Rad J (2018) Thymol, thyme, and other plant sources: Health and potential uses. Phytother Res 32:1688–1706
Satyapal US, Kadam VJ, Ghosh R (2008) Hepatoprotective activity of livobond a polyherbal formulation against CCl4 induced hepatotoxicity in rats. Int J Pharmacol 4:472–476
Shu L, Cheung K-L, Khor TO, Chen C, Kong A-N (2010) Phytochemicals: cancer chemoprevention and suppression of tumor onset and metastasis. Cancer Metastasis Rev 29:483–502
Slee EA, Harte MT, Kluck RM, Wolf BB, Casiano CA, Newmeyer DD, Wang H-G, Reed JC, Nicholson DW, Alnemri ES (1999) Ordering the cytochrome c–initiated caspase cascade: hierarchical activation of caspases-2,-3,-6,-7,-8, and-10 in a caspase-9–dependent manner. J Cell Biol 144:281–292
Sudhakar A (2009) History of cancer, ancient and modern treatment methods. J Cancer Ther 1:1
Surh Y-J (2003) Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer 3:768–780
Thomas-Charles C, Fennell H (2019) Anti-prostate cancer activity of plant-derived bioactive compounds. Curr Mol Biol Rep 5:140–151
Timmer J, Salvesen G (2007) Caspase substrates. Cell Death Differ 14:66–72
Wang H, Oo Khor T, Shu L, Su Z-Y, Fuentes F, Lee J-H, Tony Kong A-N (2012) Plants vs. cancer: a review on natural phytochemicals in preventing and treating cancers and their druggability. Anti Cancer Agents Med Chem 12:1281–1305
Warrier P, Nambiar V, Ramankutty C (1996) Indian medicinal plants: a compendium of 500 species: Orient Longman Ltd. Chennai 3:38–90
Widakowich C, de Castro JG, De Azambuja E, Dinh P, Awada A (2007) Side effects of approved molecular targeted therapies in solid cancers. Oncologist 12:1443–1455
Yue Q, Gao G, Zou G, Yu H, Zheng X (2017) Natural products as adjunctive treatment for pancreatic cancer: recent trends and advancements. Biomed Res Int:2017;2017:8412508. https://doi.org/10.1155/2017/8412508. Epub 2017 Jan 23. PMID: 28232946; PMCID: PMC5292383
Zhou T, Li Y, Yang L, Liu L, Ju Y, Li C (2017) Silencing of ANXA3 expression by RNA interference inhibits the proliferation and invasion of breast cancer cells. Oncol Rep 37:388–398
Acknowledgments
The authors are grateful to the Deanship of Scientific Research, King Saud University, for funding through Vice Deanship of Scientific Research Chairs.
Author information
Authors and Affiliations
Contributions
Conceptualization, N.A.; methodology, A.A., N.A., M.A.W., A.A.A., A.Z.; software, N.A., AA.; writing—original draft preparation, N.A. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
The experiments were approved by the Faculty of Science, Al Imam Mohammad Ibn Saud Islamic University, ethical committee (approval no. 1442-265).
Consent for publication
Not applicable
Competing interest
The authors declare no competing interests.
Additional information
Responsible Editor: Mohamed M. Abdel-Daim
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Almalki, A., Abutaha, N., Al-Doaiss, A.A. et al. Cytotoxicity, in vivo toxicity, and chemical composition of the hexane extract of Plectranthus amboinicus (Lour.) Spreng. Environ Sci Pollut Res 28, 48141–48153 (2021). https://doi.org/10.1007/s11356-021-13796-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-13796-8