Abstract
In the present study, a multi-biomarker approach was used to assess the toxicity of the coal mine effluent (CME) generated at the Rajrappa coal mine on the catfish Clarias batrachus. A core of biomarkers indicative of nutritional value, oxidative stress, and histopathology was selected to illustrate the toxic effects of CME-containing different heavy metals and other toxicants. The results of metal bioaccumulation in CME-exposed fish tissues revealed the highest metal concentration in liver (1.34–297.68 mg/kg) while lowest in muscles (1.47–23.26 mg/kg) as compared to other tissues and so was the metallothionein level. The high value of bioaccumulation observed in liver, kidney, and gills reflects their affinity for metals. In addition, the values of metal pollution index (MPI) of different fish tissues further affirmed that liver followed by kidney and gills are at greater risk than brain, skin, and muscles. Significant alterations in the activity of certain enzymes (aspartate amino transferase, alanine amino transferase, alkaline phosphatase) as well as oxidative stress markers (superoxide dismutase, catalase and lipid peroxidation) were detected in the tissues of CME-exposed fish. The tissue-specific metal accumulation and increased metallothionein levels may be associated with the biochemical and physiological activity of an organ and its constitutive antioxidant defenses. The histopathological changes in the various tissues of the CME-exposed fish justify the high metal accumulation and biochemical alterations. Overall results indicate that the Rajrappa coal mine effluent is very toxic having adverse health impact on the fish and might also affect the human health when consumed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coal is commonly called the black gold of India that contributes extensively in the economic growth of the country and is widely used for energy production (Lechner et al. 2016). With increasing energy requirement, the coal mining activities have tremendously increased in India and ranks third among top ten coal producing countries (World Coal Association, 2018). Most of the coal mines in India are situated in the states like Jharkhand, Odisha, West Bengal, Bihar, Chhattisgarh, Telangana, and Madhya Pradesh. Among these states, Jharkhand accounts for about 81.17 billion metric tons coal reserves and are the third (19.08%) largest producer of coal in the country after Chhattisgarh (21.70%) and Odisha (21.03%). The Rajrappa mining complex in the Jharkhand state is one of the major coal mining sites of India. Its mining activities generate huge quantity of effluent which is highly toxic due to presence of high levels of heavy metals, suspended particles and other toxicants (Lakra et al. 2017; Mohanty et al. 2018). Significant discharge of untreated coal mining effluent (CME) into the aquatic environment contributes to a variety of toxic effects on aquatic fauna including fish (Talukdar et al. 2017; Lakra et al. 2019a). This is because fish have the ability to absorb and concentrate water-soluble heavy metals directly from the surrounding water through epithelia, gills and skin (Authman et al. 2015). It is well known that fish is a part of a healthy diet and cheap source of protein, nutrients and essential omega-3 fatty acids (Medeiros et al. 2012). Accumulation of metals in fish tissues beyond safe limits is reported to have serious consequences that have adverse impacts not only on the fish population, but also on human (Laxmi Priya et al. 2011; Rajeshkumar et al. 2018). The catfish Clarias batrachus collected from CME-fed ponds situated in the Rajrappa coal mine areas, had high metal content in their various tissues, and also exhibited serious pathological changes in the histoarchitecture of their vital organs (Lakra et al. 2019b). These changes may be due to CME alone or might be due to complex action of CME and other aspects/elements already present in pond water due to its specific physicochemical properties of pond soil, as well as the water in fluxed as a result of run-off or leaching. Therefore, it is essential to examine the impact of CME on the fish under laboratory conditions before these dysfunctions have lethal consequences on them. The assessment of heavy metal accumulation in different fish tissues is essential not only for human consumption but also for aquatic ecosystem management point of view (Ariyaee et al. 2015; Kumar et al. 2019). The effects of toxicants on fish are multidirectional and are manifested by numerous changes in the physiological and biochemical process of their organ system. Therefore, in the present study a multi-biomarker approach has been applied since it provides an integrated detection of sensitive and early warning biological changes in living organisms after a toxicant exposure. The heavy metal stress causing an array of biochemical, physiological and histological alterations in various tissues of fishes after single or metal mixture exposure is well documented (Velma and Tchounwou 2010). Ta et al. (2018) observed that the concentration of lead and cadmium increases with exposure duration in fish Cyprinus carpio exposed to metal mixture (lead and cadmium) in laboratory conditions. Bhatkar et al. (2004) have reported Pb-induced reduction of carbohydrate, proteins and lipids in Labeo rohita. Javed and Usmani (2015) have reported alteration in serum glucose, lipid and protein levels under the impact of oxidative stress in Channa punctatus exposed to heavy metals. Ratn et al. (2018) have also reported oxidative stress and associated histopathological alterations in various fish species intoxicated with heavy metals. Studies have shown that high metal accumulation in fish impairs health by producing reactive oxygen species (ROS) or free radicals which later cause oxidative stress, cellular and tissue injury (Lushchak 2011; Jerome et al. 2017). Metal accumulation also triggers the antioxidant system of enzymes along with metallothioneins (MT), which is a metal-binding protein that provides protection against the toxic effect of metals (Saad et al. 2016; Swaleh et al. 2019). Hermenean et al. (2015) have also elucidated the antioxidant enzymes superoxide dismutase (SOD) and catalase (CAT) and lipid peroxidase (LPO) mediated defense mechanisms of reactive oxygen species (ROS) neutralization in the fish Leuciscus cephalus following exposure to heavy metals in the Tur River.
However, in literature, studies on the effect of CME comprising mixture of heavy metals on fishes in laboratory conditions are limited. Therefore, toxic effect of CME exposure on fish physiology was evaluated by analyzing different biochemical parameters (glycogen, glucose, lipids, proteins and moisture content), activities of enzymes like aspartate amino transferase (AST), alanine amino transferase (ALT), alkaline phosphatase (ALP), and markers of oxidative stress such as superoxide dismutase (SOD) and catalase (CAT) and lipid peroxidation (LPO) along with concentration of metallothionein (MT). The histopathological alterations in the skin, gills, liver and kidney were also studied simultaneously following CME exposure.
The freshwater air-breathing catfish Clarias batrachus was selected for the study as it is hardy, easily maintained under laboratory conditions, consumer preferred and well cultured catfish in India because of its rapid growth and high market price (Debnath 2011; Kumari et al. 2012; Singh and Lal 2015). The mining site at Rajrappa coal mine was selected because maximum excavation of coal in India takes place from the collieries spread mainly in the Jharkhand state, the biggest mining industry in India entrusted to Coal India (CIL) and its subsidiaries Central Coalfields Limited (CCL) with the production of about 6.1 million ton-per-annum (Lakra et al. 2017, 2019a, b; Mohanty et al. 2018).
Materials and methods
Site of collection of coal mine effluent
The coal mine effluent (CME) was collected from Rajrappa coal mine, one of the opencast coal mine extraction sites on Ramgarh coalfield 23° 37′ 55″ N and 85° 42′ 40″ E, Jharkhand, India (Fig. 1). Rajrappa coal mining project, under the Central coalfields limited (CCL), is one of the biggest coalfields of the region. It stands at the confluence of the Damodar and Bhairavi rivers. The CME samples were collected randomly from the mine field itself in 50 L plastic canes and brought to the laboratory for analyses and other experiments.
Collection of Clarias batrachus
The catfish C. batrachus (n = 150) weighing 95–100 g and 22–23 cm in length were collected from ponds in Varanasi 25° 20′ 0″ N, 83° 0′ 0″ E. The fish were acclimated to the laboratory conditions under ambient photoperiod and temperature (10.5 L:13.5D and water temperature 20.0 °C ± 2.0) for 30 days in ground tap water having pH 7.13 ± 0.03, dissolved oxygen (DO) 6.4 ± 0.11 mg/L, biochemical oxygen demand (BOD) 18.66 ± 2.66 mg/land carbon dioxide (CO2) 4.53 ± 0.53 mg/L. The fish were fed with minced goat liver once a day during acclimation and experimentation period. All experiments were carried in accordance with the guidelines of the Institutional Committee for Animal Ethics and Care of Banaras Hindu University, Varanasi, India (Ref No. F.Sc./IAEC/2016-2017/1137).
Exposure of C. batrachus to coal mine effluent
Acclimated fish (n = 25) were exposed to CME to estimate the days of fish survival. The first death in fish was observed at 22nd day of the exposure, and mortality increased gradually day by day and by the end of 28th day all fish died. Following this, the fish were divided in two groups (n = 25 fish/group) and one group was exposed to CME for 20 days, while the other was maintained in tap water for same period, that served as control. Ten fish from each group were anesthetized and sacrificed at 10th while remaining at 20th day of CME exposure to collect skin, muscles, gills, liver, kidney and brain under aseptic condition.
Analysis of metal accumulation in fish tissues
Metals (Fe, Zn, Cu, Mn, Ni, Cd, Pb, and Cr) contents in skin, muscles, gills, liver, kidney and brain tissues were analyzed following the standard methods of APHA/AWWA/WPCF (2005). In brief, 1 g of each tissue was digested in mixture containing nitric acid and per chloric acid (4:1 v/v). The samples were heated at 120 °C until all the tissues were completely dissolved. After digestion, the samples were diluted with small quantity of triple-distilled water to a final volume of 25 mL and filtered through a Whatman No.1 filter paper in acid-washed funnels. Estimation of the metals was performed on Atomic Absorption Spectrophotometer (AAS) (Perkin-Elmer Model 2380, Inc., Norwalk, CT, USA). The parameters set for analysis on AAS were (operating conditions: flame type, air-acetylene; slit width (nm): 0.2–0.7; acetylene flow (L/min): 2.50; air flow (L/min): 10; wavelengths of detection (nm): Fe = 248.3, Zn = 213.9, Cu = 324.7, Mn = 279.5, Ni = 231.6, Pb = 283.3, Cd = 228.8, and Cr = 375.9). The detection limits for metals in fish tissue (mg/kg dry weight) were 0.1 for Fe, Mn, Zn while 0.01 for Cu, Pb, Ni, 0.02 for Cr, and 0.05 for Cd, respectively. Accuracy and precision were verified by using certified reference materials (SRM NIST 1598a, Sigma-Aldrich). Analytical results of the quality control samples indicated a satisfactory performance of heavy metal determination within the range of certified values 90.2–110% recovery for the metals studied. Quality control measures were ensured to assess contamination and reliability of the data. The digests prepared in triplicate with blank digestion to quantify possible contamination during sample preparation and analysis. Blank and drift standards (Merck, Germany) were run after every five estimation to calibrate the instrument. The tissue metal concentration was expressed as mg/kg dry tissue weight.
Bioaccumulation factor
The bioaccumulation factor (BAF) is an index to quantify chemical accumulation by an aquatic organism with respect to its concentration in water or sediment (Abdallah and Abdallah 2008). It is calculated by applying the equation:
where X is the metal concentration in fish organ (mg/kg) and Y is the metal concentration in water (mg/L).
Metal pollution index
The metal pollution index (MPI) was calculated to determine the total accumulation of metals in tissues. The MPI values were calculated using the following equation of Usero et al. (1997):
(Mn is the concentration of the metal n in the fish tissue expressed in mg/kg)
Biochemical analyses
The fish were cold anesthetized prior to their sacrifice. The entire brain, liver, kidney, gills, muscles, and skin were dissected out and washed thoroughly in cold (4 °C) fish saline (64% NaCl), blot dried, weighed, and homogenized in cold (4 °C) 50 mM Tris-HCl buffer (pH 7.5) using a Potter-Elvehjem homogenizer. The homogenates were centrifuged at 4 °C for 15 min at 15,000 rpm. The respective supernatants were used for the following biochemical analyses through spectrophotometer (Thermo Scientific Spectrascan UV 2700, Double beam UV Vis spectrophotometer, USA).
Glucose
Glucose content (mg/g) was measured following the method of Seifter et al. (1950). Carbohydrate was dehydrated by sulfuric acid to form furfural. This compound forms a blue-green color product with anthrone with an absorption maximum at 620 nm.
Glycogen
Glycogen content was analyzed following the method of Carroll et al. (1956) using anthrone reagent and glucose as standard and expressed in mg/g. The anthrone reagent acts as a reducing sugar and reacts directly with polysaccharides and helps in the estimation of precipitated glycogen.
Lipid
Lipid content (mg/g) was extracted by homogenizing the tissue with the mixture of 2:1 chloroform-methanol (v/v), and filtering the homogenate following the method of Folch et al. (1957). The process was repeated twice and then the resulting filtrate was thoroughly mixed with 0.6% NaCl in separating funnel and kept in dark for overnight at room temperature. The next day lower organic phase was collected as total lipid extract and evaporated to dryness and weighed.
Protein
Protein was estimated following the method of Lowry et al. (1951). In the tissue sample (0.2 mL of the supernatant) 3 mL of alkaline solution was added and allowed to stand at room temperature for 15 min. Then, 0.3 mL of Folin-Ciocalteu reagent was added and left to stand at room temperature for 30 min and the absorbance was read at wavelength 750 nm. The same method for standard curve drawing was applied using bovine serum albumin solutions prepared at different concentrations. Results were expressed in mg/g.
Moisture content
Moisture content (mg/g) was estimated using Windsor and Barlow (1981) method. The tissue samples were dried to constant weight in an oven (NSW-143, OUA-1) which was accomplished within 24 h. Initial and final weight was recorded and the loss of weight during drying was taken as the percent moisture of the sample.
Transaminase activity
Aspartate amino transferase (AST) and alanine amino transferase (ALT) activities (μmol pyruvate/mg/h) were measured by Reitman and Frankel (1957), method using pyruvate as a standard. Enzyme activity was expressed in μmol pyruvate/mg/h. The differences in the extinction coefficients of alkaline solutions of the 2,4-dinitrophenyl-hydrazones of alpha-ketoglutarate, oxalacetate, and pyruvate, permitting the reaction rate to be measured by determining the timed change in absorbance. The enzymes were assayed using reagents obtained from enzyme assay kits (QCA Laboratories, Spain).
Alkaline phosphatase
The alkaline phosphatase (ALP) activity (μmol PNP/mg/h) was determined following the method of Bergmeyer (1956) wherein sodium p-nitrophenylphosphate was used as substrate. The ALP enzyme will convert p-nitrophenyl phosphate (pNPP) substrate to an equal amount of yellow colored p-nitrophenol (pNP) which shows maximum absorbance at 405 nm.
Antioxidant enzymes
Tissue homogenate (10%) was prepared in ice cold (4 °C) 50 mM potassium phosphate buffer saline (pH 7.4) containing 0.25 M sucrose solution. The homogenate was centrifuged at 2500 rpm for 15 min at 4 °C. A portion of supernatant was kept for the assay of lipid peroxidation. The remaining supernatant was again centrifuged at 12000 rpm for 25 min at 4 °C. The obtained supernatant was used to determine the activities of antioxidant enzymes superoxide dismutase and catalase. The superoxide dismutase (SOD) activity was estimated following the method of Das et al. (2000). The principle of assay was based on the estimation of superoxide scavenging capacity of samples. The absorbance was measured against blank at 543 nm and enzyme activity was expressed in unit of SOD/mg protein where one unit of enzyme activity was defined as the amount of SOD capable of inhibiting 50% nitrite formation under assay conditions. Catalase activity was measured in the samples according to the method of Aebi (1984) by monitoring the decrease in absorbance of H2O2 by the enzyme at 240 nm. The decrease in absorbance was recorded over a period of 3 min and change was calculated in nmol of H2O2 decomposed/min/mg protein.
Lipid peroxidation
Lipid peroxidation (LPO) in the crude homogenate was estimated as thiobarbituric acid reactive substances (TBARS) by thiobarbituric acid (TBA) assay of Ohkawa et al. 1979. The absorbance was measured against blank (without sample) at 532 nm and results were calculated from the molar extinction coefficient of TBARS 156 mM−1 cm−1and expressed as nmol of TBARS formed/mg protein in tissue.
Metallothionein
Metallothionein (MT) concentration in each tissue sample was assayed spectrophotometrically by evaluating the SH residue content with the colorimetric Ellman’s reagent following the protocol of Viarengo et al. (1997). In brief, tissue samples were homogenized in 3 volumes of the homogenization buffer having 20 mM Tris-HCl buffer (pH 8.6), 0.5 M sucrose, and 0.01% β-mercaptoethanol. The resuspended metallothionein fraction were added to 5,5-dithiobis (nitrobenzoic acid) and left for 30 min at room temperature. The concentration of reduced sulfhydryl was evaluated by reading the absorbance at 412 nm using UV spectrophotometer. Metallothionein concentrations were estimated using reduced glutathione (GSH) as standard and calculated assuming the relationship: 1 mol MT = 20 mol of cystein as described by Kagi (1991) for fish and expressed as μg MT/g wet tissue.
Histopathological analysis
Excised tissues of skin, gills, liver and kidney of the exposed and control group were rinsed in fish saline (0.65% NaCl) and fixed in aqueous Bouin’s fluid (Bancroft and Gamble 2002). Following fixation, the tissues were processed through standard histological procedure: dehydration in an ethanol series, embedding in paraffin, and cutting serial sections of 6 μm using a Leica Rotary Microtome (Model RM 2125RT, Leica Mikrosystems, Bensheim, Germany). Sections were de-paraffinized in xylene, hydrated in a descending ethanol series and stained with hematoxylin and eosine (H/E). The stained sections were examined under Leitz “Laborlux S” microscope (Ernst Leitz GmbH, Wetzlar, Germany). Images were captured by a digital camera system Leica DFC 290 (Leica Microsystems Ltd. Germany) fitted in microscope and an Intel® Pentium IV®D computer (Model dx2280 MT, Hp Compaq, CA, USA).
Statistical analysis
All values are expressed as mean ± SEM (standard error of means). The mean and standard deviation calculations were performed by Data Analysis package of MS Excel 2013 (Microsoft Inc.). Data were statistically analyzed by one-way analysis of variance (ANOVA) followed by Duncan’s multiple range test (DMRT) as multiple comparison procedure to assess whether the means of metal concentrations varied significantly among different exposure periods. Possibilities less than 0.05 were considered statistically significant (p < 0.05). All statistical calculations were performed with statistical package SPSS software version 16.0. (Chicago, IL, USA).
Results and discussion
Bioaccumulation of metals in CME-exposed fish tissues
Concentrations of heavy metals (Cu, Zn, Pb, Cd, Fe, Ni, Cr, and Mn) in the skin, muscles, gills, liver, kidney and brain of control as well as 10- and 20-day CME-exposed catfish Clarias batrachus are presented in Table 1. The pattern of metal accumulation in various organ systems of the catfish were highly varied which may be attributed to the differences in the physiological status of the organs and their detoxification abilities. The major pathways for entry of metals in fish are respiratory epithelial membranes, gills and through ingestion of water or food particles in gastrointestinal tract. Bioaccumulation of heavy metals in fish depends on the structure and function of tissues (Kotze 1997). It also depends on the concentration of metals in the water and species-specific physiological and ecological characteristics of waterbody (Omar et al. 2013; Abadi et al. 2015). In general, metabolically active tissues such as liver, kidney and gills have higher accumulations of heavy metals than other tissues such as brain, skin and muscles as evident from several folds higher values of bioaccumulation factor (BAF) (Table 2). BAF values are used as an important criterion for aquatic ecological risk assessment (Ali and Khan 2019).
The metals like Fe and Zn showed maximum accumulation in the liver. Hepatic tissue is considered to be an active and key site for uptake, storage and detoxification of metals through their natural binding proteins such as metallothionein (Gorur et al. 2012). The kidney was the next organ to accumulate Fe and Zn. Substantial amount of Cr and Ni was also stored in kidney. The concentration of almost all the metals in the liver and kidney exceeded the permissible limits of heavy metals in fish tissues recommended by WHO and FAO (World Health Organization 1989; Food and Agricultural Organization 1983). The presence of hepatic metal-binding proteins such as metallothionein has favored the accumulation of heavy metals in the kidney (Murtala et al. 2012). The gills of fish are the major site of metal ion exchange from water due to very large surface areas that facilitate rapid uptake of toxic metals into the blood stream (Dhaneesh et al. 2012). The gills accumulated maximum concentration of Fe while the concentration of Cd was lowest. These results can also be substantiated with the highest MPI values noticed in the liver followed by kidney and gills, indicating the heavy metal load in these tissues of the exposed fish (Table 3). The metal accumulation in brain was not higher than liver and kidney as evident by its low MPI value. The tight blood brain barrier might be one of the main reasons for low accumulation of metals in the brain tissue. Similar suggestion has also been proposed by Takeda (2004). Skin having extensive mucogenic and slime-sloughing activity for the removal of ambient toxicants, showed relatively less MPI value than liver, kidney and brain. The accumulation of metals in the skin was not as high as those observed in the gills, perhaps due to continuous secretion and sloughing of slime and dead cells from the body surface (Banerjee 2007). The concentration of some toxic heavy metals (Cd and Pb) investigated exceed the maximum permissible limits set by WHO (1989) and Joint FAO/WHO committee (1989) (Table 1). However, the concentration of some metals is within the acceptable limits. The level of Cu and Ni in most of the tissues were below the permissible limit of WHO and FAO (World Health Organization 1989; Food and Agricultural Organization 1983).
In fact, metals are categorized as biologically essential and non-essential classes on the basis of their need in biological process. Essential metals such as Mn, Fe, Ni, Cr, Cu, and Zn are those which are required by living organisms for different biochemical and physiological functions in the body. Deficiency in essential heavy metals may result into diseases or syndromes, if not present in adequate amounts, and may also be potentially toxic, if present in excess (Sauliute and Svecevicius 2015). On the contrary, non-essential metals like Cd and Pb are those elements, which have no known biological function in the body (Ali and Khan 2019). The deficiency of an essential metal can cause an adverse health effect, whereas its high concentration can also result in negative impacts which are equivalent to or worse than those caused by non-essential metals (Shah et al. 2009).
Among the various organ systems, the muscles which are not in direct contact with the ambient toxicants accumulated least amount of most of these metals with very low MPI value. El-Moselhy et al. (2014) have opined that the muscles are not an active tissue as a result least accumulation of metals was detected. Some studies on accumulation of heavy metals in fish have also reported low metal concentration in muscles (Begum et al. 2013). As far as human health is concerned the accumulation of heavy metals in muscles is important because this part of fish is mostly consumed by people (Hussain et al. 2014). Henceforth, the concentrations of metals were compared with the maximum allowed concentrations (MAC) in fish muscle established by the European Union (EU) and the national legislation. The level of toxic metals such as Cd and Pb exceeded the MAC of 0.05 and 0.30 mg/kg wet weight respectively prescribed by the EU legislation (European Commission 2006). The national legislation prescribed MAC for Cd and Pb at 0.05, and 0.3 mg/kg wet weight respectively that also exceeded in muscle tissue of fish. Several organizations, such as the FAO and WHO, provide guidelines on the intake of essential metals by humans. The level of Zn, Cu, and Fe did not exceed the MAC of 40, 30 and 30 mg/kg wet weight respectively prescribed by joint FAO/WHO committee (1989). The level of both Cd and Pb exceeded the MAC of 0.5 mg/kg wet weight prescribed by joint FAO/WHO committee (1989). The level of Cu, Pb and Zn exceeded the MAC of 30 mg/kg, 0.5 mg/kg and 30 mg/kg wet weight respectively, prescribed by FAO (1983). There is no national MAC set for Mn and Ni. The result obtained for toxic metals Cd and Pb in studied fish was found higher than the permissible limits for human consumption recommended by several organizations. The people who continuously consume fish as food, which accumulate metals as found in the present study, are under the target of increased health risks in the longer run. Hence, fish from this mining region may not be safe for human consumption resulting in bioaccumulation of metals and its associated detrimental effects.
CME induced biochemical alterations
The presence of toxicants in aquatic media is reported to exert their effects at the cellular and molecular levels, which are exhibited in biochemical parameters. The assessment of biomolecules helps to determine the physiological status of fish. The alterations in concentration of different biomolecules (glucose, glycogen, lipid, protein) and moisture content in vital tissues of the catfish C. batrachus exposed to CME as shown in Table 4. A significant increase in glucose concentration was observed in skin, kidney, and brain of CME-exposed fish. The increased glucose level in these tissues might help to meet the enhanced energy demands to combat CME toxicity. However, significant decrease in its content in muscles, liver, and gills were recorded in a duration dependent manner (Table 4). The decreased glucose level in muscle and liver tissues might be attributed to the increased rate of glycolysis in these tissues under CME stress. A significant decrease in glucose content of the muscle and liver tissue in the present study is similar to the earlier observations in the muscle, liver, and gills of L. rohita treated with monovalent mercury (Rajasubramaniam et al. 2006). Nanda (2014) also observed tissue-specific depletion of glucose attributing to its rapid utilization to meet the energy demands in fish Anabas testudineus on exposure to paper mill effluent.
The level of glycogen in all the tissues of CME-exposed fish decreased in the range which appears to help in providing steady supply of elevated requirement of glucose to meet energy needs (Table 4). Goswami et al. (2016) have also observed significant decrease in glycogen and protein levels in tissues of fish Tilapia mossibica exposed to cadmium chloride. After the exhaustion of glycogen, the lipid contents got depleted to maintain additional energy requirements. Apart from decline in carbohydrate and lipid moieties, the present study also revealed significant decrease in the protein contents of all the fish tissues. The decline in protein levels can again be correlated to their requirement to meet the increased energy cost of homeostasis, tissue repair and detoxification mechanisms of the stressed fish after the other sources of energy (glycogen and lipids) got exhausted. Vutukuru (2003) has reported decreased level of glycogen, lipids, and protein in gill, liver, and muscle tissue of L. rohita, exposed to chromium. However, a substantial increase in liver protein was observed in the CME-exposed fish after 20 days.
This can be attributed to increased biosynthesis of stress proteins and metallothionein against CME toxicity as a defense mechanism. Moreover, Begum (2004) has also reported an increase in liver protein contents of C. batrachus after exposure to different toxicants. The decreased moisture content of the CME-exposed fish tissues (Table 4) might be due to interference of the contaminant with the excretory and osmoregulatory tissue (kidney, gills, and skin). The changes observed in protein, glucose and lipid contents of CME-exposed fish appear to affect their nutritive value in addition to changes in the normal functioning.
Toxicity of CME on different enzyme activity
Transaminases (AST and ALT) are known for their role in the mobilization of amino acids for the gluconeogenesis and in the metabolism of carbohydrate and protein in stressed fish (Hemalatha et al. 2019). The alterations in the activity of enzymes AST, ALT and ALP in various tissues of C. batrachus exposed to raw CME is presented in Table 5. In the present study significant increase in the activity of AST and ALT were observed that suggests an increased participation of amino acids in energy metabolism to cope with the metallic stress caused by CME exposure. Significant increase in AST and ALT activity have also been reported in various studies conducted using a number of another xenobiotic (Fernandes et al. 2016; Du et al. 2017; Renuka et al. 2018).The enhanced ALP activity might be due to rapid mobilization of metabolites and necrosis of the cells in stressed condition.
A variety of xenobiotics including heavy metals exert their toxic effects on survival of fish by producing reactive oxygen species (ROS). These reactive oxygen species react with lipids, proteins and nucleic acids that cause lipid peroxidation, membrane damage and inactivation of enzymes, thus affecting the cell performance and viability that causes oxidative stress, cellular damage and lesions in tissues (Kim and Kang 2015). However, organisms have developed certain antioxidant system to counter reactive oxygen species for maintaining physiological balance and to prevent the development of pathological processes (Jerome et al. 2017). Like mammals, the antioxidant enzymes in fish such as superoxide dismutase (SOD) and catalase (CAT) help to neutralize toxic effects of reactive oxygen species (ROS). In the present study, increased SOD activity was observed in the tissues of fish C. batrachus exposed to CME for both the exposure periods (Table 5). Farombi et al. (2007) reported a significant increase in SOD activity of the African catfish, Clarias gariepinus exposed to several heavy metals (Pb, Zn, Cu, Cd, and As). This was accompanied by concomitant increase in CAT levels in all the tissues of CME-exposed fish (Table 5). Elevation in activity of catalase was also noticed by Kopp and Hetesa (2000) during the exposure to heavy metals as an adaptive response in mitigating the metal toxicity. The high level of lipid peroxidation in the tissues of the CME-exposed fish (Table 4), suggest the generation of reactive free radicals. This increase in LPO level also implies excess production of ROS/free radicals due to less effective antioxidant defense mechanism. Mugil cephalus exposed to toxic metals Cd, Cu and Zn also showed similar alterations of increased LPO level (Rajkumar and Milton 2011). It is abundantly clear that metals induce an early response in the fish as evidenced by alterations both at structural and functional levels of different organs including biochemical and enzymatic effects, thereby increasing susceptibility to multiple types of disease. This is worrisome because the consumption of wild fish on regular basis from water bodies with mining contamination may pose a greater risk to human health.
Metallothionein concentration
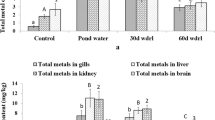
The results of metallothionein (MT) content in the control as well as raw CME-exposed fish tissues at different days of exposure are shown in Fig. 2. In our study, MT concentration was manifold higher in the liver followed by kidney, gills and lowest in muscles. Our results were consistent with Abdel-Tawwab and Wafeek (2014) who reported highest MT concentration in liver > kidney > gills > muscles of O. niloticus exposed to cadmium. The highest MT level in liver tissue suggests its function in the storage, metabolism and detoxification of metals. Amiard et al. (2006) have suggested that MT induction in fish is highest in those tissues directly involved in metal uptake, storage, and excretion, such as the gill, liver, kidney, intestine, and muscle. Hauser-Davis et al. (2012) and Min et al. (2016) have correlated the increase in MT level with metal concentration to protect the fish from deleterious effect of metals.
The metallothionein (MT) concentration in various tissues of Clarias batrachus exposed to raw coal mine effluent in comparison to control. Values are expressed in mean ± standard error of means (SEM). Different small alphabets (a–c) show significant (p < 0.05) difference between the exposure groups and identical small alphabets show insignificant difference between them
Histopathological alterations
Skin
In general, the outer most layer of the epidermis consists of single layer of epithelial cells with well-structured mucous goblet cells. The middle layer is mostly composed of large voluminous club cells and epithelial cells (Fig. 3a). After 10 days of exposure, the epidermis showed hyperplasia and hypertrophy of mucous goblet cell (MGCs) (Fig. 3b). After 20 days of CME exposure, hyperplasia and hypertrophy of MGCs (Fig. 3c) were more prominent, perhaps due to continuation of secretion of large quantity of slime. Chandra and Banerjee (2003), Kumar and Banerjee (2012) have suggested that the thick slime mucus layer on the skin are associated to protect the fish from the toxic stress of heavy metal salts. The club cells also showed progressive degenerative changes as characterized by shrinkage and swelling of their cytoplasmic contents (Fig. 3c).
Photomicrographs of cross sections (H/E) of the skin of the catfish, Clarias batrachus in control (a), 10d exposed (b), and 20d exposed (c). Scale bar = 30 μm. (a) Skin showing epidermis with well-structured mucous goblet cells (arrows) and club cells (asterisks). (b) Hyperplasia of mucous goblet cells (arrows) and vacuolated club cells (asterisks). (c) Hyperplasia of mucous goblet cells (arrows) and vacuolated club cells (asterisks). Note: shrinkage of contents of club cells (arrow heads)
Gills
Gills have large surface area and are in direct contact with the external environment. Any alteration caused by contaminants in the surrounding medium is reflected in this delicate tissue. The histological configuration of the gills of the wild catfish C. batrachus resembles with that of a typical teleostean gill (Munshi 1962) having a well-defined gill filament bearing laterally arranged secondary lamellae (SL) with prominent inter-lamellar space (Fig. 4a, b). The core of SL is characterized by presence of alternately arranged blood channels and supporting pillar cells. After 10 days of CME exposure, the gills fish showed hyperplasia and fusion of their lamellar epithelia (Fig. 4c). Swollen secondary lamellae and decrease in inter-lamellar space was also observed (Fig. 4d). After 20 days, fusion of the respiratory epithelia (Fig. 4e) appears to reduce the surface area availability for gaseous exchange, leading to respiratory dysfunction. The gills of the CME-exposed fish showed detachment of the epithelial lining of the SL from the underlying ladder-like pillar cells/blood channel system. Sloughing of extensively damaged cells at the surface was also observed (Fig. 4f), leading to significant alteration of the gill morphology. All histopathological changes obviously lead to impaired respiratory function of these fishes.
Photomicrographs of cross sections (H/E) of the gills of the catfish, Clarias batrachus in control (a, b), 10d exposed (c, d), and 20d exposed (e, f). (a) A well-structured gill filament with laterally arranged secondary lamellae (arrows). Scale bar = 50 μm. (b) Secondary lamellae with prominent inter-lamellar space (asterisks). Scale bar = 30 μm. (c) Fusion of secondary lamellae (barred arrow); shortening of the secondary lamella (arrow). Scale bar = 50 μm. (d) Swollen secondary lamellae (arrows) and decrease in inter-lamellar space (asterisk). Scale bar = 30 μm. (e) Fusion of epithelial cells of secondary lamellae (arrows) and decrease in inter-lamellar space (asterisk). Scale bar = 50 μm. (f) Sloughing of damaged cells at the surface (arrow). Scale bar = 30 μm
Liver
The liver of the control fish C. batrachus exhibits a typical architecture of a fish liver having compactly arranged hepatocytes with central nuclei (Fig. 5a, b). After 10 days of CME exposure, the liver tissue showed congestion of blood cells, extensive fibrosis and stasis inside blood vessels (Fig. 5c). Hepatocellular degeneration and congestion of blood cells around the central vein were also observed (Fig. 5d). Prolongation of exposure for 20 days aggravated the intensity of damage. The hepatocytes lost their hexagonal shape with cellular degradation, leukocyte infiltration into liver parenchyma, and infiltration of red blood cells (Fig. 5e). Vacuolization of nuclei in hepatocytes along with lateral nuclei and infiltration of red blood cells were also observed (Fig. 5f). Doaa and Hanan (2013) also observed similar pathologic changes such as rupturing and vacuolation of hepatocytes, necrosis, degeneration of blood vessels, and accumulation of pyknotic nuclei and congestion of hepatic tissue in lead treated fish. Prolonged CME exposure impairs the regulatory mechanism in liver by accumulation of toxicants which results in further structural damage as evident from our study.
Photomicrographs of cross sections (H/E) of the liver of the catfish, Clarias batrachus in control (a, b), 10d exposed (c, d), and 20d exposed (e, f). (a) Compactly arranged hepatocytes (arrows). Scale bar = 50 μm. (b) Hepatocytes with centrally placed nuclei (arrows) and sinusoids (barred arrows). Scale bar = 20 μm. (c) Congestion of blood cells (barred arrows); fibrosis (asterisks) and stasis inside blood vessels (arrows). Scale bar = 50 μm. (d) Hepatocellular degeneration (arrow); congestion of blood cells around the central vein (barred arrows). Scale bar = 20 μm. (e) Leukocyte infiltration into liver parenchyma (asterisk); infiltration of red blood cells (barred arrow); hepatocellular degeneration (arrows). Scale bar = 50 μm. (f) Vacuolization of nuclei in hepatocytes along with lateral nuclei (arrows) and infiltration of red blood cells (barred arrow). Scale bar = 20 μm
Kidney
The kidney of a typical fish showed well-defined renal tubules comprising uniformly distributed Bowman’s capsules with glomeruli in the hematopoietic tissues (Fig. 6a, b). The CME exposure of the fish drastically affected most of the components of the kidneys. After 10 days of CME exposure, the kidney tissue showed deformation of the renal tubules and increased inter-renal spaces (Fig. 6c). Hypertrophy of epithelial cells of renal tubules with narrowing of the tubular lumen and glomerular shrinkage with increased Bowman’s space was also observed (Fig. 6d). Prolonged exposure for 20 days caused deformation in the epithelial cells of the renal tubules and glomerular shrinkage with increased Bowman’s space, wide inter-renal space and degeneration of Bowman’s capsules with extensive degranulation (Fig. 6e, f). All these histopathological manifestations are suggestive of severe cellular damage of the kidney due to presence of toxicants especially of metallic origin in the CME. Heavy metals are known to induce degeneration of tubular epithelial cells with tubular necrosis (Velma and Tchounwou 2010). Similar damaged renal tubules are reported in O. niloticus facing degraded water conditions (Abdel-Khalek et al. 2016); Channa striatus and Heteropneustes fossilis exposed to heavy metals in Kali River (Fatima et al. 2015). This might lead to disruption of functioning of inter-renal tissue responsible for maintenance of body homeostasis.
Photomicrographs of cross sections (H/E) of the kidney of the catfish, Clarias batrachus in control (a, b), 10d exposed (c, d), and 20d exposed (e, f). (a) Well-structured renal tubules (arrows). Scale bar = 50 μm. (b) Figure (a) in higher magnification showing glomeruli (barred arrow) and well developed renal tubules (arrows). Scale bar = 30 μm. (c) Deformation of the renal tubules (barred arrows); increase in inter-renal spaces (arrows). Scale bar = 50 μm. (d) Hypertrophy of epithelial cells of renal tubules with narrowing of the tubular lumen (barred arrow); glomerular shrinkage with increased Bowman’s space (arrows). Scale bar = 30 μm. (e) Deformation in the epithelial cells of the renal tubules (arrows); glomerular shrinkage with increased Bowman’s space (barred arrows). Scale bar = 50 μm. (f) Hypertrophy of epithelial cells of renal tubules with narrowing of the tubular lumen (barred arrows); degeneration of the renal tubules (arrows) and increase in inter-renal spaces (asterisks). Scale bar = 30 μm
Conclusion
The present results provide experimental evidences of the toxic nature of the coal mine effluent released from Rajrappa coal mine complex. This fact was confirmed by the heavy metal analysis which revealed high metal content in fish tissues that induced oxidative stress, evident from significant elevation in the activity of antioxidant enzymes and increased metallothionein level. Furthermore, metal accumulation in fish leads to a series of biochemical and histopathological changes that deteriorated nutritional value and could be considered risk factors for several diseases. Thus, the consumption of fish from water bodies contaminated with mining effluent should be prohibited to safeguard the people health. The severity of heavy metal bioaccumulation in fish tissues calls for urgent, sustained, and targeted actions by both governmental authorities and the local scientific community of research and development of mining agency to help prevent and mitigate the situation and ensure the physical well-being of local inhabitants. Also, enforcement of legislations regarding detoxification of effluents before they are discharged into natural water bodies empowering protection of aquatic environments must be taken into considerations. There is an urgent need of stringent government policy and regular monitoring of trace metals in fishes of the mining area so that ill effects of toxic trace elements on human health can be avoided. It is abundantly clear that biomarkers can offer additional biological and ecological relevant information and could be considered a valuable tool for the establishment of guidelines for effective environment management.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abadi DRV, Dobaradaran S, Nabipour I, Lamani X, Ravanipour M, Tahmasebi R, Nazmara S (2015) Comparative investigation of heavy metal, trace, and macro element contents in commercially valuable fish species harvested off from the Persian Gulf. Environ Sci Pollut Res 22:6670–6678
Abdallah MA, Abdallah AM (2008) Biomonitoring study of heavy metals in biota and sediments in the South Eastern coast of Mediterranean Sea, Egypt. Environ Monit Assess 146:139–145
Abdel-Khalek AA, Badran SR, Marie MAS (2016) Toxicity evaluation of copper oxide bulk and nanoparticles in Nile tilapia, Oreochromis niloticus. Fish Physiol Biochem 42:1225–1236
Abdel-Tawwab M, Wafeek M (2014) Influence of water temperature and waterborne cadmium toxicity on growth performance and metallothionein–cadmium distribution in different organs of Nile tilapia, Oreochromis niloticus (L.). J Therm Biol 45:157–162
Aebi H (1984) Catalase in vitro. In: Methods Enzymol. Colowick, S.P. & Kaplane, N.O. (Eds), Vol. 105: pp 121-127. Academic Press
Ali H, Khan E (2019) Bioaccumulation of Cr, Ni, Cd and Pb in the economically important freshwater fish Schizothorax plagiostomus from three rivers of Malak and Division, Pakistan: risk assessment for human health. Bull Environ Contam Toxicol 102:77–83
Amiard JC, Amiard-Triquet C, Barka S, Pellerin J, Rainbowd PS (2006) Metallothioneins in aquatic invertebrates: their role in metal detoxification and their use as biomarkers. Aquat Toxicol 76:160–202
APHA/AWWA/WPCF (2005) Standard methods for the examination of water and wastewater, American Public Health Association, American Water Works Association and Water Pollution Control Federation (20th Ed.). Washington (DC), New York
Ariyaee M, Azadi NA, Majnoni F, Mansouri B (2015) Comparison of metal concentrations in the organs of two fish species from the Zabol Chahnimeh Reservoirs, Iran. Bull Environ Contam Toxicol 94:715–721
Authman MM, Zaki MS, Khallaf EA, Abbas HH (2015) Use of fish as bio-indicator of the effects of heavy metals pollution. J Aquac Res Dev 6:1–13
Bancroft JD, Gamble M (2002) Standard hematoxylin and eosin stain for paraffin sections. Theory and Practice of Histological Techniques. London: Churchill Livingstone, 135–136
Banerjee TK (2007) Histopathology of respiratory organs of certain air-breathing fishes of India. Fish Physiol Biochem 33:441–454
Begum G (2004) Carbofuran insecticide induced biochemical alterations in liver and muscle tissues of the fish Clariasbatrachus(Linn.) and recovery response. Aquat Toxicol 66:83–92
Begum A, Mustafa AI, Amin MN, Chowdhury TR, Quraishi SB, Banu N (2013) Levels of heavy metals in tissues of shingi fish (Heteropneustes fossilis) from Buriganga River. Bangladesh. Environ Monit Assess 185:5461–5469
Bergmeyer HU (1956) Methods for enzymatic analysis. Verlag chemie GMBH Wein Heim/BergsterAcadmic press, New York and London. pp 779-787 & 837-853
Bhatkar N, Venkhede GN, Dhande RR (2004) Heavy metal induced biochemical alterations in the fresh water fish Labeo rohita. J Ecotoxicol Environ Monit 14:1–7
Carroll NV, Longley RW, Roe JH (1956) The determination of glycogen in liver and muscle by use of anthrone reagent. J Biol Chem 220:583–593
Chandra S, Banerjee TK (2003) Histopathological analysis of the respiratory organs of the air-breathing catfish Clarias batrachus (Linn.) exposed to the air. Acta Zool Taiwanica 14:45–64
Das K, Samanta L, Chainy GBN (2000) A modified spectrophotometric assay of superoxide dismutase using nitrite formation of superoxide radicals. Indian J Biochem Biophys 37:201–204
Doaa MM, Hanan H (2013) Histological changes in selected organs of Oreochromis niloticus exposed to doses of lead acetate. J Life Sci Biomed 3:256–263
Debnath S (2011) Clarias batrachus, the medicinal fish: an excellent c &idate for aquaculture & employment generation. In International Conference on Asia Agriculture and Animal. IPCBEE (13), Singapore (pp. 32-37)
Dhaneesh KV, Gopi M, Ganeshamurthy R (2012) Bio-accumulation of metals on reef associated organisms of Lakshadweep Archipelago. Food Chem 131:985–991
Du J, Cao L, Jia R, Yin G (2017) Hepatoprotective and antioxidant effects of dietary glycyrrhiza polysaccharide against TCDD-induced hepatic injury and RT-PCR quantification of AHR2, ARNT2, CYP1A mRNA in Jian carp (Cyprinus carpio var. Jian). J Environ Sci 51:181–190
El-Moselhy KM, Othman AI, El-Azem HA, El-Metwally MEA (2014) Bioaccumulation of heavy metals in some tissues of fish in the Red Sea, Egypt. Egypt. J Basic Appl Sci1: 97–105
European Commission (2006): Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Official J Eur Union 2006, L364: 0005–0024
FAO (1983) Compilation of legal limits for hazardous substances in fish and fishery products. Food Agricultural Organization Fish, Circular No. 464: 5–100
FAO/WHO (1989) Food and Agricultural Organization/World Health Organization. Evaluation of certain food additives and the contaminants mercury, lead and cadmium. Geneva: World Health Organization. Technical Report Series No. 505
Farombi EO, Adelowo OA, Ajimoko YR (2007) Biomarkers of oxidative stress and heavy metal levels as indicators of environmental pollution in African cat fish (Clarias gariepinus) from Nigeria Ogun River. Int J Environ Res Public Health 4(2):158–165
Fatima M, Usmani N, Firdaus F, Zafeer MF, Ahmad S, Akhtar K, Husain SD, Ahmad MH, Anis E, Hossain MM (2015) In vivo induction of antioxidant response and oxidative stress associated with genotoxicity and histopathological alteration in two commercial fish species due to heavy metals exposure in northern India (Kali) river. Comp Biochem Physiol Part C: Toxicol Pharmacol 176:17–30
Fernandes S, Fernandes S, Das S, Gupta RP (2016) Toxicity study of lead nitrate on freshwater fish Cirrhina mrigala. Int J Adv Res 4:1601–1608
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Gorur FK, Keser R, Akcay N, Dizman S (2012) Radioactivity and heavy metal concentrations of some commercial fish species consumed in the Black Sea Region of Turkey. Chemosphere 87:356–361
Goswami P, Kaushik U, Damor S, Sharma P, Sharma N (2016) Effect of cadmium chloride on biochemical profile and enzyme activity in Tilapia mossibica. Int J Pharma Res Health Sci 4:1462–1465
Hauser-Davis RA, Gonçalves RA, Ziolli RL, de Campos RC (2012) A novel report of metallothioneins in fish bile: SDS-PAGE analysis, spectrophotometry quantification and metal speciation characterization by liquid chromatography coupled to ICP-MS. Aquat Toxicol 116:54–60
Hemalatha D, Rangasamy B, Nataraj B, Ramesh M (2019) Assessment of triclosan impact on enzymatic biomarkers in an Indian major carp, Catla catla. J Basic Appl Zoo 80:23
Hermenean A, Damache G, Albu P, Ardelean A, Ardelean G, Puiurdelean D, Horge M, Nagy T, Braun M, Zsuga M, Keki S, Costache M, Dinischiotu A (2015) Histopathological alterations and oxidative stress in liver and kidney of Leuciscus cephalus following exposure to heavy metals in the Tur River, North Western Romania. Ecotoxicol Environ Saf 119:198–205
Hussain M, Muhammad S, Malik RN, Khan MU, Farooq U (2014) Status of heavy metal residues in fish species of Pakistan. In Rev Environ Contam Toxicol volume (pp. 111-132). Springer, Cham
Javed M, Usmani N (2015) Stress response of biomolecules (carbohydrate, protein and lipid profiles) in fish Channa punctatus inhabiting river polluted by Thermal Power Plant effluent. Saudi J Biol Sci 22:237–242
Jerome FC, Hassan A, Omoniyi-Esan GO, Odujoko OO, Chukwuka AV (2017) Metal uptake, oxidative stress and histopathological alterations in gills and hepatopancreas of Callinectes amnicola exposed to industrial effluent. Ecotoxicol Environ Saf 139:179–193
Kagi JHR (1991) Overview of metallothionein. In: Methods of enzymology: metallobiochemistry: metallothionein and related molecules. Rierdan JF, Vallee BL (Eds.), pp. 613–626. Acadmeic Press, San Diego
Kim JH, Kang JC (2015) Oxidative stress, neurotoxicity, and non-specific immune responses in juvenile red sea bream, Pagrus major, exposed to different waterborne selenium concentrations. Chemosphere135: 46–52
Kopp R, Hetesa J (2000) Changes of haematological indices of juvenile carp (Cyprinus carpio L.) under the influence of natural populations of cyanobacterial water blooms. Acta Vet Brno 69:131–137
Kotze PJ (1997) Aspects of water quality, metal contamination of sediment and fish in the Oilfants River, Mpumalanga. M.Sc. Thesis, Rand Afr. Univ., South Africa
Kumar R, Banerjee TK (2012) Impact of sodium arsenite on certain biomolecules of nutritional importance of the edible components of the economically important catfish C. batrachus (Linn.). Ecol Food Nutr 51:114–127
Kumari B, Ahsan J, Kumar V (2012) Comparative studies of liver and brain glycogen content of male and female Clarias batrachus (L.) after exposure of different doses of arsenic. Toxicol Environ Chem 94:1758–1767
Kumar M, Gupta N, Ratn A, Awasthi Y, Prasad R, Trivedi A, Trivedi SP (2019) Biomonitoring of heavy metals in river Ganga water, sediments, plant, and fishes of different trophic levels. Biol Trace Elem Res:1–12
Lakra KC, Lal B, Banerjee TK (2017) Decontamination of coal mine effluent generated at the Rajrappa coal mine using phytoremediation technology. Int J Phytoremediat 19:530–536
Lakra KC, Lal B, Banerjee TK (2019a) Coal mine effluent-led bioaccumulation of heavy metals and histopathological changes in some tissues of the catfish Clarias batrachus. Environ Monit Assess 191:136
Lakra KC, Lal B, Banerjee TK (2019b) Application of phytoremediation technology in decontamination of coal mine effluent-fed pond water using three aquatic macrophytes. Int J Phytoremediat 21:840–848
Laxmi Priya S, Senthilkumar B, Hariharan G, Paneer Selvam A, Purvaja R, Ramesh R (2011) Bioaccumulation of heavy metals in mullet (Mugil cephalus) and oyster (Crassostrea madrasensis) from Pulicat lake, south east coast of India. Toxicol Ind Health 27(2):117–126
Lechner AM, Kassulke O, Unger C (2016) Spatial assessment of open cut coal mining progressive rehabilitation to support the monitoring of rehabilitation liabilities. Res Policy 50:234–243
Lowry OH, Rosenberg NJ, Farr AL, Randall RJ (1951) Protein measurement with Folin phenol reagent. J Biol Chem 193:265–275
Lushchak VI (2011) Environmentally induced oxidative stress in aquatic animals. Aquat Toxicol 101:13–30
Medeiros RJ, dos Santos LMG, Freire AS, Santelli RE, Braga AMC, Krauss TM, Jacob SDC (2012) Determination of inorganic trace elements in edible marine fish from Rio de Janeiro State, Brazil. Food Control 23(2):535–541
Min EY, Ahn TY, Kang JC (2016) Bioaccumulation, alterations of metallothionein, and antioxidant enzymes in the mullet Mugil cephalus exposed to hexavalent chromium. Fish Aquat Sci19:19
Mohanty AK, Lingaswamy M, Rao VG, Sankaran S (2018) Impact of acid mine drainage and hydrogeochemical studies in a part of Rajrappa coal mining area of Ramgarh District Jharkhand State of India. Groundw Sustain Dev 7:164–175
Munshi JSD (1962) The accessory respiratory organs of Clarias batrachus (Linn). J Morphol 109:115–139
Murtala BA, Abdul WO, Akinyemi AA (2012) Bioaccumulation of heavy metals in fish (Hydrocynus forskahlii, Hyperopisusbebe occidentalis and Clarias gariepinus) organs in downstream Ogun coastal water Nigeria. J Agric Sci 4(11):51–59
Nanda P (2014) Bioaccumulation of heavy metals and physiological response in anabas testudineus on exposure to paper mill effluent. J Environ Toxicol5(1): 1-8
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbutric acid reaction. Anal Biochem 95:351–358
Omar WA, Zaghloul KH, Abdel-Khalek AA, Abo-Hegab S (2013) Risk assessment and toxic effects of metal pollution in two cultured and wild fish species from highly degraded aquatic habitats. Arch Environ Contam Toxicol 65:753–764
Rajasubramaniam V, Sasikumar K, Subhasini S (2006) Effect of monovalent mercury on liver, muscle glycogen and blood glucose levels of freshwater teleost L rohita. J Ecotoxicol Environ Monit 16:215–220
Rajeshkumar S, Liu Y, Zhang X, Ravikumar B, Bai G, Li X (2018) Studies on seasonal pollution of heavy metals in water, sediment, fish and oyster from the Meiliang Bay of Taihu Lake in China. Chemosphere 191:626–638
Rajkumar JSI, Milton MJ (2011) Biochemical markers of oxidative stress in Mugil cephalus exposed to cadmium, copper, lead and zinc. Int J Pharma Bio Sci2: 41–50
Ratn A, Prasad R, Awasthi Y, Kumar M, Misra A, Trivedi SP (2018) Zn2+ induced molecular responses associated with oxidative stress, DNA damage and histopathological lesions in liver and kidney of the fish Channa punctatus (Bloch 1793). Ecotoxicol Environ Saf 151:10–20
Reitman S, Frankel S (1957) A colorimetric method for the determination of serum glutamic oxaloacetic and glutamic-pyruvic transminases. Am. J Clin Pathol 28: 56–63
Renuka S, Poopal RK, Ramesh M, Clara-Bindu F (2018) Responses of Labeo rohita fingerlings to N-acetyl-p-aminophenol toxicity. Ecotoxicol Environ Saf 157:73–80
Saad AA, El-Sikaily A, Kassem H (2016) Metallothionein and glutathione content as biomarkers of metal pollution in mussels and local fishermen in Abu Qir Bay Egypt. J Health Pollut 6:50–60
Sauliute G, Svecevicius G (2015) Heavy metal interactions during accumulation via direct route in fish: a review. Zool Ecol 25:77–86. https://doi.org/10.1080/21658005.2015.1009734
Seifter S, Novie B, Muntwyer E (1950) The estimation of glycogen with the anthrone reagent. Arch Biochem 25:191–200
Shah AQ, Kazi TG, Arain MB, Baig JA, Afridi HI, Kandhro GA, Khan S, Jamali MK (2009) Hazardous impact of arsenic on tissues of same fish species collected from two ecosystem. J Hazard Mater 167:511–515
Singh VK, Lal B (2015) Immunolocalization of nitric oxide synthase (NOS) isoforms in ovarian follicles of the catfish, Clarias batrachus and its relation with ovarian activity. Gen Comp Endocrinol 220:98–102
Swaleh SB, Banday UZ, Usmani N (2019) Comparative study of biochemical, histological and molecular biomarkers of heavy metal contamination in Cyprinus carpio collected from warm-monomictic lake and government culture pond. Chemosphere 236:124182
Ta TY, Le TT, Trinh TT, Trinh TT, Pham TMT, Pham THP (2018) Risk assessment of lead and cadmium on Juveniles of Cyprinus carpio in laboratory scale. Vietnam J Sci Tech Eng 60:78–83
Takeda A (2004) Analysis of brain function and prevention of brain diseases: the action of trace metals. J Health Sci 50:429–442
Talukdar B, Kalita HK, Basumatary S, Saikia DJ, Sarma D (2017) Cytotoxic and genotoxic effects of acid mine drainage on fish Channa punctata (Bloch). Ecotoxicol Environ Saf 144:72–78
Usero J, Gonzalez-Regalado E, Gracia I (1997) Trace metals in the bivalve molluscs Ruditapes decussatus and Ruditapes philippinarum from the Atlantic Coast of Southern Spain. Environ Int 23:291–298
Velma V, Tchounwou PB (2010) Chromium-induced biochemical, genotoxic and histopathologic effects in liver and kidney of goldfish Carassius auratus. Mutat Res 698:43–51
Viarengo A, Ponzano E, Dondero F, Fabbri R (1997) A simple spectrophotometric method for metallothionein evaluation in marine organisms: an application to Mediterranean and Antarctic molluscs. Mar Environ Res44: 69–84
Vutukuru SS (2003) Chromium induced alterations in some biochemical profiles of the Indian major carp Labeo rohita (Hamilton). Bull Environ Contam Toxicol 70(1):118–123
WHO (1989) Heavy metals-environmental aspects. Environment Health Criteria. No. 85. Geneva, Switzerland
Windsor M, Barlow S (1981) Introduction to fishery by-products. Pub Fishing News Book Ltd England pp 149
World Coal Association (2018) Coal facts. Retrieved [December 2019] from webpage http://www.worldcoal.org/resources/coal-statistics
Acknowledgments
Thanks are also due to the Rajrappa mining authorities of Central Coalfield Limited, Jharkhand for the site visit and collection of effluent. Authors also acknowledge the help of Prof. B. R. Maurya, Head, Department of Soil Science and Agricultural Chemistry, for providing atomic absorption spectrophotometer (AAS) facility.
Funding
K. C. Lakra was supported as Junior Research Fellow and Senior Research Fellow under the Maulana Azad National fellowship (vide letter no: F1-17.1/2014-15/MANF-2014-15-CHR-JHA-32885) scheme. Financial support from University Grant Commission, New Delhi, in the form of a Major Research Project (P-01/694, 42-529/2013 SR) is also acknowledged.
Author information
Authors and Affiliations
Contributions
All authors contributed extensively to the work presented in this paper. Dr. KC Lakra performed the experiments, conducted the observations and wrote the manuscript. Prof. TK Banerjee and Prof. B Lal analyzed the result and edited the manuscript. All authors discussed the results and commented on the manuscript at all stages.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All experiments were carried in accordance with the guidelines of the Institutional Committee for Animal Ethics and Care of Banaras Hindu University, Varanasi, India (Ref No. F.Sc./IAEC/2016-2017/1137).
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lakra, K.C., Banerjee, T.K. & Lal, B. Coal mine effluent-induced metal bioaccumulation, biochemical, oxidative stress, metallothionein, and histopathological alterations in vital tissues of the catfish, Clarias batrachus. Environ Sci Pollut Res 28, 25300–25315 (2021). https://doi.org/10.1007/s11356-021-12381-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-12381-3