Abstract
Cadmium pollution in the environment is ubiquitous and can be a serious health issue. Crop straw-based biochar is a promising adsorbent, yet few studies have systematically examined the effects of both feedstock and pyrolysis temperature on biochar efficacy for cadmium (Cd) sorption. Sorption-desorption experiments were conducted to explore the mechanisms of Cd sorption for biochars derived from wheat straw (WSB), rape straw (RASB), soybean straw (SSB), and peanut straw (PSB) feedstocks, which were produced by pyrolysis at 450 °C and 650 °C. For biochars pyrolyzed at 450 °C, the sorption capacities varied as PSB>SSB>RASB≈WSB, while the order changed as RASB≈WSB>PSB>SSB for 650 °C biochars. With the increase in pyrolysis temperature, for wheat straw biochar and rape straw biochars, Cd sorption capacities increased by 72% and 63%, while there were 61% and 63% decreases for peanut straw biochar and soybean straw biochar, respectively. Compared to the non-legume straw biochars (WSB and RASB), legume straw biochars (SSB and PSB) have higher sorption capacities for Cd. Peanut straw biochar (PSB) produced at 450 °C was found to be the most promising adsorbent for cadmium. Examination of the sorbed biochars using a range of analytical techniques indicated that 450 °C PSB immobilized cadmium by precipitation with non-electrostatic adsorption. The precipitation of Cd on 450 °C PSB was mainly induced by cation exchange between Cd with mineral cations, which caused Cd complexation with carboxyl functional groups.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Industrial and agricultural activities such as manufacturing, mining, wastewater irrigation, and other practices have increased the number of agricultural soils contaminated with heavy metals in many parts of the world (Houben et al. 2013; Zhang et al. 2013). Cadmium (Cd) is one of the most toxic heavy metals due to its high mobility and persistence (Loganathan et al. 2012; Cui et al. 2016; Kula et al. 2008; Mohan et al. 2007). High concentrations of Cd may lead to lung cancer, prostatic proliferative lesions, bone fractures, kidney dysfunction, and hypertensive diseases (Waalkes 2000; Han et al. 2017).
The uptake and toxicity of a metal is generally determined by the free metal ion concentrations in the soil solution (Ren et al. 2015). The uncomplexed metal ion in soil solution is considered the most available form for plant uptake (Degryse et al. 2012). The behaviors of heavy metals in contaminated soils, in terms of their impact on groundwater or effects on plant uptake, are a function of both the concentration and chemical speciation in soil solution (Cances et al. 2003). Cascades of reactions can happen between soil Cd and the surface of soil particles. The concentration of Cd in the soil solution is determined by numerous interrelated processes, including oxidation-reduction, precipitation/dissolution, adsorption/desorption, and inorganic and organic complex formation (Romic 2012).
Biochar is a carbon-rich solid material produced by pyrolyzing biomass in an oxygen-limited environment. It is added to soils in order to sequester carbon and to maintain or improve soil function (Joseph et al. 2010). Biochar has been proven to be an effective sorbent, precipitant, and surface complexation medium for cationic metals in soil solution (Van Poucke et al. 2018). The pyrolysis temperature at which biochar is produced can have a greater influence on the structural and chemical characteristics of the biochar than the biomass feedstocks used to produce it (Chen et al. 2012). Pyrolysis process conditions can impact biochar sorption capacities by modifying the characteristics of the feedstock (Jazini et al. 2018). Harvey et al. (2011) suggested that the surface charge of biochar is induced by the pyrolysis of biomass. Melo et al. (2013) compared the sorption capacities of sugar cane straw biochars pyrolyzed at 400, 500, 600, and 700 °C and found that the higher temperature biochars have higher metal sorption capacities. Kim et al. (2013) reported that a high pyrolysis temperature (> 500 °C) could increase the Cd adsorption capacity of biochar. Different pyrolysis temperatures may change biochars properties by causing alterations in surface area, porosity, pH mineral content, and functional groups (Li et al. 2017a; Kumar et al. 2011). Sun et al. (2014) reported that biochars with high surface area and high pore volume could be readily produced from crop straw at 600 °C. Except for pyrolysis temperature, the adsorption capacities of biochars varied with feedstock (Sun et al. 2014; Cao et al. 2009; Xu et al. 2013a, 2013b; Tan et al. 2015). Crop straw biochars generally exhibit a high yield, high ash content, high pH, and high alkaline and alkaline earth metal concentrations (including K+, Ca2+, Mg2+, and Na+) (Wang et al. 2013; Sun et al. 2014). According to the reported studies, sorption capacity of biochars for Cd(II) decreased in the order of crop straw>dairy manure>wood and bark (Ahmad et al. 2014; Mohan et al. 2007; Sun et al. 2014; Xu et al. 2013b; Fristak et al. 2015). Sun et al. (2014) reported that crop straw biochars displayed a well-developed pore structure and had a high sorption capacity for Cd of between 57.7 and 96.4 mg/g in aqueous solution. Currently, a detailed understanding of the influence of biomass feedstock and pyrolysis temperature on the physicochemical properties of biochars, and the associated impacts on sorption efficiency of heavy metal ions, is still limited (Sun et al. 2014; Wei et al. 2017; Shakoor et al. 2020).
To address this knowledge gap, four types of crop straw (wheat straw, rape straw, soybean straw, and peanut straw) were pyrolyzed at two temperatures (450 °C and 650 °C), and their heavy metal sorption capacities were assessed. We hypothesized that the biochar physicochemical properties will vary as a function of biomass feedstock and pyrolysis temperature. In turn, this will influence biochar Cd adsorption capacities and interaction mechanism between biochar and Cd. In this work, we aim to understand if crop straw feedstocks show similar behavior with respect to pyrolysis temperatures. This study compares the Cd sorption capacities of various biochars and elaborates the interaction mechanisms between Cd and biochar.
Materials and methods
Biochar preparation
Various crop straws (wheat, rape, soybean, and peanut) were collected and washed with deionized water to remove any attached dust or soil fragments. They were then oven-dried at 75 °C until no further weight loss was measured. The dried crop straws were crushed in a stainless steel grinder, and the resulting powders were placed in stainless steel jars, sealed and placed in a muffle furnace. The pyrolysis temperatures used were 450 °C and 650 °C. The heating rate was set at 5 °C/min. After a residence time of 3 h, the solid biochars were ground and passed through 20 and 40 mesh sieves (0.85 mm, 0.15 mm). The biochars were identified as WSB (wheat), RASB (rape), SSB (soybean), and PSB (peanut). The designation was prefixed with either 450 °C or 650 °C to indicate the pyrolysis temperature.
Chemical characterization
The quantity of biochar produced from each biomass material (yield) and the quantity of ash in each biochar were calculated from the Eqs. (1) and (2) (Zama et al. 2017; Gai et al. 2014).
where MBC is the mass of biochar, MBM is the mass of biomass, and MASH is the mass of ash.
The biochar pH was determined using a Mettler Toledo Seveneasy precision pH meter (Switzerland) with a water-biochar ratio of 20:1 (v: m), and the solutions were subsequently analyzed for electrical conductivity (EC) using a conductivity meter (DDS-307A, Rex Shanghai).
The biochar organic carbon was analyzed by wet digestion using potassium dichromate. Dissolved organic carbon (DOC) was measured using hot water (100 °C) extraction with a water-biochar ratio of 20:1 (v: m). The water/biochar mixture was shaken at 180 r/min over a 24-h contact period at 20–25 °C, and the resultant filtered solutions were subsequently analyzed using an automated total organic carbon (TOC) analyzer (Multi N/C 2100, German Analytik Jena Company). The cation exchange capacity (CEC) was assessed using the ammonium acetate (NH4OAC) method (Gaskin et al. 2010). Biochar available phosphorus (P) was extracted by sodium bicarbonate. The extract solution was filtered and analyzed using the method detailed by Murphy and Riley (1986).
The functional groups were identified by Fourier transform infrared spectroscopy (FTIR) using a Thermo Scientific Nicolet iS5 operating in potassium bromide tableting mode. The surface functional groups and chemical compositions were determined by X-ray photoelectron spectroscopy (XPS, VG Scientific West Sussex, UK). Examination of the biochar particles was carried out using a Hitachi scanning electron microscope (SEM) and a JEOL ARM200F aberration corrected scanning transmission electron microscope (STEM) with energy dispersive X-ray spectroscopy (EDS) as per the methods described by our previous studies (Archanjo et al. 2017; Joseph et al. 2013; Bian et al. 2014). The carboxyl and hydroxyl functional groups were measured using Boehm titration following the method developed by Boehm (1994).
After shaking the solution for 24 h, the biochar and solution was separated using a Brinell funnel and suction filter. Half of the liquid filtrate was collected for the determination of anion concentrations (PO43−, SO42−, SiO32−, CO32−, HCO3−). Isobutanol extraction-molybdenum blue colorimetry was used for PO43− detection, and barium chromate spectrophotometry was applied for the SO42− measurement. SiO32− was analyzed with silicon molybdenum blue colorimetry, described in detail by Lu (2000). The neutralization titration method described by Lu (2000) was applied for CO32− and HCO3− detection. The cation (Cd, Ca, Mg, Fe, Mn) concentration of the remaining filtrate was measured using a flame atomic absorption spectrometry (FAAS, A3, Persee, China).
Batch adsorption
Cd(II) stock solution was prepared by dissolving Cd(NO3)2 4H2O in Milli-Q water. The solution concentration was 1000 mg/L. The stock solution was further diluted, also with Milli-Q water, to 5, 10, 15, 25, 50, 80, 100, 150, and 200 mg/L. All the solutions with varying Cd(II) concentrations were prepared using a background electrolyte of 0.01 M NaNO3 to maintain the same ionic strength (Chen et al. 2015). 0.1 M HNO3 and NaOH solutions were used to adjust solution pH to 5.0.
Kinetic adsorption experiments were carried out in 50-mL centrifuge tubes with 50 mL of 100 mg/L Cd(II) solution (pH, 5.0) by adding 0.1 g of biochar followed by shaking at 180 r/min for a set period at 25 °C. At each sampling time (0, 0.08, 0.25, 0.5, 1, 2, 4, 8, 12 h), the suspension was filtered through 0.45 μm pore size nylon membrane filters. Cd(II) was measured in the filtrate, and the sorption was calculated as the difference between the initial and final solution concentrations of the sorbate. The residual Cd(II) concentrations in the obtained supernatants were determined using flame atomic absorption spectrometry (FAAS, A3, Persee, China). The equations of the Freundlich and Langmuir adsorption models are expressed, respectively, by
where Qe is the amount of the metal adsorbed per unit weight of biochar (mg/g), Ce is the solution concentration at equilibrium, Qmax is the maximum adsorption capacity (mg/g) of biochar, and B is the constant related to the affinity. K and N (1/n) are indicators of adsorption capacity and intensity, respectively.
Isothermal adsorption experiments were carried out by mixing 0.1 g of the biochar with 25 mL of concentrations of Cd(II) solution ranging from 5 to 500 mg/L followed by shaking at 180 r/min over a 24-h contact period at 25 °C. The sampling time, filtration method, and analysis method were the same as above. Pseudo-first-order (Lagergren 1898), pseudo-second-order (Ho and Mckay 2000), and intraparticle (Wu et al. 2009) models were applied to verify the sorption kinetics (Li et al. 2017b; Ofomaja et al. 2010). The equations are as follows:
where k1 is the pseudo-first-order (Lagergren 1898) rate constant (L/min) and k2 is the rate constant of the pseudo-second-order (Ho and Mckay 2000) adsorption (g/mg min). kp is the intraparticle (Wu et al. 2009) diffusion rate constant (mg/g min0.5). t is the contact time, and C is the intercept. Qt and Qe are Cd adsorption amounts at time t and equilibrium.
Desorption
After sorption, the biochar was filtered and washed with Milli-Q water twice. Biochar was then placed in 50-mL centrifuge tube with 25 mL of 0.1 M NaNO3 solution and then shaken at 180 r/min over a 24-h contact period at 25 °C. The concentrations of Cd(II) were detected as per the methods described for the adsorption study.
Statistical analyses
All experiments were conducted with three replicates, and the individual data were used to plot adsorption kinetics and isotherms. All data was plotted using Sigmaplot 12.5, and sorption isotherms were fitted using a Freundlich and Langmuir model. All collected data were analyzed statistically using SPSS 18 software. The data from each of the treatments were compared using a multiple-range test of Duncan, and the variability in the data was expressed as a standard deviation. A p < 0.05 was taken as statistically significant. Figures were drawn in Origin 2015.
Results
Characteristics of different biochars
Basic properties of biochars
The physicochemical properties of the biochars are summarized in Table S1. The increase in pyrolysis temperature from 450 to 650 °C reduced the yields of WSB, RASB, SSB, and PSB by 7.1%, 9.5%, 2.2%, and 2.3%, respectively, while peanut straw achieves a higher yield compared to the other feedstocks at both pyrolysis temperatures. The increase in pyrolysis temperature increases the ash contents of WSB, RASB, SSB, and PSB by 13.2%, 30.3%, 9.5%, and 8.5%, respectively. The pH of the biochars produced at both 450 °C and 650 °C ranged from 10.16 to 11.33. The higher pyrolysis temperature increased the pH of WSB and PSB by 4.3% and 4.1%, while it decreased the pH of RASB and SSB by 1% and 7.3%, relative to the lower pyrolysis temperature biochar equivalent. Biomass pyrolyzed at 650 °C had a higher EC than that at 450 °C, except for that of PSB which was 1.7% lower at 650 °C than that of 450 °C PSB. Compared to the 450 °C biochars, the organic carbons in 650 °C WSB, RASB, SSB, and PSB decreased by 16.6%, 13%, 8.6%, and 28.3%, respectively.
Mineral element contents in biochars
Results in Table S2 show the alkali cation contents in biochars. Higher pyrolysis temperature increased Na contents significantly for WSB and PSB by a factor of 9.25 and 2.61 times relative to the respective low temperature biochar. The higher pyrolysis temperature increased Ca contents for RASB, SSB, and PSB by 12.5%, 11.6%, and 10.9%, respectively, but decreased Ca contents in WSB by 31.2%. When the pyrolysis temperature increased from 450 °C to 650 °C, the cation exchange capacity (CEC) decreased by 9.7%, 2.3%, and 10.7%, for RASB, SSB, and PSB, respectively, while the CEC of 650 °C WSB was 2.2% higher than that of 450 °C WSB.
Table S3 shows the main mineral elements contents in biochars. The higher pyrolysis temperature increased CO32− contents in WSB sharply by a factor of 4.7 times relative to the low temperature equivalent. This was the largest increase observed for all the biochars. The higher pyrolysis temperature resulted in a reduction in CEC in RASB and PSB by 9.7% and 10.7%, respectively. Furthermore, the higher pyrolysis temperature sharply increased CO32−, SiO32− contents in WSB and RASB.
Functional group contents in biochar
The TOC concentrations of biochars are summarized in Fig. S1a. This showed that increasing the pyrolysis temperature from 450 to 650 °C resulted in a reduction in TOC of 23.6%, 23.5%, 27.6%, and 60.6% for WSB, RASB, SSB, and PSB, respectively. Results from Fig. S1b indicate that the concentration of carboxyl functional groups also decreased at the higher pyrolysis temperature by 66%, 61%, 90%, and 90%, for WSB, RASB, SSB, and PSB, respectively. Further analysis of functional groups with FTIR is given in Fig. S2. The absorption bands at around 1600 cm−1, which are due to stretching in carboxylates, disappeared at 650 °C, while the peaks for C–H (~ 900 cm−1) of 650 °C biochars become much stronger.
The specific surface area and porosity of biochars
The specific surface area and porosity distribution characteristics of biochars are presented in Table S4. The pore sizes of WSB are meso- (2–50 nm) and micro-pores (< 2 nm), and in RASB, are mainly micro-pores (< 2 nm). By contrast, SSB and PSB are characterized with meso- (2–50 nm) and macro-pores (> 50 nm). With increasing pyrolysis temperature from 450 to 650 °C, the concentration of micro-pores in WSB increased from 34 to 55%, respectively, while the micro-pores proportion of PSB and SSB increased with the pyrolysis temperature.
Cd sorption capacities of biochars
Adsorption isotherms
Figure 1 shows curves for the adsorption capacities of the biochars as a function of Cd(II) solution equilibrium concentration. The amounts of Cd(II) adsorption on biochars pyrolyzed at both 450 and 650 °C increased significantly when the equilibrium solution concentrations of Cd were lower than 10 mg/L. The amount of Cd(II) adsorption capacity of biochar tended to plateau when the equilibrium concentrations of Cd(II) were greater than 20 mg/L, while it was 50 mg/L for 650 °C WSB. With the increase in pyrolysis temperature, the adsorption capacities of both WSB and RASB increased, while it decreased for PBS and SSB.
Sorption isotherms for Cd(II) on wheat straw biochar (WSB), rape straw biochar (RASB), soybean straw biochar (SSB), and peanut straw biochar (PSB) pyrolyzed at (a) 450 °C and (b) 650 °C; Qe (mg/g) is the amount of metal adsorbed per unit weight of adsorbent; Ce (mg/L) is the equilibrium solution concentration of the adsorbate
Results in Table 1 demonstrate that the measured Cd maximum sorption amounts (Qmax) are close to the determined ones from the Langmuir models (except 650 °CSSB). The order of measured Cd maximum sorption amounts (Qmax) of biochars pyrolyzed at 450 °C was PSB>SSB>RASB≈WSB. Qmax of PSB is 1.58, 2.78, and 2.80 times higher than that of SSB, RASB, and WSB, respectively. The measured values of Qmax of biochar at 650 °C showed the following trend: RASB>WSB>SSB>PSB. The measured values of Qmax of RASB are 1.07, 1.58, and 1.67 times higher than that of WSB, SSB, and PSB, respectively. Cd sorption capacities increased with the pyrolysis temperature for RASB and WSB and decreased for PSB and SSB. Increasing the pyrolysis from 450 to 650 °C resulted in measured Qmax of PSB and SSB decreasing by 37.7% and 62.6%, respectively, while for WSB and RASB, it increased by 63.8% and 73.4%, respectively.
The binding strength constant (B) of Cd determined from the Langmuir model in biochars pyrolysis at 450 °C was ordered as SSB>PSB>RASB>WSB and PSB>RASB>SSB>WSB for biochars produced at 650 °C. These results indicate that PSB has higher relative affinity for Cd than other biochars. Moreover, the increase in pyrolysis temperature increased the affinity of RASB for Cd but decreased Cd adsorption affinity in SSB.
The Freundlich model is a semi-empirical equation, which describes surface sorption and multi-layer sorption under various non-ideal conditions (Ding et al. 2012). The Freundlich constant of K can be considered an indicative parameter of the adsorption strength (Tunc et al. 2009). The K values of 450 °C biochars were ordered as PSB>SSB>RASB≈WSB. Comparatively, the order of K for 650 °C biochars was as RASB>PSB>WSB>SSB. These results indicate that PSB has higher sorption capacity for Cd. The increase in pyrolysis temperature increased Cd adsorption capacity of RASB but decreased the sorption capacity of SSB for Cd. All values of N less than 1 illustrate that adsorbate is favorably adsorbed on the adsorbent (Tunc et al. 2009). The N values of 450 °C biochars were ordered as SSB<PSB<RASB<WSB, while the N values for 650 °C biochars changed as PSB<RASB<SSB<WSB. Comparatively, PSB has stronger adsorption intensity than other biochars. The increase in pyrolysis temperature decreased the adsorption intensity of SSB but increased Cd adsorption intensity of RASB.
Parameters and correlation coefficients (R2) for Langmuir (Wang and Qin 2006; Cao et al. 2009) and Freundlich models (Ho 2005; Inyang et al. 2012) presented in Table 1 show that the Langmuir model (R2 of 0.94–0.95) fits the data better than the Freundlich model (R2 of 0.74–0.85). These results indicate that Cd(II) adsorption on these biochars mainly occurred in monolayers, or through a fixed number of identical, and energetically equivalent sites on the surface.
Adsorption kinetics
Figure 2 presents the sorption kinetics for Cd(II). For the biochars pyrolyzed at 450 °C, the time required to reach the equilibrium state varied in the following order PSB<WSB<RASB<SSB, whereas for biomass pyrolyzed at 650 °C, this was WSB≈RASB<PSB<SSB.
Table 2 presents the kinetic parameters for adsorption of Cd(II) on the biochar. The rate constants of k1 and k2 for 450 °C biochars changed following the order of PSB>RASB>WSB>SSB. For 650 °C biochars, the values of k1 and k2 were ordered as WSB>RASB>PSB>SSB. The intraparticle diffusion rates (kp) of 450 °C and 650 °C biochars were in the orders of PSB>WSB>RASB>SSB and WSB≈RASB>RSB>SSB, respectively. For both 450 °C and 650 °C biochars, the intraparticle diffusion may be a rate determining step in the adsorption process. The intraparticle equation provides the best fit for 450 °C WSB, 450 °C RASB, and 450 °C SSB (R2 were 0.94–0.98), while the pseudo-second-order and pseudo-second-order models fit 450 °C PSB. The sorption kinetic characteristics of 650 °C WSB, 650 °C RASB, and 650 °C PSB are in good agreement with pseudo-second-order model (R2 of 0.93–0.97), and the intraparticle model describes 650 °C SSB better (R2 of 0.97).
It can be concluded that the sorption kinetics of WSB and RASB changed from the intraparticle equation to the pseudo-second-order models, while the sorption kinetics of SSB remain the same and PSB sorption kinetics changed from pseudo-first-order to pseudo-second-order as pyrolysis temperature increases.
Desorption study
Figure 3 provides a compilation of the range and average Cd desorption rates for the various biochars. Results in Fig. 3 indicate that WSB and RASB have higher desorption rates than SSB and PSB. The desorption rates of WSB and RASB increase with pyrolysis temperature. The respective desorption rate distribution ranges of 450 °C WSB and 450 °C RASB are 1–2% and 0.8–1.2%, while they are 2–6% and 1–3% for 650 °C WSB and 650 °C RASB, respectively. The average desorption rates of 450 °C WSB, 450 °C RASB, 650 °C WSB, and 650 °C RASB are 1.8%, 1%, 3%, and 2%, respectively. The increase in pyrolysis temperature increases the desorption rate distribution ranges of SSB and PSB, which also increases the desorption rates of SSB but decreases that of PSB. The changes of desorption rates of SSB and PSB with pyrolysis temperature are around 0.1–0.2%, which are much lower than those of WSB and RASB.
Cd adsorption on 450 °C PSB
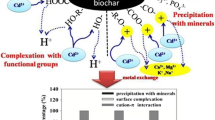
XPS analysis in Table 3 indicates that the Cd(II) has been adsorbed through cation exchange both due to π bonding with the carbon matrix and by complexation with Cl−, CO32− and OH−. 450 °C PSB contains high concentration of C–C/C–H, which decreased by 7% after Cd(II) adsorption. The percentage of C1s peaks at 284.8 and 289.2 eV for the 450 °C PSB decreases after Cd adsorption. Most notably, the proportion of O–C=O decreased by 14%. Further results in Table S5 show that there has been a reduction in the concentrations of Si2p, Al2s, K2p3/A, Ca2s, Fe2p, and Mg1s after Cd(II) adsorption.
Figure 4 a and b are high-angle annular dark field (HAADF) and bright field (BF) STEM images, respectively, of a 450 °C PSB biochar particle that has a high concentration of Cd(II) on its surface. The Cd X-ray map and average spectrum of the area (Fig. 4 c and d, respectively) show that there is a high concentration of Cd present. The area is also rich in carbon and oxygen, and the Cd phase may be CdCO3. Other compounds/minerals rich in Si, Ca, Mg, and Na may have also formed, and these appear to be bound together by organic compounds (data not shown). Figures S3a and S1b show a HAADF image and associated EDS spectrum showing the formation of CdCO3 crystals on the surface of a SiO2 particle that is embedded in the carbon matrix (data not shown). Figure S3 c and d show a HAADF image and EDS spectrum of a nanoparticle which consists of an inner crystal of CdCO3 (region labeled 1) surrounded by a porous matrix of inorganic compounds bonded with organic compounds (region labeled 2) that also have a detectable concentration of Cd. Figure S3 e is a higher magnification HAADF image of the porous region, and the EDS spectrum (Fig. S3f) indicates the existence of compounds/minerals containing Cd, Si, Na, Mg, P, Fe, and Ca. The organic compounds in this outer layer probably contain N-rich functional groups.
Discussion
Characteristics of Cd(II) adsorption on biochars
The higher pyrolysis temperature increased the Cd(II) sorption capacities for WSB and RASB, while it resulted in decreased Cd(II) sorption amounts on PSB and SSB relative to the low temperature equivalents (Table 2 and Fig.3). The increase in pyrolysis temperature increased the adsorption affinity and intensity of PSB and RASB for Cd but reduced that of SSB and had no effect on WSB. Adsorption isotherms constants (Table 1) demonstrate that the Langmuir model can fit all the adsorption isotherms of all the biochars, suggesting that Cd(II) adsorption occurs mainly as a surface monolayer. The specific surface area and the proportion of micro-pores (< 2 nm) of WSB and RASB are higher than those of SSB and PSB (Table S4), which may lead to enhanced physical adsorption of Cd onto WSB and RASB. Further, physical adsorption kinetics are usually fitted by pseudo-first-order and pseudo-second-order kinetic models (Ho and McKay 1999; Sarı and Tuzen 2009); the fitting parameters to the kinetic model (Table 2) suggest that Cd adsorption on 450 °C PSB is mainly due to physical adsorption, while the higher temperature biochar results in increased Cd physical adsorption on WSB and RASB.
Chemical adsorption processes, including electrostatic adsorption and non-electrostatic adsorption, are the main mechanisms by which Cd(II) is adsorbed onto biochars (Xu et al. 2006). To distinguish between electrostatic and non-electrostatic adsorption of Cd(II) by biochar surfaces, 1.0 M NaNO3 has been used to replace Cd(II) adsorbed by biochars electrostatically (Xu et al. 2006). As the amount of metal desorbed by KNO3 or NaNO3 was attributed to their electrostatic adsorption (Xu et al. 2005; Jiang et al. 2012), the electrostatic adsorption has a higher desorption rate of pre-adsorbed heavy metals than that of non-electrostatic adsorption (Xu and Zhao. 2013). In this study, Cd desorption rates of WSB and RASB were higher than those of SSB and PSB, indicating that the fractions of electrostatically adsorped Cd(II) on WSB and RASB were higher than that on SSB and PSB (Fig. 3). Moreover, the high temperature biochar exhibited increased electrostatic adsorption of Cd(II) for SSB, WSB, and RASB. The non-electrostatic sorption of Cd on SSB and PSB decreased with pyrolysis temperature increase.
Main factors controlling biochar sorption capacities
The presence of oxygen-containing functional groups such as carbonates, phosphates, and hydroxyls on biochar surfaces can enhance the formation of complexes and precipitates with Cd(II), which can, in turn, contribute to non-electrostatic adsorption (Yuan and Xu 2012; Khan et al. 2015; Tong et al. 2011; Cao et al. 2009). It is notable that the liming effect (the alkaline effect of biochar) can enhance Cd(II) adsorption on biochar due to both electrostatic and non-electrostatic attraction. Firstly, the liming effect can increase the total negative charge on the biochar, which will lead to more electrostatic adsorption (Tong et al. 2011). Secondly, the increase in solution pH will increase the dissociation of biochar surface functional groups, including carboxylate and hydroxyl, which will enhance non-electrostatic adsorption of Cd(II) by forming surface complexes with those deprotonated functional groups (Lu et al. 2012; Tong et al. 2011). Redundancy analysis (RDA) (Fig. S4) suggests that the critical factors controlling more than 98% of the sorption mechanisms for Cd(II) on various biochars include cations (Na, Ca, K, Mg, and Mn), anions (CO32−, SiO32−, and PO43−), EC, and pH. The liming effect, cation exchange and Cd co-deposition with anions contributed to the Cd sorption for all the biochars. As discussed above, Cd(II) adsorption on WSB and RASB was mainly caused by electrostatic adsorption, while non-electrostatic adsorption was the main reason for Cd(II) adsorption on SSB and PSB. The increase in C(II) adsorption on 650 °C WSB was mainly due to the increase in pH. The increase in concentration of meso-pores (2–50 nm) and macro-pores (> 50 nm) and anions (e.g., CO32− and SiO32−) may have increased the total negative charge on 650 °C RASB, thus enhancing Cd(II) electrostatic adsorption. The liming effect enhanced Cd(II) non-electrostatic adsorption on SSB and PSB, which is probably due to an increase in cation exchange and surface complexes, as Cd(II) sorption in 650 °C SSB and 650 °C PSB decreased with the reductions in CEC and carboxyl functional groups. The highest sorption capacity for Cd(II) was measured for 450 °C PSB, and this was due to its high concentration of surface acidic functional groups complexes.
Non-electrostatic adsorption of Cd(II) on the surface of 450 °C PSB
The maximum adsorption amount of Cd onto 450 °C PSB was 110.205(mg/g), which was much higher than many other biochars derived from different feedstocks (Table S6). Results from Tables 3 and S5 show that both functional groups and exchangeable cations change after Cd adsorption on the surface of 450 °C PSB.
Some oxygen-containing surface functional groups (e.g., carboxylate, –COOH and hydroxyl, OH) present on the surface of the biochar can have strong interactions with heavy metals by electrostatic attraction, ion-exchange, and surface complexation (Tan et al. 2015). Studies have shown that O–C=O is beneficial to heavy metal sorption, and it can be largely consumed during Cd(II) sorption process (Wang et al. 2018; Chen et al. 2015; Zhang et al. 2017). Similar findings were reported by Tan and Xiao (2009) and Chen et al. (2018). The loss of carboxylate groups with higher pyrolysis temperature may cause the reduction in Cd(II) sorption capacity of 650 °C PSB. A similar result has been reported by Fristak et al. (2015), who found that the blocking of carboxyl functional groups caused a significant decrease in biochar sorption capacity for Cd(II), as the oxygen-containing functional groups could provide more adsorption sites for Cd(II).
Uchimiya et al. (2010) stated that heavy metal could be adsorbed by biochar through specific metal-ligand complexation involving surface functional groups of chars with or without cation exchange. The reduction in surface exchangeable cations indicates that during the sorption process, cation exchange may contribute to the precipitation of Cd(II) (Sun et al. 2014). In this study, cation exchange may mainly happen between Cd and Ca through non-electrostatic adsorption. The Pauling radius of Cd2+(0.97 Å) and Ca2+(0.99 Å) are very similar (Thakur et al. 2006), but the solubility product constant of CdCO3 (log Ksp = − 12) is less than that of CaCO3 (log Ksp = − 8.35) (Novozamsky and Beek 1976). Thus, when the CaCO3 dissolves from the biochar, CO32− precipitation of CdCO3 can occur in aqueous solution. The presence of Cd 3d peak at 406.15 eV after Cd(II) adsorption for PSB (Table 3) confirms the formation of less insoluble CdCO3. This result is consistent with the studies reported by Flores-Cano et al. (2013) and Zhang et al. (2015).
Results from this study suggest that crop straw biochar sorption behavior vary with feedstock with respect to pyrolysis temperature. The interaction mechanisms between Cd and biochar change with the change in biochar surface characteristics. Non-electrostatic sorption can induce higher sorption capacity for biochar than electrostatic sorption. Overall, leguminous crop, like peanut straw and soybean straw, pyrolyzed around 450 °C may be benefit to Cd sorption in aqueous solution. It has been addressed that legume straw-derived biochars pyrolyzed at 400°Chave higher heavy metal sorption ability than non-legume straw biochars, which is mainly attributed to non-electrostatic mechanism via the formation of surface complexes between heavy metal and acid functional groups of the biochars (Tong et al. 2011; Jiang et al. 2014). This study further elaborates that the outstanding surface properties of legume straw-derived biochars, including macro-pores (> 50 nm) CEC, pH, and exchangeable cations (e.g., Ca and Mg) can contribute to the formation of Cd and carboxyl functional groups complexes.
Conclusion
Both non-electrostatic sorption and electrostatic sorption contributed to Cd(II) sorption on biochars. Biochar produced by high temperature pyrolysis (650 °C) showed a reduction in non-electrostatic sorption for SSB and PSB but an increased Cd(II) sorption for WSB and RASB with electrostatic sorption, when compared with the low temperature biochar (450 °C). The liming effect, cation exchange, and Cd(II) deposition with anions all contribute to the Cd(II) sorption on the crop straw biochars in this study, while SSB and PSB have lower desorption rates with higher non-electrostatic adsorption contribution. Further analysis results show that non-electrostatic sorption largely contributed to Cd adsorption to 450 °C PSB with a Qmax value of 110.205(mg/g). Cd(II) adsorption on this material is mostly attributed to the cation exchange assisted by the carboxylic functional groups with non-electrostatic adsorption. Therefore, optimizing biochar with feedstock and pyrolysis temperature to enhance non-electrostatic adsorption for Cd should be a priority in Cd pollution remediation.
References
Ahmad M, Rajapaksha AU, Lim JE, Zhang M, Bolan N, Mohan D, Vithanage M, Lee SS, Ok YS (2014) Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 99:19–33
Archanjo BS, Mendoza ME, Albu M, Mitchell DRG, Hagemann N, Mayrhofer C, Mai TLA, Weng Z, Kappler A, Behrens S, Munroe P, Achete CA, Donne S, Araujo JR, van Zwieten L, Horvat J, Enders A, Joseph S (2017) Nanoscale analyses of the surface structure and composition of biochars extracted from field trials or after co-composting using advanced analytical electron microscopy. Geoderma 294:70–79
Bian R, Joseph S, Cui L, Pan G, Li L, Liu X, Zhang A, Rutlidge H, Wong S, Chia C, Marjo C, Gong B, Munroe P, Donne S (2014) A three-year experiment confirms continuous immobilization of cadmium and lead in contaminated paddy field with biochar amendment. J Hazard Mater 272:121–128
Boehm HP (1994) Some aspects of the surface-chemistry of carbon-blacks and other carbons. Carbon 32:759–769
Cances B, Ponthieu M, Castrec-Rouelle M, Aubry E, Benedetti MF (2003) Metal ions speciation in a soil and its solution: experimental data and model results. Geoderma 113:341–355
Cao XD, Ma LQ, Gao B, Harris W (2009) Dairy-manure derived biochar effectively sorbs lead and atrazine. Environ Sci Technol 43:3285–3291
Chen Y, Yang H, Wang X, Zhang S, Chen H (2012) Biomass-based pyrolytic polygeneration system on cotton stalk pyrolysis: influence of temperature. Bioresour Technol 107:411–418
Chen Z, Liu T, Tang J, Zheng Z, Wang H, Shao Q, Chen G, Li Z, Chen Y, Zhu J, Feng T (2018) Characteristics and mechanisms of cadmium adsorption from aqueous solution using lotus seedpod-derived biochar at two pyrolytic temperatures. Environ Sci Pollut Res Int 25(12):11854–11866
Chen Z, Xiao X, Chen B, Zhu L (2015) Quantification of chemical states, dissociation constants and contents of oxygen-containing groups on the surface of biochars produced at different temperatures. Environ Sci Technol 49(1):309–317
Cui X, Fang S, Yao Y, Li T, Ni Q, Yang X, He Z (2016) Potential mechanisms of cadmium removal from aqueous solution by Canna indica derived biochar. Sci Total Environ 562:517–525
Degryse F, Shahbazi A, Verheyen L, Smolders E (2012) Diffusion limitations in root uptake of cadmium and zinc, but not nickel, and resulting Bias in the Michaelis constant. Plant Physiol 160:1097–1109
Ding Y, Jing D, Gong H, Zhou L, Yang X (2012) Biosorption of aquatic cadmium(II) by unmodified rice straw. Bioresour Technol 114:20–25
Flores-Cano JV, Leyva-Ramos R, Mendoza-Barron J, Guerrero-Coronado RM, Aragon-Pina A, Labrada-Delgado GJ (2013) Sorption mechanism of Cd(II) from water solution onto chicken eggshell. Appl Surf Sci 276:682–690
Fristak V, Pipiska M, Lesny J, Soja G, Friesl-Hanl W, Packova A (2015) Utilization of biochar sorbents for Cd2+, Zn2+, and Cu2+ ions separation from aqueous solutions: comparative study. Environ Monit Assess 187(1):4093
Gai XP, Wang HY, Liu J, Zhai LM, Liu S, Ren TZ, Liu HB (2014) Effects of feedstock and pyrolysis temperature on biochar adsorption of ammonium and nitrate. PLoS One 9(12):e113888
Gaskin JW, Speir RA, Harris K, Das KC, Lee RD, Morris LA, Fisher DS (2010) Effect of peanut hull and pine chip biochar on soil nutrients, corn nutrient status, and yield. Agron J 102(2):623–633
Han L, Sun H, Ro KS, Sun K, Libra JA, Xing B (2017) Removal of antimony (III) and cadmium (II) from aqueous solution using animal manure-derived hydrochars and pyrochars. Bioresour Technol 234:77–85
Harvey OR, Herbert BE, Rhue RD, Kuo LJ (2011) Metal interactions at the biochar-water interface: energetics and structure-sorption relationships elucidated by flow adsorption microcalorimetry. Environ Sci Technol 45(13):5550–5556
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Ho YS, McKay G (2000) The kinetics of sorption of divalent metal ions onto sphagnum moss peat. Water Res 34(3):735–742
Ho YS (2005) Effect of pH on lead removal from water using tree fern as the sorbent. Bioresour Technol 96(11):1292–1296
Houben D, Evrard L, Sonnet P (2013) Beneficial effects of biochar application to contaminated soils on the bioavailability of Cd, Pb and Zn and the biomass production of rapeseed (Brassica napus L.). Bioresour Technol 57:196–204
Inyang M, Gao B, Yao Y, Xue YW, Zimmerman AR, Pullammanappallil P, Cao XD (2012) Removal of heavy metals from aqueous solution by biochars derived from anaerobically digested biomass. Bioresour Technol 110:50–56
Jazini R, Soleimani M, Mirghaffari N (2018) Characterization of barley straw biochar produced in various temperatures and its effect on lead and cadmium removal from aqueous solutions. Water Environ J 32(1):125–133
Jiang T-Y, Xu R-k, Gu T-X, Jiang J (2014) Effect of crop-straw derived biochars on Pb(II) adsorption in two variable charge soils. J Integr Agric 13:507–516
Jiang J, Xu RK, Jiang TY, Li Z (2012) Immobilization of Cu(II), Pb(II) and Cd(II) by the addition of rice straw derived biochar to a simulated polluted Ultisol. J Hazard Mater 229:145–150
Joseph SD, Camps-Arbestain M, Lin Y, Munroe P, Chia CH, Hook J, van Zwieten L, Kimber S, Cowie A, Singh BP, Lehmann J, Foidl N, Smernik RJ, Amonette JE (2010) An investigation into the reactions of biochar in soil. Soil Res 48(7):501
Joseph S, Graber ER, Chia C, Munroe P, Donne S, Thomas T, Nielsen S, Marjo C, Rutlidge H, Pan GX, Li L, Taylor P, Rawal A, Hook J (2013) Shifting paradigms: development of high-efficiency biochar fertilizers based on nano-structures and soluble components. Carbon Manag 4(3):323–343
Khan S, Waqas M, Ding F, Shamshad I, Arp HPH, Li G (2015) The influence of various biochars on the bioaccessibility and bioaccumulation of PAHs and potentially toxic elements to turnips (Brassica rapa L.). J Hazard Mater 300:243–253
Kim WK, Shim T, Kim YS, Hyun S, Ryu C, Park YK, Jung J (2013) Characterization of cadmium removal from aqueous solution by biochar produced from a giant Miscanthus at different pyrolytic temperatures. Bioresour Technol 138:266–270
Kula I, Ugurlu M, Karaoglu H, Celik A (2008) Adsorption of Cd(II) ions from aqueous solutions using activated carbon prepared from olive stone by ZnCl2 activation. Bioresour Technol 99:492–501
Kumar S, Loganathan VA, Gupta RB, Barnett MO (2011) An assessment of U(VI) removal from groundwater using biochar produced from hydrothermal carbonization. J Environ Manag 92:2504–2512
Lagergren S (1898) Zur theorie der sogenannten adsorption geloster stoffe. Kungliga Svenska Vetenskapsakademiens. Handlingar 24:1–39
Li B, Yang L, Wang C-q, Zhang Q-p, Liu Q-c, Li Y-d, Xiao R (2017b) Adsorption of cd(II) from aqueous solutions by rape straw biochar derived from different modification processes. Chemosphere 175:332–340
Li H, Dong X, da Silva EB, de Oliveira LM, Chen Y, Ma LQ (2017a) Mechanisms of metal sorption by biochars: biochar characteristics and modifications. Chemosphere 178:466–478
Loganathan P, Vigneswaran S, Kandasamy J, Naidu R (2012) Cadmium sorption and desorption in soils: a review. Crit Rev Environ Sci Technol 42(5):489–533
Lu H, Zhang W, Yang Y, Huang X, Wang S, Qiu R (2012) Relative distribution of Pb2+ sorption mechanisms by sludge-derived biochar. Water Res 46:854–862
Lu R, 2000. Analysis methods of soil agricultural chemistry. China Agricultural Science and Technology Publishing House (In Chinese)
Melo LCA, Coscione AR, Abreu CA, Puga AP, Camargo OA (2013) Influence of pyrolysis temperature on cadmium and zinc sorption capacity of sugar cane straw-derived biochar. Bioresources 8(4):4992–5004
Mohan D, Pittman CU Jr, Bricka M, Smith F, Yancey B, Mohammad J, Steele PH, Alexandre-Franco MF, Gomez-Serrano V, Gong H (2007) Sorption of arsenic, cadmium, and lead by chars produced from fast pyrolysis of wood and bark duringbio-oil production. J Colloid Interface Sci 310:57–73
Murphy J, Riley JP (1986) Citation-classic-a modified single solution method for the determination of phosphate in natural-waters. Curr Cont/Agric Biol Environ Sci 12:16–16
Novozamsky I, Beek J., 1976. Chapter 6 common solubility Equilibria in soils. In: bolt GH, Bruggenwert MGM, editors. Developments in soil science. 5. Elsevier, pp. 96-125
Ofomaja AE, Unuabonah EI, Oladoja NA (2010) Competitive modeling for the biosorptive removal of copper and lead ions from aqueous solution by Mansonia wood sawdust. Bioresour Technol 101(11):3844–3852
Ren Z-L, Tella M, Bravin MN, Comans RNJ, Dai J, Garnier J-M, Sivry Y, Doelsch E, Straathof A, Benedetti MF (2015) Effect of dissolved organic matter composition on metal speciation in soil solutions. Chem Geol 398:61–69
Romic M (2012) Bioavailability of trace metals in terrestrial environment: methodological issues. Eur Chem Bull 1:489–493
Sarı A, Tuzen M (2009) Kinetic and equilibrium studies of biosorption of Pb(II) and cd(II) from aqueous solution by macrofungus (Amanita rubescens) biomass. J Hazard Mater 164:1004–1011
Shakoor MB, Ali S, Rizwan M, Abbas F, Bibi I, Riaz M, Khalil U, Niazi NK, Rinklebe J (2020) A review of biochar-based sorbents for separation of heavy metals from water. Int J Phytoremediation 22:111–126
Sun JK, Lian F, Liu ZQ, Zhu LY, Song ZG (2014) Biochars derived from various crop straws: characterization and Cd(II) removal potential. Ecotoxicol Environ Saf 106:226–231
Tan G, Xiao D (2009) Adsorption of cadmium ion from aqueous solution by ground wheat stems. J Hazard Mater 164:1359–1363
Tan X, Liu Y, Zeng G, Wang X, Hu X, Gu Y, Yang Z (2015) Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere 125:70–85
Thakur SK, Tomar NK, Pandeya SB (2006) Influence of phosphate on cadmium sorption by calcium carbonate. Geoderma 130:240–249
Tong X-J, Li J-Y, Yuan J-H, Xu R-k (2011) Adsorption of Cu(II) by biochars generated from three crop straws. J Chem Eng 172:828–834
Tunc O, Tanaci H, Aksu Z (2009) Potential use of cotton plant wastes for the removal of Remazol black B reactive dye. J Hazard Mater 163:187–198
Uchimiya M, Lima IM, Klasson KT, Chang SC, Wartelle LH, Rodgers JE (2010) Immobilization of heavy metal ions (CuII, CdII, NiII, and PbII) by broiler litter derived biochars in water and soil. J Agric Food Chem 58:5538–5544
Van Poucke R, Ainsworth J, Maeseele M, Ok YS, Meers E, Tack FMG (2018) Chemical stabilization of Cd-contaminated soil using biochar. Appl Geochem 88:122–130
Waalkes MP (2000) Cadmium carcinogenesis in review. J Inorg Biochem 79(1):241–244
Wang R-Z, Huang D-L, Liu Y-G, Zhang C, Lai C, Zeng G-M, Cheng M, Gong X-M, Wan J, Luo H (2018) Investigating the adsorption behavior and the relative distribution of Cd2+ sorption mechanisms on biochars by different feedstock. Bioresour Technol 261:265–271
Wang XS, Qin Y (2006) Removal of Ni(II), Zn(II) and Cr(VI) from aqueous solution by Altemanthera philoxeroides biomass. J Hazard Mater 138(3):582–588
Wang Y, Hu Y, Zhao X, Wang S, Xing G (2013) Comparisons of biochar properties from Wood material and crop residues at different temperatures and residence times. Energy Fuel 27(10):5890–5899
Wei S, Zhu M, Song J, Peng PA (2017) Comprehensive characterization of biochars produced from three major crop straws of China. Bioresources 12(2):3316–3330
Wu FC, Tseng RL, Juang RS (2009) Initial behavior of intraparticle diffusion model used in the description of adsorption kinetics. J Chem Eng 153(1–3):1–8
Xu R, Xiao S, Xie D, Ji G (2006) Effects of phthalic and salicylic acids on Cu(II) adsorption by variable charge soils. Biol Fertil Soils 42:443–449
Xu R, Xiao S, Zhao A, Ji G (2005) Effect of Cr(VI) anions on adsorption and desorption behavior of Cu(II) in the colloidal systems of two authentic variable charge soils. J Colloid Interface Sci 284:22–29
Xu R-k, Zhao A-Z (2013) Effect of biochars on adsorption of cu(II), Pb(II) and cd(II) by three variable charge soils from southern China. Environ Sci Pollut Res Int 20:8491–8501
Xu X, Cao X, Zhao L (2013a) Comparison of rice husk-and dairy manure-derived biochars for simultaneously removing heavy metals from aqueous solutions: role of mineral components in biochars. Chemosphere 92:955–961
Xu X, Cao X, Zhao L, Wang H, Yu H, Gao B (2013b) Removal of Cu, Zn, and Cd from aqueous solutions by the dairy manure-derived biochar. Environ Sci Pollut Res 20:358–368
Yuan JH, Xu RK (2012) Effects of biochars generated from crop residues on chemical properties of acid soils from tropical and subtropical China. Soil Res 50:570–578
Zama EF, Zhu Y-G, Reid BJ, Sun G-X (2017) The role of biochar properties in influencing the sorption and desorption of Pb(II), Cd(II) and As(III) in aqueous solution. J Clean Prod 148:127–136
Zhang F, Wang X, Yin D, Peng B, Tan C, Liu Y, Tan X, Wu S (2015) Efficiency and mechanisms of Cd removal from aqueous solution by biochar derived from water hyacinth (Eichornia crassipes). J Environ Manag 153:68–73
Zhang C, Shan B, Tang W, Zhu Y (2017) Comparison of cadmium and lead sorption by Phyllostachys Pubescens biochar produced under a low-oxygen pyrolysis atmosphere. Bioresour Technol 238:352–360
Zhang X, Wang H, He L, Lu K, Sarmah A, Li J, Bolan NS, Pei J, Huang H (2013) Using biochar for remediation of soils contaminated with heavy metals and organic pollutants. Environ Sci Pollut Res Int 20:8472–8483
Funding
This study was financially supported by National Key Research and Development Program of China (2016YFD0800306), and National Non-profit Program by Ministry of Agriculture of China (201303095-11). This research used the JEOL JEM-ARM200F funded by the Australian Research Council (ARC) – Linkage, Infrastructure, Equipment and Facilities (LIEF) grant (LE120100104) located at the UOW Electron Microscopy Centre.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Zhihong Xu
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOC 1739 kb)
Rights and permissions
About this article
Cite this article
Sui, F., Jiao, M., Kang, Y. et al. Investigating the cadmium adsorption capacities of crop straw biochars produced using various feedstocks and pyrolysis temperatures. Environ Sci Pollut Res 28, 21516–21527 (2021). https://doi.org/10.1007/s11356-020-11979-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-11979-3