Abstract
To investigate the complex Pb-Cd exposure network in school-going children, a thorough investigation of the probable exposure means (diet, water, and school micro-environments such as paint dust and school courtyard soil) and exposure route (ingestion, inhalation and dermal) was carried out in a periurban area spanning three districts in southern Assam, India. Multivariate statistical analysis was carried out to understand the complex data matrices, and the health risk assessments (carcinogenic and non-carcinogenic) based on US EPA Risk Assessment models were also made. We found the median values to be 0.9–4.0 mg Pb/kg and 0.21–6.2 mg Cd/kg in various food items. Groundwater also had Pb (0.13–0.48 mg/L) and Cd (0.11–0.29 mg/L). Pb levels in paint dust were within the permissible limits, but 50% of the samples had higher than permissible levels of Cd. Approximately 23% of the school courtyard soil had Pb above the global background levels, but all the samples had 4–27 times elevated levels of Cd in them. School micro-environment contributed significantly to the metal load in children due to their typical hand-to-mouth behavior and dietary intake (food and water) via ingestion was the most prominent route of exposure in children. The evaluation of the estimated chronic daily intake and the hazard quotient indicated hazardous exposure over a lifetime to both Pb and Cd, but only Cd posed a prominent cancer risk. It could be concluded that chronic insidious effects of metals would be a noteworthy toxicological threat to children when exposed early on.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Toxic heavy metals such as lead (Pb) and cadmium (Cd) have registered their ubiquitous presence in our environment due to activities associated with industries, such as mining, smelting, paint, pigment, and battery, or due to the natural sources or agricultural activities such as the application of pesticides and fertilizers and automobile exhausts (Liu et al. 2013a; Cao et al. 2015). These toxic metals are likely to enter into the human via soil/dust, food, drinking water, and even air (Qian et al. 2010). Chronic exposures of Pb/Cd may damage the nervous, skeletal, circulatory, enzymatic, endocrine, and immune systems of those exposed to it (Li et al. 2014). Cd (group 1) and Pb (group 2B) have been classified by the International Agency for Research on Cancer (IARC) to be potentially carcinogenic and hence a cause of concern, especially in urban settings, where both these elements have registered their prominent presence (Vainio et al. 2002).
School-going children between 6 and 16 years of age are the most vulnerable group, Pb is often associated with low IQ, while Cd is known to impair cognitive development in them (Kippler et al. 2012; Liu et al. 2019). Pb and Cd can also be carcinogenic, low Cd, and Pb intake from the diet over a period of time was associated with the increased risk of cancer (Itoh et al. 2014; Canaz et al. 2017). Children are also susceptible to Pb and Cd due to explicit physiological and behavioral patterns in them (Cao et al. 2015). In India, elevated maternal blood levels of Pb (11 μg/dl) and Cd (0.095 μg/dl) have been reported that were associated with low birth weight and poor intelligence in them (Dwivedi et al. 2013). In a quest to search the probable Pb-Cd exposure network in children, several exposure networks have been proposed. Diet as well as the water is a major source of toxic metal intake for much of the world’s population (Liu et al. 2013b). The diet of Indian children consists of staples such as rice, vegetables, fish, and egg, each of which might contribute towards the oral entry of metals, owing to the great amount of consumption. Reports on the presence of Cd and Pb in rice (0.07 mg Cd/kg and 0.79 mg Pb/kg), vegetables (2.29–23.5 mg Cd/kg and 0.8–2.9 mg Pb/kg), fish (0.004–0.85 mg Cd/kg and 0.03–0.41 mg Pb/kg), eggs (0.15 mg Pb/kg), groundwater (0.68–19.5 μg Pb/L and 0.02–1.28 μg Cd/L), and soil (19.25 mg Cd/kg and 13.12 mg Pb/kg) from India are available (Sharma et al. 2008; Tiwari et al. 2011; Giri and Singh 2014, 2017; Kumar et al. 2019; Sharafi et al. 2019). On average, Indian children between 6 and 16 years spend 800 and 1000 hours per academic year in school, which on average is 200 days (Rajadhyaksha 2014). The school environment, where they inhabit or play, is an essential route of Pb/Cd exposure in children due to their hand to mouth activities. The use of Cd/Pb-based paint is still predominant in India, and hence, the school environments in the perspective of paint dust and courtyard soil should contribute towards Pb-Cd body-burden.
The human health risk assessments, modeled by the US Environmental Protection Agency, associated with non-carcinogenic and carcinogenic risks due to heavy metal exposures have been successfully adopted all over the world to establish risks based on oral, dermal, and inhalation pathways (US EPA 2001; Mao et al. 2019). By this method, the probability of deleterious health effects in humans who might be exposed to chemicals in contaminated environments is estimated. Such type of predictive model-based assessments has a practical value as it provides quantitative evaluations of risks where there is a lack of data in human due to ethical and financial reasons.
Multivariate statistical analysis (MVA) is a collection of statistical tools that can help in better understanding and interpretation of complex data matrices and allows for their grouping based on similarities between them (Shrestha and Kazama 2007). The tool has been successfully applied to understand the water quality of rivers, in pollution monitoring, as well as in risk identification associated with metals and organic micro contaminants in vegetables (Shrestha and Kazama 2007; Margenat et al. 2019).
The present investigation is a 2-year study around a periurban area in Assam in India that has high incidences of cancer (Phukan et al. 2001). Aizawl, at a distance of 172.0 km from the study site, has the world’s highest incidence of cancers in the lower pharynx (11.5/100,000 people) and tongue (7.6/100,000 people) and also India’s highest rate of stomach cancer among men (Mudur 2005). Although some studies point towards smoking as a probable cause, pollution, as a decisive factor, could not be ruled out. Further, low IQ in school-going children is a prominent cause of concern in this zone (Dwivedi et al. 2013). In this backdrop, it would be extremely important to study the complex Pb-Cd exposure network in children via ingestion, inhalation, and dermal contact pathways and estimate the probable non-carcinogenic and carcinogenic risks associated with these potentially toxic metals, Pb and Cd, that have been reported to be present in agrochemicals (Tiwari et al. 2011). Also, there has been several industrial activities in the form of cement factory, a recently closed paper mill, several brick kilns, and moderate to high road traffic, in this region that are the probable sources of Pb/Cd in the area (Das and Choudhury 2016).
The objectives of the present study were to (1) measure the concentrations of Pb and Cd in food (rice, vegetables, fish, egg), water, and paint dust from school and school courtyard soil; (2) estimate the exposure levels and contributions from each medium by multivariate statistical analysis; and (3) estimate potential non-carcinogenic and carcinogenic health risks in children due to Pb/Cd from each medium using predictive risk assessment model based on US EPA.
Materials and methods
Study area
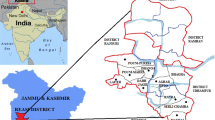
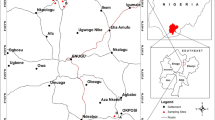
The study site is located in the Barak river valley in Assam (north-eastern India) covering an area of 7494.4 km2 and 3 districts (Cachar, Karimganj, and Hailakandi) with a population of 4.27 million. The region is agriculture based; rice and tea crops are grown extensively here. The samples were collected from the periurban areas of each district. The geographical location of the study area was provided in Fig. 1, and detailed information regarding the sampling sites was provided in Supplementary Tables S1, S2, and S3.
Sample collection
A total of 8 commonly consumed vegetable varieties (n = 160), 16 rice varieties (n = 102), 17 different fish varieties (n = 144), and 7 different varieties of poultry eggs (n = 90) were collected from various markets during the study period 2017–2019 (Tables 1 and S1). Paint dust was collected from a total of 60 schools in the urban area (30 from Cachar district and 15 each from Karimganj and Hailakandi district, n = 180) (Table S2). The paint dust was scrapped from the classroom wall by flint paper and dusted off by brush and collected in paper bags. From the same schools, a total of 300 samples (60 samples, each with 5 random subsamples) were collected. Within the same sampling unit, the subsamples were pooled for an individual composite value. In the school courtyard, a 5 × 5 grid was created, and five subsamples, one from each corner and 1 at the center, were pooled to create a composite sample. Each sample had 3 replicates. Top 15-cm soil was dug using stainless steel shovel, placed in sample pouch, and transported back to the laboratory. Groundwater samples (n = 90) were collected from 30 tube wells (24–75-m depth) that were within 1–2-km radius of the schools (Table S3). The hand pumps that were used for drinking and domestic purposes were selected. Before sampling, water was pumped for 10 min to remove the standing water. Approximately 1 L of water was collected from each site in clean laboratory grade polypropylene bottles, placed in an ice box, and transported to the laboratory.
Sample pre-treatment
All the food samples were washed thoroughly with de-ionized water several times. Samples were chopped into small pieces (wherever necessary) and oven-dried until the constant weight was achieved. Small fish (< 7 cm) were kept intact as they are generally eaten whole, while ~ 20 g flesh of large fish was taken. Dried samples were powdered with a stainless steel blender and passed through a 2-mm size sieve. Egg samples were opened carefully to separate the eggshell from egg content. The egg contents were homogenized and were kept for drying in an oven at 70 °C for 72 h and then ground with mortar to a fine powder (Giri and Singh 2017). A 50-mL groundwater sample was acidified by adding 2–3 drops of 6 M nitric acid before analysis. Soil samples were air-dried at room temperature for 3 days and sieved with a 2-mm mesh. Paint dust samples were oven-dried at 65 °C for 2 days and stored until further analysis.
Sample digestion and metal analysis
A 0.5 g of samples (food/soil) was digested with HNO3 and H2O2 using US EPA Method 3050B on a hot block. After completion of the digestion and adequate cooling, solutions were filtered through Whatman No. 42 and diluted to 50 mL with deionized water and kept at room temperature for further analysis of heavy metals. Acidified water samples were directly analyzed. A 0.1-g paint dust sample was ashed for 1 h at 500 °C in a furnace. A 4 mL of 7.5 M HNO3 was added, and the contents were heated to boiling and until reduced to half. The contents were transferred quantitatively to a 50-mL volumetric flask using distilled water and centrifuged to get clear supernatant (Searle et al. 1969). The determination of Pb and Cd in the samples was achieved by Graphite Furnace Atomic Absorption Spectrometry (GFAAS, model Vario 6, Analytikjena).
QA/QC
In the event of sample collection, transport, and storage, utmost care was ensured to minimize cross-contamination. All glassware was soaked with 10% HNO3 and HCl overnight and then rinsed with double DI-water before use. For calibration of GFAAS, the standard solution of 1, 3, and 5 ppm nitrate salts of Pb and Cd (Merck, Germany) were used. Procedural blanks and spiked internal standard every 20 samples were added to check machine stability. Standard reference materials (SRM-1570, DORM-2) were provided by Sophisticated Analytical Instrumentation Facility, North Eastern Hill University, Shillong. The results were found to be within 2% of certified values.
Risk assessment
The estimated chronic daily intake (ECDI) of Pb and Cd (mg/ kg/day) by school-going children (ages 6 to 16) was calculated as per US EPA (2001). The ECDI was predicted to cause non-carcinogenic risks in them. Since all the three exposure routes (ingestion, inhalation, and dermal) would be responsible for Pb/Cd build up in them, three separate ECDIs for each exposure route were calculated as per the following formula:
For ECDI of water by dermal contact, a minor modification of Eq. 3 was performed (Mohammadi et al. 2019):
where C is the concentration of Pb/Cd in food, soil or dust (mg/kg), or water (mg/L); Ring is the ingestion rate (rice = 0.2 kg/d; vegetables = 0.345 kg/d; fish = 0.04 kg/d; egg = 0.0075 kg/d; soil = 0.0001 kg/d; dust = 0.0002 kg/d; water = 2 L/d); Rinh is the inhalation rate (5 m3/d); PEF is the particle inhalation factor (1.36 × 109 m3/kg); SA is the surface area of the skin exposed to pollutants (18,000 cm2); SL is the skin adherence factor (0.2 mg/cm2/d); ABS is the dermal absorption factor (0.001); EF is the exposure frequency (for food items and water, 365 d/ year; for water via dermal route on bathing, 350 d/year; for paint dust, 200 d/year, the average annual school days in India); ED is the exposure duration (10 years duration of school years); CF is the conversion factor (10-6 kg/mg); Kp is the permeability coefficient (0.001 cm/hour); ET (0.58 hour/event) is the exposure time; BW is the body weight (15 kg, default for children); and AT is the average time (3650 days for non-carcinogenic risk and 25,550 days for carcinogenic risk). All the values in parenthesis are child specific and obtained from standard literature (US EPA 1997, 2001; WHO 1998; Bhuyan et al. 2017; Lin et al. 2017; NAPEP 2017; Mao et al. 2019; Mohammadi et al. 2019).
Hazard quotient (HQ), the measure of a lifetime hazard from metal, can be calculated as per the following equation (US EPA 2011a):
where RfD is the reference dose (RfDing and RfDinh = 3.5 × 10-3 mg Pb/kg; 1.0 × 10-3 mg Cd/kg. RfDderm = 5.25 × 10-4 mg Pb/kg; 5.0 × 10-5 mg Cd/kg) (Lin et al. 2017).
To measure the overall potential non-carcinogenic effects from both Pb and Cd, a summation of individual hazards was assumed:
Potential lifelong cancer risk (ILCR) of Pb and Cd, in children, was calculated as
where CSF is the cancer slope factor (CSFPb = 8.5 × 10-3and CSFCd = 3.8 × 10-1). Risks in the range of 1.0 × 10-6 to 1.0 × 10-4 are acceptable (US EPA 2011b).
Statistical analysis
Data normality (by Kolmogorov-Smirnov test), descriptive statistics, Kruskal-Wallis H test, and multivariate statistical analysis (PCA and FA) were performed using the SPSS software (ver. 18). FA was performed on the PCs by the maximum likelihood method and corresponding rotated component matrix (varimax with Kaiser Normalization), variable loadings, and explained variance were calculated. PCA was carried out to identify the dimensionality of a data set while endeavoring to keep the relationships in the original data intact (Jung et al. 2016). Kruskal-Wallis H test was performed to ascertain whether or not significant difference existed between mean Pb/Cd in various components.
Results and discussion
Concentrations of Pb and Cd in food items
A person’s diet is a regular and important exposure source of toxic heavy metals (Liu et al. 2013a). Chronic, insidious effects of metals have been a prominent toxicological threat to humans who are exposed to dietary toxic elements early on (Cao et al. 2015). In the present study, the median Pb values were between 0.9 and 4.0 mg/kg in various food items, with the first and the third quartiles between 0.23 and 1.12 mg/kg and 1.08 and 10.7 mg/kg, respectively. The median Pb followed the pattern: vegetables>rice>fish>egg. For Cd, median values were between 0.21 and 6.2 mg/kg in various food items, with the first and the third quartiles between 0.152 and 4.55 mg/kg and 0.31 and 12.4 mg/kg, respectively. The median Cd values followed the pattern: vegetables>egg> fish> rice. Except for Cd contents in fish, Pb and Cd in all the food items exceeded the maximum acceptable concentration set for the respective food items (Table 2). The metal contents above guideline values suggested a potential risk in terms of food safety and human health. Vegetables generally have high concentrations of heavy metals such as Pb or Cd, when grown in polluted soils or from aerial deposits during growing, packaging, transportation, or selling stages. Vegetables collected from polluted agricultural soil (12.2 to 13.9 mg Pb/kg and 1.6 to 2.3 mg Cd/kg) in Pakistan had high Pb (1.8–11 mg/kg) and Cd (0.2–1.6 mg/kg) (Rehman et al. 2017). From India, Pb ranged from 0.8 to 2.9 mg/kg, and Cd ranged from 0.1 to 11.2 mg/kg in various vegetables (Sharma et al. 2008). Also, the uptake of metals is dependent upon the physiological properties of crop plants (Zwolak et al. 2019). In the Indian study, cauliflower was maximally contaminated by heavy metals followed by the lady’s finger and spinach (Sharma et al. 2008). Similarly, cucumber, radish, and tomato from China had lower concentrations of Cd in their edible parts than leek and carrots (Yang et al. 2009). Our findings also showed variation in metal contents in vegetables, the median value of which followed the pattern: ridged gourd (14.4 mg/kg) > sponge gourd (10.4 mg/kg) > okra (9 mg/kg) > brinjal (7.3 mg/kg) > papaya (4.2 mg/kg) > spiny gourd (2.6 mg/kg) > bitter gourd (1.1 mg/kg) > bottle gourd (0.84 mg/kg) for Pb and ridged gourd (6.7 mg/kg) > okra (3.5 mg/kg ) > sponge gourd (2.65 mg/kg) > papaya (2.36 mg/kg) > brinjal (2.0 mg/kg) > spiny gourd (0.63 mg/kg) > bitter gourd (0.54 mg/kg) > bottle gourd (0.30 mg/kg) for Cd (Fig 2a).
Rice imposed another prominent threat in terms of Pb. Although Cd is more mobile in rice-soil complex, higher Pb in rice grains reflected greater Pb contamination. However, the rice varieties in our study still had a higher value than the global median of 0.019 mg Cd/kg (Shi et al. 2020). The locally grown Assamese varieties such as the winter rice varieties Boro, Joha, and Bao had median Pb and Cd concentration to be 0.26 mg Pb/kg and 0.20 mg Cd/kg, 1.24 mg Pb/kg and 0.40 mg Cd/kg, and 7.58 mg Pb/kg and 0.28 mg Cd/kg, respectively, and the summer rice variety Mahsuri had 8.5 mg Pb/kg and 0.06 mg Cd/kg (Fig. 2b). In the varieties from agri-intensive areas of the country, Pb and Cd enter into food crops via rampant utilization of pesticides and fertilizers (Roberts 2014).
Eggs are protein-rich wholesome food, the national per capita consumption of which in India has recently been elevated to 69 eggs (NAPEP 2017). We estimated metals from both farm-reared and country-reared poultry eggs, commonly consumed in the locality. Eggs from the farm-reared hen had a median value of 0.9 mg Pb/kg and 1.26 mg Cd/kg, while backyard-raised country hen had 1.2 mg Pb/kg and 6.8 mg Cd/kg. Similarly, backyard-raised duck and swan also had high metals, while dove and pigeon egg had the lowest levels of both the metals (Fig. 2c). Reports on the presence of Pb (4.0 mg/kg) and Cd (0.33 mg/kg) in farm-reared duck egg are also available from Thailand (Aendo et al. 2019) as well as from India (0.15 mg Pb/kg) (Giri and Singh 2017). It is predicted that the occurrence of Pb/Cd in poultry feed or additives could contribute towards the elevated levels of metals above the FAO/WHO limits found in our study (Aendo et al. 2019). High Pb and Cd in the country birds raised in the backyards indicated that the background soil of this region had higher levels of toxic metals, which is a matter of concern and needed further investigation. As the foraging hens could pick up metals from the soil along with food, consumption of such eggs raises considerable concern.
Bioaccumulation of Pb/Cd in fish tissue has been a major concern due to serious food safety-related issues in consumers (Das and Choudhury 2016). Eleven percent of the fish tested in our study had Cd levels higher than the permissible limits, while 1.5–5 times higher Pb concentrations over the permissible limit of 0.5 mg Pb/kg were observed in all the fish. Glassfish, Ambassis ranga, had the highest median Cd (4.5 mg/kg) and shad, Gudusia chapra, had the highest median value for Pb (2.69 mg/kg) (Fig 2d). Pb and Cd accumulation in fish primarily depends on their levels in the habitat that includes water, sediment, food, and also its elimination kinetics in fish (Ture et al. 2020). Several Indian fish have high contents of metals in them owing to their polluted habitat (Giri and Singh 2014). Indian freshwater systems often serve as sinks to industrial effluents that contained heavy metals which end up in fish and eventually in humans, who consume such fish (Das and Choudhury 2016). Thus, accumulation of Pb/Cd in the edible tissues raise substantial concern.
Concentrations of Pb and Cd in groundwater, paint dust from school, and courtyard soil
The median Pb and Cd values in the groundwater collected from tube wells were 0.27 and 0.148 mg/L, respectively, lower than the Indian limits for respective metals in groundwater (Table 3). In India, groundwater is widely used for drinking, bathing, domestic usages, and irrigation (Kumar et al. 2019). Apart from the geogenic presence of several metals/metalloids in Indian aquifers, rapid contamination of the same from anthropogenic sources was noted (Kumar et al. 2017). The data obtained were lower than some Indian studies for Pb (0.9–130 mg/L) and Cd (0.08–9.1 mg/L) (reviewed in Kumar et al. 2019). Thus, groundwater seemed to be a poor threat in general. But if we look into the hot and humid weather conditions of the area where drinking water by people is considerably more, consequently greater metal burden might build in the body in the long run.
To phase out Pb in paints by 2020, initiated by UNEP/WHO (2012), the presence of Pb compounds or additives in paint in excess of 90 ppm has been prohibited in India with effect from 2016 (The Gazette of India 2016). In this study, the median value for Pb was found to be 13.9 mg/kg (ranged between 1.35 and 76.8 mg/kg), which was below the permissible limit. On the other hand, 50% of the paint samples had Cd above the permissible limit of 100 mg/kg, with a median value of 108.6 mg Cd/kg (Table 3). Our paint dust had lower levels of Pb/Cd compared to that found in Nigerian paints (170–3231 mg Pb/kg and 98–1999 mg Cd/ kg) (Apanpa-Qasim et al. 2016) or in England, where extremely high median Pb of 451 mg/kg (max.152,000 mg/kg) and Cd of 252 mg/kg (max. 771 mg/kg) was reported from playground paints (Turner et al. 2016). Also, high levels of these metals were reported from nursery and primary schools in China (Chen et al. 2014). It was understood that risks from paint dust associated metal toxicity would be higher in worn-out paint-coats that deteriorate over time and metals become more mobilized into the environment (Turner et al. 2016). Therefore, regular monitoring of painted surfaces from public places, especially areas frequented by children, is recommended.
High levels of contaminants in the background soils are a major concern these days, predominantly due to their untraceable origin that occurs mainly from the non-point sources. Generally, 0.41 mg Cd/kg and 27 mg Pb/kg have been considered to be background levels in most unpolluted soils (Kabata-Pendias 2011). Twenty-three percent of the school courtyard soil sample in our study had Pb values above this level, and all the samples had 4–27 times elevated levels of Cd in them. The median value for Pb (21.3 mg/kg) and Cd (6.7 mg/kg) also reflected this (Table 3). Further, for all the exposure network/ sources of Pb/Cd, the Kruskal-Wallis H test indicated significant differences (p < 0.05) in the mean values (Tables 2 and 3).
Estimation of the exposure levels and contributions from each medium by multivariate statistical analysis
Factor analysis on the principal components (PC) and corresponding rotated component matrix (varimax with Kaiser Normalization), variable loadings, and explained variance was shown for the probable Pb-Cd exposure networks in children (Table 4). Eigenvalues > 1.0 were considered significant. After orthogonal rotation, each influencing factor was more clearly defined. A total of five PCs explained 86.3% of the total variance. PC1 explained 24.5% of the variance with high factor loadings of > 0.8 for Pb and Cd in egg and paint dust. PC 2 explained 20.9% of the variance with high factor loadings of > 0.7 for Pb and Cd in rice and Pb in soil. The same component also included Pb in water with moderate factor loading < 0.7–0.5 > and negative loadings for Cd in fish. PC 3 explained 14.88% of the variance with high factor loadings of > 0.9 for Pb and Cd in vegetables. Similarly, PC 4 and 5 explained 14.85% and 11.0% of the total variance, respectively. The applications of the multivariate statistical tool, such as PCA and factor analysis, aid in the interpretation of complex data matrices (Jung et al. 2016). The tool has been successfully applied to understand the water quality of rivers, identify the possible factors/sources that influence the quality, as well as to monitor the ecological status of the studied systems to find the rapid solution to pollution problems (Shrestha and Kazama 2007). PCA analysis has also proven to be valuable for source identification of heavy metals from wetland water and sediment in India (Das and Choudhury 2016). The varimax technique rotates the original factors such that the factors are strongly correlated with a specific set of variables, while weakly correlated with the others. As such, each variable is generally associated with one factor simplifying the interpretation of a complex data set (Jung et al. 2016). In our study, the strongly correlated factors (Pb/Cd exposure path) in a component implied greater risk in children associated with such means.
Estimated chronic daily intake
The provisional tolerable daily intake (PTDI) has been set at 0.0035 mg Pb/kg BW/day and 0.00066 mg Cd/kg BW/day for children (JECFA 1987). The ECDI for Pb and Cd exceeded the safe PTDI values owing primarily from the ingestion route; both Pb and Cd were taken in chiefly via food and water sources. Besides, ingested Cd from school paint dust is also a major concern. The median Pb/Cd levels in the ECDI of various food items such as rice (0–0.253 mg Pb/kg/d and 0.0006–0.024 mg Cd/kg/d), vegetables (0.001–2.0 mg Pb/kg/d and 0.0004–0.947 mg Cd/kg/d), and fish (0.001–0.007 mg Pb/kg/d and 0.0004–0.014 mg Cd/kg/d) along with groundwater (0.017–0.064 mg Pb/L/d and 0.014–0.038 mg Cd/L/d) demonstrated a health risk to children on a daily basis. Further, Cd ingestion from paint dust was in the range of 0–0.151 mg/kg/d; its median value was well above the permissible PTDI value (Fig. 3). Diet as well as the water is a major source of toxic metal intake for much of the world's population (Liu et al. 2013a). Rice, vegetable, and fish are considered a major staple for Assamese people. Water consumption is also high in this area owing to hot and humid weather conditions. Therefore, a considerable presence of Pb/Cd in these items posed substantial health threats. Further, Cd in paint dust is yet another concern that needed to be addressed, especially when Pb levels in paint has been regulated, similar guidelines needed to be applied for Cd in India as well.
Estimated non-carcinogenic and carcinogenic health risks in children due to Pb/Cd
Estimation of HQ is a reliable predictor of non-carcinogenic risk, which is the comparison of the estimated daily intake of toxicants and the reference dose (Yu-Jun et al. 2011). HQ > 1 indicated the considerable chronic non-cancer risk to the health of the young school-going children. It seemed that ingestion was the most dominant contributor of metal exposure in children; median HQ for Pb and Cd from all ingestion sources (diet, groundwater, school courtyard soil, and paint dust) were 2.67 and 5.2, respectively. Inhalation (only from the paint dust) and dermal routes were of nominal threat. For Pb and Cd, > 98% intake via the ingestion route was observed. Inhalation pathway contributed a nominal share in the overall risk and dermally; only 0.11% Pb and 1.89% Cd could enter the system. When several chemicals from a similar source or exposure pathway are encountered, a summation of the risk could be predicted. HI value of > 1 was, thus, an estimate of cumulative hazards from both Pb and Cd from all sources, as found here (HI = 7.9) (Table 5). In children, > 50% absorption of Pb via the ingestion pathway was also noted in a prior study (Markowitz 2000). Since both Pb and Cd are nonessential in mammals, their gastrointestinal (GI) uptake is associated with other essential elements. The GI uptake of Pb is associated with Fe-carrier protein, DMT1, and Cd with zinc transporter, ZIP8 protein (Bannon et al. 2002; Himeno et al. 2009). Interestingly, anemia, as well as dietary inadequacies, especially Zn-deficiency has been shown to increase gastrointestinal absorption of both Cd and Pb in children (McCarty 2012). This is a major cause of concern as the childhood Fe-deficiency anemia in Indian students has been associated with dietary inadequacies; ~ 60% of the school-going children are anemic (Swaminathan et al. 2019). Once in the system, the biological half-life of Pb in the blood is approximately a month and in bones, 20–30 years; their half-lives in the aforementioned tissues may be longer in children (ATSDR 2007). Similarly, the half-life of Cd is > 10 years; its level builds up in the body, predominantly in the kidneys and bones, with very little (~ 0.01–0.02%) excretion rate (ATSDR 2008). Therefore, chronically, both Cd and Pb could cause brittle bone and decreased bone density (Schoeters et al. 2006). Pb has effects on fertility, reproduction, and development, along with hypertension (JECFA 2011). On a long-term basis, daily intake over 3.5 × 10-3 mg Pb/kg was associated with a decrease of IQ point by 3 points in children, while prenatal-maternal exposure of Cd was associated with 1.7– 2.7-point reductions IQ in children from Bangladesh (Kippler et al. 2012). Cd is nephrotoxic, teratotoxic, and has effects on fertility, reproduction, and development in children exposed to it early on (Schoeters et al. 2006). Therefore, exposure of both Pb and Cd in early developmental ages poses a threat to their overall well-being.
Pb and Cd are carcinogen, low Cd, and Pb intake from the diet over a period of time was associated with the increased risk of cancer (Itoh et al. 2014; Canaz et al. 2017). The total ILCR for Pb from all exposure pathways was slightly more than 1 × 10-4, the stipulated maxima for cancer risk (Table 6). If individual exposure pathway was to be considered, ingestion alone was responsible for this value as both dermal and inhalation pathway contributed nominally. Hence, it could be inferred that Pb might not be a carcinogenic threat in school children in the study area. However, the ILCR from Cd exposure from the ingestion pathway was 69 times higher than the carcinogenic threat limit. Similar to Pb, the dermal and inhalation pathway had very little role to play in the augmentation of carcinogenic risks. Chronic Cd exposure in mammal led to DNA instability in repair and recombination, impacted the cell cycle regulation, and interfered with signal transduction, activating cellular proto-oncogenes leading to tumors and malignancy (Feki-Tounsi and Hamza-Chaffai 2014). A myriad of cancers in the lungs, GI, pancreas, kidney, and breast are associated with Cd exposure (reviewed in Bishak et al. 2015). Although there might be certain uncertainties in the estimation of the non-cancer and cancer risks based on risk assessment models due to slight overestimation of the risk, the study would nonetheless throw newer insights into the complex Pb-Cd exposure pathway in vulnerable groups such as children.
Conclusion
The investigation threw light on the complex Pb-Cd exposure network in school-going children in a periurban area in southern Assam, India. Pollution levels in rice (0–18.9 mg Pb/kg; 0.05–1.8 mg Cd/kg), vegetables (0.05–90.5 mg Pb/kg; 0.02–41.2 mg Cd/kg), fish (0.54–2.87 mg Pb/kg; 0.16–5.35 mg Cd/kg), eggs (0.26–2.06 mg Pb/kg; 0–7.12 mg Cd/kg), and groundwater (0.13–0.48 mg Pb/L; 0.11–0.29 mg Cd/L) indicated significant dietary risk. In this study, the median value for Pb in paint dust was found to be 13.9 mg/kg, which was below the permissible limit. On the other hand, 50% of the paint samples had Cd above the permissible limit of 100 mg/kg, with a median value of 108.6 mg Cd/kg. We found that the ingestion of metals was the single most dominant route of exposure in children; school microenvironment contributed significantly to the metal load due to their typical hand-to-mouth behavior. The evaluation of the estimated chronic daily intake and the hazard quotient indicated perilous exposure over a lifetime to both Pb and Cd, but only Cd posed a prominent cancer threat. It was concluded that chronic effects of metals would be a prominent toxicological risk to children when exposed early on. Thus, more attention should be paid to environmental quality, particularly to their diet, to minimize the health risks in them.
Data availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
References
Aendo P, Thongyuan S, Songserm T, Tulayakul P (2019) Carcinogenic and non-carcinogenic risk assessment of heavy metals contamination in duck eggs and meat as a warning scenario in Thailand. Sci Total Environ 689:215–222. https://doi.org/10.1016/j.scitotenv.2019.06.414
Apanpa-Qasim AF, Adeyi AA, Mudliar SN, Raghunathan K, Thawale P (2016) Examination of lead and cadmium in water-based paints marketed in Nigeria. J Health Pollut 6:43–49. https://doi.org/10.5696/2156-9614-6.12.43
ATSDR (2007) Toxicological profile for lead, vol 1. US Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry. Atlanta, GA
ASTDR (2008) Toxicological profile for cadmium. US Department of Health and Human Services, Agency for Toxic Substances and Disease Registry. Atlanta, GA
Awasthi SK (2000) Prevention of food adulteration Act No. 37 of 1954, Central and State rules as amended for 1999. Ashoka Law House, New Delhi
Bannon DI, Portnoy ME, Olivi L, Lees PS, Culotta VC, Bressler JP (2002) Uptake of lead and iron by divalent metal transporter 1 in yeast and mammalian cells. Biochem Biophys Res Commun 295:978–984. https://doi.org/10.1016/S0006-291X(02)00756-8
BIS (2012) Bureau of Indian Standards: drinking water—specification (second revision). ICS 13(060):20
Bhuyan PC, Goswami C, Kakati BK (2017) Study of fish consumption patterns in Assam for development of market-driven strategies. Res J Chem Environ Sci 5:42–52
Bishak YK, Payahoo L, Osatdrahimi A, Nourazarian A (2015) Mechanisms of cadmium carcinogenicity in the gastrointestinal tract. Asian Pac J Cancer Prev 16:9–21. https://doi.org/10.7314/APJCP.2015.16.1.9
Canaz E, Kilinc M, Sayar H, Kiran G, Ozyurek E (2017) Lead, selenium and nickel concentrations in epithelial ovarian cancer, borderline ovarian tumor and healthy ovarian tissues. J Trace Elem Med Biol 43:217–223. https://doi.org/10.1016/j.jtemb.2017.05.003
Cao S, Duan X, Zhao X, Wang B, Ma J, Fan D, Sun C, He B, Wei F, Jiang G (2015) Health risk assessment of various metal (loid) s via multiple exposure pathways on children living near a typical lead-acid battery plant. China Environ Pollut 200:16–23. https://doi.org/10.1016/j.envpol.2015.02.010
Chen H, Lu X, Li LY (2014) Spatial distribution and risk assessment of metals in dust based on samples from nursery and primary schools of Xi'an, China. Atmos Environ 88:172–182. https://doi.org/10.1016/j.atmosenv.2014.01.054
Commission Regulation (EU) 2016/217 of 16 February 2016. Amending Annex XVII to Regulation (EC) No 1907/2006 of the European Parliament and of the Council concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) as regards cadmium
Das S, Choudhury SS (2016) Analysis of heavy metals from water, sediment, and tissues of Labeo angra (Hamilton, 1822), from an Ox-box lake-an wetland site from Assam, India. J Environ Sci Health Part A 51:21–33. https://doi.org/10.1080/10934529.2015.1079102
Dwivedi D, Jain M, Jain S (2013) An association between maternal lead and cadmium levels and birth weight of the babies in North Indian population. Open J Obst Gynecol 3:331–336. https://doi.org/10.4236/ojog.2013.33061
Feki-Tounsi M, Hamza-Chaffai A (2014) Cadmium as a possible cause of bladder cancer: a review of accumulated evidence. Environ. Sci Pollut Res Int 21:10561–10573. https://doi.org/10.1007/s11356-014-2970-0
The Gazette of India (2016) Ministry Of Environment, Forest and Climate Change Notification. Regulation of lead contents in household and decorative paints rules, 201, November 1. http://egazette.nic.in/WriteReadData/2016/172451.pdf. Accessed 19 May 2020
Giri S, Singh AK (2014) Assessment of human health risk for heavy metals in fish and shrimp collected from Subarnarekha River, India. Int J Environ Health Res 24:429–449. https://doi.org/10.1080/09603123.2013.857391
Giri S, Singh AK (2017) Heavy metals in eggs and chicken and the associated human health risk assessment in the mining areas of Singhbhum copper belt, India. Arch Environ Occup Health 74:161–170. https://doi.org/10.1080/19338244.2017.1407284
Himeno S, Yanagiya T, Fujishiro H (2009) The role of zinc transporters in cadmium and manganese transport in mammalian cells. Biochimie 91:1218–1222. https://doi.org/10.1016/j.biochi.2009.04.002
Itoh H, Iwasaki M, Sawada N, Takachi R, Kasuga Y, Yokoyama S, Onuma H, NishimuraH KR, Yokoyama K, Tsugane S (2014) Dietary cadmium intake and breast cancer risk in Japanese women: a case–control study. Int J Hyg Environ Health 217:70–77. https://doi.org/10.1016/j.ijheh.2013.03.010
JECFA (1987) Toxicological evaluation of certain food additives and contaminants. WHO Food Additives Series, No. 21, Cambridge University Press, Cambridge
JECFA (2011) Evaluation of certain food additives and contaminants. Seventy-third Report. WHO Technical Report Series, No. 960World Health Organization, Geneva
Jung KY, Lee K-L, Im TH, Lee IJ, Kim S, Han K-Y, Ahn JM (2016) Evaluation of water quality for the Nakdong River watershed using multivariate analysis. Environ Technol Innov 5:67–82. https://doi.org/10.1016/j.eti.2015.12.001
Kabata-Pendias A (2011) Trace elements in soils and plants, 4th edn. CRC Press, Boca Raton
Kippler M, Tofail F, Hamadani JD, Gardner RM, Grantham-McGregor SM, Bottai M, Vahter M (2012) Early-life cadmium exposure and child development in 5-year-old girls and boys: a cohort study in rural Bangladesh. Environ Health Perspect 120:1462–1468. https://doi.org/10.1289/ehp.1104431
Kumar M, Ramanathan AL, Tripathi R, Farswan S, Kumar D, Bhattacharya P (2017) A study of trace element contamination using multivariate statistical techniques and health risk assessment in groundwater of Chhaprola Industrial Area, Gautam Buddha Nagar, Uttar Pradesh, India. Chemosphere 166:135–145. https://doi.org/10.1016/j.chemosphere.2016.09.086
Kumar A, Malyan SK, Kumar SS, Dutt D, Kumar V (2019) An assessment of trace element contamination in groundwater aquifers of Saharanpur, Western Uttar Pradesh, India. Biocatal Agric Biotechnol 20:101213. https://doi.org/10.1016/j.bcab.2019.101213
Li Z, Ma Z, Jan van der Kuijp T, Yuan Z, Huang L (2014) A review of soil heavy metal pollution from mines in China: pollution and health risk assessment. Sci Total Environ 468–469:843–853. https://doi.org/10.1016/j.scitotenv.2013.08.090
Lin M, Gui H, Wang Y, Peng W (2017) Pollution characteristics, source apportionment, and health risk of heavy metals in street dust of Suzhou, China. Environ Sci Pollut Res 24:1987–1998. https://doi.org/10.1007/s11356-016-7934-0
Liu X, Song Q, Tang Y, Li W, Xu J, Wu J, Wang F, Brookes PC (2013a) Human health risk assessment of heavy metals in soil–vegetable system: a multi-medium analysis. Sci Total Environ 463:530–540. https://doi.org/10.1016/j.scitotenv.2013.06.064
Liu J, Li L, Wang Y, Yan C, Liu X (2013b) Impact of low blood lead concentrations on IQ and school performance in Chinese children. PLoS One 8:e65230. https://doi.org/10.1371/journal.pone.0065230
Liu Z, Cai L, Liu Y, Chen W, Wang Q (2019) Association between prenatal cadmium exposure and cognitive development of offspring: a systematic review. Environ Pollut 254:113081. https://doi.org/10.1016/j.envpol.2019.113081
Mao C, Song Y, Chen L, Ji J, Li J, Yuan X, Yang Z, Ayoko GA, Frost RL, Theiss F (2019) Human health risks of heavy metals in paddy rice based on transfer characteristics of heavy metals from soil to rice. Catena 175:339–348. https://doi.org/10.1016/j.catena.2018.12.029
Margenat A, Matamoros V, Díez S, Cañameras N, Comas J, Bayona JM (2019) Occurrence and human health implications of chemical contaminants in vegetables grown in peri-urban agriculture. Environ Int 124:49–57. https://doi.org/10.1016/j.envint.2018.12.013
Markowitz M (2000) Lead Poisoning. Pediatr Rev 21:327–335. https://doi.org/10.1542/pir.21-10-327
McCarty MF (2012) Zinc and multi-mineral supplementation should mitigate the pathogenic impact of cadmium exposure. Med Hypotheses 79:642–648. https://doi.org/10.1016/j.mehy.2012.07.043
Mohammadi AA, Zarei A, Majidi S, Ghaderpoury A, Hashempour Y, Saghi MH, Alinejad A, Yousefi M, Hosseingholizadeh N, Ghaderpoori M (2019) Carcinogenic and non-carcinogenic health risk assessment of heavy metals in drinking water of Khorramabad, Iran. MethodsX 6:1642–1651. https://doi.org/10.1016/j.mex.2019.07.017
Mudur G (2005) India has some of the highest cancer rates in the world. BMJ 330:215. https://doi.org/10.1136/bmj.330.7485.215-c
NAPEP (2017) National Action Plan for Egg & Poultry-2022 for doubling farmers’ income by 2022. Department of Animal Husbandry, Dairying and Fisheries, Ministry of Agriculture and Farmers Welfare, Government of India. http://www.dahd.nic.in/sites/default/filess/Seeking%20Comments%20on%20National%20Action%20Plan-%20Poultry-%202022%20by%2012-12-2017.pdf. Accessed 12 June 2020.
Phukan RK, Ali MS, Chetia CK, Mahanta J (2001) Betel nut and tobacco chewing; potential risk factors of cancer of oesophagus in Assam, India. Br J Cancer 85:661–667. https://doi.org/10.1054/bjoc.2001.1920
Qian Y, Chen C, Zhang Q, Li Y, Chen Z, Li M (2010) Concentrations of cadmium, lead, mercury and arsenic in Chinese market milled rice and associated population health risk. Food Control 21:1757–1763. https://doi.org/10.1016/j.foodcont.2010.08.005
Rajadhyaksha M (2014) Indian kids spend too much time at school? The Times of India. https://timesofindia.indiatimes.com/home/education/news/Indian-kids-spend-too-much-time-at-school/articleshow/28452277.cms. Accessed 22 June 2020.
Rehman ZU, Khan S, Brusseau ML, Shah MT (2017) Lead and cadmium contamination and exposure risk assessment via consumption of vegetables grown in agricultural soils of five-selected regions of Pakistan. Chemosphere 168:1589–1596. https://doi.org/10.1016/j.chemosphere.2016.11.152
Roberts TL (2014) Cadmium and phosphorous fertilizers: the issues and the science. Process Eng 83:52–59. https://doi.org/10.1016/j.proeng.2014.09.012
Schoeters G, Hond ED, Zuurbier M, Naginiene R, Van den Hazel P, Stilianakis N, Ronchetti R, Koppe RJ (2006) Cadmium and children: exposure and health effects. Acta Paediatr 95:50–54. https://doi.org/10.1080/08035320600886232
Searle B, Chan W, Jensen C, Davidow B (1969) Determination of the lead in paint scrapings by atomic absorption. Atomic Absorp Newsletter 8:126–127
Sharafi K, Yunesian M, Nodehi RN, Mahvi AH, Pirsaheb M (2019) A systematic literature review for some toxic metals in widely consumed rice types (domestic and imported) in Iran: human health risk assessment, uncertainty and sensitivity analysis. Ecotoxicol Environ Saf 176:64–75. https://doi.org/10.1016/j.ecoenv.2019.03.072
Sharma RK, Agrawal M, Marshall FM (2008) Heavy metal (Cu, Zn, Cd and Pb) contamination of vegetables in urban India: a case study in Varanasi. Environ Pollut 154:254–263. https://doi.org/10.1016/j.envpol.2007.10.010
Shi Z, Carey M, Meharg C, Williams PN, Signes-Pastor AJ, Triwardhani EA, Pandiangan FI, Campbell K, Elliott C, Marwa EM, Jiujin X (2020) Rice grain cadmium concentrations in the global supply-chain. Expos Health 12:869–876. https://doi.org/10.1007/s12403-020-00349-6
Shrestha S, Kazama F (2007) Assessment of surface water quality using multivariate statistical techniques: a case study of the Fuji river basin, Japan. Environ. Modell Softw 22:464–475. https://doi.org/10.1016/j.envsoft.2006.02.001
Swaminathan S, Rajkumar H, Pandey A, Kassebaum NJ, Laxmaiah A, Longvah T, Lodha R, Ramji S, Kumar GA, Afshin A, Gupta SS, Kar A, Khera AK, Mathai M, Awasthi S, Rasaily R, Varghese CM, Millear AI, Manguerra H, Gardner WM, Sorenson R, Sankar MJ, Purwar M, Furtado M, Bansal PG, Barber R, Chakma JK, Chalek J, Dwivedi S, Fullman N, Ginnela BN, Glenn SD, Godwin W, Gonmei Z, Gupta R, Jerath SG, Kant R, Krish V, Kumar RH, Ladusingh S, Meshram II, Mutreja P, Nagalla B, Nimmathota A, Odell CM, Olsen HE, Pati A, Pickering B, Radhakrishna KV, Raina N, Rankin Z, Saraf D, Sharma RS, Sinha A, Varanasi B, Shekhar C, Bekedam HJ, Reddy KS, Lim SL, Hay SI, Dandona R, Murray CJL, Toteja GS, Dandona L (2019) The burden of child and maternal malnutrition and trends in its indicators in the states of India: the Global Burden of Disease Study 1990–2017. Lancet Child Adol Health 3:855–870. https://doi.org/10.1016/S2352-4642(19)30273-1
Tiwari KK, Singh NK, Patel MP, Tiwari MR, Rai UN (2011) Metal contamination of soil and translocation in vegetables growing under industrial wastewater irrigated agricultural field of Vadodara, Gujarat, India. Ecotoxicol Environ Saf 74:1670–1677. https://doi.org/10.1016/j.ecoenv.2011.04.029
Ture M, Kilic MB, Altinok I (2020) Relationship between heavy metal accumulation in fish muscle and heavy metal resistance genes in bacteria isolated from fish. Biol Trace Elem Res. https://doi.org/10.1007/s12011-020-02246-0
Turner A, Kearl ER, Solman KR (2016) Lead and other toxic metals in playground paints from South West England. Sci Total Environ 544:460–466. https://doi.org/10.1016/j.scitotenv.2015.11.078
UNEP/WHO (2012) Global Alliance To eliminate lead paint. United Nations Environment Program and World Health Organization. https://wedocs.unep.org/bitstream/handle/20.500.11822/14175/Annotated_Provisional_Agenda_UNEP_WHO_GAELP2_final.pdf?sequence=1&isAllowed=y. Accessed 12 June 2020
US EPA (1997) Exposure Factors Handbook (Final Report) EPA/600/P-95/002F A-c, 1997. EPA, Washington, DC
US EPA (2001) Risk Assessment Guidance for Superfund: Volume III - Part A, Process for Conducting Probabilistic Risk Assessment. US environmental protection agency [EPA 540-R-02-002], Washington, DC
US EPA (2011a) Screening level (RSL) for chemical contaminant at super-found sites. http://www.epa.gov/regshwmd/risk/human/Index.htm. Accessed 12 June 2020
US EPA (2011b) Regional screening tables. http://www.epa.gov/reg3hwmd/risk/human/rb-concentration_table/index.htm. Accessed 12 June 2020
Vainio H, Bianchini F, Heseltine E (2002) Breast Cancer Screening: IARC Handbooks of Cancer Prevention. IARC Press, Lyon
WHO (1998) Health guideline for the use of wastewater in agriculture, report of WHO Science Group. World Health Organization, Geneva, Switzerland. Technical Report Series 778
WHO/FAO (2003) Joint Expert Committee on Food Additives and Contaminants. World Health Organization, Geneva, Switzerland
Yang Y, Zhang FS, Li HF, Jiang RF (2009) Accumulation of cadmium in the edible parts of six vegetable species grown in Cd-contaminated soils. J Environ Manag 90:1117–1122. https://doi.org/10.1016/j.jenvman.2008.05.004
Yu-jun Y, Zhifeng Y, Shanghong Z (2011) Ecological risk assessment of heavy metals in sediment and human health risk assessment of heavy metals in fishes in the middle and lower reaches of the Yangtze River Basin. J Environ Pollut 159:2575–2585. https://doi.org/10.1016/j.envpol.2011.06.011
Zwolak A, Sarzyńska M, Szpyrka E, Stawarczyk K (2019) Sources of soil pollution by heavy metals and their accumulation in vegetables: a review. Water Air Soil Pollut 230:164. https://doi.org/10.1007/s11270-019-4221-y
Author information
Authors and Affiliations
Contributions
Suchismita Das: Conceptualization; methodology; data analysis; writing, reviewing and editing; and overall supervision. Akan Barhai, Moumita Nath, Agniv Laskar, Satabdi Deb Roy, Swarupa Deb, and Atiya Parveen Choudhury: Carrying out field and laboratory work.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Responsible Editor: Lotfi Aleya
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 20 kb)
Rights and permissions
About this article
Cite this article
Das, S., Nath, M., Laskar, A.K. et al. Lead and cadmium exposure network in children in a periurban area in India: susceptibility and health risk. Environ Sci Pollut Res 28, 28133–28145 (2021). https://doi.org/10.1007/s11356-021-12608-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-12608-3