Abstract
In this study, a microcosm experiment was conducted for 30 days to assess the impact of the presence of juvenile gray shrimp Crangon crangon on meiofauna. The results suggested that juvenile shrimp had a significant negative impact on the abundance of nematodes and copepods, but no effect on polychaetes. Moreover, nematodes showed a significant decline in individual weight. The collected nematodes were taxonomically identified and assigned to five functional traits: shapes of the tail and amphid, life history, feeding types, and adult length. The nematode traits were affected by the number of shrimp introduced, and descriptors followed normal or inversed bell-shaped curves. When no shrimp were present, the nematofauna had a higher species richness compared with treatments of 4, 8, and 12 shrimp. Bell-shaped curve patterns were common in relation to the two phases of feeding for C. crangon. During the first phase, C. crangon consumed the nematode species Oncholaimus campylocercoides; thereafter, shrimp fed mostly on the nematode Anticoma eberthi and copepods.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Quality assessment and monitoring of stressors’ impacts on the environment generally involves measuring physico-chemical parameters of both water and the substrate. Moreover, taking into account biological criteria in assessing the state of the system has been highlighted by several authors, such as Hagerbaumer et al. (2015) for nematodes, Van Gestel et al. (2018) for terrestrial isopods, and Hussain et al. (2020) for marine planktonic copepods. These authors define the term “stress” as any physical or chemical factor affecting the growth or survival of organisms, thus altering the structure or even composition of the entire community.
Benthic organisms and communities are proving to be particularly suitable for assessing the state of the ecosystems in which they thrive (Semprucci and Balsamo 2012). A plethora of studies have demonstrated the usefulness of meiofauna as biomonitoring tools for studying the impacts of pollutant chemical elements (PCEs) (Semprucci and Balsamo 2012). Among the meiobenthic organisms, nematodes were frequently considered in bioassays and ecotoxicological studies in relation with PCEs due to their small body (length = 1–5 mm), high abundance (up to 23 million per m2), and short generation times (few weeks to months) (Moreno et al. 2011). However, the impact of biological disturbances on nematode assemblages has gotten less attention (Bell and Coull 1978; Palmer 1988; McCall and Fleeger 1995; Feller and Coull 1995; Henry and Jenkins 1995; Gregg and Fleeger 1997) and revealed that macro-invertebrates and fish juveniles may affect the structure of the meiofauna community directly through predation. The most recent studies (Schratzberger and Warwick 1999; Feller 2006) have assessed the impact of predation by Carcinus maenas and Crangon crangon, on meiofauna through a closed microcosm experiment. These predation effects could be more complicated because macrofauna are also able to alter the physico-chemical characteristics of sediment (Federle et al. 1983; Probert 1984; Aller and Yingst 1985; Warwick et al. 1986; Warwick et al. 1990; Olafsson and Elmgren 1991).
Crangonid shrimp are common on mid-to-high latitude shorelines and are known as a potential omnivorous predators (Taylor and Peck 2004). In shallow waters, the Crangon crangon’s food items were selected according to size. Juveniles are in general epibenthic predators grazing on meiofauna, but large adults (4–5 cm) feed on relatively huge preys those may reach 6–12 cm in length like Mya arenaria, Cardium edule, Nereis diversicolor, and Corophium volutator Crangon (Pihl and Rosenberg 1984). Even larger shrimp are cannibals (Pihl and Rosenberg 1984).
Due to the fact that experimental works on biotic interactions are fewly undertaken by meiobenthologists compared to those studying effects of pollutants and the contradictory aspect of the field data, it seems that we are far from understanding well the responses of meiofauna to biological pressures. This problem can be solved through laboratory bioassays. The purpose of this work was to study the impact of the presence of the gray shrimp C. crangon larvae, on meiobenthic assemblages with focus on nematode taxonomic and functional traits through microcosm experiment.
Materials and methods
Sampling
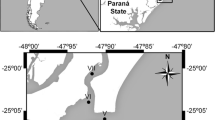
Sediment was collected on February 1, 2020, from a subtidal site at the “Old Harbor of Bizerte” (37° 16′ 435″ N; 9° 52′ 453″ E), Tunisia. Plexiglas hand cores with a section of 10 cm2 (3.6 cm inner diameter) were used to collect the sediment to a depth of 5 cm (Wakkaf et al. 2020). The juveniles of the gray shrimp C. crangon (3–4 cm in length) were caught from the shores of a close beach (37° 18′ 46″ N; 9° 52′ 07″ E) by using a net (20 × 20 cm) with 1 mm meshes, swept along the bottom, and pressed 2–3 cm into the sediment (Pihl and Rosenberg 1982).
Experimental setup
The microcosms consist of small plastic aquaria (diameter, 20 cm; height, 15 cm), each filled with 500 g of natural sediment containing infauna (height, 4 cm), topped with filtered seawater (0.1 μm) aerated by an aquarium diffuser (height, 8 cm). C. crangon juveniles were placed in each of the 3 replicates with four treatments: no shrimp (C0), 4 shrimp (C4), 8 shrimp (C8), and 12 shrimp (C12). The maximum number of C. crangon per microcosm (i.e., 12) was chosen based on previous personal tests where observations showed no disturbances due to the body contacts or fights between shrimps. The experiment was run for 30 days as reported by Essid et al. (2020). The experimental treatments were placed in a room with a controlled environment of cold light/darkness (10/14 h) and temperatures of about 15 °C/10 °C. These conditions were chosen to mimic winter conditions in the region as verified from meteorological data published at http://www.infoclimat.fr for the city of Bizerte during January 2020.
Nematode study
Sediment was sieved throughout 1 mm and 40 μm mesh sizes (Vitiello and Dinet 1979) after applying the suspension-decantation method of Wieser (1960). The retained fraction was then stained with Rose-Bengal (0.2 g/l) and fixed in 4% formalin (Essid et al. 2013). The nematodes were enumerated, and 100 individuals per replicate were sorted under a stereomicroscope (Kotta and Boucher 2001). They were then placed in 21% glycerol, evaporated to anhydrous glycerol, and mounted on slides (Seinhorst, 1959). The species were identified following Platt and Warwick (1983, 1988), Warwick et al. (1998), and Bezerra et al. (2020).
Five functional traits were defined for each specimen collected:
-
(1)
The tail shape according to Thistle et al. (1995) (short/round (s/r), elongated/filiform (e/f), conical (co), clavate/conical-cylindrical (cla))
-
(2)
The amphid shape according to Semprucci et al. (2018) (indistinct (Id); slit-like (St), blister-like (Bs); longitudinal slit (Ls); spiral (SP); rounded or elongate loop (REL); pocket-like (Pk), and circular (Cr)
-
(3)
The life history assessed through a scale from colonizers to persistent (c-p scores; from 1 to 5) (Bongers et al. 1991, 1995)
-
(4)
Feeding classification according to Wieser (1953) (selective deposit feeders (1A), non-selective deposit feeders (1B), epigrowth feeders (2A), and omnivores-carnivores (2B)),
-
(5)
Adult length (1–2 mm, 2–4 mm, and ˃ 4 mm) (Schratzberger et al. 2007).
To evaluate the body weight, the body dimensions of length and width were measured. The body volume (V, in μl) was then estimated according to the formula of Warwick and Price (1979), V = 530 L × W2, where L and W are the total body length (mm) and maximum width (mm), respectively. The wet weight (in μg WW) was calculated after considering their specific gravity (1.13 μg/nl; Wieser, 1960) and used to deduce the dry weight (in μg DW) following the ratio DW/WW is equal to 1/4 (Vanaverbeke et al. 1997).
Data processing
PRIMER 5.0 software was used to analyze the community following the methods described by Clarke (1993) and Clarke and Warwick (2001). Five univariate indices were considered in this study: abundance, species number (S), Margalef’s species richness (d), Shannon-Wiener index (H′), and Pielou’s evenness (J′). All data were first tested for normality distribution (Kolmogorov-Smirnov test) and homogeneity of variance (Bartlett test). Data were log10 (x + 1) transformed to fulfill requirements of parametric analyses (Clarke 1993 and Clarke and Gorley 2001). Then a one-way ANOVA was run followed by multiple comparisons using the Tukey HSD test with STATISTICA (v5.1) software to detect significant differences (p value ≤ 0.05) between treatments. The non-metric MultiDimensional Scaling (nMDS) ordination was applied on square root transformed species abundances and using Bray-Curtis similarity measures to represent how treatments are matched. The analysis SIMPER (SIMilarity PERcentage) was run to determine the contribution of each species to the average dissimilarity/similarity between pairs of treatments. Finally, p < 0.05 was adopted as the significance level for all analyses.
Results
Meiobenthic abundances
The meiofaunal community was dominated by nematodes (85.71 ± 0.25%) and, to a lesser degree, copepods (12.22 ± 0.50%) and polychaetes (1.43 ± 0.39%). After 30 days, the abundance of nematodes showed a significant decrease (T0 vs. C0) (Fig. 1). Moreover, the introduction of shrimp showed a significant negative impact on nematode abundances (C0 vs. C4–C12). Similar results were observed for copepods that showed a drastic decline in abundance at C0 compared to T0. However, a significant decrease in copepods was in treatment C12 compared to C0. Polychaetes abundances remained steady from the start till the end of the experiment, except for C4 where a significant decline was recorded (Fig. 1).
Individual weight and allometry for nematodes
The transition from the field to the laboratory conditions had a significant impact on biomass results (Fig. 2), and nematodes appeared larger at the end of the experiment compared to T0 and C0 (i.e., no shrimp were added). The presence of shrimp over nematodes was only significant in treatment C4 where smaller worms were collected (p value = 0.0165). The treatments with higher pressures (C8 and C12) showed similar weights to the control treatments (p value ≥ 0.364). No significant changes were noticed for relative body growth (Table 1). However, a notable significant increase in the intercepts of the regressions related to body dimensions was observed for comparisons T0 vs.C0, C0 vs. C4, and C0 vs. C12 (Table 1).
Taxonomic diversity of nematodes
The free-living marine nematodes were composed of 14 species belonging to 14 genera and 10 families. The most diverse families were Oncholaimidae, Linhomeidae, and Xyalidae (Table 2). At the onset of experiment (T0), the most dominant taxa were Oncholaimus campylocercoides (32.92 ± 2.73%) and Anticoma eberthi (27.64 ± 4.04%) and to a lesser extent Metalinhomoeus numidicus (7.84 ± 3.77%). The average relative abundance of each one of the remaining species was below 5% of the total nematofauna abundance. After being exposed to gray shrimp for 30 days, the nematofauna were dominated by either O. campylocercoides (8.66–19.33%) or A. eberthi (5.66–18%). The high presence of these two taxa was evident in all treatments (T0 and C0–12). Thus, the nMDS plot was associated with a high stress value of 0.17 (Fig. 3). Only the species number was useful in differentiating treatments T0 and C0 from C4, C8, and C12 based on species richness (Fig. 3). The diversity indices (d and H′) and evenness showed no differences between all treatments tested (Fig. 4).
Changes in diversity indices and evenness of meiobenthic nematodes at the start of the experiment (T0) and after addition of different numbers of Crangon crangon: 0 (C0), 4 (C4), 8 (C8), and 12 individuals (C12). Species number (S); evenness (J′); Margalef’s species richness (d); Shannon index (H′). Different letters above bars indicate significant differences (p < 0.05)
The dissimilarity values showed a decrease in the average dissimilarity (36.08 → 35.61 → 33.42%) between the control nematode community and those with C. crangon (C4 → C8 → C12). SIMPER results revealed that the decrease in abundance of O. campylocercoides and the increase in that of A. eberthi contributed the most to the dissimilarity in the case of the treatments C4 and C8 (Table 3). For C12, the situation was the inverse for these two taxa, and it also had an increase in abundance of Paramonohystera sp.
Functional diversity of nematodes
The initial T0 community was predominately composed of nematodes belonging to the following functional categories: omnivores-carnivores (2B) and selective deposit feeders (1A), clavate (cla) and elongated/filiform (e/f) tails, pocket-like amphids (pk), life history groups c-p 2 and c-p 4, and length intervals > 4 mm and 2–4 mm (Fig. 5). SIMPER results highlighted significant increases of the nematodes 2B, belonging to the biggest class size (˃ 4 mm), possessing clavate tails and indistinct amphids, and having the c-p score 4, compared to T0 and C0 (Table 3). The C0 treatment had a decreased abundance of members belonging to the feeding types 1B and 2A and having conical tails, spiral and circular amphids, lengths from 1 to 2 mm, and c-p scores of 3.
Graphical summary (left) and non-metric multidimensional scaling (nMDS) 2D plots (right) based on abundances of nematode functional groups at the start of the experiment (T0) and after addition of different numbers of Crangon crangon: 0 (C0), 4 (C4), 8 (C8), and 12 individuals (C12). Selective deposit feeders (1A); non-selective deposit feeders (1B); epigrowth feeders (2A); omnivores-carnivores (2B); elongated/filiform (e/f); conical (co); clavate/conical-cylindrical (cla); spiral (sp); pocket-like (pk); indistinct (id); circular (cr)
Medium C. crangon pressure (treatments C4 and C8) led to an increased presence of taxa with c-p scores 2 and 3, spiral amphids, adult lengths of 1–2 mm and 2–4 mm, elongated/filiform tails, and belonging to feeding types 1A, 2A, and 1B compared with the C0 treatment (Fig. 5). Furthermore, the nematofauna was less abundant in individuals with pocket-like amphids, c-p 4, adult lengths of less than 4 mm, clavate tails, and belonging to the group of omnivorous-carnivores. The opposite response was observed under the lowest (treatment C0) and the highest (treatment C12) C. crangon pressures (Fig. 5). The SIMPER results (Table 3) supported patterns in normal or inversed bell-shaped and did not showed a clear trend for the functional traits in relation to the increase in numbers of C. crangon, except for the nematodes with circular amphids becoming more abundant from C0 to C12. For k-dominance curves made from the functional traits (Fig. 6), no pattern is reflected against the changes in shrimp numbers, and a continuous overlapping was observed.
The nMDS second-stage ordination (Fig. 7) showed that the responses of nematode species to the increasing number of shrimp in their respective environment depended mainly to their c-p scores (70.57%), trophic groups (69.51%), and tail shapes (68.43%).
Non-metric multidimensional scaling (nMDS) second-stage ordination of inter-matrix rank correlations. For matrices included, see Figs. 4 (species) and 5 (functional traits). Values indicate average similarity percentages depicting the relationships between nMDS plots based on species and those based on functional traits
Discussion
The main objective of this work was to provide additional data about the direct or indirect trophic links between macrofauna and meiobenthic nematodes, and eventually the predation of the latter by the first organisms. This work gave a new outlook into the functional aspect, commonly underestimated by most of the meiobenthic nematologists. To test the functional changes in nematodes, gray shrimp, C. crangon, were introduced into different experimental aquaria, each filled with sediment containing its natural meiofauna.
Laboratory-related effect
The meiobenthic community at the start of the experiment seems to have been in the late stage, as evident by the dominance of nematodes and scarcity of other groups; thus, the expected taxa replacement of competitors with tolerators clearly occurred upon succession (Allouche et al. 2020). Indeed, it is well-known that nematodes are the most opportunistic groups among meiofauna (Moreno et al. 2011). A significant difference was detected between the treatments T0 and C0 after abundance reduction of nematodes belonging to the functional types with the following features: cr, sp, c-p 2, c-p 3, 1–2 mm, 2–4 mm, e/f, co, 1A, 1B, and 2A. The only way to explain such result might be that the experimental protocol imposed during 1 month in the laboratory did not include the addition of a food ration. Thus, after a while from the start, the most vulnerable taxa will see that their abundances reduced, or even they will disappear (case of Cyatholaimus prinzi), in comparison with the initial nematofauna T0. On the other hand, the increase of the individual weight appeared the result of the omnivores carnivores 2B characterized by a length higher than 4 mm. This trophic group includes opportunistic nematode taxa belonging mainly to the Oncholaimids, known as facultative predators and occasional deposit feeders (Moens and Vincx 1997).
Gray shrimp-related effect
At the end of the experiment, there was a stable abundance of polychaetes across all treatments (T0 and C0–C12). This pattern could be because of the predation pressure exerted by shrimp on or near the surface where nematodes and copepods prefer to live (Coull and Chandler 1992). Polychaetes seem to be safe as they dig deep into the sediment column. These results are consistent with those of Bell and Coull (1978), who studied the effects of grass shrimp on meiobenthic communities in a saltmarsh biotope. These authors observed significant reductions of nematode abundances and attributed it to predation. In addition, another bioassay conducted by Gregg and Fleeger (1998) found that meiofaunal abundances on Spartina alterniflora stems were significantly decreased after exposure to grass shrimp. The fact that polychaetes did not show a significant numerical response agrees with the findings of Bell and Coull (1978), who studied the predation effects of grass shrimp, Palaemonetes pugio, on the sabellid polychaete, Manayunkia aestuarina, and found that predation was only at the surface. They suggested that these annelids are disturbed by shrimp as they rework the sediment-water interface due to bioturbation activity. These results are consistent with those of Pihl and Rosenberg (1984) who put into evidence that the epibenthic feeding behavior of the gray shrimp C. crangon reworks the sediment while it forages the surface sedimentary layer to find preys. However, in case of copepods, only those inhabiting the sediment-water interface and commonly non-interstitial could be susceptible to predation (Gregg and Fleeger 1998). The sediments may act as a refuge for the burrowing copepods from predation by grass shrimp and blue crabs (Martin et al. 1989; Gregg and Fleeger 1998). It turns out that there is a reduction of all the epibenthic and infaunal meiobenthic groups that are related to the surface of the sediment (nematodes and copepods), while polychaetes did not show differences because they lived in the deeper part of the sediments.
The present study recorded no significant changes in nematode diversity indices which indicates that predation pressure, but not the interspecific competition, was important in structuring the nematode assemblage at least in the time scale of our bioassay (Bell and Coull 1978).
The SIMPER results for taxonomic and functional traits showed the overlay of two bell-shaped curves, an inversed curve ↓↑ (O. campylocercoides functional type) and a normal one ↑↓ (A. eberthi functional type). Then, two phases seemed to occur:
-
Phase 1 (C0 → C4 or C0 → C8): C. crangon feed on the nematode O. campylocercoides and globally on the functional type characterized by ˃ 4 mm, 2B, pk, c-p 4, and cla. This kind of prey would be an easy target due to its large size which would also lead to a decrease in competition for space that would benefit other nematodes such as A. eberthi. This species does not appear to be prey for the shrimp because of its small size. Such selective behavior of C. crangon in picking up large food items could explain why the individual weight of the nematofauna decreased significantly at C4. Moreover, gray shrimp numbers were low enough so that every individual seems to have had sufficient space to hunt. Under such conditions, the substrate would be enriched in pseudo-feces of C. crangon and bacterial production fuel up. Thus, the presence of A. eberthi could also be related to its bacterivorous diet and increased food availability.
-
Phase 2 (C8 → C12 or C12): C. crangon feed on the nematode A. eberthi (and globally on the functional type characterized by 1–2 mm, 2–4 mm, 1A, 2A, sp, c-p 2, c-p 3, and e/f) and copepods. The amount of space for each shrimp was smaller because of their high numbers in these treatments. Thus, their movements became detectable by O. campylocercoides. It is hypothesized that O. campylocercoides escaped the danger due to its facultative predator diet, large feeding spectra, and large size (˃ 4 mm). Furthermore, its powerful musculature would allow it to quickly burrow. Sediments have been shown to provide a refuge from predators because they provide a physically complex microhabitat and additional niche axis that increase predator search and handling time (Gregg and Fleeger 1998).
The behavior of O. campylocercoides was not adopted by A. eberthi, which were mostly prayed by gray shrimp. This small Anticomid seems to be trapped in supercial strata because of its selective feeding diet restricted to bacteria at the water-sediment surface. These results are in accordance with those of Schratzberger and Warwick (1999) where authors had suggested a modification of the nematofauna structure directly via predation (consumption) and indirectly via changing physico-chemical features of the sediment for Carcinus maenas. Indeed, C. crangon (Wilcox and Jeffries 1974) and crabs (Schratzberger and Warwick 1999) could be considered carnivorous opportunists since they randomly feed on the available infauna.
The increase of biological pressure in this experiment has been shown to have a significant role at taxonomic and functional levels which is in accordance with the results of Schratzberger and Warwick (1999) who clearly demonstrated a detectable modification in sand fauna compared to mud.
Conclusions
The present results suggested that the presence of the gray shrimp C. crangon for 30 days significantly affected the meiobenthic features through predation of nematodes and copepods. The introduction of shrimp had also significantly modified the individual weight and the taxonomic and functional traits of the nematode community depending upon the number added. At low shrimp abundances, the large nematode O. campylocercoides was targeted. However, the smaller one, A. eberthi, was targeted when shrimp became more numerous. The results obtained demonstrate that biological regulation of meiobenthic nematodes and copepods by macrofauna has indeed a profound role, as shown in case of C. crangon. These results offer additional data related to the taxonomic and functional traits of nematodes and provide new insight into benthic food webs dynamics.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Aller RC, Yingst JY (1985) Effects of marine deposit feeders Heteromastus filiformis (Polychaeta), Macoma balthica (Bivalvia) and Tellina texana (Bivalvia) on average sediment–solute transport, reaction rates and microbial distrinutions. J Mar Res 43:615–645
Allouche M, Nasri A, Harrath AH, Mansour L, Beyrem H, Bourioug M, Geret F, Boufahja F (2020) New protocols for the selection and rearing of Metoncholaimus pristiurus and the first evaluation of oxidative stress biomarkers in meiobenthic nematodes. Environ Poll 263:114529
Bell SS, Coull BC (1978) Field evidence that shrimp predation regulates meiofauna. Oecologia 35:141–148
Bezerra TN, Decraemer W, Eisendle-Flockner U, Hodda M, Holovachov O, Leduc D, Miljutin D, Mokievsky V, Pena Santiago R, Sharma J, Smol N, Tchesunov A, Enekey V, Zhao Z, Vanreusel A (2020) Nemys: world database of nematodes. Accessed at http://nemys.ugent.be
Bongers T, Alkemade R, Yeates GW (1991) Interpretation of disturbance-induced maturity decrease in marine nematode assemblages by means of the maturity index. Mar Ecol Prog Ser 76:135–142
Bongers T, de Goede RGM, Korthals GW, Yeates GW (1995) An update to the cprating of nematode genera can be found in proposed changes of c-p classification for nematodes. Russ J Nematol 3:61–62
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Aust J Ecol 18:117–143
Clarke KR, Gorley RN (2001) PRIMER v5: user manual/tutorial. PRIMER-E: Plymouth, UK 91p
Coull BC, Chandler GT (1992) Pollution and meiofauna: field, laboratory and mesocosm studies. Oceanogr Mar Biol 30:191–271
Essid N, Boufahja F, Beyrem H, Aïssa P, Mahmoudi E (2013) Effects of 17-α-estradiol on a free-living marine nematode community: a microcosm experiment. Afr J Aquat Sci 38(3):305–311
Essid N, Gharbi R, Harrath AH, Mansour L, Mahmoudi E, Beyrem H, Ansari KGMT, Boufahja F (2020) Toxicity of a chromium-enriched superfood, Spirulina platensis, assessed by taxonomic and morpho-functional diversity of marine meiofauna. Environ Poll 262:1143–1150
Federle TW, Livingston RJ, Meeter DA, White DC (1983) Modifications of estuarine sedimentary microbiota by exclusion of epibenthic predators. J Exp Mar Biol Ecol 73:81–94
Feller RJ, Coull BC (1995) Non-selective ingestion of meiobenthos by juvenile spot (Leiostomus xanthurus) (Pisces) and their daily ration. Vie Milieu 45:49–59
Gregg CS, Fleeger JW (1998) Grass shrimp Palaemonetes pugio predation on sediment- and stem-dwelling meiofauna: field and laboratory experiments. Mar Ecol Prog Ser 175:77–86
Gregg JC, Fleeger JW (1997) Importance of emerged and suspended meiofauna to the diet of the darter goby (Gobionellus bolesoma Jordan and Gilbert). J Exp Mar Biol Ecol 209:123–142
Hagerbaumer A, Hoss S, Heininger P, Traunspurger W (2015) Experimental studies with nematodes in ecotoxicology: an overview. J Nematol 47(1):11–27
Henry BA, Jenkins GP (1995) The impact of predation by the gridled goby, Nesogobius sp. 1 on abundances of meiofauna and small macrofauna. J Exp Mar Biol Ecol 191:223–238
Hussain MB, Laabir M, Daly Yahia MN (2020) A novel index based on planktonic copepod reproductive traits as a tool for marine ecotoxicology studies. Sci Total Environ 727:138621
Kotta J, Boucher G (2001) Interregional variation of freeliving nematode assemblages in tropical coral sands. Cah Biol Mar 42:315–326
McCall JN, Fleeger JW (1995) Predation by juvenile fish on hyperbenthic meiofauna: a review with data on post-larval Leiostomus xanthurus. Vie Milieu 45:61–73
Martin TH, Wright RA, Crowder LB (1989) Non-adaptive impact of blue crabs and spot on their prey assemblages. Ecology. 70:1935–1942
Moreno M, Semprucci F, Vezzulli L, Balsamo M, Fabiano M, Albertelli G (2011) The use of nematodes in assessing ecological quality status in the Mediterranean coastal ecosystems. Ecol Indic 11:328–336
Olafsson E, Elmgren R (1991) Effects of biological disturbance by benthic amphipods Monoporeira affinis on meiobenthic community structure: a laboratory approach. Mar Ecol Progr Ser 74:99–107
Palmer MA (1988) Epibenthic predators and marine meiofauna: separating predation, disturbance and hydrodynamic effects. Ecology 69:1251–1259
Platt HM, Warwick RM (1983) Free-living marine nematodes. Part I. British Enoploids. Cambridge University, London 307 p
Platt HM, Warwick RM (1988) Free-living marine nematodes. Part II. British Chromadorids. Synopsis of the British fauna (New Series). No. 38, E.J. Brill/W.Backhuys, Leiden
Probert PK (1984) Disturbance, sediment stability and trophic structure of soft-bottom assemblages. J Mar Res 42:893–921
Pihl L, Rosenberg R (1984) Food selection and consumption of the marine shrimp Crangon crangon in some shallow marine areas in western Sweden. Mar Ecol Prog Ser 15:159–168
Schratzberger M, Warwick RM (1999) Impact of predation and sediment disturbance by Carcinus maenas (L.) on free-living nematode community structure. J Exp Mar Biol Ecol 235:255–271
Schratzberger M, Warr K, Rogers SI (2007) Functional diversity of nematode communities in the southwestern North Sea. Mar Env Res 63(4):368–389
Seinhorst JW (1959) A rapid method for the transfer of nematodes from fixative to anhydrous glycerine. Nematologica 4:67–69
Semprucci F, Balsamo M (2012) Key role of free-living nematodes in the marine ecosystem. In: Boeri F, Jordan AC (eds) Nematodes: morphology, functions and management strategies. NOVA Science Publishers, Inc., Hauppauge, NY, pp 109–134
Semprucci F, Cesaroni L, Guidi L, Balsamo M (2018) Do the morphological and functional traits of free-living marine nematodes mirror taxonomicalal diversity? Mar Env Res 135:114–122
Taylor DL, Peck MA (2004) Daily energy requirements and trophic positioning of the sand shrimp Crangon septemspinosa. Mar Bio 145:167–177
Thistle D, Lambshead PJD, Sherman KM (1995) Nematode tail-shape groups respond to environmental differences in the deep-sea. Vie Milieu 45:107–115
Van Gestel CAM, Loureiro S, Zidar P (2018) Terrestrial isopods as model organisms in soil ecotoxicology: a review. In: Hornung E, Taiti S, Szlavecz K (Eds) Isopods in a changing world. ZooKeys 801:127–162
Vanaverbeke J, Martinez AP, Dahms HU, Schminke HK (1997) The metazoan meiobenthos along a depth gradient in the Arctic Laptev sea with special attentions to nematode communities. Polar Biol 18:391–401
Vitiello P, Dinet A (1979) Définition et échantillonnage du méiobenthos. Rapp. Comm. Int Expl Sci Mer Médit 25:279–283
Warwick RM, Price R (1979) Ecological and metabolic studies on free-living nematodes from an estuarine mud-flat. Estuar Coast Mar Sci 9:257–271
Warwick RM, Gee JM, Berge JA, Ambrose W (1986) Effects of the feeding activity of the polychaete Streblosoma bairdi (Malmgren) on meiofaunal abundance and community structure. Sarsia 71:11–16
Warwick RM, Clarke KR, Gee JM (1990) The effect of disturbance by soldier crabs Mictyris platycheles H. Milne Edwards on meiobenthic community structure. J Exp Mar Biol Ecol 135:19–33
Warwick RM, Platt HM, Somerfield PJ (1998) Free-living marine nematodes. Part III. British monohysterids. Synopsis of British fauna (new series) No. 53, Field Studies Council, Shrewsbury
Wieser W (1953) Die Beziehung zwischen Mundhöhlengestalt, Ernäh rungsweiseund Vorkommen bei freilebenden marinen Nematoden. Arkiv. För. Zoology 2:439–484
Wieser W (1960) Benthic studies in Buzzards Bay. II. The meiofauna. Limnol Oceanogr 5:121–137
Acknowledgements
The authors extend their appreciation to the Researchers Supporting Project number (RSP-2020/17), King Saud University (Riyadh, Saudi Arabia).
Funding
The authors extend their appreciation to the Researchers Supporting Project number (RSP-2020/17), King Saud University (Riyadh, Saudi Arabia).
Author information
Authors and Affiliations
Contributions
Mohamed Allouche: Formal analysis, validation, writing (original draft), and writing (review and editing). Ahmed Nasri: Data curation and investigation. Abdel Halim Harrath: Funding acquisition, writing (review and editing). Lamjed Mansour: Funding acquisition, writing (review and editing). Saleh Alwasel: Funding acquisition and investigation. Hamouda Beyrem: Data curation and investigation. Gabriel Plăvan: Investigation. Melissa Rohal-Lupher: Writing (review and editing). Fehmi Boufahja: Conceptualization, formal analysis, funding acquisition, investigation, methodology, supervision, validation, and writing (review and editing).
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
Not applicable.
Disclaimer
This manuscript represents the views of the authors, and not those of the Texas Water Development Board.
Additional information
Responsible Editor: Vedula VSS Sarma
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Allouche, M., Nasri, A., Harrath, A.H. et al. Do presence of gray shrimp Crangon crangon larvae influence meiobenthic features? Assessment with a focus on traits of nematodes. Environ Sci Pollut Res 28, 21303–21313 (2021). https://doi.org/10.1007/s11356-020-12069-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-12069-0