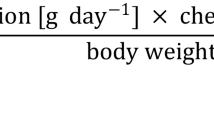Abstract
Polychlorinated dibenzo-p-dioxins and dibenzofurans (PCDD/Fs) and dioxin-like polychlorinated biphenyls (DL-PCBs) in fish samples have been investigated in many previous studies. However, the available data regarding this issue in low- and middle-income countries are very scarce, and no study has been performed in Algeria yet. Therefore, this work aimed to determine the levels of PCDD/Fs and DL-PCBs in swordfish (Xiphias gladius) caught in three regions of the Algerian coasts, and the health risk associated with these contaminants was assessed. The PCDD/Fs and DL-PCBs were analyzed in 37 fish samples from Algiers, Bejaia, and Oran using the DR-CALUX bioassay method. The overall mean (and standard deviation) of PCDD/Fs and DL-PCBs concentrations in swordfish from Algiers, Bejaia, and Oran were 0.19 (0.13), 0.27 (0.24), and 0.08 (0.05) pg TEQ g−1 wet weight (ww), respectively, which were lower than the national and European regulatory limits. Daily and weekly intake values of PCDD/F and DL-PCB were higher for the samples from Bejaia (0.45 and 3.15 pg TEQ kg−1 body weight (bw) per day and week, respectively) and for the samples from Algiers (0.31 and 2.17 pg TEQ kg−1 bw per day and week, respectively), which were higher than the tolerable weekly intake of 2 pg TEQ kg−1 bw per week recently established by the European Food Safety Authority. Overall, our findings indicate that swordfish consumption poses a risk to the Algerian consumer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fish is an integral part of a balanced diet with a high nutritional value, which production and consumption has increased in recent years (JECFA 2010; FAO 2020). It is rich in proteins of high biological value; vitamins A and D; essential minerals such as selenium, phosphorus, zinc, and iodine; and especially containing of polyunsaturated fatty acids such as omega 3 which have many beneficial health effects, e.g., anti-inflammatory, antioxidant, anticancer, neuroprotective, and antiarrhythmic effects (JECFA 2010; Li et al. 2020). Most European Food-Based Dietary Guidelines recommend a minimum of two servings of fish per week (EFSA 2014). However, fish can also be contaminated by various environmental pollutants (i.e., persistent organic pollutants (POPs)) which can increase the risk of different diseases (Li et al. 2020).
Dioxins are an important group of organic pollutants containing 75 polychlorinated dibenzo-p-dioxins (PCDDs) and 135 polychlorinated dibenzofuran (PCDF) congeners, of which 17 exhibit toxicity (Ssebugere et al. 2019). Polychlorinated biphenyls (PCBs) are a group of 209 congeners which 12 are similar to PCDD/Fs in toxicological characteristics and are referred as dioxin-like PCBs (DL-PCBs) (Hoogenboom et al. 2020; Ssebugere et al. 2019). These contaminants have a long half-life in the environment where they persist due to their high lipophilicity. Their main emission sources in the environment are the various human activities, although small quantities of these substances may be generated by natural processes, including forest fires. The main emissions are coming from municipal solid waste incinerators and various industrial processes, resulting in the deposition of these POPs in seawater and in the contamination of fish where they are found mostly in the fatty tissue and constitute the primary source of human exposure (Kanan and Samara 2018; Vuorinen et al. 2017).
PCDD/Fs and DL-PCBs have been classified by the International Agency for Research on Cancer as carcinogenic to humans (IARC 1997) and may also cause immunosuppression and behavioral changes and increase the risk of cardiovascular disease in humans and animals (Cui et al. 2018). An emerging body of evidence measured these pollutants in different water bodies worldwide (Castro-Jiménez et al. 2011; Howell et al. 2008; Kalantzi et al. 2017). However, the available data for low- and middle-income countries is very scarce yet, and we are not aware of any study on the investigation of PCDD/Fs and DL-PCBs in the fish of the Algerian sea and other seas of Africa. However, it is important to carry out studies on persistent organic pollutants in fish caught in Algerian waters since they are close to large urban areas possibly highly polluted. Therefore, this study aimed to measure the concentrations of the total PCDD/Fs and DL-PCBs in the flesh of swordfish (Xiphias gladius) fished in the littoral Algerian and compare the results obtained with the Algerian and European regulatory thresholds. Moreover, the health risk of consumption of this fish was investigated.
Materials and methods
Sampling and preparation of samples
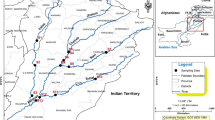
A total of 37 samples of swordfish (Xiphias gladius) (n = 37) were randomly sampled in three market stalls in Algeria. Fifteen samples were collected in Algiers (North Centre, NC), 12 samples in Bejaia (North East, NE), and 10 samples in Oran (North West, NW) (Fig. 1). Sampling was conducted over a 6-month period from April to September 2017. The frequency of sampling was not regular, and was depending on the availability of fish in the market stalls. The samples were transferred directly to the laboratory for preparation.
Once in the laboratory, the fish has been prepared, of which only the flesh has been kept for analysis; the swordfish skin was removed. The swordfish flesh matrix has been crushed by a grinder (Retsch Grindomix 200, Germany), labeled, and packaged in polyethylene bags and stored at − 20 °C until analysis.
Fat extraction
In order to have 2 g of fat, 40.8 g of swordfish meat was weighed with a scale (METTLER AE 200, Germany) and placed in a 250-mL glass flask (SCHOTT DURAN, Germany). Fat was extracted from fish samples (13 to 40 g, according to the amount available) using a shaken solvent extraction method, as follows: 30 mL of water (HiPerSolv CHROMANORM, VWR, Leuven, Belgium) and 30 mL of isopropanol (Sigma-Aldrich, Germany) were added to the flask containing the fish flesh and shaken in a rotary shaker (Heidolph REAX2, Germany) for 10 min. Then, 60 mL of extraction solvent n-hexane/diethyl ether (97:3, v/v) (Merck, Darmstadt, Germany) was added and the flasks were shaken for 1 h. After sedimentation, the supernatant was transferred to a plugged-in Erlenmeyer flask (SCHOTT DURAN, Germany) using an automatic pipette (Piptus®-akku, Germany). The operation of adding the extraction solvent and stirring (for 30 min) was repeated twice. Five grams of anhydrous sodium sulfate (Fluka, Germany) was added to the extract. The extract was then filtered through a pleated filter paper and the solvent was evaporated using a rotavapor (LABOROTA 4000, Germany), at 35 °C and 90 rpm until its volume reached 2 mL. The fat content of the samples varied from 0.2 to 9%.
Purification
The PCDD/F and DL-PCBs were separated from the fat using a multilayer silica column containing 20 g of 33% acid silica, 20 g of acid silica 20%, and 1 g of anhydrous sodium sulfate (Fluka, Germany). The columns were conditioned with 55 mL of the n-hexane/diethyl ether (97:3, v/v) mixture before loading the extracted fat. PCDD/Fs and DL-PCBS were eluted using 125 mL n-hexane/diethyl ether (97:3, v/v). The eluates were evaporated using a TurboVap II (Zymark®, Germany) and then transferred into conical bottom tubes before continuing evaporation under nitrogen flow up to a volume of 50 μL. Twenty-five microliters of dimethyl sulfoxide (DMSO) (Acros Organics, Belgium) was then added and the remaining hexane was evaporated under nitrogen.
Analysis by the Dioxin Responsive Chemical-Activated LUciferase gene eXpression (DR-CALUX) bioassays
The DR-CALUX® was developed by Wageningen University (Aarts 1993) and is distributed by BioDetection System (BDS, NL). This assay involves the rat hepatoma H4IIE cell line stably transformed with an AhR-controlled luciferase reporter gene construct.
The cells were cultivated in MEMα (Thermo Fisher Scientific, Massachusetts, USA) completed with 10% v/v fetal bovine serum (Greiner, Kremsmünster, Austria), 50 IU/mL penicillin and 50 mg/mL streptomycin (Sigma-Aldrich, Missouri, USA), and incubated in a H20-saturated atmosphere with 5% CO2, at 37 °C. The DR-CALUX (Dioxin Responsive Chemical-Activated LUciferase gene eXpression) bioassay methodology was detailed elsewhere (Scippo et al. 2004; Doan et al. 2019). Shortly, cells were initially inoculated in white clear-bottomed 96-well microplates (Greiner, Kremsmünster, Austria) and incubated for 24 h to reach about 90% of confluence in the well. The cells were exposed for 24 h, in triplicates, to either the sample extracts or to standard solutions of tetrachlorodibenzo-p-dioxin (TCCD) diluted in αMEM (Biowhittker®, Belgium), with a final concentration of 0.4% DMSO.
The cells were washed with a phosphate-buffered saline solution (Sigma-Aldrich, Missouri, USA) after 24 h of exposure, then treated with a lysis solution (with Triton X100, Sigma-Aldrich, Missouri, USA). Finally, luciferin (Promega, Wisconsin, USA) and ATP (Roche Diagnostics, Rotkreuz, Switzerland) were added to the cell lysate to obtain luminescence, which was determined with a luminometer (ORION II, Berthold Detection System, Pforzheim, Germany). Concentrations, expressed as TCDD TEQ, were measured from a TCDD standard calibration curve, ranging from 0 (blank DMS0) to 20 pg per well.
Quality controls
For quality control of the method (QC), we used a procedure blank (complete procedure under the same conditions without sample) and “in-house” QC samples consisting of beef fat fortified with the 17 PCDD/F and the 12 DL-PCBs to have a content of 2 pg TEQ g−1 fat. The analysis of procedure blanks and reference samples (QC) were performed in duplicate.
The limit of quantification of this assay is typically 0.06 pg TCDD/well, corresponding roughly to 0.05 pg TEQ g−1 wet weight (ww) of fish, according to the weighted samples and their fat content.
Statistical study
Mean concentrations, standard deviation, and minimum and maximum of values were measured using Microsoft Excel® (2010). The statistical study was performed using R software (version 3.6.0). The distribution of data was tested using the Shapiro-Wilk normality test. For the comparison of the difference in concentrations of the total PCCD/Fs and DL-PCBs in different studied areas, the test of Kruskal-Wallis was utilized, and the pairwise Wilcoxon test was used to determine which of these areas have a significant difference. A significant level of 0.05 was used for all analyses.
Health risk assessment
The exposure risk assessment for the adult population was carried out by estimating dietary intake (EDI) as follows (Rauscher-Gabernig et al. 2013):
where EDI is the dietary intake of PCDD/Fs and DL-PCBs in pg TEQ kg− 1 bw day−1 week−1, C is the mean concentration of PCCD/Fs and DL-PCBs in fish flesh (pg TEQ g−1 ww), and O is the fish portion per day in grams. Since there is data about this fish species consumption in Algeria, it was decided to use a 100-g portion. W is the mean body weight (kg) which was set to 60 kg for an Algerian adult (MFFR 2018).
Results and discussion
Concentrations of PCCD/Fs and DL-PCBs
Descriptive statistics of measured PCCD/Fs and DL-PCBs are presented in Fig. 2. The lowest minimum value of PCCD/Fs and DL-PCBs was measured in the swordfish of Bejaia and Oran with similar values of 0.03 pg TEQ g−1 ww. The highest maximum value was observed in the swordfish of Bejaia (0.72 pg TEQ g−1 ww). This can be explained by the heavy pollution of the sea in this area due to the discharge of the urban waste from the commercial port and industrial areas, as well as from the rivers. The lowest mean value was recorded in the swordfish of Oran (0.08 pg TEQ g−1 ww) and the highest mean value was measured in the swordfish of Bejaia (0.27 pg TEQ g−1 ww). The concentrations of sum PCDD/Fs and DL-PCBs studied in the swordfish captured in the Algerian sea were lower than the threshold level of Algerian and European regulations (i.e., 8 and 6.5 pg TEQ g−1 ww respectively) (OJPDRA 2011; EU 2011). The mean concentrations of PCDD/F and DL-PCB obtained in the flesh of swordfish (Xiphias gladius) in the present study were lower than those reported by Perelló et al. (2015) and Bocio et al. (2007) (0.71 and 0.48 pg TEQ g−1 ww respectively) in Spain and by Diletti et al. (2018) (1.84 pg TEQ g−1 ww) in Italy.
The Kruskal-Wallis test showed a significant difference in the PCCD/Fs and DL-PCBs values between different sampling areas (P value = 0.006). The results of pairwise Wilcoxon test indicated a significant difference between the PCCD/Fs and DL-PCBs values in the Oran swordfish and those of Algiers (P value = 0.016), as well as Oran and Bejaia (P value = 0.037). However, no significant difference was observed between the PCCD/Fs and DL-PCBs values in Algiers and Bejaia (P value = 0.904).
Previous studies indicated that the concentration of PCCD/Fs and DL-PCBs in fish samples were significantly depended on the concentration of these pollutants in the marine waters where they were caught (Domingo et al. 2007). Other physiological and biological factors of the fish species, e.g., age, size, and reproductive status, also could influence the concentration of PCCD/Fs and DL-PCBs in fish (Mezzetta et al. 2011). Moreover, these pollutants are lipophilic; therefore, higher lipid level in fish could lead to higher accumulation of these pollutants in the fish (Corsolini et al. 2005). The trophic position of fish species could also influence the level of these pollutants. It has been reported that fish with the high trophic position tend to accumulate more PCCD/Fs and DL-PCBs due to biomagnification. Furthermore, metabolic activity has been reported as another important factor in the bioaccumulation of pollutants in fish (Corsolini et al. 2005). Another important factor in concentrations of PCCD/Fs and DL-PCBs variation was the method of analysis. Vromman et al. (2012) obtained two different means concentrations in fish from Belgium after analysis with two analytical methods: GC-HRMS and CALUX; the mean concentration of 4.62 pg TEQ g−1 ww was reported in the case of analysis by the CALUX method, which was higher than that reported in the case of analysis by the GC-HRMS method (2.93 pg TEQ g−1 ww). This discrepancy can come from the difference in WHO-TEF and CALUX response potencies (REP) of some of the 29 congeners, in particular the DL-PCBs (Scippo et al. 2004).
Health risk assessment
Table 1 presents the results of estimated dietary intake (pg TEQ kg−1 bw day−1 week−1) for the intake of PCCD/Fs and DL-PCBs due to consuming swordfish. Results indicated that the highest daily and weekly intake was observed for Bejaia samples (0.45 and 3.15 pg TEQ kg−1 bw day−1 week−1, respectively). Moreover, the lowest daily and weekly intake was observed for Oran (0.13 and 0.91 pg TEQ kg−1 bw day−1 week−1, respectively).
The dietary intakes (Table 1) estimated in the region of Bejaia and Algiers were higher than the tolerable weekly intake (2 pg TEQ kg−1 bw week−1) established by the European Food Safety Authority (EFSA 2018) but lower than the tolerable daily intake fixed earlier by the World Health Organization (1–4 pg TEQ kg−1 bw day−1) (Anonymous 2000). The dietary intakes estimated in Oran do not exceed these tolerable values.
The values of daily intakes estimated through the consumption of swordfish in Algeria (Table 1) were higher than that reported by Bocio et al. (2007) and Perelló et al. (2015) (0.03 and 0.04 pg WHO-TEQ day−1, respectively) in Spain. These values are not only influenced by the concentrations of total PCCD/fs and DL-PCBs but also by the amount of fish consumed (Corsolini et al. 2005).
This study had some limitations that should be considered in future researches. Due to limitations in our budget, the number of samples in our study was too small. Moreover, we did not sample swordfish according to biometers of the fishes, which may be the effect of results. Also, we did not investigate the concentrations of PCCD/Fs and DL-PCBs in the water body of studied areas. Finally, seasonal variation in the concentrations of PCCD/Fs and DL-PCBs was not assessed in this study.
Conclusion
In this study, the concentrations of PCCD/Fs and DL-PCBs in swordfish from the Algerian coastline were determined based on the bioassay technique. The concentrations of these pollutants in the studied areas were lower than threshold levels recommended by the Algerian and European Union. The concentrations of PCCD/Fs and DL-PCBs were significantly different in the study area indicating that fishing areas were important factors in the variation of these pollutants. The dietary intake obtained from swordfish of Bejaia and Algiers exceeded the tolerable weekly intake fixed by the European Food Safety Authority (EFSA 2018) but was lower than the tolerable daily intake proposed by the World Health Organization (Anonymous 2000). However, our findings showed that levels of PCCD/Fs and DL-PCBs in swordfish were not safe levels. Hence, regular controls and permanent monitoring are needed in these areas. Further studies with larger sample numbers in different seasons and different fish species are recommended.
References
Aarts J (1993) Ah receptor-mediated luciferase expression: a tool for monitoring dioxin-like toxicity. Organohalogen Compd 13:361–364 http://dioxin20xx.org/wp-content/uploads/pdfs/1993/93-1.pdf. Accessed 01 Jan 2020
Anonymous (2000) Assessment of the health risk of dioxins: re-evaluation of the tolerable daily intake (TDI). Geneva, Switzerland, 25-29 May 1998. Food Addit Contam Part A 17:223–240
Bocio A, Domingo JL, Falcó G, Llobet JM (2007) Concentrations of PCDD/PCDFs and PCBs in fish and seafood from the Catalan (Spain) market: estimated human intake. Environ Int 33:170–175. https://doi.org/10.1016/j.envint.2006.09.005
Castro-Jiménez J, Mariani G, Vives I, Skejo H, Umlauf G, Zaldívar JM, Dueri S, Messiaen G, Laugier T (2011) Atmospheric concentrations, occurrence and deposition of persistent organic pollutants (POPs) in a Mediterranean coastal site (Etang de Thau, France). Environ Pollut 159:1948–1956. https://doi.org/10.1016/j.envpol.2011.03.012
Corsolini S, Ademollo N, Romeo T, Greco S, Focardi S (2005) Persistent organic pollutants in edible fish: a human and environmental health problem. Microchem J 79:115–123. https://doi.org/10.1016/j.microc.2004.10.006
Cui L, Wang S, Yang X, Gao L, Zheng M, Wang R, Qiao L, Xu C (2018) Fatty acids, polychlorinated dibenzo-p-dioxins and dibenzofurans, and dioxin-like polychlorinated biphenyls in paired muscle and skin from fish from the Bohai coast, China: Benefits and risks associated with fish consumption. Sci Total Environ 639:952–960. https://doi.org/10.1016/j.scitotenv.2018.05.195
Diletti G, Scortichini G, Abete MC, Binato G, Candeloro L, Ceci R, Chessa G, Conte A, Di Sandro A, Esposito M (2018) Intake estimates of dioxins and dioxin-like polychlorobiphenyls in the Italian general population from the 2013-2016 results of official monitoring plans in food. Sci Total Environ 627:11–19. https://doi.org/10.1016/j.scitotenv.2018.01.181
Doan T, Berntsen H, Zimmer K, Verhaegen S, Ropstad E, Connolly L, Igout A, Muller M, Scippo M (2019) A realistic mixture of persistent organic pollutants (POPs) inhibits the transactivation activity of the aryl hydrocarbon receptor (AhR) in vitro. Environ Pollut 254:113098. https://doi.org/10.1016/j.envpol.2019.113098
Domingo JL, Bocio A, Falcó G, Llobet JM (2007) Benefits and risks of fish consumption: Part I. A quantitative analysis of the intake of omega-3 fatty acids and chemical contaminants. Toxicology 230:219–226. https://doi.org/10.1016/j.tox.2006.11.054
European Food Safety Authority (EFSA) (2014) Scientific opinion on health benefits of seafood (fish and shellfish) consumption in relation to health risks associated with exposure to methylmercury. E J 12:3761
European Food Safety Authority (EFSA) (2018) Risk for animal and human health related to the presence of dioxins and dioxin-like PCBs in feed and food. E J 16(11):5333. https://doi.org/10.2903/j.efsa.2018.5333
European Union (EU) (2011) Amending Regulation (EC) No1881/2006 setting maximum levels for certain contaminants in foodstuffs, Commission Regulation No 420/2011 of 29 April 2011, Official Journal of the European Union L 111/3. https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2011:111:0003:0006:EN:PDF. Accessed 2 Jan 2020
Food and Agriculture Organization (FAO) (2020) The State of World Fisheries and Aquaculture 2020. Sustainability in action. Rome. https://doi.org/10.4060/ca9229en. Accessed 15 Aug 2020
Hoogenboom RLAP, Malisch R, Van Leeuwen SPJ, Vanderperren H, Hove H, Fernandes A, Schächtele A, Rose M (2020) Congener patterns of polychlorinated dibenzo-p-dioxins, dibenzofurans and biphenyls as a useful aid to source identification during a contamination incident in the food chain. Sci Total Environ 746:141098. https://doi.org/10.1016/j.scitotenv.2020.141098
Howell NL, Suarez MP, Rifai HS, Koenig L (2008) Concentrations of polychlorinated biphenyls (PCBs) in water, sediment, and aquatic biota in the Houston Ship Channel, Texas. Chemosphere 70:593–606. https://doi.org/10.1016/j.chemosphere.2007.07.031
International Agency for Research on Cancer (IARC) (1997) Polychlorinated dibenzo-para-dioxins and polychlorinated dibenzofurans, IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. IARC Press, France (FR) https://publications.iarc.fr/87. Accessed 19 Jaune 2020
Joint FAO/WHO Expert Committee on Food Additives (JECFA) (2010) Report of the Joint FAO/WHO Expert Consultation on the risks and benefits of fish consumption. FAO Fisheries and Aquaculture Report. No 978. Rome. http://www.fao.org/3/ba0136e/ba0136e00.pdf. Accessed 15 Aug 2020
Kalantzi I, Mylona K, Sofoulaki K, Tsapakis M, Pergantis SA (2017) Arsenic speciation in fish from Greek coastal areas. J Environ Sci 56:300–312. https://doi.org/10.1016/j.jes.2017.03.033
Kanan S, Samara F (2018) Dioxins and furans: a review from chemical and environmental perspectives. Trends Environ Anal Chem 17:1–13. https://doi.org/10.1016/j.teac.2017.12.001
Li N, Wu X, Zhuang W, Xia L, Chen Y, Wu C, Rao Z, Du L, Zhao R, Yi M (2020) Fish consumption and multiple health outcomes: umbrella review. Trends Food Sci Technol 99:273–283. https://doi.org/10.1016/j.tifs.2020.02.033
Mezzetta S, Cirlini M, Ceron P, Tecleanu A, Caligiani A, Palla G, Sansebastiano GE (2011) Concentration of DL-PCBs in fish from market of Parma city (north Italy): estimated human intake. Chemosphere 82:1293–1300. https://doi.org/10.1016/j.chemosphere.2010.12.028
Ministry of Fisheries and Fishery Resources (MFFR) (2018) Statistical data of the consummation of fish in Algeria
Official Journal of the People’s Democratic Republic of Algeria (OJPDRA) (2011) Inter-ministerial Order of 30 Moharram 1432 corresponding to 5 January 2011. Fixing the presence of threshold levels of chemical contaminants, microbiological and toxicological in fishery products and aquaculture. http://extwprlegs1.fao.org/docs/pdf/alg106236.pdf. Accessed 10 Jan 2020
Perelló G, Díaz-Ferrero J, Llobet JM, Castell V, Vicente E, Nadal M, Domingo JL (2015) Human exposure to PCDD/Fs and PCBs through consumption of fish and seafood in Catalonia (Spain): temporal trend. Food Chem Toxicol 81:28–33. https://doi.org/10.1016/j.fct.2015.04.010
Rauscher-Gabernig E, Mischek D, Moche W, Prean M (2013) Dietary intake of dioxins, furans and dioxin-like PCBs in Austria. Food Addit Contam Part A 30:1770–1779. https://doi.org/10.1080/19440049.2013.814169
Scippo M-L, Eppe G, De Pauw E, Maghuin-Rogister G (2004) DR-CALUX® screening of food samples: evaluation of the quantitative approach to measure dioxin, furans and dioxin-like PCBs. Talanta 63:1193–1202. https://doi.org/10.1016/j.talanta.2004.05.037
Ssebugere P, Sillanpää M, Matovu H, Mubiru E (2019) Human and environmental exposure to PCDD/Fs and dioxin-like PCBs in Africa: a review. Chemosphere 223:483–493. https://doi.org/10.1016/j.chemosphere.2019.02.065
Vromman V, Baert K, Vanderperren H, Goeyens L, André Huyghebaert A, Maghuin-Rogister G, Scippo M-L, Moons E, Vinkx C, Saegerman C, Pussemier L (2012) Evaluation of the use of CALUX results for dioxins and dioxin-like PCBs analysis for quantitative human exposure assessments. Food Control 27:314–321. https://doi.org/10.1016/j.foodcont.2011.10.047
Vuorinen PJ, Roots O, Keinänen M (2017) Review of organohalogen toxicants in fish from the Gulf of Finland. J Mar Syst 171:141–150. https://doi.org/10.1016/j.jmarsys.2016.12.002
Acknowledgments
The authors thank Haifa Ben Mesaoud for their help.
Funding
The authors would like to extend their thanks to the Institute of Veterinary Sciences of Blida, having funded the internship.
Author information
Authors and Affiliations
Contributions
Fetta Mehouel: Conceptualization and writing—original draft preparation. Leila Bouayad and Ali Berber: Visualization and methodology. Samiha Boutaleb: Conducted the experiments. Mohammad Miri: revised the original and the final draft, and analyzed the data obtained. Marie-Louise Scippo: Investigation, methodology, and revised the final draft.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mehouel, F., Bouayad, L., Berber, A. et al. Health risk assessment of exposure to polychlorinated dibenzo-p-dioxins/dibenzofurans (PCDD/Fs) and dioxin-like polychlorinated biphenyls (DL-PCBs) in swordfish from the Mediterranean Sea. Environ Sci Pollut Res 28, 26106–26111 (2021). https://doi.org/10.1007/s11356-021-12479-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-12479-8