Abstract
Contamination of soils with heavy metals (HMs) caused serious problems because plants tend to absorb HMs from the soil. In view of HM hazards to plants as well as agro-ecosystems, we executed this study to assess metal toxicity to mung bean (Vigna radiata) plants cultivated in soil with six treatment levels of cadmium (Cd) and nickel (Ni) and to find metal tolerant variety, i.e., M-93 (V1) and M-1(V2) with multifarious plant biochemical and physiological attributes. Increasing doses of Cd and Ni inhibited plant growth and photosynthesis and both varieties showed highly significant differences in the morpho-physiological attributes. V2 showed sensitivity to Cd and Ni treatments alone or in combination. Tolerance indices for attributes presented a declined growth of Vigna plants under HM stress accompanied by highly significant suppression in gas exchange characteristics. Of single element applications, the adverse effects on mung bean were more pronounced in Cd treatments. V1 showed much reduction in photosynthesis attributes except sub-stomatal CO2 concentration in all treatments compared to V2. The yield attributes, i.e., seed yield/plant and 100-seed weight, were progressively reduced in T5 for both varieties. In combination, we have observed increased mobility of Cd and Ni in both varieties. The results showed that water use efficiency (WUE) generally increased in all the treatments for both varieties compared to control. V2 exhibited less soluble sugars and free amino acids compared to V1 in all the treatments. Similarly, we recorded an enhanced total free amino acid contents in both varieties among all the metal treatments against control plants. We conclude that combinatorial treatment proved much lethal for Vigna plants, but V1 performed better than V2 in counteracting the adverse effects of Cd and Ni.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil contamination with metals is one of the most prominent and serious disturbances to the soil micro- and macro-environment. Starting from perturbance in life cycle of primary producers, metal retention in food chain as well as mixing up in soils has drastically affected the stability of ecosystem (Rai et al. 2019). In recent times, biosphere pollution due to heavy metals (HMs) has attained significant attention to find new ways to maintain, restore, and manage the environment (Khalid et al. 2019b; Noman and Aqeel 2017; Noman et al. 2017; Wahid et al. 2008). Heavy metals accumulate in the soils and atmosphere either through natural components or by human activities including smelting, electroplating, fossil fuel combustion, and waste disposal on land (Figlioli et al. 2019; Tchounwou et al. 2012). Some metals are considered essential ones like copper, manganese, and zinc (Cu, Mn, Zn), and needed for several activities and vital cellular processes while the others like lead, cadmium, or nickel (Pb, Cd, Ni) are considered non-essential and highly toxic (Khalid et al. 2019a; Rizwan et al. 2016; Tchounwou et al. 2012). High concentration of these metals not only cause serious problems in plants but also severely affect animals and human beings by entering into the food chain (Figlioli et al. 2019).
Nickel (Ni) and cadmium (Cd) are the potential phyto-toxic metals particularly when prevailing in higher concentrations. Incremented concentrations of Ni disturb numerous processes inside the plants such as germination inhibition and seedling growth (Ahmad et al. 2009; Ashraf et al. 2011), reduction in dry mass production, and inhibition of photosynthetic activity (Ahmad et al. 2011), as well as it also deleteriously affects the plant respiration, mineral nutrition acquisition, and transport of assimilates (Sreekanth et al. 2013). Besides Ni, Cd in nature co-exist in combination with other elements, e.g., sulfur and chloride. Cadmium is usually extracted from zinc, lead, and copper ores as minor component (Ashraf et al. 2016; Cwielag-Drabek et al. 2020). Due to its higher water solubility, Cd is one of the highly dangerous pollutants present in soil-plant biosystems (Bahmani et al. 2012; Wahid et al. 2008; Zhang et al. 2020). Increase in soil Cd contents and its accumulation in plants results in an inhibition of photosynthesis, respiration, growth, and plant development, and even cause plant death (Bahmani et al. 2012). Chlorosis, leaf rolls, and stunting are clearly observable symptoms of Cd toxicity in plants (Wahid et al. 2008). Due to its high mobility in the soil-plant system, it is a metal of major concern with respect to plant exposure and accumulation in food chain subsequently affecting human health (Cwielag-Drabek et al. 2020; Rai et al. 2019). Interestingly, toxic effects of Cd are directly coupled with imbalance in many macro- and micro-nutrient levels (Ahmad et al. 2011). It has been reported that growth of several major crops such as wheat, maize, mung bean, sunflower, and barley is adversely affected by Cd and Ni contamination (Bahmani et al. 2012; Dong et al. 2005; Noman and Aqeel 2017).
Legumes are cultivated on a large area in Pakistan and are commonly used in annual rotations with other crops. Among all legumes, mung bean (Vigna radiata L.) is a valuable legume crop with immense nutritional quality (Sundari 2009; Wahid and Ghani 2008). It is regarded as the principal source of cheaper protein as it contributes substantially to total protein intake and can be used as a substitute for the more expensive animal protein foods (Bartholomae et al. 2019; Hou et al. 2019; Yi-Shen et al. 2018). It has great value as food, fodder, and green manure. Like other legume crops, it can improve soil fertility through biological nitrogen fixation (Ilyas et al. 2018). In Pakistan, the climate is very suitable for its growth and cultivation and it is usually grown during two growing seasons spring and autumn (Ashraf et al. 2016; Ilyas et al. 2018). Unfortunately, soil pollution with HMs, i.e., Cd and Ni, has not only adversely affected crop production but also human health. It is, therefore, direly needed to evaluate survival potential of crops other than staple food crops, e.g., legumes especially mung bean on metal-contaminated soils. Survey of literature reveals paucity of data with reference to the survival strategies of mung bean as well as soil remediation of Ni- and Cd-polluted soils. The present study investigated the morpho-physio-biochemical potential as well as the yield responses of mung bean varieties under Cd and Ni stress regarding alterations in its tolerance index and survival capacity.
Materials and methods
Experimental conditions and plant material
The study was conducted to optimize the influence of cadmium (Cd) and nickel (Ni) either individually or in combination on morpho-physio-biochemical and yield attributes of mung bean. Seeds of two mung bean varieties [M-93 (V1) and M-1 (V2)] were acquired from the Pulse Department, Ayub Agricultural Research Institute (AARI), Faisalabad, Pakistan. The seeds were sown in plastic pots lined with polyethylene bags each containing 8-kg mixture of soil and plant manure (3:1, v/v) at Botanical Garden, University of Agriculture, Faisalabad, Pakistan (31.25 °N, 73.04 °E, 183.2-m elevation). Twelve seeds were sown in each pot. Following germination, the plants were thinned and six plants were retained in each pot.
Treatment evaluation
Twenty-day-old plants of uniform height, number of leaves, and stem diameter were selected for treatment with doses for Cd and Ni and further experimentation. Treatment schedule is presented in Table 1.
Evaluation of morphology, biomass, and yield
Data regarding various morphological traits was recorded 28 days after Cd and Ni application to evaluate the effects caused by metal exposure. Three, out of six plants, were carefully harvested (per replicate) and roots were thoroughly washed to remove any traces of soil. Subsequently, shoots and roots were separated for assessing fresh biomasses. Meanwhile, plant height (from root to the tip of the youngest leaf) and number of leaves per plant were recorded. Later, the harvested plants were oven-dried at 75 °C until constant weight was achieved for the evaluation of root and shoot dry weights. Afterwards, these dried plants were used for the estimation of different minerals and metal elements. The remaining plants were kept on growing under the prevailing growth conditions to record the final yield (seed yield/plant and 100-seed weight). Leaf area was calculated by using laboratory leaf area meter (LI-3000C, LICOR Inc. Biosciences). Leaf chlorosis (expressed in % age of the total area) was determined by intervening chlorosed area with the help of Vernier calipers following the methodology of (Wahid and Ghani 2008).
Photosynthetic and biochemical attribute
Gas exchange parameters
Measurements of gas exchange attributes such as net photosynthetic rate (Pn), transpiration rate (E), stomatal conductance (gs), sub-stomatal CO2 concentration (Ci), and water use efficiency (WUE) were made on a fully expanded flag leaf of each plant by using a portable infra-red gas analyzer (IRGA) (Model Cl-340; Analytical development company, Hoddesdon, England). Measurements were recorded during full sun shine from 1000 to 1300 h with the following specifications/adjustments: molar flow rate of air 402.3 mmol/m2/s1, atmospheric pressure 98.8–99.9 kPa, water vapor pressure in chamber ranged from 6.0 to 8.9 mbar, PAR at leaf surface was maximum up to 1711 μmol/m2/s1, temperature of leaf arranged from 29.4 to 32.3 °C, ambient temperature ranged from 23.2 to 28.8 °C, and ambient CO2 concentration was 352 μmol/mol1.
Leaf chlorophyll content (SPAD)
Measurement of total chlorophyll contents in plants was made 10 days after metal treatment application with soil and plant analyzer development (SPAD) value on the third top fully expanded leaf by using a chlorophyll meter (Model SPAD-502, Konica Minolta Sensing, Inc., Japan).
Soluble proteins, total free amino acids, and total soluble sugar estimation
For the estimation of soluble proteins, 0.5 g fresh plant material was standardized in 10 ml of ice-chilled 50 mM sodium-phosphate buffer (pH 7.0). The homogenized plant material was centrifuged at 600 rpm for 30 min. The supernatant was separated and used for the estimation of total soluble proteins following the methodology of Bradford (Bradford 1976). Total free amino acids and total soluble sugars were estimated by protocols established by Hamilaton and Van Slyke (1943) and Riazi et al. (1985), respectively.
Root and shoot elemental quantification
Oven-dried and ground plant material (0.1 g shoot and root) was used for digestion as described by Wolf (1982). Digested plant material was later filtered for elemental quantification. Calcium (Ca) and potassium (K) were quantified by using flame photometer (JENWAY PFP 7), while phosphorus (P) was measured with the help of spectrophotometer (JENWAY 6300) at 470 nm following Wolf (1982). Concentrations of magnesium (Mg), iron (Fe), cadmium (Cd), and nickel (Ni) were determined by using an atomic absorption spectrophotometer (Perkin Elmer, Model Analyst 3000, Norwalk). Chloride (Cl) contents were estimated using chloride analyzer (Model 926, Sherwood Scientific Ltd., and Cambridge, UK) by water extraction method (Jackson 1962).
Statistical data analysis
Current experiment was laid out in completely randomized design (CRD) with three replicates for each treatment. Two-way analysis of variance (ANOVA) for each attribute was performed through statistical software COSTAT v6.303 (Cohort software, Monterey, CA, USA). Means were separated and compared by using Duncan’s multiple range test (DMRT) at p ≤ 0.001 significance level. Graphs were developed by using Origin Pro 9.1, and statistical program “R-studio (v4.0.3)” was used for estimating Pearson’s correlation coefficient, and principal component analysis among various attributes.
Results
Growth, morphology, and biomass production under metal stress
Severely affected growth and morphology of mung bean varieties was observed under the adverse impacts of Cd and Ni. Increasing treatment doses of Cd and Ni inhibited plant growth and both varieties depicted highly significant differences (p < 0.001) in the number of leaves, plant height, leaf area, and leaf chlorosis (Table 2). However, shoot fresh weight and root dry weights seemed to have non-significant difference. The growth inhibition in both varieties was significantly (p < 0.001) impacted by different treatments. V1 showed the lowest plant height in T5 followed by T6 (Table 3) that was proportional to the decline in the number of leaves and leaf area in T5 and T6 in comparison with control. Decline in shoot dry weight was maximum, i.e., 45.8% and 58.5% in V1 and V2, respectively. Leaf chlorosis was significantly visible and higher in T5 for both varieties. Of the single element treatments, we observed greater signs of chlorosis in T2 as compared to T1 and in T4 as compared to T3. V2 also had the lowest values of plant height, number of leaves, and leaf area in T5 and T6. Moreover, yield biomass, i.e., seed yield/plant and 100-seed weight, was also affected by Cd and Ni treatment undergoing progressive reduction in both of these attributes in T5 for both varieties. The highest reduction (69.7%) in yield per plant was recorded for V2 in T5 (Table 3).
Gas exchange attributes and leaf chlorophyll level (SPAD value)
The statistical analysis of data regarding photosynthetic attributes of plants under applied metal stress exhibited perturbation in these attributes. The decline in growth attributes of mung bean plants under HM stress was accompanied with highly significant (p < 0.001) suppression of gas exchange characteristics (Table 2). Increasing doses of Cd and Ni caused steady reduction in photosynthetic rate of both varieties and the lowest value of this attribute was observed under T5 treatment (Fig. 1a–f). However, V2 depicted 14% lower photosynthesis under T5 treatment as compared to V1 at the same treatment level. As a consequence of metal stress, transpiration rate was declined in both varieties and minimum value of this attribute was recorded under T5 treatment followed by T6. Under single elemental treatments, Cd application caused more reductions in transpiration rate in both varieties when compared with Ni stress. For instance, 34% reduction in transpiration rate was caused by Ni in T1 for both varieties. And for T2, this reduction was up to 53.2% and 60.3% in V1 and V2, respectively. A similar trend was also observed for other physiological characteristics. Sub-stomatal CO2 concentration was highest in T5 for both varieties. The control plants had the highest stomatal conductance. We noted a trend in the reduction of stomatal conductance with increasing doses of metals for both varieties. However, the lowest value of stomatal conductance for V1 was observed under T6 and for V2, it was under T5 treatment. Likewise, readings of WUE (water use efficiency) also showed an increasing trend for both treatments. WUE generally increased in all the treatments for both varieties as compared with control. However, V1 exhibited greater WUE as compared to V2. The leaf chlorophyll contents (SPAD value) were also found to be significantly (p < 0.001) reduced by the adverse effects of Ni and Cd in both varieties (Table 2, Fig. 1a–f). This reduction was prominent in all the treatment groups as compared to the control. However, the highest reduction of SPAD value was observed in T5 for both varieties. Cd treatments in T2 and T4 caused 15% more reduction in SPAD as compared to Ni treatments in T1 and T3. The reduction in SPAD was greater in V2 than the corresponding V1.
a–i Photosynthetic attributes, total soluble sugars, total free amino acids, and soluble proteins of two mung bean (Vigna radiata) varieties grown under different treatments of Cd and Ni. Data are mean ± SE (n = 3). Different letters above the bars indicate significant differences at p ≤ 0.001 (Duncan’s multiple range test). Mean with similar letters in each plot are statistically not significant
Soluble proteins, total free amino acids, and total soluble sugar estimation
Different concentrations of Cd and Ni significantly (p < 0.001) influenced total soluble sugars, soluble proteins, and free amino acids (Table 2). Both varieties were differentially significant (p < 0.001) from each other for the mentioned attributes. In comparison with the control, V1 and V2 revealed a 166% and 192% increase in total soluble sugars at T5, respectively (Fig. 1g–i). The effect of T2 treatment was higher than T3 as it caused 42% and 51% increase in soluble sugars in V1 and V2, respectively. V1 exhibited less soluble sugars as compared to V2 in all the treatments because of the adverse impacts of Cd and Ni. Similarly, we recorded an enhanced total free amino in both varieties among all the metal treatments against the plants growing in soil without metals. Total soluble proteins were also interfered by soil Cd and Ni treatments. The analysis of data depicted HM dose-dependent decline in total soluble proteins and lowest value was noted in T5 for both varieties (Fig. 1g–i).
Quantification of Cd and Ni
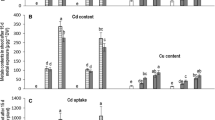
Mung bean varieties accumulated significantly high concentrations of Cd in both roots and shoots with increasing levels of Cd (Fig. 2a, b). Under all the treatments of Cd, V2 exhibited higher concentrations of Cd than V1. The highest concentration of Cd in root and shoot was recorded in T4 treatment for varieties, i.e., 4.0 mg kg−1 and 4.4 mg kg−1 in shoots of V1 and V2, respectively. Roots of both varieties accumulated even more Cd than the shoot in T4, i.e., 5.0 mg kg−1 and 5.7 mg kg−1 in V1 and V2, respectively. The concentration of Cd in shoots was found to be positively correlated with Cd contents in roots as represented by the value of the correlation coefficient (R2 = 0.987). Similarly, Ni concentration was also found to be higher in shoots and roots of both varieties with increasing concentration of Ni (Fig. 2a, b, Tables 4, 5). Mung bean varieties varied significantly (p < 0.01) for Ni concentration in roots and shoots. Treatments also significantly (p < 0.001) affected both varieties. Unlike Cd, Ni concentration was higher in shoots of both studied varieties. For instance, the maximum Ni in roots of V1 and V2 was 35.6 mg kg−1 and 39.0 mg kg−1 under T5 treatment. And the shoots of V1 and V2 under T5 treatment showed 40.9 mg kg−1 and 48.2 mg kg−1 Ni, respectively.
a–d Ionic (cadmium, nickel, chloride, calcium) concentration of the root and shoot of two mung bean (Vigna radiata) varieties grown under different treatments of Cd and Ni. Data are mean ± SE (n = 3). Different letters above the bars indicate significant differences at p ≤ 0.001 (Duncan’s multiple range test). Mean with similar letters above the bars in each plot are statistically not significant
Elemental quantification
The concentration of chloride (Cl) was highly significant (p < 0.001) in all the applied treatments (Fig. 2c), and the lowest Cl content was seen for control. We observed increasing Cl contents with increments in Cd and Ni Concentration. Although T6 depicted the highest reading for Cl contents in roots and shoots of both varieties, V2 exhibited differentially high accumulation of Cl than V1 for all the treatments. Ca, Fe, K, P, and Mg ions depicted reduction in all the metal treatments in both varieties as compared to control plants (Fig. 2d, Fig. 3a–d, Tables 4, 5). However, Cd in T2 and T4 caused much more reductions in these essential ion concentrations as compared to Ni effects under T1 and T3 in both varieties. Lowest values of these essential ions were recorded under T5 and T6 treatment levels. Overall, V2 was found to be more adversely impacted by Ni and Cd treatments with respect to the studied nutrient elements (Ca, Fe, K, P, and Mg).
a–d Ionic (iron, potassium, phosphorus, magnesium) concentration of the root and shoot of two mung bean (Vigna radiata) varieties grown under different treatments of Cd and Ni. Data are mean ± SE (n = 3). Different letters above the bars indicate significant differences at p ≤ 0.001 (Duncan’s multiple range test). Mean with similar letters above the bars in each plot are statistically not significant
Relationship
Pearson’s correlations revealed the significantly positive correlations of Cd and Ni concentrations in shoots and in roots with water use efficiency, sub-stomatal CO2 concentration, total soluble sugars, total free amino acids, leaf chlorosis, and Cl contents (Fig. 4). However, a significantly negative correlation is evident for Ni and Cd concentrations in roots and shoots of both varieties with reference to photosynthetic and transpiration rates, stomatal conductance, SPAD, soluble proteins, Ca, Fe, K, P, Mg, and other growth as well as yield-related attributes like plant height, number of leaver/plant, leaf area, seed yield/plant, and 100-seed weight. For further validation of Pearson’s correlation results and evaluation of inter-varietal responses of mung bean plants to different Cd and Ni applications, principal component analysis was executed (Fig. 5). The results obtained from Pearson’s correlation were attested by the PCA-biplot figure, and it represented inverse relationship between stress levels and plant survival. We imaged the direction of the cases on a factor plane in PCA with the biplot. A clear separation of all different parameter of V1 and V2 in response to different levels of Cd and Ni was exhibited by Dim1 and Dim2. The contribution of Dim1 to the total variance was 78.9%, in comparison of contribution of Dim2, i.e., 15.5%. A clear separation between both varieties with respect to their different attributes under HMs was observed in PC analysis (Fig. 5).
Pearson correlation between different attributes of two mung bean varieties reveals impact of Cd and Ni treatments on plant growth and productivity. Pn: photosynthetic rate; E: transpiration rate; WUE: water use efficiency; gs: stomatal conductance; Ci: sub-stomatal CO2 concentration; SPAD: total chlorophyll contents; SP: soluble proteins; TFAA: total free amino acids; TSS: total soluble sugars; R_Cd: root Cd; S_Cd: shoot Cd; R_Ni: root Ni; S_Ni: shoot Ni; R_Cl: root Cl; S_Cl: shoot Cl R_K: root K; S_K: shoot K; R_P: root P; S_P: shoot P; R_Ca: root Ca; S_Ca: shoot Ca; R_Mg: root Mg; S_Mg: shoot Mg; R_Fe:root Fe; S_Fe: shoot Fe; LA: leaf Area; LC: leaf chlorosis; SFW: shoot fresh weight; SDW: shoot dry weight; RFW: root fresh weight; RDW: root dry weight; PH: plant height; NL: number of leaves/plant; SYP: seed yield/plant; X100SY: 100-seed weight
Principal component analysis (PCA) showing the relationship among different measured variables of two mung bean varieties under Cd and Ni applications. Pn: photosynthetic rate; E: transpiration rate; WUE: water use efficiency; gs: stomatal conductance; Ci: sub-stomatal CO2 concentration; SPAD: total chlorophyll contents; SP: soluble proteins; TFAA: total free amino acids; TSS: total soluble sugars; LA: leaf area; LC: leaf chlorosis; PH: plant height; NL: number of leaves/plant; SYP: seed yield/plant; X100SY: 100-seed weight
Discussion
Our study has confirmed the adversaries caused by soil HM pollution. It has been observed that V. radiata become vulnerable to Cd and Ni presence in the soil and exhibit continuous deterioration in growth and development with incremented metal doses. Our findings about decrease in plant biomass directly coupled with significant decline in photosynthetic attributes. These characteristics were particularly observed in V2 and showed its sensitivity to metal doses in soil. Besides, translocation of both metals, i.e., Cd and Ni was comparatively low in V1 as compared to V2. In combined treatment of Cd and Ni, a difference was found in the translocation of both metals but the combinatorial effect of Cd and Ni was more drastic, e.g., T5 and T6, as compared to single dose of Cd or Ni. Translocation factor for both metals indicate relatively high uptake of Ni as compared to Cd. The metabolic and morphological changes are indices of destruction for plant health and life cycle in different plants (Ahmad et al. 2009; Ashraf et al. 2016; Bahmani et al. 2012; Dong et al. 2005; Noman et al. 2019; Wahid et al. 2008). Our observations regarding decreased growth attributes are directly coupled with metal toxicity. The obliteration in growth as well as photosynthetic pigments is the main reason for suppressed photosynthetic activity (Chen et al. 2017). In fact, elevated uptake of metals regardless of plant parts exerts negative influence upon growth and development. The metal toxicity symptoms revealed by biomass reduction, pigment deterioration, and alteration in photosynthesis activity describe metal toxicity–based striking interference in both varieties used in this study. The low growth response and decomposition of pigments especially in plants grown under T5/T6 level is the main reason for decline in photosynthesis. In parallel with growth, reduction in biomass was also caused by the uptake of Cd and Ni in the plant body which creates nutrient imbalance. The suppression in photosynthetic characteristics, i.e., decreased photosynthesis, gas exchange, and transpiration as recorded in the present study, emerges as a main reason for decline in growth and other associated characteristics. This fact is advocated by different studies conducted by using different plants (Yadav 2010). Khalid et al. (2017a) had presented the resembling reason, i.e., HM uptake and accumulation in plant body, as a major reason to lower the plant biomass and overall yield quality. The rate of photosynthesis is directly proportional to the concentration of chlorophyll. Actually, inhibition of chlorophyll or its degradation in plants can reportedly be held responsible for the change in photosynthesis and growth ultimately by HMs (Khalid et al. 2017a; Sandalio et al. 2001). We are of the opinion that sensitivity of photosynthesis and chlorophyll to varying metal concentrations can be coupled to the functional interruption in organelles hosting process of photosynthesis. Here, we propose similar sensitivity of enzymes taking part in fixing carbon. Such collective events lead to productivity losses (De Filippis and Ziegler 1993) as observed in this study.
Deficiency or imbalance of certain essential ions under adverse conditions in plants can lead to chlorosis (Mengel et al. 2001). V2 showed significantly higher foliar chlorosis, which might be due to less tolerance to Cd and Ni toxicity showed by this variety compared to its counterpart V1. Under our experimental conditions, the increased chlorosis in both varieties in response to metal treatments is one of the reasons that hampered photosynthesis. Such decrease in photosynthetic activity can be due to the reduction in chlorophyll content caused by the Cd or Ni treatment. The kinetics of metal adsorption/absorption is directly linked with concentration in growth media for plants. The discrepancy in response to metals is being best explained by the presented metal tolerance index and survival.
According to Khalid et al. (2018), reduction in photosynthetic rate is a definite consequence of HM toxicity. In fact, our results for both Vigna varieties are in the same order of magnitude as that assessed from data obtained by other workers where they have found reduced photosynthetic activity in soybean (Li et al. 2013), Brassica (Shah et al. 2011), maize (Figlioli et al. 2019), wheat (Ci et al. 2010), spinach, and fenugreek (Younis et al. 2015) under metal stress. More reduction in sub-stomatal conductance in V2 under T5 and T6 is a reason for disturbance in photosynthesis under combined HM treatments. Such declined sub-stomatal conductance means reduced intake of CO2 and thus limiting the photosynthesis. Cd stimulates the stomata to close (Sandalio et al. 2001). On the other hand, the stomatal closure helps plant to conserve water under stress conditions in order to reduce the transpiration rate and to enhance WUE. The high WUE and tolerance index among plants experiencing combination of Cd and Ni treatments reflects survival strategy of these plants (Tables 3, 4). This is simply attestation of impairments in plant attributes and tackling scheme. Nonetheless, the effects of collective Cd and Ni on transpiration rate are evident and directly related with other findings of this study. It seemingly depends upon plant species, type of metal, its concentration, and treatment period (Greger and Johansson 1992; Irshad et al. 2020; Rucińska-Sobkowiak 2016; Schlegel et al. 1987). As metal toxicity is associated with reduced root growth thus lowering water uptake through roots, reduced K ions in the plant as a result of metal treatments could have also caused the closure of stomata leading to reduced gas exchange and photosynthesis.
The physiological reactions of plants to HM stress include synthesis as well as maintenance of compatible solutes and ROS scavenging. Normally, during HM, the soluble proteins and starch content inside the plant cells are degraded with a consequent increase in soluble sugars and free amino acids (Ashraf et al. 2010; Khalid et al. 2017a). We found significantly greater concentrations of soluble sugars in V2 among different treatment groups as compared to V1. Inverse relationships between total soluble proteins and total soluble sugars have also been witnessed in this study. The reason for this increase in soluble sugars is based upon the degeneration of enzymes involved in starch-sugar conversions (Amirjani 2012). As stated earlier, the decreased biomass in stress conditions might suppress cell expansion and growth because of low turgor pressure. This maintenance of osmotic and other functions is performed by various organic substances inside the cells, and the reduction in their concentrations hampers plant growth (Osman et al. 2004). Although the combined treatment of Cd and Ni more adversely affected total soluble sugars and protein, Cd alone also affected total soluble proteins more negatively. In addition, individual application of Cd caused more reduction in yield as compared to that of Ni but their combined application, i.e., T5, proved more lethal for yield parameters. This is directly linked to reduced rate of photo-assimilation triggered by Cd and Ni together. Hence, it is evident that combination of both HMs even in different doses proved more hazardous for plant growth and productivity than presence of single metal in soil. It was observed that both Cd and Ni did not interfere with translocation of each other. V2 showed more sensitivity than V1 and translocation of Ni in combination with Cd was a bit more in V2. Cd translocation was also high in V2 as compared to V2 when applied alone too.
Disproportionate experience of plants to HMs decreases macro- and micronutrient concentration in plant tissues. We also noted a reduction in the concentration of other essential ions such as Mg, Fe, and P both in the roots and shoots. These are crucial ions for the plant metabolic activities (Khalid et al. 2017b). Ni displays features analogous to Mg2+, Fe2+, and Zn2+and possess the same ionic charge (Kazemi et al. 2010). Low concentrations of Fe2+ as well as Mg2+ in shoots curtail synthesis of chlorophyll pigments and ultimately results in chlorosis under Ni stress. Such reduction in the chlorophyll content is one of the main reasons for the declined dry mass (Kotapati et al. 2017). It is on record that Cd or Ni treatments caused a decline in essential ions like K, Ca, and Mg levels in rice (Irshad et al. 2020; Rubio et al. 1994). Interestingly, such decline in nutrient level was proportionately higher than growth depression in rice plants. Therefore, it is evident that both Cd and Ni interfere with uptake of nutrients and their distribution also in different parts of plants. Rubio and co-workers (1994) have stated that such Cd/Ni-based interference in nutrient homeostasis as a reason inhibited xylem transport due to low transpiration. It is pertinent to mention that we have also recorded disturbance in transpiration rate coupled with altered nutrient balance. Supporting our stance, for instance, Mg is directly involved in the formation of chlorophyll; thus, its reduced quantity could have affected photosynthesis. In addition to restricting the uptake of essential nutrient ions, heavy metals also inhibit the transport of essential nutrients by altering the structure of plasma membranes (Shamsi et al. 2007). Metal toxicity seemed to be more harmful and adversely affecting the essential ions in V2 compared to V1 under different HM treatment levels. This also shows the less adaptive/non-tolerable nature of V2 in this study. We saw a significantly higher concentration of Cl in all the treatments compared to control in both varieties (Fig. 2). This might have been due to the application of chloride salts of Cd and Ni which we used for making different treatments. Cd and Ni concentrations were substantially higher in the metal treatment groups. The concentration of Cd was greater in roots than in shoots; however, Ni concentration was higher in shoots than in roots (Figs. 2, 4). Previous studies have also reported the higher accumulation of Cd in the roots than the aboveground plant parts in mung bean which could be attributed to localization of Cd in the plant body (Wahid and Ghani 2008). V2 accumulated high Cd and Ni in roots and shoots compared to V1.
Conclusion
The results reveal that the sensitive variety, i.e., V2, showed reduced growth and metabolic attributes caused by its inability to control the uptake of heavy metals and nutrient imbalance. Both of these metals significantly decreased the ability of mung bean plants to grow successfully in metal-contaminated rhizosphere. Cadmium showed more toxic effect than nickel and both the metals caused great damage to mung bean at their higher concentration levels. Therefore, the relatively low physiological and biochemical responses in V2 synchronous with the decrease in other attributes reveal that V1 is more tolerant to Cd and Ni. On the other hand, V1 accumulated less Cd and Ni and performed better than V2 as it is evident from its photosynthetic rate, status of essential nutrient ions, and yield, and can be used in the future for obtaining optimum yield under different metal stress conditions. Cd/Ni-based differential loss of metabolites in both varieties and their relative responses reveal that such metabolites have crucial significance for their survival strategy under HM stress. Thus, further studies are required to explicit the mechanism of damages caused by both of these metals separately or in combination and the elucidation of the mechanism of target enzymes or metabolites in plants.
Data availability
Not applicable
Change history
23 February 2021
A Correction to this paper has been published: https://doi.org/10.1007/s11356-021-13093-4
References
Ahmad MS, Ashraf M, Hussain M (2011) Phytotoxic effects of nickel on yield and concentration of macro- and micro-nutrients in sunflower (Helianthus annuus L.) achenes. J Hazard Mater 185:1295–1303. https://doi.org/10.1016/j.jhazmat.2010.10.045
Ahmad MSA, Hussain M, Ashraf M, Ahmad R, Ashraf MY (2009) Effect of nickel on seed germinability of some elite sunflower (Helianthus annuus L.) cultivars. Pak J Bot 41:1871–1882
Amirjani M (2012) Effects of cadmium on wheat growth and some physiological factors. Int J For Soil Eros 2:50–58
Ashraf MY, Ashraf M, Arshad M (2010) Major nutrients supply in legume crops under stress environments. Climate change and management of cool season grain legume crops. Springer, In, pp 155–169
Ashraf MY, Roohi M, Iqbal Z, Ashraf M, Öztürk M, Gücel S (2016) Cadmium (Cd) and lead (Pb) induced changes in growth, some biochemical attributes, and mineral accumulation in two cultivars of mungbean [Vigna radiata (L.) Wilczek]. Commun Soil Sci Plant Anal 47:405–413
Ashraf MY, Sadiq R, Hussain M, Ashraf M, Ahmad MSA (2011) Toxic effect of nickel (Ni) on growth and metabolism in germinating seeds of sunflower (Helianthus annuus L.). Biol Trace Elem Res 143:1695–1703
Bahmani R, Bihamta M, Habibi D, Forozesh P, Ahmadvand S (2012) Effect of cadmium chloride on growth parameters of different bean genotypes (Phaseolus vulgaris L.). ARPN J Agric Biol Sci 7:35–40
Bartholomae E, Incollingo A, Vizcaino M, Wharton C, Johnston CS (2019) Mungbean protein supplement improves muscular strength in healthy, underactive vegetarian adults. Nutrients 11:2423
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chen Q, Zhang X, Liu Y, Wei J, Shen W, Shen Z, Cui J (2017) Hemin-mediated alleviation of zinc, lead and chromium toxicity is associated with elevated photosynthesis, antioxidative capacity; suppressed metal uptake and oxidative stress in rice seedlings. Plant Growth Regul 81:253–264
Ci D, Jiang D, Wollenweber B, Dai T, Jing Q, Cao W (2010) Cadmium stress in wheat seedlings: growth, cadmium accumulation and photosynthesis. Acta Physiol Plant 32:365–373
Cwielag-Drabek M, Piekut A, Gut K, Grabowski M (2020) Risk of cadmium, lead and zinc exposure from consumption of vegetables produced in areas with mining and smelting past. Sci Rep 10:1–9
De Filippis L, Ziegler H (1993) Effect of sublethal concentrations of zinc, cadmium and mercury on the photosynthetic carbon reduction cycle of Euglena. J Plant Physiol 142:167–172
Dong J, Wu FB, Zhang GP (2005) Effect of cadmium on growth and photosynthesis of tomato seedlings. J Zhejiang Univ Sci B 6:974–980. https://doi.org/10.1631/jzus.2005.B0974
Figlioli F, Sorrentino MC, Memoli V, Arena C, Maisto G, Giordano S, Capozzi F, Spagnuolo V (2019) Overall plant responses to cd and Pb metal stress in maize: growth pattern, ultrastructure, and photosynthetic activity. Environ Sci Pollut Res 26:1781–1790. https://doi.org/10.1007/s11356-018-3743-y
Greger M, Johansson M (1992) Cadmium effects on leaf transpiration of sugar beet (Beta vulgaris). Physiol Plant 86:465–473
Hamilaton P, Van Slyke D (1943) Amino acids determination with ninhydrin. J Biol Chem 150:223–231
Hou D et al (2019) Mungbean (Vigna radiata L.): bioactive polyphenols, polysaccharides, peptides, and health benefits. Nutrients 11:1238
Ilyas N, Ambreen F, Batool N, Arshad M, Mazhar R, Bibi F, Saeed M (2018) Contribution of nitrogen fixed by mungbean to the following wheat crop. Commun Soil Sci Plant Anal 49:148–158
Irshad MK, Chen C, Noman A, Ibrahim M, Adeel M, Shang J (2020) Goethite-modified biochar restricts the mobility and transfer of cadmium in soil-rice system. Chemosphere 242:125152
Jackson ML (1962) Soil chemical analysis vol 497. Constable and Co Ltd., London
Kazemi N, Khavari-Nejad RA, Fahimi H, Saadatmand S, Nejad-Sattari T (2010) Effects of exogenous salicylic acid and nitric oxide on lipid peroxidation and antioxidant enzyme activities in leaves of Brassica napus L. under nickel stress. Sci Hortic 126:402–407
Khalid N, Aqeel M, Noman A (2019a) System biology of metal tolerance in plants: An integrated view of genomics, Transcriptomics, metabolomics, and Phenomics. Plant Metallomics and Functional Omics, In, pp 107–144. https://doi.org/10.1007/978-3-030-19103-0_6
Khalid N, Hussain M, Hameed M, Ahmad R (2017a) Physiological, biochemical and defense system responses of Parthenium hysterophorus to vehicular exhaust pollution. Pak J Bot 49:67–75
Khalid N, Masood A, Noman A, Aqeel M, Qasim M (2019b) Study of the responses of two biomonitor plant species (Datura alba & Ricinus communis) to roadside air pollution. Chemosphere 235:832–841. https://doi.org/10.1016/j.chemosphere.2019.06.143
Khalid N, Noman A, Aqeel M, Hadayat N, Anjum S (2017b) NPK could alleviate the adverse effects of simulated acid rain in sunflower (Helianthus annuus L.). J Plant Nutr 41:584–595. https://doi.org/10.1080/01904167.2017.1406108
Khalid N, Noman A, Sanaullah T, Akram MA, Aqeel M (2018) Vehicle pollution toxicity induced changes in physiology, defence system and biochemical characteristics of Calotropis procera L. Chem Ecol 34:565–581. https://doi.org/10.1080/02757540.2018.1452917
Kotapati KV, Palaka BK, Ampasala DR (2017) Alleviation of nickel toxicity in finger millet (Eleusine coracana L.) germinating seedlings by exogenous application of salicylic acid and nitric oxide. Crop J 5:240–250. https://doi.org/10.1016/j.cj.2016.09.002
Li Q, Lu Y, Shi Y, Wang T, Ni K, Xu L, Liu S, Wang L, Xiong Q, Giesy JP (2013) Combined effects of cadmium and fluoranthene on germination, growth and photosynthesis of soybean seedlings. J Environ Sci 25:1936–1946
Mengel K, Kirkby EA, Kosegarten H, Appel T (2001) Nitrogen. In: Principles of plant nutrition. Kluwer Academic Publishers, Springer, Dordrecht, pp 397–434. https://doi.org/10.1007/978-94-010-1009-2_7
Noman A, Aqeel M (2017) miRNA-based heavy metal homeostasis and plant growth. Environ Sci Pollut Res 24:10068–10082. https://doi.org/10.1007/s11356-017-8593-5
Noman A, Fahad S, Aqeel M, Ali U, Amanullah, Anwar S, Baloch SK, Zainab M (2017) miRNAs: major modulators for crop growth and development under abiotic stresses. Biotechnol Lett 39:685–700. https://doi.org/10.1007/s10529-017-2302-9
Noman A, Sanaullah T, Khalid N, Islam W, Khan S, Irshad MK, Aqeel M (2019) Crosstalk between plant miRNA and heavy metal toxicity. Plant Metallomics and Functional Omics, In, pp 145–168. https://doi.org/10.1007/978-3-030-19103-0_7
Osman ME, El-Naggar AH, El-Sheekh MM, El-Mazally EE (2004) Differential effects of Co2+ and Ni2+ on protein metabolism in Scenedesmus obliquus and Nitzschia perminuta. Environ Toxicol Pharmacol 16:169–178
Rai PK, Lee SS, Zhang M, Tsang YF, Kim K-H (2019) Heavy metals in food crops: health risks, fate, mechanisms, and management. Environ Int 125:365–385
Riazi A, Matsuda K, Arslan A (1985) Water-stress induced changes in concentrations of proline and other solutes in growing regions of young barley leaves. J Exp Bot 36:1716–1725
Rizwan M, Ali S, Rizvi H, Rinklebe J, Tsang DCW, Meers E, Ok YS, Ishaque W (2016) Phytomanagement of heavy metals in contaminated soils using sunflower: a review. Crit Rev Environ Sci Technol 46:1498–1528
Rubio M, Escrig I, Martinez-Cortina C, Lopez-Benet F, Sanz A (1994) Cadmium and nickel accumulation in rice plants. Effects on mineral nutrition and possible interactions of abscisic and gibberellic acids. Plant Growth Regul 14:151–157
Rucińska-Sobkowiak R (2016) Water relations in plants subjected to heavy metal stresses. Acta Physiol Plant 38:257
Sandalio L, Dalurzo H, Gomez M, Romero-Puertas M, Del Rio L (2001) Cadmium-induced changes in the growth and oxidative metabolism of pea plants. J Exp Bot 52:2115–2126
Schlegel H, Godbold DL, Hüttermann A (1987) Whole plant aspects of heavy metal induced changes in CO2, uptake and water relations of spruce (Picea abies) seedlings. Physiol Plant 69:265–270
Shah SS, Mohammad F, Shafi M, Bakht J, Zhou W (2011) Effects of cadmium and salinity on growth and photosynthesis parameters of Brassica species. Pak J Bot 43:333–340
Shamsi IH, Wei K, Jilani G, G-p Z (2007) Interactions of cadmium and aluminum toxicity in their effect on growth and physiological parameters in soybean. J Zhejiang Univ Sci B 8:181–188
Sreekanth T, Nagajyothi P, Lee K, Prasad T (2013) Occurrence, physiological responses and toxicity of nickel in plants. Int J Envrion Sci Technol 10:1129–1140
Sundari T (2009) Morphological and physiological characteristics of shading tolerant and sensitive mungbean genotypes. HAYATI J Biosci 16:127–134
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Heavy metal toxicity and the environment. Molecular, clinical and environmental toxicology. Springer, In, pp 133–164
Wahid A, Ghani A (2008) Varietal differences in mungbean (Vigna radiata) for growth, yield, toxicity symptoms and cadmium accumulation. Ann Appl Biol 152:59–69. https://doi.org/10.1111/j.1744-7348.2007.00192.x
Wahid A, Ghani A, Javed F (2008) Effect of cadmium on photosynthesis, nutrition and growth of mungbean. Agron Sunstain Develop 28:273–280. https://doi.org/10.1051/agro:2008010
Wolf B (1982) A comprehensive system of leaf analyses and its use for diagnosing crop nutrient status. Commun Soil Sci Plant Anal 13:1035–1059
Yadav S (2010) Heavy metals toxicity in plants: an overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S Afr J Bot 76:167–179
Yi-Shen Z, Shuai S, Fitzgerald R (2018) Mungbean proteins and peptides: nutritional, functional and bioactive properties. Food. Nutr Res 62:1290. https://doi.org/10.29219/fnr.v62.1290
Younis U, Qayyum MF, Shah MHR, Danish S, Shahzad AN, Malik SA, Mahmood S (2015) Growth, survival, and heavy metal (cd and Ni) uptake of spinach (Spinacia oleracea) and fenugreek (Trigonella corniculata) in a biochar-amended sewage-irrigated contaminated soil. J Plant Nutr Soil Sci 178:209–217
Zhang H, Zhang LL, Li J, Chen M, An RD (2020) Comparative study on the bioaccumulation of lead, cadmium and nickel and their toxic effects on the growth and enzyme defence strategies of a heavy metal accumulator, Hydrilla verticillata (L.f.) Royle. Environ Sci Pollut Res 27:9853–9865. https://doi.org/10.1007/s11356-019-06968-0
Funding
The Deanship of Scientific Research, King Khalid University, funded this work through research groups program under grant number R.G.P. 2/101/41.
Author information
Authors and Affiliations
Contributions
MA, AN: conceived the idea, designed the experiment, and drafted the manuscript; MA, NK, MH, AN: writing, review, editing; AT, RZA, ML, MKI: performed experiment, gathered literature; MSA, MTJ, SA: analyzed the data, and helps in interpretation of results; SA, MH, AN: critically revised the manuscript; all authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Gangrong Shi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aqeel, M., Khalid, N., Tufail, A. et al. Elucidating the distinct interactive impact of cadmium and nickel on growth, photosynthesis, metal-homeostasis, and yield responses of mung bean (Vigna radiata L.) varieties. Environ Sci Pollut Res 28, 27376–27390 (2021). https://doi.org/10.1007/s11356-021-12579-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-12579-5