Abstract
The present investigation is an attempt to assess the impact of untreated methyl orange and Oedogonium subplagiostomum AP1 treated methyl orange dye solutions on Labeo rohita. The behavioural response, mortality, haematological (red blood corpuscles (RBC), packed cell volume (PCV), haemoglobin (Hb), white blood corpuscles (WBC), mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH) and mean corpuscular haemoglobin concentration (MCHC)), biochemical (plasma glucose and protein), enzymological (aspartate amino transaminases (AST) and alanine amino transaminases (ALT)) and histological examination (gills, liver and kidney) of Labeo rohita are exposed to untreated and treated methyl orange dye solutions were assessed on 7th day. The fish exposed to tap water and treated dye solution showed normal behavioural response whereas abnormal behaviour was noted in fish exposed to untreated dye solution. Similar trend was recorded in the mortality rate of the fishes. Fish exposed to untreated dye solution showed reduction in RBC, PCV, Hb, MCHC, plasma glucose and plasma protein, increased level of WBC, MCV and MCH and also alteration in AST and ALT thereby indicating the toxicity of the dye. No such reduction and alteration were observed in haematological, biochemical and enzymological levels of fishes exposed to tap water and treated dye solution indicating the non-toxic nature of the degraded metabolites of dye. Histological examination of fishes exposed to methyl orange dye revealed necrosis and haemorrhage in the gills and hepatocytes, congested and shrunken glomeruli in kidney thereby indicating the toxicity of the dye. The histoarchitecture of control and algae-treated fishes showed no structural changes indicating the non-toxic nature of the degraded metabolites of the dye. The results concluded that methyl orange dye solution treated with O. subplagiostomum AP1 can be explored for aquacultural purposes owing to its non-toxic nature.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rapid industrialization and market demands have substituted natural dyes with synthetic dyes due to their better fastness, brightness, wide ranges of colours and easy application as well as its cost-efficiency. Dyes are not only used by textile industries but also utilized in many other industries such as leather, tanning, paper and pulp, food, colour and paint, photography, pharmaceuticals and medicine, cosmetic, hair colourings, wood staining, agricultural and also in biological and chemical research (Jagruti 2018; Shabban et al. 2020). Enhanced industrial and anthropogenic activities with technological advancement along with increased population have led to the extreme release of textile dye contaminated wastewater into the ecosystems. Particularly, azo dyes used in textile dyeing industries are highly toxic, and its discharge into aquatic ecosystem has modified them as a sink for water pollution with serious impacts on aquatic faunal resources (Athira and Jaya 2018).
Methyl orange, a water-soluble anionic azo dye, was chosen in the study due to its wide application in textile, paper manufacturing, pharmaceutical, printing, food industries and research laboratories. When methyl orange unintentionally enters the body, it causes cancer, allergy, hypersensitivity and dermatitis and eventually gets metabolized into aromatic amines by intestinal microbes (Mittal et al. 2007; Chen et al. 2010, 2011; Pillai 2017). Exposure of untreated dyes into water bodies may cause behavioural changes, biological and physiological alterations, allergic, mutagenic, carcinogenic and detrimental effects to the living creatures especially the fishes (Amte and Mhaskar 2013; Amaya et al. 2018; Parmar and Shah 2020; Vigneshpriya et al. 2017). Aspiring the results of the various researchers on the impact of toxicants on fishes, meagre studies have been carried out to assess the impact of dye solution treated with algae on Indian carps. Thus, this study gains significance through zootoxicity assay which would provide complete and effective solution to be implemented in the polluted riverine systems without any hazard to aquatic fauna. Environmental toxicologists extensively employ zootoxicity tests using fish as a bioindicator for assessing the effect of pollutants on the molecular, biochemical, enzymological and histological responses which have been employed as the biomarkers of various environmental stresses (Tufekci et al. 2007). The treated wastewater of dyeing industries has been used in aquacultural practices around the world for the production of fish biomass which is the primary goal with marginal concern in wastewater renovation (Das et al. 2012).
Oedogonium subplagiostomum AP1, a freshwater dominant, unbranched, filamentous non-toxic green alga economically obtained on a large scale from the nearby water resources was experimented as a biosorbent in the removal of methyl orange from aqueous solutions and used for fish bioassay studies (Maruthanayakam et al. 2020). The Indian major carp Labeo rohita, a bottom feeder which mainly consumes algae and aquatic plants was selected as the experimental animal in the present study due to its rapid growth, high sustainability, tolerance at high stocking density and survival ability in oxygen-depleted water. Studies associated to the exposure of Indian major carps to methyl orange treated with O. subplagiostomum AP1 are meagre. Keeping this in view, the present study aims in assessing the haematological, biochemical, enzymological activities and histopathology of L. rohita.
Materials and methods
Zootoxicity assay
Procurement and acclimatization of the experimental fish
Freshwater fingerlings of L. rohita (length 7.2 ± 0.4 cm and weight 8.3 ± 1.0 g) were procured and transported to laboratory from National fish seed farm, Department of Fisheries, Bhavanisagar, Erode, Tamil Nadu, India, in clean aerated polythene bags. The collected fishes were safely brought to the laboratory and acclimatized under laboratory conditions for 20 days in fish tank disinfected with potassium permanganate solution to prevent from fungal infection. Dechlorinated tap water with pH 7, temperature 25 °C, 5.3 mg/L of dissolved oxygen and 20 mg/L of total hardness (APHA 1998) was filled in the fish tank. During the acclimatization period, the fishes were fed daily with rice bran and groundnut oil cake (2:1) which had no detectable amount of dye. The water in the acclimatization tank was renewed daily to remove the excess amount of feed and excretory materials. Fishes showing abnormal characteristic behaviour was removed from the tank. At the end of the acclimatization period, healthy fingerlings were separated and subjected to experimental study.
Preparation of treated dye solution
Oedogonium subplagiostomum AP1 at a concentration of 400 mg/L was introduced into the aqueous solution amended with methyl orange (500 mg/L), and the decolourization efficiency was recorded at room temperature (Maruthanayakam et al. 2020). The physico-chemical characterization (colour, odour, pH, electrical conductivity, total dissolved solids (TDS), total suspended solids (TSS), total solids (TS), biological oxygen demand (BOD), chemical oxygen demand (COD), total hardness, total alkalinity, chloride, sulphate and nitrate) of untreated and treated methyl orange dye solution was assessed as per the standard method (APHA 1998), and the decolourized/treated dye solution is used for the zootoxicity study (Alaguprathana and Poonkothai 2018).
Experimental design
Fifteen fishes were introduced into the tubs filled with 20 L of dechlorinated tap water which served as control (T1), untreated dye (T2) and treated dye (T3) solutions separately. For each treatment, triplicates were maintained, and the experimental water samples were renewed daily. The feeding level of the fishes was 5% of their body weight. Dead fishes were removed immediately to prevent the contamination and to maintain desired concentration of oxygen. The fishes were maintained at 12:12 light: dark cycle.
Mortality rate
The mortality of L. rohita was recorded for every 24 h during the observation period of 168 h. If there is no visible movement (e.g. gill movements) or no reaction while touching the caudal peduncle of the fish, it is considered to be dead and removed. The mortality of the fishes were recorded as per the cumulative percentage of fishes dead (Amte and Mhaskar 2013). At the end of the experimental period, the fishes from each treatment were collected and subjected to toxicological studies.
Collection of samples for toxicological studies
The blood was collected from the experimental fishes by cardiac puncture using heparinized syringes and transferred into heparin-coated vials. The whole blood was used for haematological studies, and the remaining was centrifuged (Remi, Mumbai) at 10,000 rpm for 20 min to separate the plasma and used for biochemical assays. After drawing blood from fishes, they were washed with distilled water and blotted dry with absorbent paper. The liver was isolated from the control and experimental fishes. One hundred milligrams of each tissue were weighed and homogenized with 2.5 ml of 0.25 M sucrose solution in ice-cold condition. The homogenates were centrifuged at 6000 rpm for 20 min, and the clear supernatant fluid was taken for enzyme assay. The collected whole blood, plasma and the supernatant obtained from the liver samples were subjected haematological, biochemical and enzymological analyses.
Haematological, biochemical and enzymological analyses
RBC and WBC were counted by haemocytometer (Rusia and Sood 1992), packed cell volume was determined by microhematocrit reader (Nelson and Morris 1989) and the haemoglobin concentration was estimated by cyanmethemoglobin method (Drabkin 1946). Erythrocyte indices like MCV, MCH and MCHC were determined by calculation method (Blaxhall and Daisley 1973). Plasma glucose and plasma protein (Trinder 1969) and Doumas et al. 1971) and liver AST and ALT activities (Reitman and Franckel 1957) were estimated using spectrophotometer (Labman, Chennai).
Statistical analyses
The data obtained were statistically analysed by mean ± SD. The data obtained was statistically analysed by one-way analysis of variance (P < 0.05) using statistical software Sigma stat 3.1.
Histological examination
For histological investigation, with the fishes exposed to tap water, untreated and treated dye solutions were dissected to collect the tissues of the gills, liver and kidney. The dissected tissues were immediately fixed in 10% formalin and washed in distilled water, dehydrated in graded ethanol series (30%, 50%, 70%, 85% and 100%), infiltrated with xylene and embedded in paraffin wax at 56–60 °C. The tissues embedded in paraffin wax were sectioned using a rotator microtome (5 μm) and treated with xylene to remove paraffin and subsequently washed in 90%, 70%, 50% and 30% alcohol (Bancroft and Cook 1994). Finally, paraffin-free sections were washed with distilled water, stained with haematoxylin for 3 min and washed in running tap water for 1 min. Finally, the tissues were stained in eosin for 45 s, examined under microscope (Labomed, California) and photographed. The changes in the tissues of the experimental fishes were compared with the control fishes.
Results and discussion
Behavioural responses and mortality of Labeo rohita
The levels of the physico-chemical parameters analysed in the untreated and treated methyl orange dye solution were within the BIS limit prescribed for the discharge of industrial effluent (Alaguprathana and Poonkothai 2018). The L. rohita exposed to different treatments selected for the present study showed a variety of behavioural changes. The irregularities observed prior to mortality are a signal of depleted oxygen content due to high concentration of toxicant (Dahunsi and Oranusi 2012). Based on visual observation and the physical behaviour, the fishes exposed to tap water (T1) exhibited normal swimming, vision and proper body motion whereas those exposed to untreated dye solution (T2) initially showed abnormal movements such as erratic swimming, hyperexcitation and variation in opercular beat rates. After 24 h, the fishes became weaker, showed sluggish movement and started to settle at the bottom of the trough. At the end of 168 h, morphological changes such as reddening in the gills, faded body colour, gulping of air and shedding of scales from the skin were observed. Thus, methyl orange directly affects the fishes by reducing the reflex actions and equilibrium thereby causes abnormalities in growth and its behaviour. Similar such observation was noticed in Oreochromis niloticus exposed to acid dye 53 (Amwele et al. 2015) and L. rohita to direct green 6 (Barot and Bahadur 2013). Kane et al. (2005) also noticed the absorption of environmental pollutants onto the skin of fish causing damage in the dermal layer and thereby lead to shedding of the scales. The fishes exposed to treated dye solution (T3) showed normal swimming and stable movement indicating its tolerance level and depicting the reduction in the toxicity of dye.
Labeo rohita being a fresh water fish can tolerate disturbances and contaminants in the aquatic environment, and the mortality depends on the sensitivity and duration of exposure to the toxicant (Cavas and Gozukara 2005). However, introduction of fishes into the toxicant medium leads to severe damage of organ systems and causes mortality (Suvetha et al. 2015). The percentage mortality of fishes was high in untreated dye solution (88%) when compared with fishes exposed to treated dye solution (33%) and tap water (1%), respectively (Fig. 1). Roopadevi and Somashekar (2012) observed the death of fishes stocked in higher effluent concentration for longer period, and also, the homeostatic behaviour of the test organism was eventually disturbed. This confirms with the results of the present study stating the toxicity of methyl orange to the experimental fish, L. rohita.
Haematological parameters
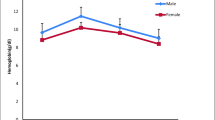
Blood is a pathophysiological reflector of the whole body, and therefore, its parameters are important in diagnosing the altered physiological status of fish exposed to toxicants. The haematological elements of fish are widely used as an indicator in toxicological research and environmental monitoring studies (Carvalho and Fernandes 2006). The results revealed that L. rohita exposed to untreated dye solution (T2) showed a notable decrease in the levels of RBC, PCV, Hb and MCHC when compared with T1 and T3 fishes. The WBC, MCV and MCH levels increased in the T2 fishes when compared with T1 and T3 fishes (Table 1). The haematological profile, plasma glucose and plasma protein levels and enzyme activities were significant (P < 0.05) in fishes exposed to treated dye solution when compared with untreated dye solution. Decrease in the haematological parameters in T2 fishes depicts its anaemic condition which might have resulted from the haemolysis or damage caused in the gills leading to impaired respiratory capacity by the pollutant, methyl orange. The significant reduction of RBC content in T2 fishes might be due to the failure of erythrocyte production, impaired osmoregulation, internal haemorrhage and inhibition of oxygen production during stress conditions (Kaoud et al. 2011; Kavitha et al. 2010). Tariq et al. (1996) observed an increase in the treatment and carbon dioxide content in the blood of fishes exposed to pollutants. He also noticed the swelling of RBCs, cytotoxic effect on erythropoietic tissues leading to disturbances in bone marrow and alteration in the cell cycle and erythropoiesis.
PCV is a measure of percentage of RBCs present in a volume of whole blood. Decrease in the PCV of fishes exposed to untreated dye solution may be due to anaemia or destruction of red blood cells or nutritional deficiency, disturbances occurred in metabolic and haemopoietic activities leading to impairment in haemopoietic organs (Sharma and Langer 2014). Thus, PCV appears to be positively correlated with erythrocyte count where decrease in the number of RBCs followed by PCV confirms anaemia in Labeo rohita (Palanisamy et al. 2011). The results are in agreement with the findings of Srivastav and Roy (2015) who observed a significant decrease in PCV of fishes exposed to malachite green when compared with the control and treated fishes.
Hb seems to be reliable and best blood indicator of environmental stress. Increase in its concentration could be a persistent sign of an adaptational improvement in oxygen-transporting capacity of blood (Khalesi et al. 2014). Lower level of haemoglobin in T2 fishes might decrease the ability to enhance the activities required to meet demands such as seeking of food and escaping from predators (Barot and Bahadur 2013). The depletion or reduction in Hb content in T2 fishes could also be attributed to the production of reactive oxygen species under the influence of toxicant resulting in the destruction of red blood cell membrane and its function, inhibition of the enzymes involved in Hb synthesis (Pamila et al. 1991), impaired intestinal absorption of iron or transferrin dysfunction (Joshi et al. 2002) and impairment in the immunological reactions to produce antibodies to cope up with stress induced by the toxicant (Ramdas 2013). Similar decrease in the amount of RBC, Hb and PCV could be corroborated with the findings of earlier investigations in Tilapia mossambica exposed to textile dyeing effluent (Deepika and Noorjahan 2018), Catla catla exposed to acid red 97 (Avni and Jagruti 2017) and Carassius auratus gibelio exposed to azo red 120 (Al-Sabti 2000), respectively.
Leucocytes or white blood corpuscles are cells of immune system which play a vital role in both specific and non-specific immune responses in protecting the body against toxicants. One of the most elementary ways to assess the immune system is to explore the changes in WBC count (Bujjamma and Padmavathi 2018). The WBC count was observed to be high in T2 fishes when compared with T1 and T3 fishes where the count was decreased or lowered. Increase in the WBC count in T2 fishes can be attributed to the stimulation of the immune system in response to tissue damage caused by the dye. Tiago et al. (2008) reported that the increase in antibody production helps in the survival and recovers the fishes exposed to toxicants. Significant increase in the total WBC count was observed in Heteropneustes fossilis exposed to malachite green and pyceze (Srivastav and Roy 2015) which supports the findings of the present investigation.
For examining the health status, blood index analyses have proven to be a valuable method, which provides reliable information on metabolic ailments, deficiency and chronic stress status. The RBC indices, namely, MCV, MCH and MCHC are the part of whole blood count, which express the size and Hb content of erythrocyte. MCV is one of the important blood parameters, which gives an indication of the status of the size of RBC and reflects the normal or abnormal cell division during erythropoiesis (Zhou et al. 2009).
In the present study, fishes exposed to untreated dye solution showed a higher level of MCV and MCH when compared with the control and treated fishes. This increase might be due to the enlargement or swelling of RBCs because of osmotic disturbances or hypotonic condition or uptake of electrolytes and water into the cells accompanied by acidification of the cytoplasm of RBCs leading to macrocytic anaemia in fishes (Suvetha et al. 2015). Ferrando and Moliner (1991) also reported that higher concentration of smaller immature erythrocytes in the circulation due to hyperplasia in erythrocyte forming sites leads to increased MCV. The decreased MCHC value in the present study may be due to the binding of the pollutant to Hb which prevents the oxygen-carrying capacity and leads to metabolic stress and death of fishes (Lemly 1993). These results coincide with the findings of Amte and Mhaskar (2013), Barot and Bahadur (2014) and Afaq and Rana (2009) who observed an increase in the values of MCV and MCH in Oreochromis mossambicus, Labeo rohita and Cirrhinus mrigala exposed to textile dyeing effluent, direct green 6, Bismarck brown and acid leather brown, respectively. In contrast, brilliant green treated with Sargassum wightii did not induce any observable hazardous health effects on the fish (Vigneshpriya et al. 2020). Thus, haematological parameters also determine the changes in the levels of biomarkers namely the enzymes, normal functioning and histomorphology of the organs.
Biochemical parameters
To monitor the presence of toxicants in aquatic media, biochemical analysis offers as an important bioindicator. Carbohydrates and proteins serve as nutrient and energy stores in all-biological processes, but under severe stress condition, they provide energy in metabolic pathways and biochemical reactions according to the need of an organism (Olaganathan and Patterson 2013). Plasma glucose has been extensively used as a sensitive biochemical indicator to study the stress of fish under unfavourable environment (Sancho et al. 2000). Glucose is one of the most important compounds of carbohydrates, which serve as an immediate and major metabolic fuel for all the biological activities (Pal and Reddy 2018). The decreased level of plasma glucose in untreated fishes (T2) might be due to the hypoxic condition caused by the toxicant that reflects an excess utilization of stored carbohydrate during treatment period (Agrahari et al. 2007). In addition, the accumulation of the untreated dye in the kidney may cause renal injury which in turn reflects the concentration of glucose during stress conditions. Protein is an important biochemical parameter, which has been used to understand the general state of health and biological mechanism and metabolism under the stress of a toxicant (Saravanan and Ramesh 2013). Decrease in the plasma protein in T2 fishes might be due to necrosis or liver cirrhosis or alteration in the enzyme involved in the biosynthesis of proteins (Palaniappan and Vijayasundaram 2009). The decrease in the plasma glucose and plasma protein in the present investigation coincides with the studies carried out in Clarias gariepinus and Oreochromis niloticus exposed to textile dye industry wastewater (Agbon et al. 2014), Oncorhynchus mykiss treated with malachite green (Atamanalp 2007) and Cyprinus carpio with textile industrial effluent (Dhanalakshmi et al. 2018) respectively.
Enzymological parameters
Various physiological activities are regulated by the vital organ, the liver, which performs metabolism, storage, secretion and detoxification in fishes. Enzyme activities in the blood serum are considered an important biochemical indicator in hepatic dysfunction and damage (Jung et al. 2003). Hence, enzyme assays are widely used to assess the health of an organism in aquatic toxicology (Gul et al. 2004). Amongst the battery of enzymes, aspartate transaminase and alanine transaminase are widely used as pathological and potential markers to detect the function of liver or toxicant-induced hepatotoxicity (Huang et al. 2006). In the present study, the activities of serum AST and ALT were high in T2 fishes when compared with T3 and control fishes. When the fishes were exposed to untreated dye solution, the hepatic parenchyma cells were damaged, liberated AST and ALT into the blood stream, and their activities were elevated. Increased or enhanced AST and ALT indicated increased or active transamination of amino acids, possibly providing ketoacids which are utilized for energy synthesis through TCA cycle to favour gluconeogenesis (Beyer et al. 1996; Javed et al. 2016; Naveed et al. 2010; Neelimal et al. 2013).
Histopathological examination of Labeo rohita
Histological changes in the gill
Gills are a good indicator of water quality and possess respiratory, osmoregulatory and excretory functions. Absorption of pollutants from an aquatic medium through gills makes fish a vulnerable target to its toxicity (Fant et al. 2003). Histological observation on the gills of L. rohita exposed to tap water (T1) showed normal architecture of gill filaments such as primary and secondary gill lamella (Fig. 2a). The gills of Labeo rohita fingerlings exposed to untreated dye solution (T2) showed necrosis of secondary lamella and severe haemorrhage and erosion in the lamella of gills (Fig. 2b). These histopathological changes may be a reaction to pollutant intake or an adaptive response to prevent their entry through the gill surface. The necrosis of respiratory epithelial cells can adversely affect the gaseous exchange and ionic regulation in fishes. Such similar necrosis and haemorrhage in the gills of fishes exposed to untreated dye solution were also evidenced in Catla catla to reactive red 120 (Avni and Jagruti 2016) and Labeo rohita to textile mill effluent (Nikalje et al. 2012), respectively. However, the fingerlings exposed to treated dye solution did not show any deformities in the gills, and the regeneration of respiratory epithelium of the gills was observed (Fig. 2c). Such similar findings were reported in Catla catla exposed to treated sago effluent (Ramesh and Nagarajan 2014) and Etheostoma olmstedi exposed to Bacillus pumilus–treated textile wastewater (Watharkar et al. 2014), respectively.
Histology of gills exposed to control (T1), untreated (T2) and treated (T3) methyl orange dye solution at × 45 magnification (PL—primary lamellae, SL—secondary lamellae, ILE—inter-lamellar epithelium, ILS—inter-lamellar space, LS—lamellar space, GL—gill filament, SILE—swelling of inter-lamellar epithelium, NRE—necrosis of respiratory epithelial cells, SHEL—severe haemorrhage and erosion in the lamella of gills and RRE—regeneration of respiratory epithelium). a Gill section of L. rohita—control (T1). b Section of gills in L. rohita exposed to untreated dye solution (T2). c Section of gills in L. rohita exposed to treated dye solution (T3)
Histological changes in the liver
The liver plays an important role in the detoxification of contaminants because it acts as a main storage organ for many substances. Hence, the accumulation of toxicants may affect its functions and thereby decreases the blood supply to all parts of the body (Nagai et al. 2002). The histology of liver in control fishes showed normal hepatocytes arrangement. The hepatocytes were located amongst the sinusoids that form a cord-like structure and possess large nuclei cords (Fig. 3a). No remarkable changes were observed in the hepatocytes of fishes exposed to treated dye solution, and the histoarchitecture was similar to that of control fishes (Fig. 3c). Fishes exposed to untreated dye solution showed vacuolar degeneration, sinusoidal congestion, necrosis and haemorrhage of hepatocytes (Fig. 3b). Intoxication of the pollutant in the fish changes the general architecture of liver indicating the degree of structural heterogeneity that enhances with the inhalation of the toxicant. The histoarchitecture of the fishes exposed to untreated dye is in confirmation with the findings of Poecilia reticulata exposed to textile dyeing industry effluent (Selvaraj et al. 2015), and Channa punctatus exposed to vat blue 4 and vat green 1 (Olaganathan and Patterson 2012), respectively.
Histology of liver exposed to control (T1), untreated (T2) and treated (T3) methyl orange dye solution at × 45 magnification (CV—central vein, NH—normal hepatocytes, NS—normal sinusoids, SC—sinusoidal congestion, NHC—necrosis of hepatic cells, VDH—vacuolar degeneration of the hepatocytes). a Liver section of L. rohita—control (T1). b Section of liver in L. rohita exposed to untreated (T2) dye solution. c Section of liver in L. rohita exposed to treated (T3) dye solution
Histological changes in the kidney
The kidney is a primary organ important for the elimination of waste and osmoregulation in fish. Histology of kidney in control fishes showed well-characterized glomeruli and renal tubules (Fig. 4a). Fishes exposed to untreated dye solution showed highly degenerative and necrotic changes in the renal tubules and congested and shrunken glomeruli (Fig. 4b). Degeneration of renal tubules and necrotic changes in the kidney has also been observed in Labeo rohita exposed to direct green 6 (Barot and Bahadur 2013), Clarias lazera exposed to dyestuff wastewater (Abdel-Moneium et al. 2008), Cyprinus carpio exposed to textile industrial effluent (Dhanalakshmi et al. 2018) and Labeo rohita exposed to textile dyeing effluent (Rana and Raizada 2000), respectively. El-Neweshy and Srag (2011) observed necrotic changes in the tubular epithelium of the kidney exposed to acid orange 7. The intoxication of the dye may result in necrotic cell death, which might be caused by the swelling and eventual failure of cell membrane, leading to rupture of the renal cells and release of its content into the extracellular space. The renal tubules and glomeruli were regenerated in the fishes exposed to treated dye solution (Fig. 4c). The structural features were found to be alike of those observed in the control fishes. Literature on haematological, biochemical, enzymological and histological parameters analysed in fishes exposed to treated dye solution are meagre, and the present study forms a platform to carry out research in this area.
Histology of kidney exposed to control (T1), untreated (T2) and treated (T3) methyl orange dye solution at × 45 magnification (NG—normal glomeruli, NRT—normal renal tubules, DRT—degeneration of renal tubules, CSG—congested and shrunken glomeruli, RG—regeneration of glomeruli). a Kidney section of L. rohita—control (T1). b Section of kidney in L. rohita exposed to untreated (T2) dye solution. c Section of kidney in L. rohita exposed to treated (T3) dye solution
Conclusions
The present study infers that methyl orange treated with O. subplagiostomum AP1 does not have profound influence on haematological, biochemical and enzymological profiles of the Indian major carp L. rohita, hence portraying the less toxic nature of the degraded products. On the contrary, unusual conditions were noticed in fish exposed to untreated methyl orange indicating the toxic nature of the dye. Histopathological lesions in the gills, liver and kidney tissues of fish exposed to untreated dye solution might be due to the stress induced by the dye. The parameters studied could be utilized as potential biomarkers in assessing the toxicity of the freshwater fish in the field of environmental biomonitoring and can also be used as a warning indicator for dye exposure to aquatic fauna. Thus, methyl orange–treated O. subplagiostomum AP1 could be explored for aquacultural purpose and paves a platform for an ecofriendly approach to mankind.
Data availability
All data generated or analysed during this study are included in this published article.
References
Abdel-Moneium AM, Abou Shabana NM, Khadre SEM, Abdel-Kader HH (2008) Physiological and histopathological effects in catfish (Clarius lazera) exposed to dyestuff and chemical wastewater. Int J Zool Res 4(4):189–202
Afaq S, Rana K (2009) Toxicological effects of leather dyes on total leukocyte count of fresh water teleost, Cirrhinus mrigala (Ham). Biol Med 1(2):134–138
Agbon AO, Arowolo T, Bakare W (2014) Local textile industry wastewater effect on freshwater fish species. J Aquat Sci 29(2):401–410
Agrahari AS, Kashev C, Pandey A, Gopal K (2007) Biochemical alteration induced by monocrotophos in the blood plasma of fish, Channa punctatus (Bloch). Pestic Biochem Physiol 88:268–272
Alaguprathana M, Poonkothai M (2018) Impact of untreated and bioremediated methyl orange on seed germination, growth and yield of flower plants - marigold (yellow and orange), Celosia argentea. International Journal for Innovative research in Science and Technology 5(7):22–28
Al-Sabti K (2000) Chlorotriazine reactive azo red 120 textile dye induces micronuclei in fish. Ecotoxicol Environ Saf 47(2):149–155
Amaya VS, Priyatha CV, Chitra KC (2018) Haematological responses in the freshwater fish, Anabas testudineus (Bloch,1792) exposed to sublethal concentration of acid orange 7. Journal of Global Biosciences 7(8):5536–5549
Amte GK, Mhaskar TV (2013) Impact of textile - dyeing industry effluent on some haematological parameters of freshwater fish Oreochromis mossambicus. Nat Environ Pollut Technol 12(1):93–98
Amwele HR, Papirom P, Chukanhom K, Beamish FHW, Petkam R (2015) Acute and subchronic toxicity of metal complex azo acid dye and anionic surfactant oil on fish Oreochromis niloticus. J Environ Biol 36:199–205
APHA (1998) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association, American Water Works Association and Water Environmental Federation, Washington DC
Atamanalp M (2007) The effects of a disinfectant (malachite green) on blood biochemistry of rainbow trout Oncorhynchus mykiss. J Fish Aquat Sci 2(1):82–85
Athira N, Jaya DS (2018) The use of fish biomarkers for assessing textile effluent contamination of aquatic ecosystems: a review. Nat Environ Pollut Technol 17(1):25–34
Avni P, Jagruti B (2016) Determination of genotoxic effect of azo dye C.1. RR 120 on fish Catla catla. Biotechnol Res 2(2):77–80
Avni P, Jagruti B (2017) Haematotoxic potential of acid red 97 on Catla catla (Ham.). Biosci Discov 8(2):198–201
Bancroft JD, Cook HC (1994) Manual of histological techniques and their diagnostic application. Churchill Livingstone, London, pp 289–305
Barot J, Bahadur A (2013) Behavioural and histopathological effects of azo dye on kidney and gills of Labeo rohita fingerlings. J Environ Biol 34:147–152
Barot J, Bahadur A (2014) Toxic effect of azo dye (CI direct green 6) on blood parameters of freshwater fish Labeo rohita (Ham.). Cell Tissue Res 14(2):4251–4254
Beyer J, Sandvik M, Hylland K, Field E, Egaas E, Aas E et al (1996) Contaminant accumulation and biomarker responses in flounder (Platichthys flesus L.) and Atlantic cod (Cadus morhua L.) exposed by caging to polluted sediments in Sorfjorden, Norway. Aquat Toxicol 36:75–98
Blaxhall P, Daisley KW (1973) Routine haematological methods for use with fish blood. J Fish Biol 5:771–781
Bujjamma P, Padmavathi P (2018) Effect of cadmium on haematological changes in a freshwater catfish, Heteropneustes fossilis. Int J Zool Stud 3(1):132–141
Carvalho CS, Fernandes MN (2006) Effect of temperature on copper toxicity and hematological responses in the neotrophical fish, Prochilodus scrofa at low and high pH. Aquaculture 251:109–117
Cavas T, Gozukara SE (2005) Induction of micronuclei and nuclear abnormalities in Oreochromis niloticus following exposure to petroleum refinery and chromium processing plant effluents. Aquat Toxicol 74:264–271
Chen S, Zhang J, Zhang C, Yue Q, Li Y, Li E (2010) Equilibrium and kinetic studies of methyl orange and methyl violet adsorption on activated carbon derived from Phragmites australis. Desalination 252:149–156
Chen ZX, Jin XY, Chen Z, Megharaj M, Naidu R (2011) Removal of methyl orange from aqueous solutionusing bentonite-supported nanoscale zero-valent iron. J Colloid Interface Sci 363(2):601–607
Dahunsi SO, Oranusi US (2012) Acute toxicity of synthetic resin effluent to African catfish, Clarias gariepinus [Burchell, 1822]. American Journal of Food Science and Nutrition 2(2):42–46
Das S, Unni B, Bhattacharjee M, Wann SB, Rao PG (2012) Toxicological effects of arsenic exposure in a freshwater teleost fish, Channa punctatus. Afr J Biotechnol 11(19):4447–4454
Deepika T, Noorjahan CM (2018) Impact of untreated and treated textile effluent on haematological parameters of fresh water fish, Tilapia mossambica. Int J Adv Sci Res Manag 3(6):24–28
Dhanalakshmi G, Dhanapakiam P, Reniprabha A (2018) Biochemical alternations induced by textile industrial effluent on common carp Cyprinus carpio. Int J Dev Res 8(10):23183–23188
Doumas BT, Watson WA, Biggs HG (1971) Albumin standards and the measurement of serum albumin with bromocersol green. Clin Chim Acta 31(1):87–96
Drabkin DL (1946) Spectrometric studies, XIV: the crystallographic and optimal properties of the haemoglobin of man in comparison with those of other species. J Biol Chem 164:730–723
El-Neweshy MS, Srag MAA (2011) Chronic malachite green toxicity in Nile tilapia: pathological and hematological studies with special reference to quantitative histopathological assessment. Researcher 3(4):55–64
Fant E, Rios FSA, Romao S, Vianna ACC, Freiberger S (2003) Histopathology of the fish Corydora spaleatus contaminated with sublethal levels of organophosphorus in water and food. Ecotoxicol Environ Saf 54:119–130
Ferrando MD, Moliner EA (1991) Changes in selected biochemical parameters in the brain of the fish, Anguilla anguilla (L.) exposed to lindane. Bull Environ Contam Toxicol 47(3):459–464
Gul S, Kurutas EB, Yildiz E, Sahan A, Doran F (2004) Pollution correlated modifications of liver antioxidant systems and histopathology of fish (Cyprinidae) living in Seyhan Dam Lake, Turkey. Environ Int 30:605–609
Huang XJ, Choi YK, Im HS, Yarimaga O, Yoon E, Kim HS (2006) Aspartate aminotransferase (AST/GOT) and alanine aminotransferase (ALT/GPT) detection techniques. Sensors (Basel) 6(7):756–782
Jagruti B (2018) Evaluation of azo dye toxicity using some haematological and histopathological alterations in fish Catla catla. International Journal of Biological, Food, Veterinary and Agricultural Engineering 9(5):2015
Javed M, Ahmad I, Ahmad A, Usmani N, Ahmad M (2016) Studies on the alterations in haematological indices, micronuclei induction and pathological marker enzyme activities in Channa punctatus (spotted snakehead) perciformes, channidae exposed to thermal power plant effluent. SpringerPlus 5(1):761–770
Joshi PK, Bose M, Harish D (2002) Haematological changes in the blood of Clarias batrachus exposed to mercuric chloride. J Ecotoxicol Environ Monit 12:119–122
Jung H, Wilson DB, Walker LP (2003) Binding and reversibility of Thermobifida fusca Cel5A, Cel6B, and Cel48A and their respective catalytic domains to bacterial microcrystalline cellulose. Biotechnol Bioeng 84(2):151–159
Kane AS, Salierno JD, Brewer SK (2005) Fish models in behavioral toxicology: automated techniques, updates and perspectives. In: Ostrander GK (ed) Methods in aquatic toxicology, (chapter 32), volume (32). Lewis Publishers, Boca Raton, pp 559–590
Kaoud HA, Zaki MM, El-Dahshan AR, Saeid S, Zorba HYE (2011) Amelioration the toxic effects of cadmium-exposure in nile tilapia (Oreochromis niloticus) by using Lemnagibba L. Life Sci J 8(1):185–195
Kavitha C, Malarvizhi A, Kumaran SS, Ramesh M (2010) Toxicological effects of arsenate exposure on hematological, biochemical and liver transaminases activity in an Indian major carp, Catla catla. Food Chem Toxicol 48(10):2848–2854
Khalesi S, Sun J, Buys N, Jayasinghe R (2014) Effect of probiotics on blood pressure novelty and significance. Hypertension 64(4):897–903
Lemly AD (1993) Teratogenic effects of selenium in natural populations of fresh water fish. Ecotoxicol Environ Saf 26(2):181–204
Maruthanayakam A, Poonkothai M, Kalaiarasi K, Sudhakar C (2020) In vitro and in silico studies on the removal of methyl orange from aqueous solution using Oedogonium subplagiostomum AP1. Water Air Soil Pollut 231:232. https://doi.org/10.1007/s11270-020-04585-z
Mittal A, Malviya A, Kaur D, Mittal J, Kurup L (2007) Studies on the adsorption kinetics and isotherms for the removal and recovery of methyl orange from wastewaters using waste materials. J Hazard Mater 148:229–240
Nagai T, Yukimoto T, Suzuki N (2002) Glutathione peroxidase from the liver of Japanese sea bass Lateolabrax japonicus. Z Naturforsch C J Biosci 57(1–2):172–176
Naveed A, Janaiah C, Venkateshwarlu P (2010) The effects of lihocin toxicity on protein metabolism of the freshwater edible fish, Channa punctatus (Bloch). J Toxicol and Environ Hlth Sciences 3(1):18–23
Neelimal P, Kumar CA, Gopalarao N, Rao CJ (2013) Studies on activity levels of aspartate amino transferase (ASAT) and alanine amino transferase (ALAT) in the tissues of Cyprinus carpio (Linn.) exposed to cypermethrin (25% EC). Int J Pharm Pharm Sci 5(3):566–570
Nelson DA, Morris MW (1989) Basic methodology, haematology and coagulation, part IV. In: Nelson DA, Henry JB (eds) Clinical diagnosis and management by laboratory methods, 17th edn. W.B. Saunder Company, Philadelphia, pp 578–625 (Chapter 27)
Nikalje SB, Muley DV, Angadi SM (2012) Histopathological changes in liver of freshwater major carp Labeo rohita after acute and chronic exposure to textile mill effluent. The Bioscan 7(2):215–220
Olaganathan R, Patterson J (2012) Histological changes in the target organs of Channa punctatus after exposure to anthraquinone vat dyes. J Ecotoxicol Environ Monit 22(5):443–449
Olaganathan R, Patterson J (2013) Effect of anthraquinone dyes on the carbohydrate, protein and lipid content in the muscle of Channa punctatus and Cyprinus carpio. Int J Pharm Appl 4(1):11–18
Pal S, Reddy PB (2018) Biophenol a (BPA) induced histopathological and biochemical alterations in the liver and kidney of stinging cat fish Heteropneustes fossilis. Trends in Fisheries Research 7(1):67–73
Palaniappan PLRM, Vijayasundaram V (2009) The effect of arsenic exposure and the efficacy of DMSA on the proteins and lipids of the gill tissues of Labeo rohita. Food Chem Toxicol 47(8):1752–1759
Palanisamy P, Sasikala G, Mallikaraj D, Bhuvaneshwari N, Natarajan GM (2011) Haematological changes of fresh water food fish Channa striata on exposure to Cleistanthus collinus suicidal plant extract. Res J Pharm, Biol Chem Sci 2:812–816
Pamila D, Subbaiyan PS, Ramaswamy M (1991) Toxic effects of chromium and cobalt on Sarotherodan mossambicus (Peters). Indian J Environ Health 33:218–225
Parmar AI, Shah AI (2020) Haematological parameters and histopathological alterations in the gills of fish, Catla catla exposed to azo dye acid red – 97. Advances in Zoology and Botany 8(4):342–350. https://doi.org/10.13189/azb.2020.080406
Pillai HPJS (2017) Optimization of process conditions for effective degradation of azo blue dye by Streptomyces DJP15. J Pure Appl Microbiol 11:1757–1765. https://doi.org/10.22207/JPAM.11.4.14
Ramdas BV (2013) Protective role of ascorbic acid on the cadmium induced changes in hematology of the freshwater fish, Channa orientalis (Schneider). Adv Appl Sci Res 4:305–308
Ramesh F, Nagarajan K (2014) Influence of treated sago effluent on the growth rates of the fresh water fish Catla catla. Int J Bioassays 3(4):2013–2016
Rana KS, Raizada S (2000) Histopathological alterations induced by tannery and textile dyeing effluents in kidney of Labeo rohita. J Environ Biol 21(4):301–304
Reitman S, Franckel S (1957) A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminase. Am J Clin Pathol 28(1):56–63
Roopadevi H, Somashekar RK (2012) Assessment of the toxicity of waste water from a textile industry to Cyprinus carpio. J Environ Biol 33(2):167–171
Rusia V, Sood SK (1992) Routine haematological tests. In: Mukerjee, Kanai L (eds) Medical Laboratory Technology, Vol. I (Fifth reprint). Tata McGraw Hill Publishing Company Limited, New Delhi, pp 252–258
Sancho E, Ceron JJ, Ferrando MD (2000) Cholinesterase activity and hematological parameters as biomarkers of sublethal molinate exposure in Anguilla anguilla. Ecotoxicol Environ Saf 46(1):81–86
Saravanan M, Ramesh M (2013) Short and long-term effects of clofibric acid and diclofenac on certain biochemical and ionoregulatory responses in an Indian major carp, Cirrhinus mrigala. Chemosphere 93:388–396
Selvaraj D, Leena R, Kamal DC (2015) Toxicological and histopathological impacts of textile dyeing industry effluent on a selected teleost fish Poecilia reticulata. Asian Journal of Pharmacology and Toxicology 3(10):26–30
Shabban OA, Jahin HS, Mohamed GG (2020) Removal of anionic and cationic dyes from wastewater by adsorption using multiwall carbon nanotubes. Arab J Chem 13:4797–4810
Sharma J, Langer S (2014) Effect of manganese on haematological parameters of fish, Garra gotyla gotyla. J Entomol Zool Stud 2(3):77–81
Srivastav KA, Roy D (2015) Effects of malachite green (Triarylmethane dye) and Pyceze (Bronopol) on the hematological parameters of a freshwater catfish Heteropneustes fossilis (Bloch). Int J Fish Aquac 2(6):119–122
Suvetha L, Saravanan M, Hurb JH, Ramesh M, ClaraBindu F (2015) Responses of the Indian major carp Labeo rohita to deltamethrin at acute and sublethal concentrations. Toxicol Environ Chem 97(2):186–199
Tariq J, Ashraf M, Jaffar M, Afzal M (1996) Pollution status of the Indus river, Pakistan, through heavy metal and macronutrient contents of fish, sediment and water. Water Res 30(6):1337–1344
Tiago SFH, Ive MA, Iwana GK, Johnson SC, Moracs G, Afonson LOB (2008) Impairment of the stress response in matrinxa juvenile Brycon amazonicus exposed to low concentration of phenol. Comp Biochem Physiol 147:416–423
Trinder P (1969) Determination of blood glucose using an oxidase-peroxidase system with a non-carcinogenic chromogen. J Clin Pathol 22(2):158–161
Tufekci N, Sivri N, Toroz I (2007) Pollutants of textile industry wastewater and assessment of its discharge limits by water quality standards. Turk J Fish Aquat Sc 7(2):97–103
Vigneshpriya D, Krishnaveni N, Renganathan S (2017) Effect of textile effluent on growth and germination of cowpea Vigna unguiculata L. Indian J Environ Pro 37(2):163–168
Vigneshpriya D, Krishnaveni N, Renganathan S, Priyadarshini RSS (2020) Impact of untreated and Sargassum wightii-treated brilliant green dye exposure on Indian major carp, Labeo rohita Ham.: hematology, biochemistry, enzymology and histopathology. Int J Phytoremediation. https://doi.org/10.1080/15226514.2019.1710816
Watharkar AD, Khandare RV, Waghmare PR, Jadhav JP (2014) Treatment of textile effluent in a developed phytoreactor with immobilized bacterial augmentation and subsequent toxicity studies in Etheostoma olmstedi fish. J Hazard Mater 283:1–12
Zhou X, Li Q, Arita A, Sun H, Costa M (2009) Effects of nickel, chromate and arsenite on histone 3 lysine methylation. Toxicol Appl Pharmacol 236:78–84
Acknowledgements
The authors wish to place their record of thanks to the authorities of Avinashilingam Institute for Home Science and Higher Education for Women, Coimbatore, for the support given to conduct the study and DST CURIE (No. SR/CURIE/PHASE II/01/2014) to utilize the chemicals required for the study.
Author information
Authors and Affiliations
Contributions
MP contributed to the study conception and design. MA performed experiment and data collection. The data was analysed and interpreted by MP and MA. The draft of the manuscript was written by MP. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alaguprathana, M., Poonkothai, M. Haematological, biochemical, enzymological and histological responses of Labeo rohita exposed to methyl orange dye solution treated with Oedogonium subplagiostomum AP1. Environ Sci Pollut Res 28, 17602–17612 (2021). https://doi.org/10.1007/s11356-020-12208-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-12208-7