Abstract
Firefighting water additives are used to increase the rate at which fires can be extinguished. The majority of ecotoxicological research has focused on firefighting formulations containing perfluorinated compounds as additives, due to the persistence and bioaccumulative nature of the perfluorinated constituents. A number of relatively new additives have come on the market to replace the products containing perfluorinated compounds. The potential effect of these new additives on the environment has been largely unstudied. This study investigated the toxicity of six firefighting water additives: Eco-Gel™, ThermoGel 200L™, FireAde™, Fire-Brake™, Novacool Foam™, and F-500™ to terrestrial biota. Terrestrial organisms could be exposed to firefighting water additives through leaching into soil and/or runoff following a firefighting event or through direct aerial application during a forest fire. Toxicity to three plant species was assessed through seedling germination and emergence tests: Fagopyrum esculentum (buckwheat), Raphanus raphanistrum subsp. sativus (radish), and Rudbeckia hirta (black-eyed Susan). The effects of firefighting water additives on three soil invertebrates, the collembolan Folsomia candida, the earthworms Eisenia andrei, and Dendrodrilus rubidus, were also investigated using static acute tests to estimate EC50/LC50s. The concentration that resulted in a 50% reduction in survival (LC50) for the acute toxicity tests conducted with F. candida ranged from 3 (Eco-Gel) to 0.175% (Novacool) by volume. Comparatively, the acute toxicity of two firefighting water additives to D. rubidus could not be determined, as a 50% reduction in survival was not observed. A number of firefighting water additives were found to pose a hazard to terrestrial organisms based on a worst-case exposure scenario of direct application at the greatest recommended application rate for a class A fire (e.g., wood, paper). The firefighting water additive F-500 was found to pose a hazard (HQ ≥ 1) for all species tested, except for the acute test conducted with D. rubidus. Comparatively, Eco-Gel posed a hazard for only the acute and chronic tests with F. candida. This study represents the first comparative deterministic risk assessment of firefighting water additives to terrestrial ecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
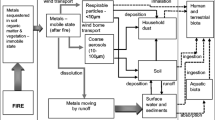
To more efficiently control the spread and reduce potential damages of fires, firefighters mix firefighting additives with the water used to fight a variety of fire types (e.g., residential, industrial, forest). The use of water additives in firefighting could result in contamination of terrestrial ecosystems (Bridge et al. 2005; Lui et al. 2017; Song et al. 2014). In forest fire scenarios, firefighting water additives can be applied through aerial drops from an aircraft that can release hundreds of liters in each application (Liu et al. 2017; Satoh et al. 2005). Firefighting water additives used to enhance the efficacy of water for dousing fires could adversely affect soil organisms directly, which could cause a reduction in species richness, and ecosystem services (Chagnon et al. 2015; Semenzin et al. 2009). In addition to many physical processes, the microbial community in soil helps to regulate biological processes like nutrient cycling (Chagnon et al. 2015). Plants constitute the base of the food web, contributing to and promoting species diversity within the terrestrial ecosystem, and they are intimately involved with the nutrient cycling of the soil (Arts et al. 2015; Chagnon et al. 2015).
In the past, firefighting water additives contained perfluorinated compounds, which have been determined to be highly persistent in the environment (Janie 1995; Liu et al. 2017; Xiao et al. 2015). The compounds of greatest concern, perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA), have been detected in a variety of environmental matrices (Montagnolli et al. 2017). Firefighting additives have been identified as a major source of PFOS to soils (Liu et al. 2017), and PFASs have also been shown to have adverse effects on soil biota, including terrestrial invertebrates like earthworms (Liu et al. 2017, Yuan et al. 2017).
The earthworms Eisenia fetida and Eisenia andrei are extremely important indicators of soil health and environmental contamination due to their role in decomposition and nutrient cycling (Chagnon et al. 2015; Yuan et al. 2017). Numerous studies have shown that earthworms exposed to subchronic concentrations of PFOS and PFOA experience negative effects as indicated by changes in biochemical indicators (e.g., superoxide dismutase and cellulase activity) and impaired decomposition activity Lankadurai et al. 2013; Xu et al. 2013; Yuan et al. 2017).
PFASs have also been found to have bioaccumulative properties (Ghisi et al. 2019). When PFASs are present in soil, they have shown to accumulate in the tissues of soil biota (Ghisi et al. 2019). Although differences in bioaccumulation among species have been observed, all plants can accumulate PFOS and PFOA to some extent, and the difference could potentially be due to the ability of the plant to take up and translocate the constituents (Ghisi et al. 2019). The difference in bioaccumulation can also be attributed to the composition and surface area of the plants’ roots (Ghisi et al. 2019). The manufacturing of firefighting water additives and other consumer products that contain PFOS and PFOA has been restricted, but there is limited information available on the effect of emerging firefighting water additives and these require investigation (Liu et al. 2017).
The objective of this study was to investigate the toxicity of six firefighting water additives to three plant species (Fagopyrum esculentum, Raphanus raphanistrum subsp. sativus, and Rudbeckia hirta) and three terrestrial invertebrates (Folsomia candida, a species of Collembola, and two species of earthworm, E. andrei and Dendrodrilus rubidus). Following a characterization of the concentration–response relationship of the firefighting water additives to these six species, a hazard assessment was completed in order to assess the impact that these additives could have on terrestrial ecosystems.
Materials and methods
Test chemicals
Six firefighting water additives were investigated in this study; Eco-Gel™, ThermoGel 200L™, FireAde™, Fire-Brake™, Novacool Foam™, and F-500™ (Table 1). All firefighting water additives are proprietary mixtures, and all known chemical components are listed in Table 1. The additives are sold in liquid form and are soluble in water; consequently, exposure solutions for testing were generated by mixing the additives in deionized water (DI). The concentrations of exposure solutions were expressed as a percentage, calculated as the volume of firefighting water additives divided by the total volume of the additive solution. These concentrations could be expressed as parts-per-million; for example, 2% could be expressed as 20,000 ppm. The application rate recommended by each manufacturer varies depending on the class of fire that is being treated (Table 1). The species tested in this study, as well as the firefighting water additives and the concentrations tested, are presented in Table 2.
Plant species
Seeds of R. hirta (black-eyed Susan), F. esculentum (buckwheat), and R. raphanistrum subsp. sativus (radish) were ordered from William Dam Seeds (Dundas, ON) and stored at 4 °C prior to use. The dicotyledonous species F. esculentum and R. raphanistrum are historically used in plant testing according to the Organization for Economic Co-operation and Development (OECD) 208 and the Environment and Climate Change Canada (ECCC) EPS/RM/56 protocol (OECD 2006; ECCC 2013). Rudbeckia hirta was included along with the two species traditionally used in plant toxicity testing because exposure of boreal species to firefighting water additives is relevant from a forest fire perspective.
Germination test
Seed germination and root elongation tests (120 h) were conducted following the American Society for Testing and Materials (ASTM) E1963-09 guideline (ASTM 2014). The firefighting water additives were mixed directly into DI water to produce exposure solutions with nominal concentrations ranging from 0 to 5%, expressed as volume of firefighting water additives per total volume of solution. Ten seeds of R. hirta, F. esculentum, or R. raphanistrum were placed into lidded Petri dishes with a diameter of 9.0 cm on top of two pieces of P8-creped filter paper saturated with 4 mL of exposure solution. Five concentrations of water additive treatments and a negative control of deionized water were prepared for each experiment (Table S1). Each treatment consisted of five replicate Petri dishes. Petri dishes were incubated for 120 h in the dark at 24 ± 2 °C. The pH was measured in exposure solutions at initiation of the test (Table S1). At 120 h, germination success, determined to be when the radicle of the seedling was visible, was recorded (ASTM 2014) (Table S2–S4). Root length from the base of the seed to the end of the radicle was measured after 120 h of incubation (Table S5).
Emergence toxicity test
Seedling emergence and growth tests (14 days; 21 days) were conducted following the OECD 208 guideline (OECD, 2006). Firefighting water additives were mixed with DI water to create five nominal concentrations ranging from 0 to 5%. Percentages were expressed as volume of additives per total volume of solution.
Seeds of F. esculentum and R. raphanistrum were germinated prior to planting. Seeds were placed into Petri dishes (9.0-cm diameter) on top of two pieces of P8-creped filter paper saturated with 4 mL of DI water and incubated for approximately 48 h in the dark at a temperature of 24 ± 2 °C. Five successfully germinated seeds, where the seedling radicle was visible, were used for each replicate. For emergence tests conducted with R. hirta, ten un-germinated seeds were used for each replicate, as the seeds for this species take more time to germinate and have a relatively low success rate. When a minimum of five seedlings had emerged, any additional R. hirta plants were cut at the root upon emergence and not included in the counts.
Plastic pots with a 3-in. diameter were lined with unbleached paper towel and filled with 30 ± 5 g of MiracleGro™ potting soil (properties provided in Table S6). The moisture content and water holding capacity was determined using the ECCC EPS/RM/56 protocol (ECCC 2013). The F. esculentum and R. raphanistrum seedlings or R. hirta seeds were placed below 0.5 cm of soil, and 25 mL of treatment solutions was applied to the soil surface. The firefighting water additives were mixed directly into DI water at nominal concentrations ranging from 0 to 5% and added until the soil of each pot reached a WHC of 75%. This volume (25 mL) corresponded with an application rate of 3 L/m2, which would be representative of an aerial drop for a forest firefighting scenario. For each test, there were five concentrations (plus a negative control), and each concentration had five replicate test vessels.
The pH was measured in exposure solutions at initiation of the test using an Orion™ Versa Star Pro™ Multiparameter Benchtop Meter (Table S7). The vessels were covered with clear plastic to minimize moisture loss and incubated in a growth chamber at 20 ± 2 °C with a photoperiod of 16-h light:8-h dark and a light intensity of 1500 lx. For F. esculentum and R. raphanistrum, plants were incubated for 14 days, and for R. hirta, plants were incubated for 21 days. During the growth period, plants were watered daily with DI water, until it seeped through the bottom of the pot. The clear plastic that covered each test unit was removed once plants reached the plastic cover.
At test completion, emergence was recorded, and the plants were photographed (growth data are provided in Tables S8–S10). All plant species tested had one shoot per plant, for a total of five shoots per replicate, and each shoot was cut at the base of the shoot to measure shoot length, from the base of the stem to the terminal bud of the plant, using a ruler. The number of leaves per shoot was recorded, and the aboveground tissue was placed in aluminum weigh boats and weighed using an analytical balance to determine the fresh weight. The weigh boats and plant tissue were placed into a drying oven at 65 °C for 1 week and weighed to determine the dry mass of the tissue (Table S11–S13).
Folsomia candida
Folsomia candida eggs were isolated from a culture maintained at the University of Guelph. Eggs were placed into a Petri dish lined with moistened filter paper and a substrate made from plaster of Paris and activated charcoal. A total of 0.004 g of Fleischmann’s ® Traditional Active Dry Yeast was placed onto the substrate as a source of food for young springtails. To moisten the substrate, DI water was sprayed on the surface daily.
Acute toxicity test
Acute toxicity tests (14 days) were conducted following the ECCC EPS 1/RM/47 guideline (ECCC, 2017). Each experiment consisted of six treatments including a negative control; each treatment contained five replicate vessels. Test vessels (125-mL glass mason jars) were filled with 46 g of field soil. The field soil was collected in 2012 from a field that had been fallow for 10 years outside of Guelph, Ontario. The physicochemical properties of the field soil can be found in Table S14.
DI water was then added to each test vessel until the soil reached a WHC of 70%, as determined using the ECCC EPS 1/RM/47 protocol (ECCC 2007). The treatments of firefighting water additives were created by mixing the additives with DI water at nominal concentrations from 0 to 5%. The volume of treatment solution that was added to the surface of each test vessel with a graduated cylinder is based on an application rate of 3 L/m2. The application rate is reflective of an aerial drop of firefighting water additive during a forest fire. The volume added to each vessel was calculated using the diameter of the jar opening to calculate area. The pH and conductivity of the exposure solutions were measured at test initiation (Table S15). Test vessels were then allowed to rest covered for 24 h before adding juvenile springtails.
After 24 h, ten juvenile (12 days old) F. candida were placed into the test vessels with approximately 0.004 g of Fleischmann’s® Traditional Active Dry Yeast. The vessels were randomized using a random number table and incubated in an environmental chamber at 20 ± 2 °C with a photoperiod of 16-h light:8-h dark and light intensity of 1500 lx.
At day seven, a visual inspection of each test vessel was completed, and mold coverage, surface activity of springtails, and uneaten yeast were recorded. Any uneaten yeast was removed, and approximately 0.004 g of Fleischmann’s ® Traditional Active Dry Yeast was added. On day fourteen (test termination), mortality was assessed by floating springtails with deionized water (Table S16).
Chronic toxicity test
Chronic toxicity tests (27 days) with springtails were conducted according to the ECCC EPS 1/RM/47 guideline (ECCC 2014). Each experiment consisted of five treatments and a negative control in five replicate vessels. Approximately 30 g of field soil (as described above) was placed into each test vessel (125-mL glass mason jars).
For the chronic tests with F. candida, the firefighting water additive Eco-Gel was tested at nominal concentrations ranging from 0 to 4%. The moisture content and WHC for the field soil was calculated using the ECCC EPS 1/RM/47 protocol, and the treated solutions were added to the soil to a WHC of 70% (ECCC 2007). At the start of the test, pH and conductivity of the exposure solutions were measured.
Ten F. candida were placed into the test vessels 24 h after application of exposure solutions with 0.004 g of Fleischmann’s ® Traditional Active Dry Yeast. The vessels were placed randomly using a random number table and incubated in an environmental chamber at 20 ± 2 °C with a photoperiod of 16-h light:8-h dark and light intensity of 1500 lx. At the completion of the test (day 27), mortality was assessed by using distilled water to float the organisms, and the number of adults and progeny was counted using the ImageJ analysis software (Table S17).
Eisenia andrei
Eisenia andrei were obtained from an established culture at the University of Guelph. Cultures were maintained according to the Environment Canada EPS 1/RM/43 guideline (ECCC 2007). Once or twice per week depending on the culture size, each culture bin was fed a mixture of 4–6 tablespoons of prepared Quaker Instant Oats and PC Organic Quick Oats, amended with Magic Products, Inc. Worm Food®. This was done once or twice per week, according to the number of worms in each culture bin and how much excess food remained after each feeding. DI water was sprayed onto the surface of the soil until it reached a crumbly consistency if the moisture content of the soil in each culture bin failed the “squeeze” test, which is described in the Environment Canada EPS 1/RM/43 guideline (ECCC 2007).
Acute toxicity tests (14 days) were conducted with guidance from the ECCC EPS 1/RM/43 (ECCC, 2004). There were five treatments and one negative control for each experiment, and each treatment contained three replicate test vessels. There was also an additional replicate for each treatment to measure the pH at test initiation (day 0) and conclusion of the test (day 14).
Test vessels (300-mL glass wide-mouth mason jars) were filled with approximately 200 g dry weight of artificial soil, which was prepared according to the ECCC EPS 1/RM/43 guideline (ECCC 2007). Using the same protocol as a guide, the WHC and moisture content of the soil were also measured. Nominal concentrations of Eco-Gel ranging from 0 to 4% were prepared by mixing the additive with DI water. The soil was then hydrated to 70% of its WHC with DI water. Exposure solutions were added to the surface of each test vessel with a graduated cylinder at application rate of 3 L/m2 based on the application rate for an aerial drop as determined from the diameter of the opening of the test vessel. Test vessels were covered and allowed to rest for 24 h before adding worms. The pH and conductivity of the treated soils were measured at test initiation and test conclusion (Table S18). At 24 h post-application, five sexually mature worms, identified by the presence of a clitellum, were added to each test vessel. All vessels were then placed into an environmental chamber set at 20 ± 2 °C with a photoperiod of 16-h light:8-h dark and light intensity of 1500 lx. After 14 days of incubation, the test vessels were emptied and assessed for worm mortality (Table S19).
Dendrodrilus rubidus
Dendrodrilus rubidus were taken from established laboratory cultures, which were maintained in the same manner as described for Eisenia andrei. Acute toxicity tests (14 days) were conducted with guidance from the ECCC EPS 1/RM/43 guideline (ECCC, 2004). There were five treatments and one negative control of deionized water, each with three replicates. There was also an additional replicate for each treatment to measure the pH at test initiation and conclusion of the test.
Approximately 200 g of dry artificial soil was prepared according to ECCC EPS 1/RM/43 guidelines and was placed into 300-mL glass wide-mouth mason jars (ECCC 2007). The WHC and moisture content had been previously determined for the artificial soil. Nominal concentrations of 0–4% and 0–2% (% by volume of additive per total volume of exposure solution) were prepared with F-500 and Eco-Gel, respectively. DI water was added to achieve 70% of the soil’s WHC, and then a volume of exposure solution was added based on an application rate of 3 L/m2 and the diameter of the jar opening. The soil was tested for pH and conductivity following application and at the conclusion of the test (Table S20). The test vessels were left to incubate for 24 h before test organisms were added post-application.
For the tests with Eco-Gel and F-500, five and four sexually mature worms were added to each test vessel, respectively. The test organisms were deemed to be sexually mature when a clitellum was observed. The environmental chamber was set to 20 ± 2 °C with a photoperiod of 16-h light:8-h dark and light intensity of 1500 lx, and test vessels were incubated for 14 days. Following 14 days of incubation, each vessel was assessed for mortality of worms (Table S21).
Statistical analysis
The firefighting water additive concentration resulting in a 50% reduction in survival (LC50) and 50% reduction in all other endpoints (EC50) was estimated using drc package in R Studio version 1.1.456 along with associated 95% confidence intervals (CI) (Ritz et al. 2015; RStudio 2016). Nominal concentrations (% by volume) were used to establish ECx and LCx values for 10, 25, and 50% effect. A 4-parameter logistic model was fit to the data from the toxicity tests. For mortality, emergence, and germination success, upper and lower limits were fixed to 100 and 0, and for reproduction and growth, the lower limit was fixed to 0. The no-observed-effect concentration (NOEC) values were identified using one-way analysis of variance (α = 0.05) in SigmaPlot 14.0. Normality and equal variance among treatments were determined using the Shapiro–Wilk and Brown–Forsythe tests before deciding to use a parametric ANOVA. Any significant differences between the treatments and control were determined using Dunnett’s test. A Kruskal–Wallis test on ranks was completed if the normality and/or equal variance test failed in order to test for significant differences among treatments followed by Dunn’s multiple comparison test.
Hazard assessment
A hazard assessment was conducted by determining the hazard quotients for each species. Hazard quotients were calculated by comparing effect endpoints (EC50 for R. hirta, F. esculentum, R. raphanistrum subsp. sativus; LC50 for F. candida, E. andrei, D. rubidus) to the highest concentration at application (%) for each firefighting water additive recommended by each manufacturer. To be conservative, it was also assumed that the concentration of firefighting water additive in the water dropped by a water bomber was at the highest percentage recommended by the manufacturer for a class A fire (e.g., wood, paper). For example, Eco-Gel recommends an application rate of 6% for class A fires. For this investigation, if the hazard quotient was greater or equal to one, it indicated a potential hazard.
Results
Plant species
Germination tests
For the three plant species, Eco-Gel had the greatest EC50 (germination), with firefighting water additive values of > 4% for R. sativus and F. esculentum and > 5% for R. hirta (Table 3). FireAde also had the greatest EC50 (germination) for R. sativus at a value of > 4%. F-500 had the lowest EC50 for R. sativus and R. hirta, 0.24% (95% CI of 0.19–0.29 and 0.18–0.29, respectively) (Table 3). Novacool had the lowest EC50 for R. hirta, which was 0.11% (0.07–0.15) (Table 3).
With the EC50 values as an effect concentration, it was determined that F-500 could pose a hazard (hazard quotient ≥ 1) for the germination of every species tested and was the only additive to pose a hazard to R. sativus (Table 4). In addition to F-500, FireAde and Fire-Brake could also pose a hazard to F. esculentum (Table 4). For R. hirta, all firefighting additives except Eco-Gel could pose a hazard (Table 4). With the NOEC values as an effect concentration, the only firefighting water additive that did not pose a hazard to all species was Fire-Brake, and Eco-Gel and ThermoGel indicated a hazard for all three species (Table 5).
Emergence tests
The mean control seedling emergence for R. sativus was > 70%, which met the criterion of the OECD 208 protocol (OECD, 2006). Of the six firefighting water additives tested, Eco-Gel and FireAde had the highest EC50 (emergence). The EC50 values ranged from > 4 to 0.36%; F-500 had the lowest EC50 (Table 6). Four firefighting water additives were tested with F. esculentum, and EC50 (emergence) for all firefighting water additives ranged from > 5 to 0.36% (Table 6). For R. hirta, the EC50 (emergence) values for Eco-Gel and F-500 were > 5% and 1.33%, respectively (Table 6). Based on emergence EC50 value, the hazard assessment determined that the only firefighting water additive to pose a hazard to R. sativus, F. esculentum, and R. hirta was F-500 (Table 4). Novacool was the only firefighting water additive that could pose no hazard for any of the species based on the NOEC values.
Folsomia candida
In the 14-day tests with F. candida, the mean survival of the controls was > 80%, which met the criterion of the ECCC EPS 1/RM/47 guideline (ECCC 2014). Eco-Gel had the highest LC50 (mortality), and values ranged from 3.0 to 0.329%, and F-500 had the lowest (Table 7). All firefighting water additives except for FireAde were found to pose a hazard to F. candida survival using the LC50 values (Table 4). The hazard assessment using the NOEC values determined that all firefighting water additives could pose a hazard (Table 5).
In the 28-day test with F. candida, the mean number of live offspring for the controls was > 100, which met the criterion of the ECCC EPS 1/RM/47 guideline (ECCC 2014). Eco-Gel, the only firefighting additive tested for 28 days with F. candida, was found to have an EC50 (reproduction) of 1.68% (1.03–2.34) (Table 6). From the hazard assessment using the EC50 and NOEC values, Eco-Gel could pose a hazard to reproduction of F. candida (Tables 4 & 5).
Eisenia andrei
The mean control survival for the controls in the acute test with E. andrei was 100%, which exceeded the criterion of > 90% (ECCC 2007). For E. andrei, Eco-Gel had an LC50 (mortality) value of > 4.0% as the highest concentration tested did not result in 50% mortality. Consequently, a hazard quotient could not to be determined using the LC50 values; however, the hazard assessment completed with the NOEC values found that Eco-Gel could pose a hazard (Tables 4, 5 and 8).
Dendrodrilus rubidus
The mean control survival was 100%, which met the criterion of ≥ 90% (ECCC, 2007). The LC50 values (mortality) for D. rubidus for Eco-Gel and F-500 were greater than the highest concentration tested, 4% and 2%, respectively (Table 5). Thus, hazard quotients for both firefighting water additives could not be determined with the LC50 values; however, F-500 would not pose a hazard using the NOEC values (Table 3).
Discussion
Although the toxicities of the firefighting water additives varied among species, the endpoints for the terrestrial plant R. sativus (radish) indicated that this plant was not sensitive to the additives. The EC50 value for germination of this species could only be determined for F-500, as less than 50% reduction in germination was observed for the other firefighting water additives at the highest concentration tested. Additionally, Eco-Gel, FireAde, Fire-Brake, Novacool, and ThermoGel were found not to pose a hazard to R. sativus germination and emergence. Previous studies have determined that certain terrestrial plant species were not sensitive to firefighting water additives (Song et al. 2014). Song et al. (2014) found that in the field, firefighting water additives Forexpan S, PhosChek-WD881, and Silv-ex did not negatively affect seed germination of Pinus desniflora (Korean red pine), Pinus rigida (pitch pine), and Brassica campestris (rapeseed). Rudbeckia hirta was the most sensitive of the species tested in this study. For the germination tests, the EC50 values ranged from 0.11 to > 4%. In comparison, the EC50 values for the germination tests conducted with R. sativus ranged from 0.24 to > 4%.
A conservative approach was taken when carrying out the hazard assessment with the toxicity data from the terrestrial data. The application rate of 3 L/m2 was used to develop the hazard quotients, and this was considered to be the worst-case scenario. The volume of firefighting water additive that would reach the soil is dependent on a variety of factors, including the height or speed of the plane during application, weather conditions, and interception by vegetation (Satoh et al. 2004; Satoh et al. 2005). Additionally, environmentally relevant exposure would likely be a single application, rather than repeated exposures over a short period of time. The results from this hazard assessment confirm that it is extremely important to understand the fate and effects of the firefighting water additives being released into the terrestrial environment, as some could pose a hazard to terrestrial organisms.
The hazard of firefighting water additives was observed to vary among endpoints and species. For example, F-500 posed a hazard (hazard quotient ≥ 1) for every species except D. rubidus (acute). The firefighting water additive Eco-Gel only posed a hazard to F. candida (acute and chronic). Similar trends were observed in an aquatic hazard assessment of firefighting water additives (Graetz et al. 2020). In the aquatic assessment, F-500 posed the greatest hazard quotients to the greatest number of aquatic species, while Eco-Gel posed a hazard to the fewest species (Graetz et al. 2020). It is likely that variation in toxicity across species and endpoints reflects differences in the chemical composition among the different firefighting water additives tested in this study. Unfortunately, limited information was available on the constituents of each of the firefighting water additives (Table 1), making it difficult to explain the variation in toxicity among additives.
Conclusion
This investigation determined that there was large variation in toxicity between the firefighting water additives towards terrestrial plant and animal species. The hazard that each firefighting water additive might pose to terrestrial biota differed for each species and firefighting water additive. It was clear that certain additives posed a greater hazard to terrestrial biota relative to other additives. When the toxicity of the firefighting water additives was ranked from most toxic to least toxic for each test where all six products could be tested, the mean ranking was the greatest for F-500 and the lowest mean ranking was for Eco-Gel (Table 9). The results of this study clearly show that certain water additives should be avoided for use in natural areas. This study is the first to assess and compare toxicity and hazard of these six firefighting water additives to terrestrial biota. This assessment provides important data for the evaluation of the potential risk of firefighting water additives to terrestrial ecosystems.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Arts GHP, Dollinger M, Kohlschmid E, Maltby L, Ochoa-Acuña H, Poulsen V (2015) An ecosystem services approach to pesticide risk assessment and risk management of non-target terrestrial plants: recommendations from a SETAC Europe workshop. Environ Sci Pollut Res 22:2350–2355
ASTM-E1963-09 (2014) Standard guide for conducting terrestrial plant toxicity tests. ASTM International, West Conshohocken, PA
Bridge SRJ, Miyanishi K, Johnson EA (2005) A critical evaluation of fire suppression effects in the boreal forest of Ontario 10. Retrieved from http://people.ucalgary.ca/~johnsone/pub/Bridge_etal_2005.pdf
Chagnon M, Kreutzweiser D, Mitchell EAD, Morrissey CA, Noome DA, Van der Sluijs JP (2015) Risks of large-scale use of systemic insecticides to ecosystem functioning and services. Environ Sci Pollut Res 22:119–134
Environment Canada and Climate Change (2007) Biological test method: tests for toxicity of contaminated soil to earthworms (Eisenia andrei, Eisenia fetida, and Lumbricus terrestris). Environmental Technology Centre, Report EPS 1/RM/43, Environment Canada, Ottawa, Ontario
Environment Canada and Climate Change (2013) Biological test method: test for growth in contaminated soil using terrestrial plants native to the boreal region. Environmental Technology Centre, Report EPS 1/RM/56, Environment Canada, Ottawa, Ontario
Environment Canada and Climate Change (2014) Biological test method for measuring survival of springtails exposed to contaminants in soil. Environmental Technology Centre, Report EPS 1/RM/47, Environment Canada, Ottawa, Ontario
Ghisi R, Vamerali T, Manzetti S (2019) Accumulation of perfluorinated alkyl substances (PFAS) in agricultural plants: a review. Environ Res 169:326–341
Graetz S, Ji M, Hunter S, Sibley PK, Prosser RS (2020) Deterministic risk assessment of firefighting water additives to aquatic organisms. Ecotoxicology 29(9):1377–1389
Janie Y (1995) FoamVSFire_Aerial. 29. Retrieved from https://www.fs.fed.us/t-d/pubs/pdf/hi_res/95511209hi.pdf
Lankadurai BP, Furdui VI, Reiner EJ, Simpson AJ, Simpson MJ (2013) 1H NMR-based metabolomic analysis of sub-lethal perfluorooctane sulfonate exposure to the earthworm, Eisenia fetida, in soil. Metabolites 3:718–740
Liu Z, Lu Y, Wang P, Wang T, Liu S, Johnson AC, Sweetman AJ, Baninla Y (2017) Pollution pathways and release estimation of perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) in central and eastern China. Sci Total Environ 580:1247–1256
Montagnolli RN, Matos Lopes PR, Matos Cruz J, Marina Turini Claro E, Quiterio GM, Bidoia ED (2017) The effects of fluoride based fire-fighting foams on soil microbiota activity and plant growth during natural attenuation of perfluorinated compounds. Environ Toxicol Pharmacol 50:119–127
OECD (Organization for Economic Cooperation and Development). (2006) OECD guidelines for the testing of chemicals: terrestrial plant test: seedling emergence and seedling growth test, No. 208, Paris, France
Ritz C, Baty F, Streibig JC, Gerhard D (2015) Dose-response analysis using R. PLoS One 10:e0146021
RStudio (2016) RStudio: integrated development for R. RStudio, Inc., Boston, MA
Satoh K, Kuwahara K, Yang KT (2004) A numerical study of forest fire progression and fire suppression by aerial fire fighting. Energy Conversion and Resources: Fuels and Combustion Technologies, Energy, Nuclear Engineering 2004:79–86
Satoh K, Maeda I, Kuwahara K, Yang K (2005) A numerical study of water dump in aerial fire fighting. Fire Safety Science 8:777–787
Semenzin E, Critto A, Marcomini A (2009) Ecosystem impairment evaluation on biodiversity and functional diversity for contaminated soil assessment. Integr Environ Assess Manag 5:399
Song U, Mun S, Waldman B, Lee EJ (2014) Effects of three fire-suppressant foams on the germination and physiological responses of plants. Environ Manage 54:865–874
Xiao F, Simcik MF, Halbach TR, Gulliver JS (2015) Perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in soils and groundwater of a U.S. metropolitan area: migration and implications for human exposure. Water Res 72:64–74
Xu D, Li C, Wen Y, Liu W (2013) Antioxidant defense system responses and DNA damage of earthworms exposed to perfluorooctane sulfonate (PFOS). Environ Pollut 174:121–127
Yuan Z, Zhang J, Zhao L, Li J, Liu H (2017) Effects of perfluorooctanoic acid and perfluorooctane sulfonate on acute toxicity, superoxide dismutase, and cellulase activity in the earthworm Eisenia fetida. Environ Sci Pollut Res 24:18188–18194
Funding
Funding for this study was provided through the National Science and Engineering Research Council of Canada’s Discovery grant (RGPIN 401357) awarded to RS Prosser.
Author information
Authors and Affiliations
Contributions
SG, WM, NW, and JA conducted the toxicity tests. SG analyzed the data generated from the toxicity tests. SG wrote the original draft of the manuscript. PKS and RSP conceptualized the study; acquired the funding for the study; provided guidance to SG, WM, NW, and JA on the toxicity testing; and prepared the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 125 kb)
Rights and permissions
About this article
Cite this article
Graetz, S., Martin, W., Washuck, N. et al. Deterministic risk assessment of firefighting water additives to terrestrial organisms. Environ Sci Pollut Res 28, 20883–20893 (2021). https://doi.org/10.1007/s11356-020-12061-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-12061-8