Abstract
This work explored the use of porous carbon (PC) materials converted from waste lignin as raw materials for the removal of chloramphenicol (CAP) in water. The PC with controllable pores was prepared through a facile, cost-effective one-step method. The physical and chemical properties of the material were characterized by BET, SEM, FT-IR, and XRD, and the best conditions for preparation were selected based on the results of adsorption experiments. The PC, which was prepared at reaction temperature of 800 °C and the K2CO3/sodium lignosulfonate mass ratio of 4, namely PC-800-4, had a high specific surface area (1305.5 m2 g−1) and pore volume (0.758 cm3 g−1). At a lower initial concentration of CAP (C0 = 120 mg L−1), the maximum adsorption capacity of this adsorbent was 534.0 mg g−1 at 303 K. In addition, PC-800-4 maintained good adsorption performance in a wide pH range and strongly resisted the interference of ions and humic acid. The results showed that the adsorption removal CAP was based on physical adsorption and chemical adsorption as a process supplement. The advantages of wide sources, high efficiency and speed, wide application, and rich oxygen-containing functional groups made the adsorbent have great application potential for removal chloramphenicol from water.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent decades, reports of antibiotics detected in water have become more frequent (Wei et al. 2019). Antibiotics are considered as new pollutants due to their trace, bioaccumulative, hardly degradable, and persistent properties (Wang and Chu 2016). Most of the antibiotics administered into the living organism cannot be completely absorbed (Carvalho and Santos 2016; Wang et al. 2020), causing a large quantity of antibiotics to be discharged into environment. Chloramphenicol is a broad-spectrum antibiotic that is widely used in human and veterinary medicines because of its low cost and inhibitory effect on a variety of bacterial infections, including gram-negative and typhoid bacteria. It is a stronger bactericidal agent than penicillin and tetracycline (Liu et al. 2017a). CAP has many side effects on the human body, including gray baby syndrome (Mauer et al. 1980), aplastic anemia (Clesham et al. 2019), and bone marrow suppression (Jimenez et al. 1990). Therefore, many countries including China (Liu et al. 2017b), the USA (George and Hall 2002), and Japan (Kikuchi et al. 2017) strictly prohibit the use of CAP for livestock breeding. However, CAP is still commonly used in many developing countries because it is inexpensive and effective. In order to solve the problem of antibiotic pollution, especially in water, many treatment technologies have been developed such as membrane separation (Srinivasa Raghavan et al. 2018), redox (Ye et al. 2020), adsorption (LÜ et al. 2012), and catalysis (Lu et al. 2019). In a variety of technologies, adsorption is most preferable and widely used because of its low cost, high efficiency, and simple operation (Li et al. 2019).
Porous carbon materials were widely used for pollutant removal due to their large specific surface area, rich porosity, and high corrosion resistance (Awual and Hasan. 2015; Awual. 2017a). Biomass materials have attracted widespread attention due to their abundance, availability, and environmental friendliness (Chen et al. 2021; Liu et al. 2019). Till date, there have been numerous reports on the usage of different biomass materials as carbon sources to develop carbon materials for specific uses. For instance, bovine bones are abundant in inorganic hydroxyapatite and organic collagen, which can be carbonized by self-template carbonization and then in-situ alkali-activation to produce porous carbon materials (Dai et al. 2018a). Facts have proved that the prepared material has significant adsorption capacity for sulfamethazine and CAP antibiotics. Furthermore, biomass, including shiitake mushroom (Cheng et al. 2015), coconut shell (Dissanayake Herath et al. 2019), and auricularia (Xie et al. 2019a), have also been used as raw materials for making porous carbon. Optically, conjugated porous materials have high selectivity and sensitivity to pollutants (Awual. 2019a; 2017b; 2016a), while porous carbon materials have the advantages of rapid and high adsorption capacity.
Studies have found that oxygen-containing functional groups such as carboxyl, carbonyl, phenols, lactones, and quinones could greatly affect the unique adsorption performance of activated carbon to pollutants (Bhatnagar et al. 2013). They can combine heavy metal ions and organic small molecule pollutants through chelation, coordination, and hydrogen bonding (Zhang et al. 2015). Agricultural by-products not only have functional groups such as carboxyl and hydroxyl groups, but also have the characteristics of loose and porous structure, which are good precursors for adsorption materials (Dai et al. 2018b; Zhou et al. 2015). Lignin is a renewable carbon source and is the second largest biopolymer on earth after cellulose. Despite the potential for large-scale use of lignin, it is worth noting as at today that humans use only 3% of lignin. Sodium lignosulfonate (SLS), as a by-product of lignin used in the paper industry, generates approximately 70 million tons per year (Liu et al. 2015). Compared with lignin, SLS still contains more oxygen-containing functional groups, such as α-O-4′ and β-O-4′ linkages (Abukhadra et al. 2019; Galkin and Samec 2016), which can significantly improve water solubility and expand the scope of application. Hence, SLS may be the most promising carbon raw material. Literature evidence indicates that lignin-based porous carbon materials have been successfully used to remove heavy metals and dyes from water (Wu et al. 2020; Zhang et al. 2019). Therefore, it makes great sense to use SLS for the preparation of porous carbons to remove CAP. As far as we know, there have been no such reports before.
Based on the International Union of Pure and Applied Chemistry (IUPAC) specifications, pores have been divided into three types based on their diameter: micropores (< 2 nm), mesopores (2–50 nm), and macropores (> 50 nm). Research has shown that micropores and small mesopores can expand the specific surface area of porous carbon, while large mesopores and macropores are conducive for the transport of target molecules (Yang et al. 2019; Lv et al. 2016; Awual and Hasan. 2014). Therefore, in order to improve the ability of porous carbon to absorb pollutants, it is necessary to reconfigure its pore structure. Carbon raw materials can be converted into activated carbon by physical or chemical activation. Compared with the former, carbon produced by chemical activation method has better pore structure and specific surface area (Gao et al. 2013). However, common chemical activation methods for the preparation of porous carbon require the use of templates. The process involves a series of time-consuming template carbonization, template removal, and subsequent activation, which will greatly increase production costs (Xie et al. 2019b). For non-template method, Oginni et al. (Oginni et al. 2019) showed that the one-step method produced porous carbon with larger specific areas, total pore volumes, and average pore diameters than the two-step method, which were carbon-carbonized and then activated. More importantly, the porous carbon prepared by one-step method exhibited higher adsorption capacity.
In this work, a simple method was proposed for making layered, porous carbons (PCs) by using SLS as carbon precursor. By adjusting temperature and the mass ratio of activator K2CO3 to SLS, porous carbons with different pore structures were prepared through the one-step method. With CAP as the target pollutant, the removal mechanism and applicability of PCs were explored. This study showcased several advantages derivable from the fabrication of lignin porous carbon by the one-step method. These include (1) the one-step method greatly simplifies the manufacturing process of porous carbon materials and reduces the preparation cost and time and (2) abundance and availability of economical carbon raw materials make PCs promising as a large-scale practical applications adsorbent. With these advantages, PCs can be used as a viable, economical, and efficient adsorbent for the removal of antibiotics in the environment.
Materials and methods
Materials and reagents
CAP (C11H12C12N2O5, 98%), SLS, and potassium carbonate (K2CO3, 99%) were purchased from Aladdin Industrial Corporation (Shanghai, China). All chemicals were of analytical grade, without any further purifications.
Preparation of PCs
PCs were prepared as follows: 4.0 g of SLS with different masses of K2CO3 (0 g, 4.0 g, 8.0 g, 12.0 g, 16.0 g) were ground, mixed, and placed in a tube furnace. Under the protection of N2 flow, the temperature was raised from room temperature to 750 °C, 800 °C, 850 °C at the rate of 5 °C min−1 and maintained for 2 h. The reaction mixture was then cooled to room temperature. Afterwards, the black powder was washed with dilute hydrochloric acid and hot deionized water to remove inorganic impurities such as potassium compounds. The resulting powder was purified by washing with water several times and subsequently dried at 105 °C. The product was expressed as PC-T-x, where T and x represent activation temperature and mass ratio of K2CO3/SLS, respectively.
Characterization methods
The topographic characteristics of the synthesized PCs were determined by scanning electron microscopy (SEM, S-4800, Hitachi, Japan) and X-ray diffraction (XRD, D8 ADVANCE, Bruker AXS, Germany) technique. The Fourier transform infrared spectroscopy (FT-IR) was performed on a Spectrum Two spectrophotometer (PerkinElmer, USA) with KBr pellets. N2 adsorption-desorption isotherms were determined by ASAP 2020 PLUS (Micromeritics, USA) volumetric instrument at 77 K.
Batch adsorption experiments
To investigate the effect of the mass ratios of K2CO3 and SLS on the adsorption properties of the synthesized PCs, 3.0 mg of PC-800-x adsorbent was used to adsorb 20 mL CAP solution (100 mg L−1) for 12 h in water. After filtration, the CAP concentration in the supernatant was calculated from the absorbance readings taken at 278 nm in a UV spectrophotometer. Subsequently, in order to study the effect of pyrolysis temperature on adsorption, the above experiment was repeated using PC-750-4 and PC-850-4. The adsorption and BET results showed that PC-800-4 had the second highest microporosity and adsorption capacity for CAP. In order to obtain accurate data, all experiments were performed three times, and the average data was obtained for the next analysis.
In another experiment, the effects of pH (2–12), initial CAP concentration, metal ion concentration, humic acid, and temperature on the adsorption of CAP by PC-800-4 were investigated. The pH affects the form of ions in the solution (Awual. 2019b; 2016b). We first explored the effect of pH on adsorption and determined the best adsorption conditions for subsequent experiments. For the adsorption isotherm experiment, 3.0 mg of PC-800- 4 was added to the CAP aqueous solution with different initial concentrations (50, 75, 100, 120, 135 mg L−1). After equilibration in a 303 K water bath for 12 h, the adsorption equilibrium was reached. The adsorbent PC-800-4 was separated by filtration, and the concentration of non-adsorbed CAP molecules was measured at 278 nm using an ultraviolet-visible spectrophotometer. The equilibrium adsorption capacity for CAP was calculated by the following formula.
where C0 and Ce (mg L−1) represent the initial and adsorption equilibrium concentrations of the CAP solution, respectively; V (mL) is the solution volume and M (mg) is the weight of adsorbent.
The influence of solution pH on adsorption was investigated. The CAP solution (120 mg L−1) was adjusted to 2.0–12 with 0.1 M dilute HCl and NaOH solution. At 303 K, 3.0 mg of PC-800-4 was put into 20 mL of CAP solution of different pHs for adsorption. In addition, the effects of 0.1 M common metal ions (K+, Na+, Ca2+, Mg2+, Al3+) and different concentrations (10, 30, 50, 70 mg L−1) of humic acid on the adsorption of CAP solution (100 mg L−1) were also studied.
Adsorption kinetics was used to study the effect of contact time on the amount of adsorption (Awual. 2019). In the study, 3.0 mg of PC-800-4 and 20 mL of CAP aqueous solution (C0 = 120 mg L−1) were added to the centrifuge tube. At 303 K, CAP in the supernatant was measured in a regular time (5, 10, 15, 30, 45, 60, 90, 120, 180, 240, 300, and 420 min). In addition, the above experiment was repeated using PC-750-4 and PC-850-4 for comparison. At time t (min), the adsorption capacity Qt (mg g−1) is calculated as follows.
where Qt (mg g−1) is the quantity of CAP absorbed on the adsorbent at the setting time t.
Adsorption isotherms
Adsorption isotherms are useful for understanding the interaction between PC-800-4 and CAP. Langmuir, Freundlich, and Temkin adsorption isotherm models were used to fit the data in this experiment. The nonlinear expressions are as follows.
where Qm (mg g−1) is the maximum adsorption for CAP per unit mass of carbon; KL (L mg−1), KF (mg g−1) ((L mg−1)1/n) are Langmuir (3) and Freundlich (4) constant, respectively; 1/n is a measure of the adsorption intensity; b related to the adsorption heat is the Temkin (5) constant, and Kt is the equilibrium-binding constant.
Adsorption kinetics
To figure out the adsorption mechanism of CAP, pseudo-first-order, pseudo-second-order, and intra-particle diffusion kinetic models were used to fit and analyze the experimentally obtained kinetic data. The nonlinear equations for the three models are as follows.
where Qe and Qt (mg g−1) are the quantity of CAP absorbed on the PC-800-4 at equilibrium and setting time t; K1 (min−1), K2 (g mg−1 min−1), and Kpi (mg (g min1/2)−1) are the pseudo-first-order, pseudo-second-order, and intra-particle diffusion rate constant; Ci is a constant of Eq. (8).
Adsorption thermodynamics
Changes in thermodynamic parameters, Gibbs free energy change (ΔG), enthalpy change (ΔH), and entropy change (ΔS) during the adsorption process were monitored in order to understand the adsorption behavior and determine its mechanism. Quantitative changes were measured using the following equations.
where, ΔG is the adsorption free energy change, kJ mol−1; ΔH is the adsorption enthalpy change, kJ mol−1; ΔS is the adsorption entropy change, kJ mol−1 K−1; R is the ideal gas constant (8.314 J (mol K)−1); T is the thermodynamics temperature (K); and Kd is the equilibrium distribution coefficient of adsorption.
Mean free adsorption energy
The Dubinin-Radushkevitch (D-R) adsorption isotherm model has been widely used to measure adsorption, especially for microporous carbon. The nonlinear form of the D-R isotherm equation is as follows.
where Qmax is the amount of adsorption in the saturated state; β is the correlation constant with the average free energy of adsorption. ε is Polanyi potential energy, J mol−1. E is the mean free energy of adsorbate unit molecule from the solution to the surface of the adsorbent.
E is an important index to distinguish between physical adsorption and chemical adsorption. If the value of E is less than 8 kJ mol−1, or is between 8 and 16 kJ mol−1 or greater than 20 kJ mol−1, it indicates that the adsorption process is physical adsorption, ion exchange chemical adsorption, and chemical adsorption, respectively (Sarkar and Paul 2020).
The average relative error analysis
The average relative error (ARE) (%) is used to evaluate which model is most appropriate to describe the actual data. ARE (%) can be calculated by the following formula.
where Qi, exp and Qi, cal (mg g−1) are the experimental and fitted adsorption quantity, respectively.
Results and discussion
Material characterization
Figure 1 a–c are the SEM images of PC-800-0, PC-800-2, and PC-800-4, respectively. It was observed that as the mass of K2CO3 increased, carbon particles became finer and more porous morphologically. This is because K2CO3 decomposes into K2O and CO2 at any temperature greater than 700 °C, thereby initiating a reaction of K2O and CO2 with carbon (C) to form potassium (K) vapor and carbon monoxide (CO), respectively. More porous carbons are formed when K vapor is inserted into the carbon matrix. The reaction sequence of these processes can be depicted as follows (Sayğılı and Sayğılı 2019).
Figure 1 d is a high-resolution SEM image of PC-800-4. It was evident that a large number of pore structures were evenly distributed on the surface of the carbon particles, which served as transportation channels and provided a large surface area for adsorption of target pollutants in wastewater. The above results showed that PC-800-4 was a porous carbon with excellent pores.
The FT-IR spectra of SLS, PC-800-0 and PC-800-4 are shown in Fig. 2. The broad peak at 3401 cm−1 is caused by the stretching vibration of the hydroxyl group. After SLS was carbonized and activated, the products PC-800-0 and PC-800-4 showed gradually weaker peaks for the hydroxyl stretching vibration. In the SLS and PC-800-0 spectra, the absorption peaks at 2938 and 2843 cm−1 correspond to the C–H stretching vibration. The characteristic peaks of the aromatic structure skeleton vibration in the SLS molecule are located at 1599, 1517, 1463, 1427, and 1328 cm−1; and the stretching vibration peaks of C–O in carboxylic acids, alcohols, phenols, and esters are located at 1219 and 1037 cm−1 (Zhang et al. 2011). In addition, the absorption peak of β-O-4′ ether bond is located at 1140 cm−1. Compared with the infrared spectrum of SLS, most of the characteristic peaks in the spectrum of the carbonized PC-800-0 showed greatly reduced intensity or disappeared. In the spectrum of PC-800-4, the absorption peaks at 1599 and 1043 cm−1 confirmed the existence of C=O and sulfonic acid groups, respectively (Xie et al. 2019a). The results showed that the carbonization and activation steps affected the surface groups and chemical properties of the product.
In order to explore the crystalline/amorphous properties of PC-800-4, XRD characterization was performed over the 2θ range of 10–80°. As shown in Fig. 3, the (002) peak of hexagonal graphitic carbon appeared at 2θ = 29° (Yang et al. 2020a). Compared with PC-800-0, the (002) peak of PC-800-4 was higher and narrower, indicating that the latter had a higher degree of graphitization. The peak at 43° corresponds to the (100) plane of single-layer graphene honeycomb lattice (Tian et al. 2017), indicating the existence of short-range ordered and parallel-stacked graphite crystallites. During the activation process, SLS (carbon source) was converted into a mixture of defective carbon and graphitic carbon. Defective carbon and graphitic carbon help to enrich pores and promote crystal structure, respectively.
Figure 4 a shows the N2 adsorption-desorption isotherm of PC-T-x. According to the IUPAC classification, all isotherm curves are of type I, indicating that PCs have microporous structures. Table 1 lists the structural parameters of PC-T-x. The results showed that increasing the temperature or the ratio of K2CO3/SLS was beneficial to improve specific surface area and pore volume. At the same temperature, when x increased from 0 to 4, the specific surface area increased from 581.8 to 1305.5 m2 g−1, and the pore volume increased from 0.328 to 0.758 cm3 g−1. When the weight ratio was 4, the specific surface areas of PC-750-4 and PC-850-4 were 1044.1 m2 g−1 and 1522.6 m2 g−1, respectively. It was worth noting that PC-850-4 showed the highest pore volume (0.864 cm3 g−1). This may be because more potassium vapor and carbon dioxide were released at higher temperatures during the activation process. However, the microporosity of PC-850-4 was lower than that of PC-800-4 and PC-750-4, which can be attributed to the destruction of pore structure caused by high activation temperature. It can be seen from Fig. 4b that the pore diameters of the five porous carbons are mainly less than 2.5 nm. Thus, the porous carbons with abundant mesopores and micropores would have potential applications for CAP adsorption.
Effect of activation parameters
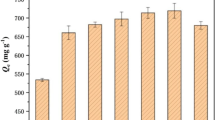
Figure 5 shows the adsorption capacity of PC-T-x for CAP (C0 = 100 mg L−1) at 303 K. It can be seen that at the same activation temperature (800 °C), increasing the amount of K2CO3 significantly improved the adsorption capacity of porous carbon materials. For example, the adsorption capacities of PC-800-1, PC-800-2, PC-800-3, and PC-800-4 were 200.1, 392.8, 409.6, and 511.9 mg g−1, respectively. Similarly, the adsorption capacity increased as the activation temperature was increased under the same experimental conditions. For example, at the K2CO3/SLS mass ratio of 4, the adsorption capacity of porous carbon materials for CAP increased from 444.5 to 589.6 mg g−1 as the activation temperature rose from 750 to 850 °C. The adsorption capacity was consistent with the BET results, indicating that the highly porous structure was the key to improved adsorption. In short, the activation conditions had a significant influence on the adsorption capacity of the adsorbent by affecting its specific surface area, pore volume, surface chemical properties, and product pore structure.
Effects of solution pH, ion strength, and humic acid
The influence of pH on CAP adsorption was studied under the condition of C0 = 120 mg L−1. As shown in Fig. 6a, pH has a slight effect on the adsorption of PC-800-4. The adsorption capacity increased slightly at pH 2–4, but decreased slightly in the range of pH 6–9, indicating that the electrostatic interaction between PC-800-4 and CAP affected the adsorption capacity of the adsorbent. This implied that PC-800-4 could maintain its high-efficiency adsorption capacity for CAP in acid-base environment, which expanded the practical application range of the adsorbent. Under the naturel pH (4.86), the adsorption capacity did not change much, so the original pH was selected as the best condition for subsequent experiments.
Most often, sewage is a mixed solution containing metal ions and humic acid. Therefore, we also investigated the effects of common metal ions and humic acid concentrations on the adsorption of CAP on PC-800-4. As shown in Fig. 6b, at high concentration of 0.1 M, monovalent metal ions (K+, Na+) had little effect on adsorption. However, the adsorption capacity of PC-800-4 was increased by multi-valent metal ions in the order Al3+ > Mg2+ > Ca2+, which was consistent with the order of metal reactivity. This could also have been be caused by the strong electrostatic interactions between the PC-800-4 and the multi-valent cations. Figure 6 c shows that the presence of humic acid has a significant effect on the adsorption capacity of PC-800-4 for CAP. This may be caused by the competitive adsorption between humic acid and CAP molecules in the solution through hydrogen bonding and π-π EDA interactions. It is noteworthy also that under high humic acid conditions (70 mg L−1), the amount of CAP adsorbed on PC-800-4 was as high as 263.8 mg L−1. This implied that the humic acid molecules were not effectively absorbed by the pores inside PC-800-4, perhaps due to large molecular size of the acid. Based on the above results, we believe that PC-800-4 has a broad application prospect in the treatment of CAP-containing wastewater.
Adsorption isotherm analysis
The equilibrium adsorption isotherm model can be used to explain the adsorption mechanism of the adsorbent for the target. The Langmuir, Freundlich and Temkin isotherm models are suitable for single-layer physical adsorption on uniform surfaces, multi-layer adsorption on non-uniform surfaces, and strong electrostatic interactions between positive and negative charges. Figure 7 and Table 2 show the nonlinear fitting curves and parameters for PC-800-4 adsorption of CAP at different temperatures. It can be seen that the adsorption capacity of PC-800-4 was enhanced with the increasing temperature. The determination coefficient (R2) values for the Langmuir, Freundlich, and Temkin models were in the range of 0.9976–0.9992, 0.9786–0.9942, and 0.9650–0.9750, respectively. According to higher R2 and the lower values of ARE, the correlation of isotherms was as follows: Langmuir > Freundlich > Temkin. Therefore, it can be deduced that the single-layer physical adsorption played a dominant role in the CAP adsorption by PC-800-4. At the same time, using the D-R isotherm equation to calculate E = 7.514 < 8 kJ mol−1, it was verified that physical adsorption was the dominant mechanism. The KL value and KF value increased with temperature, which may be related to the decrease in the inherent chemical properties of the adsorbent and the heterogeneity factor, respectively. In addition, the ratio 1/n can be used to judge the difficulty of adsorption. In the range of 298–308 K, 1/n was less than 0.5, indicating that PC easily adsorbed to CAP (Liang et al. 2020). Moreover, as the temperature increasing, the 1/n value gradually decreased, indicating that the temperature rise was conducive to increased adsorption. This was consistent with the chemical adsorption behavior. Therefore, it can be inferred that chemical adsorption played a role in promoting the removal of CAP by PC-800-4.
Analysis of adsorption kinetics of PC-800-4
The adsorption rate is a key parameter that reflects the adsorption performance of the adsorbent. Computations based on different adsorption kinetic models can give better understanding of the performance and adsorption mechanism of the adsorbent. Figure 8 a shows the distribution of PC-T-4 (T = 750, 800, 850 °C) adsorption profile overtime at 303 K and CAP concentration of 120 mg L−1. The non-linear fitting results and dynamic parameters by pseudo-first-order and pseudo-second-order models are shown in Table 3. The fitting results show that the pseudo-second-order model can better describe the experimental data (higher R2 and the lower values of ARE) than the pseudo-first-order model. It can be inferred that PC-T-4 had some chemical adsorption to CAP, and the mechanism may be attributed to its π-πEDA interaction (Sun et al. 2020).
The migration of the adsorbate from the solution to the surface of the adsorbent usually involves external diffusion, surface diffusion, and pore diffusion processes. For the intra-particle diffusion model, a multilinear graph means multiple rate-controlling steps. The fitting of the intra-particle diffusion with the coefficients is shown in Table 4. Figure 8 b shows that the adsorption process includes three diffusion steps: (1) CAP molecules rapidly diffuse from solution to the surface of PC-T-4; (2) CAP molecules diffuse into the interior of PC-T-4; and (3) an equilibrium is established.
Thermodynamic analysis of CAP adsorption
To investigate the effect of temperature on the adsorption of CAP, three temperatures of 298 K, 303 K, and 308 K were selected for thermodynamic experiments. As shown in Table 5, a positive value of ΔH indicates that the reaction is endothermic, which is consistent with the analysis described by kinetics and isotherms. A negative ΔG indicates that adsorption was a spontaneous process. As the temperature increased, the ΔG value gradually decreased from − 6.2419 to − 6.9136 kJ mol−1, indicating that the adsorption reaction was favorable at high temperatures. In addition, the value of ΔG is in the range of − 20 to 0 kJ mol−1, indicating that the adsorption process was dominated by physical adsorption (Zhang et al. 2018), which is supported by the value of Langmuir isotherm R2. ΔS is an indicative measure of the system’s randomness. In this case, the value of ΔS indicates that the degree of system randomness increases after loading CAP molecules to PC-800-4.
Comparison with other adsorbents
The adsorption capacity is an important indicator for evaluating the performance of adsorbents. Table 6 compares the CAP removal capacity of this work and other developed adsorbents. It can be seen that the porous carbon prepared by this work still had a high adsorption capacity under the conditions of relatively low initial concentration and dosage. Therefore, PC is a kind of adsorbent with great potential.
Conclusion
This work successfully transformed SLS into porous carbons through one-step activation method. It not only simplified the production process of porous carbon materials, but also realized the efficient adsorption of CAP, achieving the effect of treating wastes with wastes. At 303 K, the maximum adsorption capacity of the synthesized PC-800-4 at a lower initial concentration of CAP (120 mg L−1) was 534.0 mg g−1, which was a great improvement compared to the work of others. Analysis of the adsorption data showed that the Langmuir isotherm model and pseudo-second-order kinetics perfectly described the adsorption isotherm. Thermodynamic analysis of adsorption isotherm showed that the adsorption process of CAP was spontaneous and endothermic. An evaluation of the adsorption isotherm indicated that the physical and chemical synergistic effects favored the adsorption of CAP. It was exciting that the synthesized adsorbents maintained a high adsorption capacity in a complex water environment. PC-800-4 was considered to be a simple, low-cost, and efficient biomass-based porous carbon with broad application prospects.
Data availability
The dataset used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abukhadra MR, Adlii A, Bakry BM (2019) Green fabrication of bentonite/chitosan@cobalt oxide composite (BE/CH@Co) of enhanced adsorption and advanced oxidation removal of Congo red dye and Cr (VI) from water. Int J Biol Macromol 126:402–413. https://doi.org/10.1016/j.ijbiomac.2018.12.225
Awual MR (2016a) Assessing of lead(III) capturing from contaminated wastewater using ligand doped conjugate adsorbent. Chem Eng J 289:65–73. https://doi.org/10.1016/j.cej.2015.12.078
Awual MR (2016b) Solid phase sensitive palladium(II) ions detection and recovery using ligand based efficient conjugate nanomaterials. Chem Eng J 300:264–272. https://doi.org/10.1016/j.cej.2016.04.071
Awual MR (2017a) New type mesoporous conjugate material for selective optical copper(II) ions monitoring & removal from polluted waters. Chem Eng J 307:85–94. https://doi.org/10.1016/j.cej.2016.07.110
Awual MR (2017b) Novel nanocomposite materials for efficient and selective mercury ions capturing from wastewater. Chem Eng J 307:456–465. https://doi.org/10.1016/j.cej.2016.08.108
Awual MR (2019a) An efficient composite material for selective lead(II) monitoring and removal from wastewater. J Environ Chem Eng 7:103087. https://doi.org/10.1016/j.jece.2019.103087
Awual MR (2019b) Efficient phosphate removal from water for controlling eutrophication using novel composite adsorbent. J Clean Prod 228:1311–1319. https://doi.org/10.1016/j.jclepro.2019.04.325
Awual MR, Hasan MM (2014) Novel conjugate adsorbent for visual detection and removal of toxic lead(II) ions from water. Micropor Mesopor Mat 196:261–269. https://doi.org/10.1016/j.micromeso.2014.05.021
Awual MR, Hasan MM (2015) Fine-tuning mesoporous adsorbent for simultaneous ultra-trace palladium(II) detection, separation and recovery. J Ind Eng Chem 21:507–515. https://doi.org/10.1016/j.jiec.2014.03.013
Bhatnagar A, Hogland W, Marques M, Sillanpää M (2013) An overview of the modification methods of activated carbon for its water treatment applications. Chem Eng J 219:499–511. https://doi.org/10.1016/j.cej.2012.12.038
Carvalho IT, Santos L (2016) Antibiotics in the aquatic environments: a review of the European scenario. Environ Int 94:736–757. https://doi.org/10.1016/j.envint.2016.06.025
Chen A, Xie Y, Wei X, Chen B, Pang J (2021) One-step preparation of sodium alginate-based porous carbon for the adsorption of Bisphenol A in water. J Chem Eng Data. https://doi.org/10.1021/acs.jced.0c00894
Chen X, Jiang X, Yin C, Zhang B, Zhang Q (2019) Facile fabrication of hierarchical porous ZIF-8 for enhanced adsorption of antibiotics. J Hazard Mater 367:194–204. https://doi.org/10.1016/j.jhazmat.2018.12.080
Cheng P, Gao S, Zang P, Yang X, Bai Y, Xu H, Liu Z, Lei Z (2015) Hierarchically porous carbon by activation of shiitake mushroom for capacitive energy storage. Carbon 93:315–324. https://doi.org/10.1016/j.carbon.2015.05.056
Clesham K, Bhatnagar N, Samarasinghe S (2019) Diagnosis and management of childhood aplastic anaemia. Paediatr Child Health 29(8):327–333. https://doi.org/10.1016/j.paed.2019.05.001
Dai J, Qin L, Zhang R, Xie A, Chang Z, Tian S, Li C, Yan Y (2018a) Sustainable bovine bone-derived hierarchically porous carbons with excellent adsorption of antibiotics: equilibrium, kinetic and thermodynamic investigation.F Powder Technol 331: 162-170. https://doi.org/10.1016/j.powtec.2018.03.005
Dai Y, Sun Q, Wang W, Lu L, Liu M, Li J, Yang S, Sun Y, Zhang K, Xu J, Zheng W, Hu Z, Yang Y, Gao Y, Chen X, Zhang X, Gao F, Zhang Y (2018b) Utilizations of agricultural waste as adsorbent for the removal of contaminants: a review. Chemosphere 211:235–253. https://doi.org/10.1016/j.chemosphere.2018.06.179
Dissanayake Herath GA, Poh LS, Ng WJ (2019) Statistical optimization of glyphosate adsorption by biochar and activated carbon with response surface methodology. Chemosphere 227:533–540. https://doi.org/10.1016/j.chemosphere.2019.04.078
Galkin MV, Samec JSM (2016) Lignin valorization through catalytic lignocellulose fractionation: a fundamental platform for the future biorefinery. ChemSusChem 9(13):1544–1558. https://doi.org/10.1002/cssc.201600237
Gao Y, Yue Q, Gao B, Sun Y, Wang W, Li Q, Wang Y (2013) Preparation of high surface area-activated carbon from lignin of papermaking black liquor by KOH activation for Ni(II) adsorption. Chem Eng J 217:345–353. https://doi.org/10.1016/j.cej.2012.09.038
George AM, Hall RM (2002) Efflux of chloramphenicol by the CmlA1 protein. FEMS Microbiol Lett 209(2):209–213. https://doi.org/10.1016/S0378-1097(02)00558-X
Jimenez JJ, Jimenez JG, Daghistani D, Yunis AA (1990) Interaction of chloramphenicol and metabolites with colony stimulating factors: possible role in chloramphenicol-induced bone marrow injury. Am J Med Sci 300(6):350–353. https://doi.org/10.1097/00000441-199012000-00002
Jin E, Lee S, Kang E, Kim Y, Choe W (2020) Metal-organic frameworks as advanced adsorbents for pharmaceutical and personal care products. Coordin Chem Rev 425:213526. https://doi.org/10.1016/j.ccr.2020.213526
Kikuchi H, Sakai T, Teshima R, Nemoto S, Akiyama H (2017) Total determination of chloramphenicol residues in foods by liquid chromatography-tandem mass spectrometry. Food Chem 230:589–593. https://doi.org/10.1016/j.foodchem.2017.03.071
Li M, Liu Y, Zeng G, Liu N, Liu S (2019) Graphene and graphene-based nanocomposites used for antibiotics removal in water treatment: a review. Chemosphere 226:360–380. https://doi.org/10.1016/j.chemosphere.2019.03.117
Liang H, Sun R, Song B, Sun Q, Peng P, She D (2020) Preparation of nitrogen-doped porous carbon material by a hydrothermal-activation two-step method and its high-efficiency adsorption of Cr(VI). J Hazard Mater 387:121987. https://doi.org/10.1016/j.jhazmat.2019.121987
Liu J, Zhou D, Xu Z, Zheng S (2017b) Adsorptive removal of pharmaceutical antibiotics from aqueous solution by porous covalent triazine frameworks. Environ Pollut 226:379–384. https://doi.org/10.1016/j.envpol.2017.03.063
Liu WJ, Jiang H, Yu HQ (2015) Thermochemical conversion of lignin to functional materials: a review and future directions. Green Chem 17(11):4888–4907. https://doi.org/10.1039/C5GC01054C
Liu X, Steele JC, Meng X (2017a) Usage, residue, and human health risk of antibiotics in Chinese aquaculture: a review. Environ Pollut 223:161–169. https://doi.org/10.1016/j.envpol.2017.01.003
Liu Z, Adewuyi YG, Shi S, Chen H, Li Y, Liu D, Liu Y (2019) Removal of gaseous Hg0 using novel seaweed biomass-based activated carbon. Chem Eng J 366:41–49. https://doi.org/10.1016/j.cej.2019.02.025
Lü G, Wu L, Wang X, Liao L, Wang X (2012) Adsorption of chlortetracycline from water by rectories. Chinese J Chem Eng 20(5):1003–1007. https://doi.org/10.1016/S1004-9541(12)60429-7
Lu J, Sun J, Chen X, Tian S, Chen D, He C, Xiong Y (2019) Efficient mineralization of aqueous antibiotics by simultaneous catalytic ozonation and photocatalysis using MgMnO3 as a bifunctional catalyst. Chem Eng J 358:48–57. https://doi.org/10.1016/j.cej.2018.08.198
Mauer SM, Chavers BM, Kjellstrand CM (1980) Treatment of an infant with severe chloramphenicol intoxication using charcoal-column hemoperfusion. J Pediatr 96(1):136–139. https://doi.org/10.1016/S0022-3476(80)80350-7
Lv K, Han J, Yang C, Cheng C, Luo Y, Wang X (2016) A category of hierarchically porous tin (IV) phosphonate backbone with the implication for radioanalytical separation. Chem Eng J 302:368–376. https://doi.org/10.1016/j.cej.2016.05.061
Mohd Din AT, Ahmad MA, Hameed BH (2015) Ordered mesoporous carbons originated from non-edible polyethylene glycol 400 (PEG-400) for chloramphenicol antibiotic recovery from liquid phase. Chem Eng J 260:730–739. https://doi.org/10.1016/j.cej.2014.09.010
Oginni O, Singh K, Oporto G, Dawson-Andoh B, Mcdonald L, Sabolsky E (2019) Influence of one-step and two-step KOH activation on activated carbon characteristics. Bioresource Technology Reports 7:100266. https://doi.org/10.1016/j.biteb.2019.100266
Sarkar A, Paul B (2020) Analysis of the performance of zirconia-multiwalled carbon nanotube nanoheterostructures in adsorbing As(V) from potable water from the aspects of physical chemistry with an emphasis on adsorption site energy distribution and density functional theory calculations. Micropor Mesopor Mat 302:110191. https://doi.org/10.1016/j.micromeso.2020.110191
Sayğılı H, Sayğılı GA (2019) Optimized preparation for bimodal porous carbon from lentil processing waste by microwave-assisted K2CO3 activation: spectroscopic characterization and dye decolorization activity. J Clean Prod 226:968–976. https://doi.org/10.1016/j.jclepro.2019.04.121
Srinivasa Raghavan DS, Qiu G, Ting Y (2018) Fate and removal of selected antibiotics in an osmotic membrane bioreactor. Chem Eng J 334:198–205. https://doi.org/10.1016/j.cej.2017.10.026
Sun S, Jiao C, Xu Z, Yang Z, Peng S (2020) KOH activated ZIF-L derived N-doped porous carbon with enhanced adsorption performance towards antibiotics removal from aqueous solution. J Solid State Chem 289:121492. https://doi.org/10.1016/j.jssc.2020.121492
Tian S, Dai J, Jiang Y, Chang Z, Xie A, He J, Zhang R, Yan Y (2017) Facile preparation of intercrossed-stacked porous carbon originated from potassium citrate and their highly effective adsorption performance for chloramphenicol. J Colloid Interf Sci 505:858–869. https://doi.org/10.1016/j.jcis.2017.06.062
Wang T, Wei X, Zong Y, Zhang S, Guan W (2020) An efficient and stable fluorescent sensor based on APTES-functionalized CsPbBr(3)perovskite quantum dots for ultrasensitive tetracycline detection in ethanol. J Mater Chem C 35:12196–12203. https://doi.org/10.1039/D0TC02852E
Wei C, Li X, Xie Y, Wang X (2019) Direct photo transformation of tetracycline and sulfanomide group antibiotics in surface water: kinetics, toxicity and site modeling. Sci Total Environ 686:1–9. https://doi.org/10.1016/j.scitotenv.2019.04.041
Wu Z, Huang W, Shan X, Li Z (2020) Preparation of a porous graphene oxide/alkali lignin aerogel composite and its adsorption properties for methylene blue. Int J Biol Macromol 143:325–333. https://doi.org/10.1016/j.ijbiomac.2019.12.017
Xie A, Cui J, Chen Y, Lang J, Li C, Yan Y, Dai J (2019a) Simultaneous activation and magnetization toward facile preparation of auricularia-based magnetic porous carbon for efficient removal of tetracycline. J Alloy Compd 784:76–87. https://doi.org/10.1016/j.jallcom.2018.12.375
Xie A, Dai J, Chen Y, Liu N, Ge W, Ma P, Zhang R, Zhou Z, Tian S, Li C, Yan Y (2019b) NaCl-template assisted preparation of porous carbon nanosheets started from lignin for efficient removal of tetracycline. Adv Powder Technol 30(1):170–179. https://doi.org/10.1016/j.apt.2018.10.020
Yang F, Zhang Q, Jian H, Wang C, Xing B, Sun H, Hao Y (2020c) Effect of biochar-derived dissolved organic matter on adsorption of sulfamethoxazole and chloramphenicol. J Hazard Mater 396:122598. https://doi.org/10.1016/j.jhazmat.2020.122598
Yang J, Dai J, Wang L, Ge W, Xie A, He J, Yan Y (2019) Ultrahigh adsorption of tetracycline on willow branche-derived porous carbons with tunable pore structure: isotherm, kinetics, thermodynamic and new mechanism study. J Taiwan Inst Chem E 96:473–482. https://doi.org/10.1016/j.jtice.2018.12.017
Yang J, Ji G, Gao Y, Fu W, Irfan M, Mu L, Zhang Y, Li A (2020b) High-yield and high-performance porous biochar produced from pyrolysis of peanut shell with low-dose ammonium polyphosphate for chloramphenicol adsorption. J Clean Prod 264:121516. https://doi.org/10.1016/j.jclepro.2020.121516
Yang V, Arumugam Senthil R, Pan J, Rajesh Kumar T, Sun Y, Liu X (2020a) Hierarchical porous carbon derived from jujube fruits as sustainable and ultrahigh capacitance material for advanced supercapacitors. J Colloid Interf Sci 579:347–356. https://doi.org/10.1016/j.jcis.2020.06.080
Ye Q, Xu H, Zhang J, Wang Q, Zhou P, Wang Y, Lu J (2020) Enhancement of peroxymonosulfate activation for antibiotics removal by nano zero valent tungsten induced Cu(II)/Cu(I) redox cycles. Chem Eng J 382:123054. https://doi.org/10.1016/j.cej.2019.123054
Zhang L, Song X, Liu X, Yang L, Pan F, Lv J (2011) Studies on the removal of tetracycline by multi-walled carbon nanotubes. Chem Eng J 178:26–33. https://doi.org/10.1016/j.cej.2011.09.127
Zhang W, Chen J, Hu Y, Fang Z, Cheng J, Chen Y (2018) Adsorption characteristics of tetrabromobisphenol A onto sodium bisulfite reduced graphene oxide aerogels. Colloids Surf A Physicochem Eng Asp 538:781–788. https://doi.org/10.1016/j.colsurfa.2017.11.070
Zhang X, Li Y, Hou Y (2019) Preparation of magnetic polyethylenimine lignin and its adsorption of Pb(II). Int J Biol Macromol 141:1102–1110. https://doi.org/10.1016/j.ijbiomac.2019.09.061
Zhou Y, Zhang L, Cheng Z (2015) Removal of organic pollutants from aqueous solution using agricultural wastes: a review. J Mol Liq 212:739–762. https://doi.org/10.1016/j.molliq.2015.10.023
Funding
This work was supported by the National Postdoctoral Science Foundation (No. 2017M610618), Postdoctoral Science Foundation Funded Project of Shaanxi Province (No. 2017BSHEDZZ64), and Fundamental Research Funds for the Central Universities of Chang’ an University (No. 300102290104).
Author information
Authors and Affiliations
Contributions
Conception and design of the research: Jiaju Pang and Xiao Wei. Acquisition of data: Aixia Chen, Bei Chen, Jiaju Pang, and Yaping Xie. Analysis and interpretation of the data: Jiaju Pang and Xiao Wei. Writing of the manuscript: Jiaju Pang and Aixia Chen. Critical revision of the manuscript for important intellectual content: Aixia Chen and Jiaju Pang. AC and JP contributed equally to this work.
Corresponding authors
Ethics declarations
Ethical approval and consent to participate
Not applicable.
Consent to publish
The authors confirm that the final version of the manuscript has been reviewed, approved, and consented for publication by all authors.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Tito Roberto Cadaval Jr
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
Porous carbon can be quickly prepared by one-step method.
PC-800-4 had a high specific surface area (1305.5 m2 g−1) and pore volume (0.758 cm3 g−1).
PC-800-4 had ultrahigh adsorption capacity of 534.0 mg g−1 for chloramphenicol at 303 k.
The strong anti-interference ability and high adsorption capacity made PC-800-4 have huge application potential.
Rights and permissions
About this article
Cite this article
Chen, A., Pang, J., Wei, X. et al. Fast one-step preparation of porous carbon with hierarchical oxygen-enriched structure from waste lignin for chloramphenicol removal. Environ Sci Pollut Res 28, 27398–27410 (2021). https://doi.org/10.1007/s11356-021-12640-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-12640-3