Abstract
Hexavalent chromium (CrVI) is an environmental pollutant and an endocrine-disrupting metal. Se and Zn are essential trace elements, known to play a crucial role in thyroid homeostasis. However, there is a lack of data reporting thyrotoxicity during gestation. In this study, we investigated the protective effects of selenium and zinc against potassium dichromate–induced thyrotoxicity in pregnant Wistar rats. Thirty pregnant Wistar rats were divided into control and four treated groups receiving subcutaneously (s.c) on the 3rd day of pregnancy, K2Cr2O7 (10 mg/kg, s.c) alone, or in association with Se (0.3 mg/kg, s.c), ZnCl2 (20 mg/kg, s.c), or both of them simultaneously. The hormonal profile, oxidative stress biomarkers, DNA damage, and histological modifications were evaluated. Our main findings showed that K2Cr2O7 promoted hypothyroidism, oxidative stress, genotoxicity, and histological alterations in the thyroid gland. The co-treatment with Se or ZnCl2 has mitigated K2Cr2O7-induced thyrotoxicity in pregnant Wistar rats by exhibiting antioxidant and genoprotective effects. However, the combined co-treatment of both of them was less thyroprotective, and therefore, further investigations on the synergetic interaction of Se and Zn against CrVI toxicity using different doses and exposure routes are required.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The thyroid gland is known to be prone to endocrine toxins due to its complex histological structure and function (Ben Amara et al. 2009; Ben Hamida et al. 2001; Buha et al. 2013; Rodrigues-Pereira et al. 2015). These toxins can affect the gland homeostasis at many levels, including iodine uptake, thyroid hormone biosynthesis, conversion, metabolism, and degradation; also, they can interrupt hormone-receptor binding and modulate hormonal actions in the target tissues (Gilbert et al. 2012; Miller et al. 2009).
One of the alleged thyroid-disrupting metals is hexavalent chromium (CrVI); it is widespread in the environment, either naturally or by its excessive usage in anthropogenic activities and improper disposal of industrial wastes (Cohen et al. 1993; Richelmi and Baldi 1984). The amounts of CrVI that have been dispersed through water, soil, and air continue to increase in the last decades, promoting its bioaccumulation in living beings through contaminated food and water (IARC 1990). CrVI-induced toxicity is linked to its oxidizing potency; it generates reactive oxygen species (ROS) and Cr intermediates via its cellular reduction, which interact with biomolecules and cause their oxidative damage (Valko et al. 2006). Although it is generally recognized that cells comprise many antioxidant enzymes, such as catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPx), and glutathione-S-transferase (GST) that are responsible for neutralizing free radicals and restoring cellular redox homeostasis, the oxidative damage occurs to membrane lipids, proteins, and nucleic acids if ROS are overproduced and the detoxification processes are overwhelmed (Flora 2009). Therefore, several studies reported that CrVI induced cytotoxicity and mutagenicity (Levis and Bianchi 1982). Evenly, CrVI compounds are known to be hematotoxic (Adjroud 2010), hepatotoxic (Soudani et al. 2011), nephrotoxic (Goodarzi et al. 2017), reprotoxic (Jahnabi et al. 2017), and genotoxic (Khorsandi and Rabbani-Chadegani 2013; Monteiro et al. 2019).
Trace elements, namely selenium (Se) and zinc (Zn), are essential for thyroid hormone synthesis and activation (Betsy et al. 2013; Triggiani et al. 2009). Se is mainly incorporated in selenoproteins (Kryukov et al. 2003), which play a definite role in the endocrine system; they are implicated in several physiological processes and biochemical pathways, for instance, spermatogenesis, thyroid hormone deiodination, regulating the genomic transcription, and protecting the cell from ROS by peroxide degradation (Dumitrescu et al. 2005). Regarding the chemopreventive mechanisms of Se, it has been shown that Se prevented free radical formation and diminished lipid peroxidation to overcome CrVI-induced renal injury (Soudani et al. 2010). Additionally, the reduction of DNA-adduct formation and chromosome breaks is considered to be among Se-induced anticarcinogenic effects (Yildiz et al. 2019).
Zn is a potent antioxidant; it is required for many biological functions such as reproduction, fetal development, immunity, growth, and DNA synthesis (Barceloux 1999; Prasad 2013). Also, Zn is known for playing an integral role in the hormonogenesis of thyroid-hypophysis-hypothalamus axis (Brandao-Neto et al. 2006; Farooqi et al. 2000; Pekary et al. 1991). In addition to its well-known functions in several cellular processes, Zn acts as an effective antiradical and anti-inflammatory agent; it inhibits NADPH oxidases, boosts SOD activity, and promotes ROS scavenging by causing the induction of cysteine-rich metallothionein protein expression to keep the cell redox homeostasis (Prasad et al. 2004). Furthermore, Zn yielded antagonistic effects on tumorigenesis by regulating many DNA repair genes via Zn-finger transcription factors (Yildiz et al. 2019).
Although the imperative role of thyroid functions during gestation in animals and humans, there is a lack of studies reporting the oxidative, genotoxic, and endocrine disruptive effects of environmental pollutants in the thyroid gland. Herein, we aimed through this study to investigate the potential protective effects of both Se and Zn against the hazardous effects of potassium dichromate (K2Cr2O7) on thyroid hormonogenesis, redox status, and DNA integrity in pregnant Wistar albino rats.
Materials and methods
Materials
K2Cr2O7, Se, and ZnCl2 were purchased from Sigma-Aldrich (Chemie Gmbh, Taufkirchen, Germany). All chemicals were dissolved in sterile saline (NaCl 0.9%) and the pH was adjusted when necessary to 7.5. Low melting point agarose and normal melting point agarose were from Sigma-Aldrich (St. Louis, MO, USA). 1-Butanol and pyridine were from BDH-ProLabo (Fontenay-sous-Bois, France). Thiobarbituric acid (TBA), trichloroacetic acid (TCA), Tris-HCl, glacial acetic acid, sodium dodecyl sulfate, phosphate-buffered saline (PBS), hydrogen peroxide solution, glutathione reduced, 5, 5-dithiobis-2-nitrobenzoic acid (DTNB), 1-chloro-2, 4-dinitrobenzene (CDNB), 2, 4-dinitrophenylhydrasine (DNPH), and ethidium bromide were supplied by Sigma-Aldrich (Steinheim, Germany). DMSO (Sharlau, Barcelona, Spain), EDTA and ethyl acetate (Thermo Fisher Scientific, Strasbourg, France). BSA solution, nitro blue tetrazolium (NBT), riboflavin, methionine, guanidine hydrochloride, NAOH, Triton X-100, formalin, paraffin, ethanol, xylene, eosin, and hematoxylin (Merck KGaA, Darmstadt, Germany) were used.
Animals
This study was carried out on healthy female Wistar albino rats purchased from Pasteur Institute, Algiers, Algeria, weighing 180–250 g. All animals were maintained in polypropylene cages, under controlled conditions of light/dark (12/12 h) and temperature (23 ± 1 °C) and had free access to food and water. Female rats were caged with males overnight and those with a positive mating smear were considered to be at day zero of gestation. The standards established by the Institutional Animal Care and Use Committee at Batna University were followed.
Methods
Experimental design
Thirty pregnant Wistar albino rats were used and allocated into five groups of six animals each; they were housed separately from day zero of pregnancy. All rats in different groups were injected on day 3 of pregnancy by subcutaneous route. Each animal was anesthetized with diethyl ether.
The control group received a single injection (s.c) of saline solution 0.9%.
The 2nd group was treated with a single dose of 10 mg/kg, s.c, bw of K2Cr2O7; this dose was selected based on the results of our previous studies (Adjroud 2009, 2010).
The remaining groups 3rd, 4th, and 5th were co-treated respectively by K2Cr2O7 and Se (0.3 mg/kg, s.c. bw) or with ZnCl2 (20 mg/kg, s.c. bw) or with the three of them simultaneously. The selected dose of Se has antagonized the reprotoxic effects of nickel in male and female rats and their progeny (Käkelä et al. 1999). In addition, it counterbalanced nickel chloride–induced toxic effects in preimplanted Wistar rats (Adjroud 2013). Also, the dose of ZnCl2 was chosen based on its protective efficacy on heavy metal–induced toxic effects (Paksy et al. 1996; Nasiry Zarrin Ghabaee et al. 2017). On the 20th day of gestation, rats were anesthetized with diethyl ether and blood samples were collected into heparinized tubes from the jugular vein for hormonal quantification. Thyroid glands were quickly excised after the sacrifice (Hadie et al. 2013), rinsed in ice-cold physiological saline solution, and conserved either at − 20 °C to assess thyroid oxidative stress markers and DNA damage, or in 10% neutral buffered formalin for the histological examination.
Evaluation of organ and maternal body weight
The maternal body weight of rats was measured on the 3rd and 20th day of gestation, while the thyroid weight was recorded directly after the sacrifice.
Hormone quantification
At the 20th day of pregnancy, blood was collected in heparinized tubes and centrifuged at 1500×g for 15 min at 4 °C. Plasma samples were drawn and kept at − 20 °C until hormone analysis. Total plasma concentrations of triiodothyronine (T3), thyroxine (T4), and thyroid-stimulating hormone (TSH) were measured using Roche Cobas E411 analyzer and electrochemiluminescent immunoassay (ECLIA) commercial kits (Elecsys® and Cobas E411 analyzers, Roche Diagnostics, Germany), according to the manufacturer’s instructions.
Preparation of thyroid protein homogenates
Thyroid samples were homogenized with a potter (glass-Teflon) in 500 μl of cold Tris-HCl (10 mM, pH 7.4). After, they were centrifuged (12000 rpm, 30 min, 4 °C), the supernatants were recovered and aliquoted in Eppendorf tubes and stored at − 20 °C until the analysis of stress oxidative biomarkers.
Estimation of protein concentrations in thyroid extracts
Protein concentrations were measured according to the Bio-Rad Protein Assay. To 5 μl of each extract, 95 μl of water and 900 μl of Bradford solution were added, the mixture was allowed to stand at dark for 10 min, and absorbances were read using Biochrom (LIBRA) spectrophotometer (Serlabo technologies, Vedène, France) at 595 nm, where a BSA solution was used as a standard (Bradford 1979).
Superoxide dismutase activity
The measurement of SOD activity is based on a photoreduction method using the NBT. The appearance of the blue color indicates the reduction of NBT by the superoxide anion generated by the riboflavin and methionine mixture in an aerobic environment under the white light. To 50 μl of each thyroid extract, 950 μl of phosphate buffer (pH 7.0), 1 ml of EDTA-methionine (pH 7.8), 85.2 μl of NBT (1 mM), and 22.6 μl of riboflavin (1 mM) were added; the reaction mixture was allowed to stand under white light for 30 min in an ambient temperature. Then, it was read at 540 nm. In addition, two control tubes were prepared; the same reagents were introduced except thyroid extract supernatants which were replaced by the phosphate buffer. The first was incubated under white light and served to SOD total activity, while the second was incubated in the dark in order to set the spectrophotometer at zero. Activity refers to the enzyme amount that inhibits the NBT oxidation by 50%, which is equivalent to one unit of SOD. Results were expressed in IU/mg of protein (Beauchamp and Fridovich 1971).
Estimation of lipid peroxidation status (malondialdehyde assay)
The lipid peroxidation level was determined according to the method of Ohkawa et al. (1979), which is based on the colorimetric reaction between TBA and malondialdehyde resulting in the formation of a colored complex (MDA-TBA2) which will be assessed spectrophotometrically. To 250 μl of each thyroid extract, 100 μl of 8.1% sodium dodecyl sulfate, 750 μl of both acetic acid (20%) and TBA (0.8%), and 2 ml of water were added, the mixture was heated at 90 °C for 2 h, then cooled for 10 min in an ice bath. Finally, 2.5 ml of butanol pyridine (15: 1) was added to the mixture. After centrifugation at 4000 rpm for 10 min, the organic layer was recovered and the optical density was read at 540 nm against the control.
Estimation of catalase activity
Our method was based on monitoring the degradation of hydrogen peroxide by catalase action in a quartz spectrophotometer cuvette containing 780 μl of phosphate buffer solution (pH 7.0), 20 μl of each thyroid extract, and 200 μl of H2O2 (1 M). Then, catalase activity was measured at 240 nm for 1 min against an appropriate blank, where the sample was replaced by the phosphate buffer. CAT activity was expressed as millimole of degraded H2O2/min/mg of proteins (Clairbone 1985).
Protein carbonyl assay
Proteins and amino acids are targets of oxidation and fragmentation by the ROS attack. Our method consists of the measurement of the reactivity of carbonyl groups by the DNPH. The initial reaction mixture that contains 200 μl of each thyroid extract and 800 μl of DNPH was incubated in the dark at room temperature for 1 h. Then, we performed a series of washes. First, we added 1 ml of 20% TCA. The tubes were allowed to stand 10 min in an ice bath. Then, they were centrifuged at 4000 rpm for 5 min in order to prevent the denaturation of proteins. The second wash was done by adding 1 ml of 10% TCA, and then it was vortexed in order to break the protein precipitate. Finally, the pellets were washed for the third time with 800 μl of ethanol-ethyl acetate (1:1, v/v) to remove any residue of free DNPH. The final precipitates were solubilized in 500 μl of guanidine hydrochloride (6 M) and left for 10 min at 37 °C. Then, they were centrifuged at 11000 rpm for 10 min. The optical density was read at 340 nm (Mercier et al. 2004).
Glutathione peroxidase assay
Cytosolic glutathione peroxidase activity was assayed following the method of Flohe and Gunzler (1984). Five milliliters of reaction mixture contained 200 μl of each thyroid extract, 400 μl of reduced glutathione (GSH; 0.1 mM), and 200 μl of phosphate buffer (pH 7.8). After an incubation for 5 min at 25 °C, 200 μl of H2O2 (1.3 mM) and 1 ml of 1% TCA were added and the tubes were allowed to set in ice bath for 30 min. Then, they were centrifuged at 3000 rpm/10 min/4 °C in order to recover the supernatants. Finally, 2.2 ml of Na2HPO4 (0.32 M, pH 7.4) and 320 μl of DTNB (0.1 M) were added to 480 μl of each supernatant and the absorbances were read at 412 nm for an interval of 5 min against controls, which contain all the same compounds except the samples. GPx activity was expressed in μmol of oxidized GSH/min/mg of protein.
Glutathione-S-transferase assay
GST activity was assayed based on the method described by Elia et al. (2006, 2007). In a spectrophotometer cuvette, 830 μl of phosphate buffer (100 mM, pH 7.0), 50 μl of CDNB (20 mM), 100 μl of GSH (100 mM), and 20 μl of thyroid extract were mixed using a vortex. The reaction between the reduced glutathione and the CDNB produced a chromophore (1-glutathione-2, 4-dinitrobenzene from 1-Cl-2, 4-dinitrobenzene) which was read by the spectrophotometer at 340 nm for 1 min against a proper blank.
Single-cell gel electrophoresis (comet assay)
The assay is based on the migration of denatured DNA fragments subjected to an electric field. The alteration of DNA integrity depends on the length of the comet tail.
The cell suspension of each thyroid gland was prepared by homogenizing the sample with a potter (glass-Teflon) in PBS solution (pH 7.4); containing DMSO (1%) and EDTA (20 mM). The final cell-agarose suspension was obtained by adding 60 μl of 0.5% low melting agar to 60 μl of each cell suspension. A total of 120 μl of the final cell-agarose suspension was spread over on a 1% normal-melting agar pre-coated microscope slide. The cell lysis was processed by submerging the slides for 24 h at 4 °C in a buffer containing 2.5 M NaCl, 100 mM EDTA, Tris (10 mM, pH 10.0) with freshly prepared 1% Triton X-100 and 10% DMSO. Then, the slides were washed with deionized water and placed in a horizontal electrophoresis unit (Bio-Rad, California, USA) for 20 min in an alkaline solution containing 300 mM NaOH and 1 mM EDTA (pH > 13.0). Thus, allowing the DNA to unfold. The DNA was electrophoresed for 15 min at 300 mA and 25 V (0.9 V/cm). The slides were neutralized with Tris (0.4 M, pH 7.5). Comets were visualized after staining with ethidium bromide (20 μg/ ml) with Nikon Eclipse TE 300 fluorescence microscope (Nikon, Tokyo, Japan). 100 comets on each slide were scored visually. Based on the fluorescence intensity in the tail, the comets are classified into five classes; each comet class was given a value of 0, 1, 2, 3, and 4 from undamaged, 0, to highly damaged, 4, as described by Collins et al. (1996). The total score was calculated by the following equation (percentage of cells in class 0 × 0) + (percentage of cells in class 1 × 1) + (percentage of cells in class 2 × 2) + (percentage of cells in class 3 × 3) + (percentage of cells in class 4 × 4) and it ranged for 100 comets from 0 to 400 (Singh et al. 1988).
Histopathological examination
Thyroid tissues were fixed previously in 10% neutral buffered formalin and processed for histological procedures. The pieces were dehydrated in graded ethanol baths, cleaned in xylene, and embedded in paraffin. Five-micrometer-thick paraffin wax sections were stained with hematoxylin and eosin (H&E) and examined using a light microscope from ZEISS Axioscope, Göttingen, Germany (Bancroft and Gamble 2008).
Statistical analysis
All data were expressed as mean ± SEM (n = 6). The statistical comparisons were carried out by using one-way analysis of variance (ANOVA) and followed by Tukey’s post hoc test. The statistical analysis was performed using GraphPad Prism 7. The differences were considered significant when p < 0.05.
Results
Effects of Se and ZnCl2 on maternal body weight, absolute and relative thyroid weights in K2Cr2O7-treated Wistar albino pregnant rats
During the experiment, no mortality was recorded among pregnant Wistar albino rats in any group. Results in Table 1 showed that K2Cr2O7 decreased significantly (p < 0.001) the maternal body weight and increased the absolute and relative weights of thyroid glands when compared with the control group. The three co-treatments increased significantly the maternal body weight on the 3rd and 20th day of gestation, whereas the simultaneous administration of Se and ZnCl2 decreased significantly (p < 0.001) the thyroid relative weight when compared with the K2Cr2O7-treated group.
Effects of Se and ZnCl2 on TSH level and thyroid hormonal profile of K2Cr2O7-treated Wistar albino pregnant rats
The administration of K2Cr2O7 (10 mg/kg, s.c) induced a significant difference of T3 (nmol/L), T4 (nmol/L), and TSH (mIU/L) levels at the 20th day of pregnancy when compared with the control group.
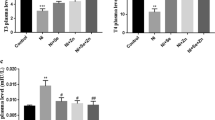
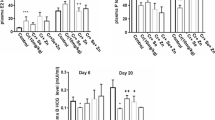
The obtained results showed that K2Cr2O7 decreased the T3 plasma level significantly (− 48.3 %, p < 0.001) when compared with the control group (Fig. 1a).
Effects of Se and ZnCl2 on plasma T3 (a), T4 (b), and TSH levels in K2Cr2O7-treated Wistar albino pregnant rats. Values are expressed as mean ± SEM, (n = 6). p < 0.05 considered statistically significant, (*) statistically significant compared with the control. (+) statistically significant compared with the K2Cr2O7 treated group
The plasma T4 level was decreased significantly (− 29.4 %, p < 0.001) when compared with the control group (Fig. 1b).
Conversely to the decrease of thyroid hormones levels, the level of plasma TSH was increased significantly (+ 1187.5 %, p < 0.001) on the 20th day of gestation (Fig. 1c).
The co-administration of K2Cr2O7 and Se induced at the 20th day of pregnancy an insignificant increase in plasma T3 level compared with the K2Cr2O7-treated group (Fig. 1a), whereas plasma T4 level was increased significantly (+ 26.5 %, p< 0.01) at the 20th day of pregnancy (Fig. 1b).
On the other hand, the co-treatment with Se has significantly decreased the level of plasma TSH (− 24.2 %, p < 0.001) on day 20 of gestation (Fig. 1c).
In addition, the combined administration of ZnCl2 with K2Cr2O7 prevented the hypothyroidism induced by K2Cr2O7-exposure, the plasma T3 level increased significantly (+ 47.1 %, p < 0.01) when compared with the K2Cr2O7-treated group (Fig.1a).
Furthermore, the plasma T4 level was also increased in a significant way (+ 29.9 %, p < 0.01) (Fig.1b). Additionally, TSH plasma level decreased significantly (− 25.2 %, p < 0.001) at the 20th day of gestation when compared with the K2Cr2O7-treated group (Fig. 1c).
The simultaneous administration of Se and ZnCl2 decreased the TSH plasma level significantly (− 17.4 %, p < 0.05) when compared with the K2Cr2O7-treated group (Fig. 1c). On the other hand, the combination of Se and ZnCl2 did not improve significantly the decreased T3 and T4 plasma levels induced by K2Cr2O7, even though the two elements play an essential role in thyroid hormone synthesis. The concentrations of T3 and T4 increased but the differences stayed insignificant when compared with the K2Cr2O7-treated group (Fig. 1a, b).
Effects of Se and ZnCl2 on SOD activity in the thyroid of K2Cr2O7-treated Wistar albino pregnant rats
SOD is one of the most important enzymes involved in the antioxidant defense of biological organisms; it catalyzes the dismutation of the superoxide radical into hydrogen peroxide and molecular oxygen to protect the organism against the oxidative action of free radicals. Results indicate that the subcutaneous administration of K2Cr2O7 in pregnant rats caused a significant increase in SOD activity in the thyroid gland as compared with the control group (p < 0.001). The co-treatment of K2Cr2O7 with ZnCl2 or with Se and ZnCl2 concomitantly induced a significant decrease in the enzyme activity (p < 0.001). However, the co-administration of K2Cr2O7 with Se decreased the activity of SOD insignificantly (Table 3).
Effects of Se and ZnCl2 on lipid peroxidation level in the thyroid of K2Cr2O7-treated Wistar albino pregnant rats
MDA is generated by the oxidative degradation of membrane lipids, which is an indicator of cell death. Results indicate that K2Cr2O7 increased significantly the level of MDA in the thyroid as compared with the control group (p < 0.001). The three co-treatments reduced significantly (p < 0.001) the MDA levels in the thyroid of pregnant rats as shown in Table 2.
Effects of Se and ZnCl2 on CAT activity in the thyroid of K2Cr2O7-treated Wistar albino pregnant rats
CAT is one of the enzymes that play a major role in the antioxidant cell defense system; it catalyzes the dismutation of H2O2 to protect the cell against oxidative stress. Results in Table 3 show that K2Cr2O7 increased the activity of CAT in the thyroid of pregnant rats compared with the control group (p < 0.001). The co-treatment of K2Cr2O7 with Se, ZnCl2, or simultaneously with both of them decreased markedly the CAT activity (p < 0.001).
Effects of Se and ZnCl2 on protein carbonyl level in the thyroid of K2Cr2O7-treated Wistar albino pregnant rats
The obtained results in Table 2 show that K2Cr2O7 induced protein oxidation by increasing the formation of carbonyl groups in the thyroid of pregnant Wistar rats (p < 0.01). However, the formation of carbonyl groups in the thyroid was reduced when K2Cr2O7 was co-administered with Se (p < 0.01) or ZnCl2 (p < 0.001) or with both of them simultaneously (p < 0.05).
Effects of Se and ZnCl2 on GPx activity in the thyroid of K2Cr2O7-treated Wistar albino pregnant rats
GPx participates effectively in the cellular antioxidant defense and involves in maintaining redox homeostasis. Results indicate that K2Cr2O7 is able to increase GPx activity in the thyroid of pregnant rats compared with the control group (p < 0.001). However, GPx activity showed a significant decrease in the three co-treated groups with Se (p < 0.001), ZnCl2 (p < 0.01) or simultaneously with both of them (p < 0.001) (Table 3).
Effects of Se and ZnCl2 on GST activity in the thyroid of K2Cr2O7-treated Wistar albino pregnant rats
GST is a key enzyme that plays a crucial role in initiating the neutralization of alkylating agents. K2Cr2O7 treatment caused a significant increase in GST activity in thyroid of pregnant Wistar rats, when compared with the control group (p < 0.001). The co-treatment of K2Cr2O7 with Se or ZnCl2 or concomitantly with both of them showed a significant decrease of GST activity as compared with K2Cr2O7-treated group (p < 0.001) (Table 3).
Effects of Se and ZnCl2 on DNA fragmentation in the thyroid of K2Cr2O7-treated Wistar albino pregnant rats
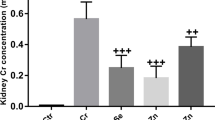
The alkaline comet assay is widely used to test the genotoxicity of chemicals; results of the total scoring of DNA damage are illustrated in Fig. 2. Results showed that K2Cr2O7 induced significant DNA damage compared with the control group (p < 0.001); it increased from 62.167 ± 0.749 to 311 ± 8.008. The three co-administrations, Cr + Se (p < 0.01), Cr + Zn (p < 0.001), and Cr + Se + Zn (p < 0.05), have reduced significantly the DNA fragmentation compared with the K2Cr2O7-treated group (311 ± 8.008 vs. 277.33 ± 5.457 or 210.833 ± 8.4 or 282.833 ± 5.474, respectively).
Effects of Se and ZnCl2 on thyroid DNA fragmentation induction in K2Cr2O7-treated Wistar albino pregnant rats. Values are expressed as mean ± SEM, (n = 6). p < 0.05 considered statistically significant, (*) statistically significant compared with the control. (+) statistically significant compared with the K2Cr2O7-treated group
Effects of Se and ZnCl2 on thyroid histoarchitecture in K2Cr2O7-treated Wistar albino pregnant rats
In order to evaluate the histological changes caused by K2Cr2O7 exposure and the potential protective effects of Se and ZnCl2 in restoring the thyroid gland histoarchitecture, we used H&E staining, The microscopic analysis of thyroid slices from the control group demonstrated normal thyroid parenchyma composed mainly by follicles of variable sizes with the predominance of larger ones (macrofollicles), filled with eosinophilic colloid composed mostly of thyroglobulin and surrounded by a monolayer of flattened epithelium lining composed of thyrocytes. The interfollicular space was thin contained connective tissue with capillaries beds and clustered parafollicular cells. The epithelial cell height was normal (Fig. 3a).
Photomicrographs of thyroid gland sections of preimplanted Wistar rats stained with H&E; thyroid parenchyma appeared well organized; covered by a capsule (c) and composed of follicles with the preponderance of macrofollicles (F), parafollicular cells (pF) and connective tissue (star) in the control group (a). thyroid sections of Cr-treated group (b–f) revealed disorganized thyroid parenchyma with the presence of microfollicles with swollen epithelial cells (SF), reduced colloidal matter (RC), wide interfollicular space (IFS), vacuolated colloidal matter (thin arrow), squamous hyperplasic epithelial cells (arrow head), collapsed and scattered follicles with exfoliated epithelium (CF), the dissolution of the connective tissue (CT), desquamated epithelial cells in colloid (double-headed arrow), degenerated follicles filled with squamous cells (DF), disruption of the epithelial lining (blue thick arrow). Thyroid sections of Cr + Se (g, h), Cr + Zn (i, j), and Cr + Se +Zn (k, l) showed remarkable improvement with the persistence of vacuolated colloid and thyrocytes, and some collapsed follicles
In the Cr-treated group, the thyroid parenchyma appeared less well organized, it was characterized by the predominance of microfollicles, the desquamation of the epithelial lining, and disruption of the follicular walls led to the collapse of many follicles. In addition, their colloid matter was filled by exfoliated cells. The epithelial cells seemed hyperplasic and vacuolated, the colloid was diluted and reduced remarkably, and the presence of the resorption vacuoles was evident at the periphery of follicles. The connective tissue was disintegrated, the follicular area was reduced and the interfollicular space was massively enlarged (Fig. 3b–f)
In the Cr + Se-treated group, the histopathological findings showed an improvement in restoring the normal architectural pattern of the thyroid gland, the follicles were rather large and filled with rich colloid, they were lined with a flat epithelium, and the interfollicular space was not apparent. However, there are some follicles where the lumen is filled with squamous cells and microfollicles with slightly vacuolated epithelial cells (Fig. 3g, h).
In the Cr + Zn-treated group, the microscopic analysis showed that apart from the presence of some vacuoles at the edges of the colloidal matter, the administration of ZnCl2 has almost restored the thyroid histoarchitecture of pregnant Wistar rats exposed to K2Cr2O7 (Fig. 3i, j).
In the Cr + Se + Zn-treated group, the thyroid slices showed the presence of microfollicles, their epithelial cells height was increased, and the colloid was exceedingly vacuolated. The collapsed follicles merged with neighboring ones due to the desquamation of the epithelial lining. Whereas the administration of ZnCl2 or Se alone in the thyroid of K2Cr2O7-treated pregnant rats exhibited marked protective effects, the concomitant administration of both of them was less effective in restoring thyroid histoarchitecture based on our findings (Fig. 3k, l).
Discussion
The results of the hormonal profile TSH, T3 and T4 revealed that the subcutaneous exposure to K2Cr2O7 provoked peripheral hypothyroidism as indicated by a significant increase in plasma TSH and a significant decrease in plasma T3 and T4 levels, even though during gestation, the thyroid hormonal status witnesses hyperthyroidism that accommodates the presence of the fetal and placental tissues. Indeed, the gestational exposure to methimazole induced maternal hypothyroidism, which affected thyroid function and growth rate in suckling pups (Ben Amara et al. 2009). Consistently, multiple related studies have shown that K2Cr2O7 caused hypothyroidism by disturbing T3, T4, and TSH levels in Wistar albino male rats (ElBakry and Tawfik 2014; Mahmood et al. 2010). Additionally, K2Cr2O7 can affect the anterior pituitary gland and alters the hypothalamic-pituitary axis along with the proper functioning of the endocrine system (Quinteros et al. 2007).
Indeed, the reduction of the T4 plasma level observed in this study can be attributed to the inhibition of its synthesis by the thyroid. Thyroid iodine uptake is an essential step in gland hormonogenesis. Hence, it may be vulnerable to the damaging effects of thyroid disruptors (Boas et al. 2006). It was reported that methimazole induced a decrease in the intrathyroidal iodine pool through impairing its uptake in the thyroid gland of lactating Wistar rats (Ben Amara et al. 2009). Further, it was indicated in several studies that methimazole exhibits hypothyroidism by depleting the enzymatic activity of thyroid peroxidase (TPO) in catalyzing the incorporation of iodide into tyrosine residues on thyroglobulin (Dorea 2002). Instead, methimazole competes with thyroglobulin and acts as a substrate for TPO. Once iodinated, it is metabolized peripherally, resulting in thyroid iodine store reduction (Mitchell and Pearce 2019). In addition to that, several chemicals have disturbed the human TPO activity in vitro (Song et al. 2012). Furthermore, both TPO and dual oxidase are inactivated by the increased amounts of H2O2 generated via oxidative stress, causing a conformational change and leading to a less active form of the enzymes (Fortunato et al. 2010). As well, cadmium, another thyroid-disrupting heavy metal have altered gene expression related to the hypothalamic-pituitary-thyroid axis by upregulating TSH and thyroglobulin mRNA, and downregulating the expression of both thyroid hormone receptors ThRβ and ThRα in fish (Li et al. 2014). Moreover, many authors suggested that cadmium influences the production and /or the secretion of T4 by the induction of oxidative phosphorylation disorders in the mitochondria of thyrocytes (Hammouda et al. 2008; Prakash et al. 1997; Yoshizuka et al. 1991).
The declined plasma T3 levels demonstrated by our results, suggest that K2Cr2O7 influences negatively the enzymes implicated in the extrathyroidal metabolism of thyroid hormones such as the 5′ monodeiodinase, which catalyzes the conversion of T4 to the active T3. This can be confirmed by previous investigations on rats and mice treated with cadmium (Chaurasia et al. 1996; Gupta et al. 1997). Consequently, the inhibition of the 5′ monodeiodinase is associated with cadmium-induced lipid peroxidation, which affects the cell membrane integrity including the enzyme’s binding sites (Chaurasia et al. 1996). This hormonogenesis impairment might be explained, according to previous studies, by the fact that CrVI exerts its toxicity by generating oxidative stress. Indeed, thyrocytes are supposed to be highly sensitive to disruptions in the endoplasmic reticulum homeostasis due to their secretory function. Therefore, the expression of genes involved in thyroid hormone synthesis and their transcriptional regulators are downregulated in thyrocytes during endoplasmic reticulum stress, causing hormone synthesis depletion (Wen et al. 2017). Accordingly, the present study revealed that K2Cr2O7-induced thyroidal oxidative stress in pregnant Wistar rats.
Consistently, it was reported that K2Cr2O7 induced hepatic oxidative stress by mitochondrial dysfunction and antioxidant defense system depletion (Garcia-Nino et al. 2013). Lipids and fatty acids are sensitive to the oxidative action of free radicals. Therefore, the amplified production of free radicals associated with CrVI toxicity is responsible for the increased lipid peroxidation in the uterus, ovary, kidney, and liver of rabbit does (Mary-Momo et al. 2019), which concurs with the increased malondialdehyde level in our study. Hence, metal ions are thought to exacerbate the oxidation of polyunsaturated fatty acids by the means of generated ROS; they attack fatty acids in cellular membranes and induce lipid peroxidation, which leads to membrane structural and functional deterioration (Sole et al. 1990; Zhang et al. 2009).
In addition to their cytotoxic potential, lipid peroxidation products along with free radicals can alter protein structure (Poli et al. 2008). According to our study, K2Cr2O7 increased protein carbonyl level in the thyroid. Protein carbonylation is the outcome of proteins’ oxidative modification by free radicals, which leads to their structural integrity and catalytic activity impairment (Stadtman and Levine 2006); this could be attributed to heavy metal-induced oxidative stress, which is considered to be a prime pathway for protein homeostasis disturbance (Winterbourn and Hampton 2008). Indeed, CrVI is known to trigger oxidative protein damage. In addition, it affects mRNA translation thus results in a defective protein structure (Holland et al. 2007; Sumner et al. 2005).
The interaction of free radicals, aldehydes derived from lipid peroxidation, and protein carbonyls with DNA may lead to the hydrolysis of chemical bonds, resulting in DNA fragmentation (De Bont and van Larebeke 2004). Hence, metals tend to bind primarily with DNA and nuclear proteins, thus leading to the oxidative deterioration of biomolecules (Flora et al. 2008). Indeed, it has been reported that DNA is potentially susceptible to Fenton reaction substrates. Consistently, increased DNA damage and oxidized nucleosides were detected in both nuclear and mitochondrial DNA of porcine thyroid under oxidative stress conditions (Stepniak et al. 2013; Karbownik-Lewińska et al. 2012). CrVI imposes its genotoxicity and mutagenicity by different mechanisms; it binds to DNA and affects genome stability by interacting with base pairing and stacking process which allows mutations to occur (Fang et al. 2014). Thus, DNA oxidative damage has been observed in several tissues after CrVI oral exposure in mice (Sekihashi et al. 2001); these findings coincided with the genotoxic effect of K2Cr2O7 observed in the current study. Interestingly, the increased oxidative damage to macromolecules in the thyroid gland upon exposure to exogenous or endogenous prooxidants is considered to be a substantial contributor to the development of different thyroid diseases (Karbownik-Lewinska and Kokoszko-Bilska 2012).
Thyroid cells are thought to have an important antioxidant enzymatic system that is responsible for degrading and scavenging free radicals (Bjijrkman and Ekholm 1995). In response to oxidative stress against toxicants, the activities of the antioxidant enzymes are upregulated to protect cellular components against oxidation (Kubrak et al. 2010), which is confirmed by our findings, the activities of CAT, SOD, GPx, and GST were increased significantly in the thyroid of pregnant rats.
The detoxifying process of ROS commonly implies the conversion of superoxide to hydrogen peroxide catalyzed by SOD (Fridovich 1986). Hydrogen peroxide is either decomposed to water and disulfide glutathione by GPx (Kesheri et al. 2014) or metabolized to water and free oxygen by CAT (Knight, 1997). Thus, the restorative effect of cell redox homeostasis exhibited by these enzymes could explain their increased activities upon K2Cr2O7 exposure in our study. Similarly, CrVI exposure increased SOD and GPx mRNA levels in zebra fish to eliminate free radicals, which concurs with our results (Jin et al. 2015). Moreover, the activities of the antioxidant enzymes SOD and CAT were increased upon CrVI administration in rats and fish tissues (Patlolla et al. 2009; Kumari et al. 2014). GST plays an essential role in initiating the neutralization of alkylating agents by catalyzing the conjugation of toxicants with the SH group of glutathione, thereby facilitating their elimination (Dorval et al. 2003). Regarding the increased GST activity determined in the present study, a similar increase in GST activity upon CrVI exposure was determined in fish (Ciacci et al. 2012; Kim and Kang 2016).
Concordantly to K2Cr2O7-induced thyroidal oxidative stress observed in the present study, the results of the histological examination showed that K2Cr2O7 has altered the thyroid histoarchitecture. Indeed, the thyroid parenchyma appeared highly disorganized, with unevenness in follicles diameter and form. The presence of microfollicles was more preponderant than the larger ones. These findings were in accordance with other studies (Mahmood et al. 2010; Elbakry and Tawfik 2014). In addition, the desquamation of the epithelial lining of the follicles was noticed, which led to the disruption of the follicular walls and provoked their collapse. Moreover, the colloid that serves as a pool for thyroid hormones seemed to be diluted and reduced considerably, which may explain the decline in thyroid hormone levels in pregnant Wistar rats and as reported previously by Elbakry and Tawfik 2014. Owing to the degeneration of follicles and the reduction of the follicular area, the interfollicular space has been enlarged massively. This widening has been seen in the thyroid of male, nonpregnant rats and mice, respectively, treated with K2Cr2O7 (Hala et al. 2016), aluminum (Aktac and Bakar 2002), and cadmium (Pilat Marcinkiewicz et al. 2003). As regards thyrocytes, they appeared hyperplasic and highly vacuolated, as reported also by Hala et al. 2016. Thyrocytes hyperplasia may be due to the resultant increase in circulating levels of TSH. Indeed, this later is known as the primary stimulus for thyroid hormonogenesis. Herein, the overstimulation of thyroid follicular epithelium may lead to thyrocytes hypertrophy and hyperplasia (Gaide Chevronnay et al. 2015). Additionally, thyroid weight has increased significantly upon K2Cr2O7 exposure. Indeed, several thyroid-disrupting chemicals have caused an increase in thyroid absolute weight (Ben Amara et al. 2009; Yang et al. 2013). It is well known that thyroid hormones promote growth. Thus, the decrease in maternal body weight observed in the current study could be attributed to K2Cr2O7-induced hypothyroidism. Consistently, similar findings were reported in nickel chloride–exposed preimplanted Wistar rats and pups of methimazole-treated pregnant rats (Adjroud 2013; Ben Amara et al. 2009).
Previous studies have shown that the administration of antioxidant substances has mitigated CrVI-induced toxicity (Chandra et al. 2007; Goodarzi et al. 2017; Saber et al. 2015). Thus, the co-administration of Se and ZnCl2 or both of them combined has counterbalanced K2Cr2O7-induced thyroid hormonal disruption, oxidative stress, DNA fragmentation, and histological alterations in pregnant Wistar rats. ZnCl2 cotreatment improved plasma TSH, T3, and T4 levels significantly; this could be explained by the indispensability of Zn, not only for the activity of the enzymes that convert T4 to its active form through its antioxidant properties but also for the synthesis of TSH in the anterior hypophysis and the thyrotropin-releasing hormone in the hypothalamus (Brandao-Neto et al. 2006; Danforth and Burger 1989; Pekary et al. 1991). Moreover, thyroid structural integrity was restored remarkably in the Cr + Zn group. Similarly, it was reported that Zn administration in pregnant and lactating rats has attenuated arsenic-induced kidney histological lesions in their pups (Nassiry Zarrin Ghabaee et al. 2017). Moreover, supporting our results, it was reported that Zn supply alone or in combination with Se has improved body weight gain and reversed relative thyroid weight changes in cadmium-treated Wistar rats (Hammouda et al. 2008). Further to this, Zn was found to exhibit a protective effect against nickel-induced glutathione and lipid peroxidation in brain cells of mice (Šulinskienė et al. 2019). As well, Zn plays a vital role in cell defense against free radicals; as being a cofactor of Cu/Zn SOD, a regulator of GPx activity, and a potent inducer of metallothionein expression (Cruz et al. 2015; Lima and Sampaio 2011; Oteiza et al. 1996), the antiradical efficacy of Zn against cadmium-induced oxidative stress, apoptosis, and necrosis was reported by several studies (Jacquillet et al. 2006; Jihen et al. 2010; Rogalska et al. 2009). Furthermore, Zn exhibited a marked impact in maintaining DNA integrity by preventing its oxidative damage and promoting its repair (Ho et al. 2003). Thereby, the prophylactic treatment with Zn promoted DNA repair in HeLa cells by revoking the inhibition of DNA-protein interactions exerted by cadmium (Hartmann and Hartwig 1998).
Analogously, based on our study, Se cotreatment enhanced thyroid hormonal and histological status and exhibited antioxidant and genoprotective effects. Indeed, this improvement can be explained by the fact that Se is a key trace element that takes a major part in maintaining thyroid homeostasis. Thus, enhanced T3 plasma concentration in Se-cotreated group could be attributed to an upregulation of DIOI (deiodinase I) and an increase in the conversion of T4 to T3 (Thomson et al. 2009). Therefore, the alterations in the quantity of DIOI mRNA in rat liver caused DIOI activity impairment and thyroid dysfunction (Berry et al. 1991). Likewise, when Se was supplemented to the diet of methimazole-treated rats, the T4 plasma level was ameliorated partially, while the T3 level was returned to normal. As well, Se promoted a partial recovery in body weights of both mothers and pups, which concurs with our findings (Ben Amara et al. 2009). Additionally, in agreement with our results, Hala et al. (2016) reported that Se prevented CrVI-induced morphological alterations in the thyroid gland. Further, it was reported that Se counterbalanced lead acetate–induced lipid peroxidation and glutathione reduction in the thyroid of male rats (Atteia et al. 2018). Similarly, Hassanin et al. (2013) highlighted the antioxidant and anti-apoptotic effects of Se against CrVI-induced oxidative stress and fibrosis, this concurs with the fact that Se participates actively in the antioxidant system. It may be considered an exogenous scavenger of ROS formed in the thyroid after CrVI exposure (Hala et al. 2016). Moreover, it is incorporated in many Se-dependent antioxidant enzymes that protect thyrocytes from oxidative damage (Winther et al. 2015). Therefore, improving free radical scavenging, antioxidant defense, and metal chelating were proven to be major contributors to Se-induced protection against K2Cr2O7 toxicity (Soudani et al. 2010).
Although the synergistic effect of Se and Zn was more effective against cadmium-induced thyroid dysfunction and kidney oxidative damage in male Wistar rats (Hammouda et al. 2008; Messaoudi et al. 2009), the combined administration of Se and ZnCl2 in this study exhibited less thyroprotective effects in pregnant rats exposed to K2Cr2O7 compared with the co-treatment with Se or ZnCl2 alone. In this way, several studies reported that the interaction between exogenous antioxidants may exert prooxidants effects at high doses or in the presence of metal ions (Bouayed and Bohn 2010).
Conclusions
In conclusion, the present study revealed that the acute exposure to K2Cr2O7 via the subcutaneous administration induced hypothyroidism, oxidative stress, DNA damage, and histological alterations in the thyroid gland. However, the cotreatment of Se and ZnCl2 has mitigated K2Cr2O7-induced thyrotoxicity by promoting free radical scavenging and restoring thyroid homeostasis in pregnant Wistar rats. Owing to the growing use of Cr compounds in the industrial field, the exposure risk to CrVI in workers increased. Thus, Se and Zn could be used as protective agents to counterbalance CrVI-induced toxic effects. However, effective thyro-protection requires further investigations using different doses and routes of exposure on the synergetic interaction of Se and Zn.
Data availability
The data that support the findings of this study are available from the corresponding author upon request.
References
Adjroud O (2009) Effects of potassium dichromate on haematological parameters in female and male Wistar albino rats. Ass Univ Bull Environ Res 12:2
Adjroud O (2010) Protective effects of selenium against potassium dichromate-induced hematotoxicity in female and male Wistar albino rats. Ann Toxicol Anal 22:165–172
Adjroud O (2013) The toxic effects of nickel chloride on liver, erythropoiesis, and development in Wistar albino preimplanted rats can be reversed with selenium pretreatment. Environ Toxicol 28(5):290–298. https://doi.org/10.1002/tox.20719
Aktac T, Bakar E (2002) The histopathological changes in the mouse thyroid depending on the aluminium. Cell Mol Biol 1:69–72
Atteia HH, Arafa MH, Prabahar K (2018) Selenium nanoparticles prevent lead acetate-induced hypothyroidism and oxidative damage of thyroid tissues in male rats through modulation of selenoenzymes and suppression of miR-224. Biomed Pharmacother 99:486–491
Bancroft JD, Gamble M (2008) Theory and practice of histological techniques, 6th edn. Churchill Livingstone, Elsevier, China
Barceloux DG (1999) Zinc. J Toxicol Clin Toxicol 37(2):279–292
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Ben Amara I et al (2009) Effect of selenium on hypothyroidism induced by methimazole (MMI) in lactating rats and their pups. Acta Biol Hung 61(2):145–157
Ben Hamida F, Soussia L, Guermazi F, Rebai T, Zeghal N (2001) Effets de deux antithyroïdiens (propyltiouracile et perchlorate) sur la fonction thyroïdienne de la souris en période d’allaitement. Ann Endocrinol 62:446–453
Berry MJ, Banu L, Larsen PR (1991) Type I iodothyronine deiodinase is a selenocysteine-containing enzyme. Nature 349(6308):438–440
Betsy A, Binitha MP, Sarita S (2013) Zinc deficiency associated with hypothyroidism: an overlooked cause of severe alopecia. Int J Trichology 5(1):40–42
Bjijrkman U, Ekholm R (1995) Hydrogen peroxide degradation and glutathione peroxidase activity in cultures of thyroid cells. Mol Cell Endocrinol 111:99–107
Boas M, Feldt-Rasmussen U, Skakkebæk NE, Main KM (2006) Environmental chemicals and thyroid function. Eur J Endocrinol 154:599–611
Bouayed J, Bohn T (2010) Exogenous antioxidants-double-edged swords in cellular redox state: health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxidative Med Cell Longev 3(4):228–237
Bradford M (1979) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72:248–254
Brandao-Neto J et al (2006) Lack of acute zinc effect on thyrotropin-releasing hormone–stimulated thyroid-stimulating hormone secretion during oral zinc tolerance test in healthy men. Nutr Res 26:493–496
Buha A, Antonijević B, Bulat Z, Jaćević V, Milovanović V, Matović V (2013) The impact of prolonged cadmium exposure and co-exposure with polychlorinated biphenyls on thyroid function in rats. Toxicol Lett 221:83–90
Chandra KA et al (2007) Effect of curcumin on chromium-induced oxidative damage in male reproductive system. Environ Toxicol Pharmacol 24(2):160–166
Chaurasia SS, Gupta P, Kar A, Maiti PK (1996) Free radical mediated membrane perturbation and inhibition of type-I iodothyronine 59-monodeiodinase activity by lead and cadmium in rat liver homogenate. Biochem Mol Biol Int 39:765–770
Ciacci C et al (2012) Effects of sublethal, environmentally relevant concentrations of hexavalent chromium in the gills of Mytilus gallo-provincialis. Aquat Toxicol 120–121:109–118
Clairbone A (1985) Catalase activity. Press Boca Rton FL, Handbook of methods for oxygen radical research. CRC, pp 283–284
Cohen M et al (1993) Mechanisms of chromium carcinogenicity and toxicity. Crit Rev Toxicol 23:255–281
Collins AR, Dusinská M, Gedik CM, Stĕtina R (1996) Oxidative damage to DNA: do we have a reliable biomarker? Environ Health Perspect 104:465–469
Cruz KJ, de Oliveira AR, Marreiro Ddo N (2015) Antioxidant role of zinc in diabetes mellitus. World J Diabetes 6(2):333–337
Danforth E Jr, Burger AG (1989) The impact of nutrition on thyroid hormone physiology and action. Annu Rev Nutr 9:201–227
De Bont R, van Larebeke N (2004) Endogenous DNA damage in humans: a review of quantitative data. Mutagenesis 19(3):169–185
Dorea JG (2002) Iodine nutrition and breast feeding. J Trace Elem Med Biol 16:207–220
Dorval J, Leblond VS, Hontela A (2003) Oxidative stress and loss of cortisol secretion in adrenocortical cells of rainbow trout (Oncorhynchusmykiss) exposed in vitro to endosulfan, an organochlorine pesticide. Aquat Toxicol 63:229–241
Dumitrescu AM, Liao XH, Abdullah MSY, Lado-Abeal J, Majed FA, Moeller LC, Boran G, Schomburg L, Weiss RE, Refetoff S (2005) Mutations in the SBP2 gene produce abnormal thyroid hormone metabolism in man. Nat Genet 37(11):1247–1252
ElBakry RH, Tawfik SM (2014) Histological study of the effect of potassium dichromate on the thyroid follicular cells of adult male albino rat and the possible protective role of ascorbic acid (vitamin C). J Microsc Ultrastruct. Environ Monit 14:2121–2126
Elia A, Dörr A, Mastrangelo C, Prearo M, Abete M (2006) Glutathione and antioxidant enzymes in the hepatopancreas of crayfish Procambarus clarkii (Girard, 1852) of Lake Trasimeno (Italy). Bulletin Francais de la Pêche ET de la Protection des Milieux Aquatiques, pp 1351–1361. https://doi.org/10.1051/kmae:2006040
Elia A, Dörr A, Prearo M, Taticchi M, Abete M (2007) Detoxification enzymes of freshwater crayfish Procambarusclarkii fed a diet enriched in selenium: preliminary results. Marine Freshwater Physiol Behav 40:195–199
Fang Z, Zhao M, Zhen H, Chen L, Shi P, Huang Z (2014) Genotoxicity of tri- and hexavalent chromium compounds in vivo and their modes of action on DNA damage in vitro. PLoS One 9(8):e103194
Farooqi L, Mazeto GMFS, Shuhama T, Brandão-Neto J (2000) Effects of a single venous dose of zinc on thyroid status in healthy individuals and patients with Graves’ disease. Metal-Based Drugs 7:151–155
Flohe L, Gunzler W (1984) Assays of glutathione peroxidase. Methods Enzymol 105:114–121
Flora SJ (2009) Structural, chemical and biological aspects of antioxidants for strategies against metal and metalloid exposure. Oxidative Med Cell Longev 2(4):191–206
Flora SJS, Mittal M, Mehta A (2008) Heavy metal induced oxidative stress and its reversal by chelation therapy. Indian J Med Res 128:501–523
Fortunato RS, Lima de Souza EC, Hassani RAE, Boufraqech M, Weyemi U, Talbot M, Lagente-Chevallier O, de Carvalho DP, Bidart JM, Schlumberger M, Dupuy C (2010) Functional consequences of dual oxidase-thyroperoxidase interaction at the plasma membrane. J Clin Endocrinol Metab 95(12):5403–5411
Fridovich I (1986) Biological effects of the superoxide radical. Arch Biochem Biophys 247(1):1–11
Gaide Chevronnay HP, Janssens V, van der Smissen P, Liao XH, Abid Y, Nevo N, Antignac C, Refetoff S, Cherqui S, Pierreux CE, Courtoy PJ (2015) A mouse model suggests two mechanisms for thyroid alterations in infantile cystinosis: decreased thyroglobulin synthesis due to endoplasmic reticulum stress/unfolded protein response and impaired lysosomal processing. Endocrinology 156(6):2349–2364. https://doi.org/10.1210/en.2014-1672
García-Niño WR et al (2013) Curcumin pretreatment prevents potassium dichromate- induced hepatotoxicity, oxidative Stress, decreased respiratory complex I activity, and membrane permeability transition pore opening. Evid Based Complement Alternat Med 2013:424692
Gilbert ME, Rovet J, Chen Z, Koibuchi N (2012) Developmental thyroid hormone disruption: prevalence, environmental contaminants and neurodevelopmental consequences. Neurotoxicol 33:842–852
Goodarzi Z, Karami E, Ahmadizadeh M (2017) Simvastatin attenuates chromium-induced nephrotoxicity in rats. Nephropathol 6(1):5–9
Gupta P, Chaurasia S, Maiti P, Kar A (1997) Cadmium induced alterations in extrathyroidal conversion of thyroxine to triiodothyronine by type-I iodothyronine 59-monodeiodinase in male mouse. Horm Metab Res 29:151–152
Hadie SNH, Abdul-Manan H, Abdulla S (2013) Thyroid gland resection in euthanised rat: a practical guide. Intern Med J 20(1):1–4
Hala ZE, Ibrahim KR, Hemmat HG (2016) A histological study on the possible protective effect of selenium against chromium-induced thyrotoxicity in adult male albino rats. The Egyptian Journal of Histology 39(1)
Hammouda F, Messaoudi I, el Hani J, Baati T, Saïd K, Kerkeni A (2008) Reversal of cadmium-induced thyroid dysfunction by selenium, zinc, or their combination in rat. Biol Trace Elem Res 126:194–203
Hartmann M, Hartwig A (1998) Disturbance of DNA damage recognition after UV-irradiation by nickel (II) and cadmium(II) in mammalian cells. Carcinogenesis 19:617–621
Hassanin KM, Abd El-Kawi SH, Hashem KS (2013) The prospective protective effect of selenium nanoparticles against chromium-induced oxidative and cellular damage in rat thyroid. Int J Nanomedicine 8:1713–1720
Ho E, Courtemanche C, Ames BN (2003) Zinc deficiency induces oxidative DNA damage and increases P53 expression in human lung fibroblasts. J Nutr 133(8):2543–2548
Holland S, Lodwig E, Sideri T, Reader T, Clarke I, Gkargkas K, Hoyle DC, Delneri D, Oliver SG, Avery SV (2007) Application of the comprehensive set of heterozygous yeast deletion mutants to elucidate the molecular basis of cellular chromium toxicity. Genome Biol 8(12):R268
IARC (International Agency for Research on Cancer) (1990) Chromium, nickel and weldings. Monographs on the evaluation of carcinogenic risks to humans. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans 49: 1- 648.
Jacquillet G et al (2006) Zinc protects renal function during cadmium intoxication in the rat. Am J Physiol Ren Physiol 290:127–137
Jahnabi S, Choudhuri S, Choudhuri D (2017) Effect of subchronic exposure to chromium on hematological and biochemical parameters of male albino rat. Asian J Pharm Clin Res 10(5):345–348
Jihen H et al (2010) Cadmium retention increase: a probable key mechanism of the protective effect of zinc on cadmium-induced toxicity in the kidney. Toxicol Lett 196(2):104–109
Jin Y, Liu Z, Liu F, Ye Y, Peng T, Fu Z (2015) Embryonic exposure to cadmium (II) and chromium (VI) induce behavioral alterations, oxidative stress and immunotoxicity in zebrafish (Danio rerio). Neurotoxicol Teratol 48:9–17
Käkelä R, Käkelä A, Hyvärinen H (1999) Effects of nickel chloride on reproduction of the rat and possible antagonistic role of selenium. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol 123:27–37
Karbownik-Lewińska M et al (2012) High level of oxidized nucleosides in thyroid mitochondrial DNA; damaging effects of Fenton reaction substrates. Thyroid Res 5:24
Karbownik-Lewińska M, Kokoszko-Bilska A (2012) Oxidative damage to macromolecules in the thyroid - experimental evidence. Thyroid Res 5:25
Kesheri M, Kanchan S, Sinha RP (2014) Isolation and in silico analysis of Fe-superoxide dismutase in the cyanobacterium Nostoc commune. Gene 553(2):117–125
Khorsandi K, Rabbani-Chadegani A (2013) Studies on the genotoxic effect of chromium oxide (Cr VI): Interaction with deoxyribonucleic acid in solution. Mutat Res 750(1-2):105–110
Kim JH, Kang JC (2016) Oxidative stress, neurotoxicity, and metallothionein (MT) gene expression in juvenile rock fish Sebastes schlegelii under the different levels of dietary chromium(Cr6+) exposure. Ecotox Environ Safe 125:78–84
Knight JA (1997) Reactive oxygen species and the neuro-degenerative disorders. Ann Clin Lab Sci 27:11–25
Kryukov GV, Castellano S, Novoselov SV, Lobanov AV, Zehtab O, Guigó R, Gladyshev VN (2003) Characterization of mammalian selenoproteomes. Science 300:1439–1443
Kubrak OI et al (2010) Chromium effects on free radical processes in gold fish tissues: comparison of Cr (III) and Cr (VI) exposures on oxidative stress markers, glutathione status and antioxidant enzymes. Comp Biochem Phys C 152(3):360–370
Kumari K, Khare A, Dange S (2014) The applicability of oxidative stress biomarkers in assessing chromium induced toxicity in the fish Labeo rohita. Biomed Res Int 2014:782493
Levis AG Bianchi V (1982) Mutagenic and cytogenetic effects of chromium compounds, in S.Langard (Ed.), Biological and environmental aspects of chromium, Amsterdam 171-208.
Li ZH, Chen L, Wu YH, Li P, Li YF, Ni ZH (2014) Effects of waterborne cadmium on thyroid hormone levels and related gene expression in Chinese rare minnow larvae. Comp Biochem Physiol Part C Toxicol Pharmacol 161:53–57
Lima VBS, Sampaio FA (2011) Parameters of glycemic control and their relationship with zinc concentrations in blood and with superoxide dismutase enzyme activity in type 2 diabetes patients. Arq Bras Endocrinol Metabol 55:701–707
Mahmood T, Qureshi IZ, Iqba MJ (2010) Histopathological and biochemical changes in rat thyroid following acute exposure to hexavalent chromium. Histol Histopathol 25(11):1355–1370
Mary Momo CM, Ferdinand N, Omer Bebe N, Alexane Marquise M, Augustave K, Bertin Narcisse V, Herve T, Joseph T (2019) Oxidative effects of potassium dichromate on biochemical, hematological characteristics, and hormonal levels in rabbit doe (Oryctolagus cuniculus). Vet Sci 6(1):30
Mercier Y, Gatellier P, Renerre M (2004) Lipid and protein oxidation in vivo, and antioxidant potential in meat from Charolais cows finished on pasture or mixed die. Meat Sci 66:467–473
Messaoudi I, El Heni J, Hammouda F et al (2009) Protective effects of selenium, zinc, or their combination on cadmium-induced oxidative stress in rat kidney. Biol Trace Elem Res 130:152–161. https://doi.org/10.1007/s12011-009-8324-y
Miller MD, Crofton KM, Rice DC, Zoeller RT (2009) Thyroid-disrupting chemicals: interpreting upstream biomarkers of adverse outcomes. Environ Health Perspect 117(7):1033–1041
Mitchell AL, Pearce SHS (2019) Autoimmune thyroid diseases. In: Rich RR, Fleisher TA, Shearer WT, Schroeder HW, Frew AJ, Weyand CM (eds) Clinical immunology, principles and practice, 5th edn. Elsevier, Philadelphia, pp 947–956
Monteiro C, Conceição S, Bastos V, Oliveira H (2019) Cr (VI) -induced genotoxicity and cell cycle arrest in human osteoblast cell line MG-63. J Appl Toxicol 1–9.
Nasiry Zarrin Ghabaee D, Talebpour Amiri F, Esmaeelnejad Moghaddam A, Khalatbary AR, Zargari M (2017) Administration of zinc against arsenic-induced nephrotoxicity during gestation and lactation in rat model. J Nephropathol 6(2):74–80
Ohkawa H, Ohishi N, Yagi K (1979) Assay of lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Oteiza PL, Olin KL, Fraga CG, Keen CL (1996) Oxidant defense systems in testes from zinc deficient rats. Exp Biol Med 213:85–91
Paksy K, Varga B, Lázár P (1996) Zinc protection against cadmium-induced infertility in female rats. Effect of zinc and cadmium on the progesterone production of cultured granulosa cells. BioMetals 10:27–36
Patlolla AK, Barnes C, Yedjou C, Velma VR, Tchounwou PB (2009) Oxidative stress, DNA damage, and antioxidant enzyme activity induced by hexavalent chromium in Sprague-Dawley rats. Environ Toxicol 24(1):66–73
Pekary AE, Lukaski HC, Mena I, Hershman JM (1991) Processing of TRH precursor peptides in rat brain and pituitary is zinc dependent. Peptides 12:1025–1032
Pilat-Marcinkiewicz B et al (2003) Structure and function of thyroid follicular cells in female rats chronically exposed to cadmium. Bulletin-Veterinary Institute in Pulwy 47:157–163
Poli G, Biasi F, Leonarduzzi G (2008) 4-hydroxynonenal—protein adducts: a reliable biomarker of lipid oxidation in liver diseases. Mol Asp Med 29:67–71
Prakash P et al (1997) Superoxide anion radical production as a cadmium-mediated mechanism of toxicity in avian thyroid: An electron spins resonance study by spin trapping. Comp Biochem Physiol Part C Pharmacol Toxicol Endocrinol 118:89–95
Prasad S (2013) Discovery of human Zinc deficiency: Its impact on human health and disease. Adv Nutr 2:176–190
Prasad AS, Bao B, Beck FWJ, Kucuk O, Sarkar FH (2004) Antioxidant effect of zinc in humans. Free Radic Biol Med 37:1182–1190
Quinteros F, Poliandri A, Machiavelli L, Cabilla JP, Duvilanski BH (2007) In vivo and in vitro effects of chromium VI on anterior pituitary hormone release and cell viability. Toxicol Appl Pharmacol 218(1):79–87
Richelmi P, Baldi C (1984) Blood levels of hexavalent chromium in rats. “In vitro” and “in vivo” experiments. Int J Environ Anal Chem 17(3-4):181–186
Rodrigues-Pereira P et al. (2015) Influence of Organotin on thyroid morphophysiological status. J Environ Health Sci.
Rogalska J, Brzóska MM, Roszczenko A, Moniuszko-Jakoniuk J (2009) Enhanced zinc consumption prevents cadmium-induced alterations in lipid metabolism in male rats. Chem Biol Interact 177:142–152
Saber TM, Farag MR, Cooper RG (2015) Ameliorative effect of extra virgin olive oil on hexavalent chromium-induced nephrotoxicity and genotoxicity in rats. Rev Med Vet 166(1-2):11–19
Sekihashi K, Sasaki T, Yamamoto A, Kawamura K, Ikka T, Tsuda S, Sasaki YF (2001) A comparison of intraperitoneal and oral gavage administration in comet assay in mouse eight organs. Mutat Res-Gen Tox En 493(1-2):39–54
Singh NP, McCoy MT, Tice RR, Schneider EL (1988) A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 175:184–191
Sole J et al (1990) In vivo effects of nickel and cadmium in rats on lipid peroxidation and ceruloplasmin activity. Bull Environ Contam Toxicol 44:686–691
Song M, Kim YJ, Park YK, Ryu JC (2012) Changes in thyroid peroxidase activity in response to various chemicals. J Environ Monit 14(8):2121–2126
Soudani N, Sefi M, Ben Amara I, Boudawara T, Zeghal N (2010) Protective effects of Selenium (Se) on Chromium (VI) induced nephrotoxicity in adult rats. Ecotoxicol Environ Saf 73(4):671–678. https://doi.org/10.1016/j.ecoenv.2009.10.002
Soudani N, Ben Amara I, Sefi M, Boudawara T, Zeghal N (2011) Effects of selenium on chromium (VI)-induced hepatotoxicity in adult rats. Exp Toxicol Pathol 63(6):541–548
Stadtman ER, Levine RL (2006) Protein Oxidation. Ann N Y Acad Sci 899(1):191–208
Stepniak J et al (2013) Membrane lipids and nuclear DNA are differently susceptive to Fenton reaction substrates in porcine thyroid. Toxicol in Vitro 27:71–78
Šulinskienė J et al. (2019) Effect of zinc on the oxidative stress biomarkers in the brain of nickel-treated mice. Oxidative Med Cell Longev
Sumner ER, Shanmuganathan A, Sideri TC, Willetts SA, Houghton JE, Avery SV (2005) Oxidative protein damage causes chromium toxicity in yeast. Microbiology 151:1939–1948
Thomson CD, Campbell JM, Miller J, Skeaff SA, Livingstone V (2009) Selenium and iodine supplementation: effect on thyroid function of older New Zealanders. Am J Clin Nutr 90:1038–1046
Triggiani V et al (2009) Role of iodine, selenium and other micronutrients in thyroid function and disorders. Endocr Metab Immune 9(3):277–294
Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M (2006) Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact 160(1):1–40
Wen G, Ringseis R, Eder K (2017) Endoplasmic reticulum stress inhibits expression of genes involved in thyroid hormone synthesis and their key transcriptional regulators in FRTL-5 thyrocytes. PLoSONE 12(11):e0187561
Winterbourn CC, Hampton MB (2008) Thiol chemistry and specificity in redox signalling. Free Radic Biol Med 45(5):549–561
Winther KH, Bonnema SJ, Cold F, Debrabant B, Nybo M, Cold S, Hegedüs L (2015) Does selenium supplementation affect thyroid function? Results from a randomized, controlled, double-blinded trial in a Danish population. Eur J Endocrinol 172:657–667
Yang H, Zhang W, Kong Q, Liu H, Sun R, Lin B, Zhang H, Xi Z (2013) Effects of pubertal exposure to thiazole-Zn on thyroid function and development in female rats. Food Chem Toxicol 53:100–104
Yildiz A, Kaya Y, Tanriverdi O (2019) Effect of the interaction between selenium and zinc on DNA repair in association with cancer prevention. J Cancer Prev 24(3):146–154. https://doi.org/10.15430/JCP.2019.24.3.146
Yoshizuka M, Mori N, Hamasaki K, Tanaka I, Yokoyama M, Hara K, Doi Y, Umezu YI, Araki H, Sakamoto Y, Miyazaki M, Fujimoto S (1991) Cadmium toxicity in the thyroid gland of pregnant rats. Exp Mol Pathol 55:97–104
Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, Dong MQ, Han J (2009) RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 325(5938):332–336
Funding
This work was supported by the DGRSDT/MESRS (code: N ° E2212600).
Author information
Authors and Affiliations
Contributions
Anfal Fedala and Ounassa Adjroud designed the experiment; Anfal Fedala and Rim Timoumi performed the experiment; Anfal Fedala, Ounassa Adjroud, Salwa Abid-Essefi, and Rim Timoumi analyzed the data; Anfal Fedala and Ounassa Adjroud wrote the manuscript; Ounassa Adjroud and Salwa Abid Essefi revised the manuscript; and all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
All procedures were approved by the Institutional Animal Care and Use Committee of Batna University.
Informed consent
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Responsible editor: Mohamed M. Abdel-Daim
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fedala, A., Adjroud, O., Abid-Essefi, S. et al. Protective effects of selenium and zinc against potassium dichromate–induced thyroid disruption, oxidative stress, and DNA damage in pregnant Wistar rats. Environ Sci Pollut Res 28, 22563–22576 (2021). https://doi.org/10.1007/s11356-020-12268-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-12268-9