Abstract
The aim of this study was to evaluate the genotoxic and morphological systemic effects of both an acute and a chronic exposure of bullfrog tadpoles to fipronil. Lithobates catesbeianus tadpoles had morphological biomarkers (skin, liver, and blood) analyzed at Gosner stages 36–38, when exposed to four different concentrations of Regent® 800 WG (80% fipronil): 0.00 (control), 0.04, 0.08, 0.4 mg/L, and four experimental times: 4, 8, 12, and 16 days. Body darkness responded directly to the treatment and exposure time. There was a treatment-dependent decrease in darkness of heads and tails. In relation to the biometric analysis, fipronil induced a decrease in the individual weight and liver mass at the end of the experiments, whereas the hepatosomatic index did not vary according to the treatment. For the exposed animals and for the control group, the area of hepatic melanin increased as exposure time increased. Fipronil has genotoxic effects on L. catesbeianus tadpoles even after short exposure times (e.g., 4 and 8 days), and the main nuclear abnormality is in the anucleate cells. A relevant correlation was observed between genotoxic biomarkers and cutaneous and internal melanin. The frequency of nuclear abnormalities is inversely correlated both with the hepatic melanin area and with the cutaneous melanin of animals. Fipronil has distinct systemic effects on tadpoles based on its concentration, as well as on its exposure time. Such alterations (pigmentation level and rate of erythrocyte abnormality) result in morphological and physiological effects, which may compromise the behavior and survival of the anurans.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the last 10 years, Brazil has become the biggest toxic agrochemical market in the world, corresponding to a 190% increase in the national market (Lopes and Albuquerque 2018; Anvisa 2019; MMA 2018). The increase in the use of such chemical substances results in water resource contamination (Thompson et al. 2007; Ribeiro et al. 2013) and may affect natural ecosystems (Konstantinou et al. 2006; Hildenbrandt et al. 2008; Foit et al. 2010) along with the non-target species which live in these habitats (Mann et al. 2009).
Fipronil (C12H4Cl2F6N4OS) is a new-generation insecticide known worldwide (Roberts and Reigart 2013); besides being used in agriculture, it is a veterinary medication for the control of pests, parasites, and larvae (Zhao and Salgado 2010; Amaral 2012). Fipronil is responsible for causing endocrine disruption (Silva et al. 2011); thus, it is classified as carcinogenic for humans (Fent 2014). It also causes genotoxicity (Tomiazzi et al. 2018), hepatotoxicity (Ferreira et al. 2012), reproductive toxicity (Josende et al. 2015), behavioral, immunological, and mortality alterations (Pochini and Hoverman 2017). Among the products in which Fipronil is the active ingredient, one of which, Regent 800 WG, is classed as a highly toxic—red label (Anvisa 2019). It acts by means of contact or ingestion and is delivered by land or air (Agrofit 2020). Thus, it may be transported and found in aquatic environments where it causes great damage to the local biota (Albuquerque et al. 2016; Bernabò et al. 2016). Simon-Delso et al. (2015) summarized some reasons for the success of systemic insecticides (fipronil and neonicotinoids) based on the similarities of their properties, e.g., toxicity and physicochemical profiles, and also reviewed their trends, uses, mode of action, and their metabolites, in addition to documenting their share of the global market in the last 25 years. In amphibians, fipronil may spread once or twice faster than in mammals (Wagner et al. 2016) and its effects in anurans are related to enzymatic alterations (Reynaud et al. 2012; Gripp et al. 2017) and metamorphosis time (Boscolo et al. 2017).
Experiments regarding the concentrations of fipronil in aquatic environments are lacking, mainly in the ponds that serve as a transient environment for amphibians that live close to the cultivation areas, especially during application periods. These concentrations in a realistic condition may be higher than those that have been described for freshwater rivers. However, according to de Toffoli et al. (2015), in the soil close to agricultural areas of Northwest Brazil, environmentally relevant concentrations of fipronil, fipronil sulfone, and fipronil sulfide are present, ranging from 35 to 180 μg/kg, which is very close to the concentration in the water used in our experimental protocols.
The acute toxicity for pesticides on aquatic organisms can be measured by establishing the LC50 or the concentration in water (e.g., mg/L) which is toxic to the test organisms. According to a review of the effects of fipronil on vertebrate wildlife (Gibbons et al. 2015), there is a marked variation among vertebrate’s taxa and different systemic insecticides in acute toxicity. In that study, neonicotinoid and fipronil insecticides could exert their impact on vertebrates either directly, through their overt toxicity, or indirectly, for example by reducing their food supply. For amphibians, no fipronil toxicity experiments have been reported; however, for some fish, the toxicity of fipronil varies according to species, with lethal concentrations (LC50) ranging from 0.042 mg/L in Oreochromis niloticus (African tilapia) to 0.43 mg/L in Cyprinus carpio (European carp; Qureshi et al. 2016). The LC50 in Rhamdia quelen (silver catfish) is 0.81 mg/L at 48 h of study (Fredianelli et al. 2019).
Anurans are biomarkers of the environment quality, since they have a quick response to environmental stress; therefore, they are vulnerable to terrestrial and aquatic contamination (Brodeur and Candioti 2017). Their organs and internal membranes contain melanin (Franco-Belussi et al. 2016)—an endogenously extracted polymer (Césarini 1996) which absorbs and neutralizes free radicals and other potentially toxic agents derived from the cellular catabolism (Zuasti et al. 1989; Agius and Roberts 2003). In the skin, melanin is the first barrier against UV radiation (Montagna and Carlisle 1991) and also enables the adaptive interaction of animals and the environment (e.g., camouflage, cryptic coloration) (Aspengren et al. 2009; Sköld et al. 2016). Researchers have found evidence of internal melanin alterations and skin coloration due to environmental contaminants (Franco-Belussi et al. 2018; Pérez-Iglesias et al. 2019; Scaia et al. 2019); these are correlated to genotoxic effects (Franco-Belussi et al. 2018), which show that aquatic contaminants may cause damage to the adaptive capacity of animals to their environment.
Melanomacrophages (MMs) are cells which are present in the hematopoietic organs of fish and anurans. Such cells, with a phagocytic activity similar to that of macrophages (Agius 1980), contain melanin and substances of the cellular catabolism, such as hemosiderin and lipofuscin (Agius and Roberts 2003). MMs are morphological biomarkers of effects and are used as tools to evaluate the effects of contaminants, e.g., glyphosate (Pérez-Iglesias et al. 2016; Bach et al. 2018; Pérez-Iglesias et al. 2019), benzo[α]pyrene (Fanali et al. 2017, 2018), flutamide (Gregorio et al. 2016), pyrethroid (Oliveira et al. 2016), carbaryl (Çakici 2015), and sexual steroid hormones (Zieri et al. 2015). Hence, these cells are important in order to detect tissue alterations as a result of possible xenobiotics present in the environment. However, there are few studies that evaluate the role of these cells in tadpoles and there are no reports on the possible effects that fipronil may have on them.
Therefore, the objective of this study was to evaluate the systemic effects of both acute and chronic exposure of bullfrog tadpoles to fipronil. In order to do so, morphological biomarkers such as morphological nuclear alterations in erythrocytes were examined, in addition to histological analyses of the liver and animal coloration. Moreover, a comparative approach was used regarding genotoxic responses, by assaying the melanin present both in the liver and the skin of the animal, in order to test the hypothesis that it plays a role in protecting tissues (De Oliveira et al. 2017).
Materials and methods
Animal sampling and experimental design
In total, 128 Lithobates catesbeianus (Shaw 1802) tadpoles were studied, at Gosner stages 36–38; they were obtained from UNESP’s Centro de Aquicultura (CAUNESP) in Jaboticabal, São Paulo, Brazil. Animals were kept in aquaria containing 1 L water/animal, with constant aeration, controlled temperature (27.0 ± 1 °C), and natural photoperiod (~ 12-h light:12-h dark). Animals were fed with a protein meal every day for 7 days before experiments began.
After that acclimatation period, animals were randomly rearranged in 32 aquaria with 4 specimens in each tank; the proposed population density was 1 animal per liter of water. The specimens were exposed to four different concentrations of Regent® 800 WG (80% fipronil): 0.00 (control), 0.04, 0.08, and 0.4 mg/L, and four periods of exposure: 4, 8, 12, and 16 days. These concentrations are in accordance with Toffoli et al. (2015) agricultural soil of Northwest of Brazil (i.e., 35 to 180 μg/kg). Each group was analyzed in duplicate, with a total of N = 8/concentration/time. The water was totally renewed every 48 h with the replacement of compound concentrations in the treated groups. These animals were also fed every 48 h.
After the experimental periods, animals were photographed and then euthanized with benzocaine (10 g/L); their blood was sampled through cardiac puncture with a heparinized syringe and needles for the obtention of blood extensions. Subsequently, animals were desiccated and had their livers removed. All manipulation and care of animals followed the NIH Guide for Care and Use of Laboratory Animals; all procedures were previously approved by the Ethical Committee and Animal Experimentation from IBILCE/UNESP (CEUA #199/2018).
Biometric analyses
Following the 7-day acclimatization period, an exploratory analysis of the biomass that composed each replicate was conducted, immediately before the beginning of the experiments. The biomass behavior was evaluated for each subgroup (control and treated), both before and after the experiments, aiming to observe possible sub-lethal alterations of anatomic or developmental character. Animals were weighed on an analytical balance with 0.001-g precision and measured with a pachymeter (0.01 mm); the livers were also weighed. Based on this data, it was possible to calculate the hepatosomatic index (HSI = liver weight (g)/animal weight (g) × 100).
Body darkness analyses
In order to quantify body darkness, we used Photoshop® to standardize the area measured. Landmarks were used to standardize measurements in each animal (Franco-Belussi et al. 2018). All image analyses were performed in accordance with Svensson et al. (2005). The digital images were converted to CIE L*a*b*, which consisted of three parameters: L* (lightness) represents the relative lightness ranging from total black to total white; a* (redness) represents the balance between red and green; and b* (yellowness) represents the balance between yellow and blue. Afterwards, the following formula was used: 100 − (100 × L*/255), considering the relative area of L* (Svensson et al. 2005; Franco-Belussi et al. 2018).
Hepatic melanin
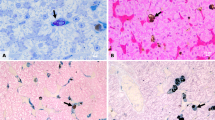
The liver was fixed in Methacarn (60% methanol, 30% chloroform, and 10% acetic acid) for 3 h, then dehydrated and immersed in historesin (Leica). Cuts of 2 μm were stained in hematoxylin-eosin and photographed in a microscope coupled to the image capturing system (Leica DM4B). The melanin area present in the liver was measured through the ImagePro-plus 6.0 software by using a differential staining technique (Santos et al. 2014). In order to do so, 25 photomicrographs were used for each animal.
Nuclear abnormalities in erythrocytes
Blood smears were stained with Giemsa 7.5% for 15 min and subsequently washed in running water. Following this, 1000 erythrocytes were analyzed for each animal through a light microscope (Leica DM4000). The nuclear abnormalities were evaluated according to Franco-Belussi et al. (2016).
Statistical analysis
In order to model the ratio of nuclear abnormalities, a generalized linear model (GLM) was built with a binomial distribution and a log link function, including treatment and time of exposure; each one containing a categorical predictor with 4 levels, in addition to their interactions. We tested our model assumptions by using diagnostic plots though the R (R Core Team 2017) package sjplot (Lüdecke 2016). Residuals had homogeneity of variance and normal distribution.
In order to model the darkness (response variables), a generalized linear mixed-effects models (GLMM) with beta distribution was fitted, including treatment and time as categorical predictor variables with four levels each, along with their interactions (Franco-Belussi et al. 2018). In order to model the area of hepatic melanin, a GLMM was used, but instead with a Gaussian distribution.
Pearson correlation was used in order to test for a correlation between the ratio of nuclear abnormalities and body darkness. In addition, nuclear abnormalities were tested with hepatic melanin. All analyses were conducted through R v. 3.4.0 (R Core Team 2017).
Results
Biometrics
Table 1 contains the assayed biometric variables. No anatomical alteration was verified qualitatively. The 16-day group had an increase in biomass, compared to their biomass at the beginning of the experiment (P < 0.001; Table S1). By assaying the individual’s variables, fipronil was observed to cause a decrease in the individual weight in 4-days of 0.04 mg/L (P = 0.03; Table S2) and liver mass in 4 days of 0.04-mg/L and 0.08-mg/L treatments (P < 0.05; Table S3). Although the treatments decreased liver weight, the hepatosomatic index (HSI) did not vary according to the treatment (Table S4). Similarly, total length did not vary among control and treatment groups (Table S5). All biometric variables result in significant differences in at least one experimental time (Table 1; S1-S5) showing the effects of time of exposure.
Body darkness
The color of L. catesbeianus tadpoles responds directly to the treatment and exposure time. There was a decrease in the darkness of heads and tails, thus indicating it is both time- and treatment-dependent (Fig. 1).
The head pigmentation at initial times (i.e., 4 and 8 days) decreased at the lowest concentration (0.04 mg/L; Fig. 2; Table S6); such response persisted during the other times. The tail darkness decreased in 8 days, only at the 0.08-mg/L concentration, whereas for 12 and 16 days, there was a decrease in pigmentation at the lowest concentration (0.04 mg/L; Figs. 1 and 2; Table S7).
Hepatic melanin
The area of hepatic melanin increased as exposure time increased in all treatments, including in the control group (Figs. 3 and 4; Table S8). For the exposed animals, hepatic melanin decreased at a 0.04-mg/L concentration of fipronil after 4 days of exposure (P = 0.0001; Table S8); however, after 8 days at the same concentration, there was a substantial increase (P = 0.0009; Table S8), which indicates an acute response to the treatment. For the other exposure times, only 12 days had a treatment effect, i.e., there was an increase in hepatic pigmentation at 0.08-mg/L concentration, thus suggesting a chronic effect of such concentration (P < 0.0001; Fig. 4; Table S8). By considering the effects of time and treatment together, it is noteworthy that after 16 days of exposure, all concentrations led to the same response (Fig. 4; Table S8).
Nuclear abnormalities
Fipronil presents genotoxic effects on L. catesbeianus tadpoles in short exposure times (e.g., 4 and 8 days) (P < 0.05; Figs. 5 and 6; Table S9). That may be due to the fact that in tadpoles, the erythrocyte replacement rate tends to be higher at initial developmental stages. The main nuclear abnormality observed was that of anucleate cells (Fig. 5). At the 4-day exposure time, it was possible to observe an increase in the nuclear abnormalities in animals exposed to 0.04 and 0.08 mg/L fipronil (P < 0.0001; Table S9); when the time was 8 days, the increase was observed in all the exposed animals (P < 0.005; Fig. 6; Table S9). For 16-day exposure, the increase in nuclear abnormality frequency was observed at the concentrations of 0.08 and 0.4 mg/L (P < 0.01; Fig. 6; Table S9) showing a dependency of time and treatment.
Compared analysis between nuclear abnormalities and melanin
Although significant, the frequency of nuclear abnormalities is weakly correlated with both hepatic melanin area (r = − 0.23; P = 0.03), and cutaneous melanin (r = − 0.22; P = 0.04).
Discussion
Exposure to the commercial insecticide Regent® 800 WG caused a decrease in the individual’s weight (4 days–0.04 mg/L) and liver mass (4 days–0.04, 0.08 mg/L), whereas the total length and hepatosomatic index did not suffer significant effects when comparing control and treatment groups. Physalaemus nattereri tadpoles exposed to the same insecticide (0.5–1.5 mg/L) had an accelerated development (Boscolo et al. 2017), which may result in individuals with a lower mass (Cauble and Wagner 2005) and size (Denver and Crespi 2006). Similar results have been observed for the insecticide endosulfan, which caused a weight decrease in Bufo bufo (Brunelli et al. 2009). In turn, the insecticides carbaryl, malathion, and permethrin have led to an increase in the mass of Bufo americanus and Rana clamitans; moreover, there was a developmental delay (Boone 2008), which was also observed for endosulfan (Brunelli et al. 2009).
Even though there were effects regarding the biometric parameters of L. catesbeianus, those responses were occasional and nonlinear and they may be related to the intrinsic properties of each animal, which have then culminated in body weight and liver mass reduction. Considering that there was no mortality and that animals after 16 days had biomass gain (Table 1), we assume that a normal development of the tadpoles occurred. However, other effects at morphological and cellular levels were observed in this study.
Fipronil caused the agglomeration of melanin granules, which decreased the skin pigmentation of the head and tail of L. catesbeianus. The dispersion of granules, which led to a darkening of the body, was observed for tadpoles exposed to different contaminants, such as insecticides (Pandey and Tomar 1985), herbicides (Pérez-Iglesias et al. 2019), and endocrine disruptors (Scaia et al. 2019). Fish exposed to nitrate/phenol/hexachlorocyclohexane had decreased pigmentation (Daiwile et al. 2015). This difference may be explained due to the mechanism of action of the compounds and the physiological responses of each species. Another determining factor in the response of chromatophores is the exposure time. Fish treated with arsenic suffered an initial reduction in their pigmentation (i.e., 7–60 days of exposure), followed by an increase during longer periods (i.e., 90 days), suggesting a physiological adaptation mechanism of melanophores (Allen et al. 2004). Therefore, pigmentation is an important biomarker and, once conserved within the various animal groups, can provide an intrinsic characteristic to their cells and/or melanin. The conservation of pigmentation has been reported for some amphibian taxa (Provete et al. 2012).
Hepatic melanin was also responsive to fipronil exposure, with a decrease after 4 days and subsequent increase after 8 and 12 days. Hence, time is a relevant factor, because the same concentration (0.04 mg/L) resulted in an inverse response as days advanced. Alterations in this hepatic pigment occur as a response to different chemical compounds and to contrasting land use patterns, i.e., agriculture activities (Franco-Belussi et al. 2020), in which there may be an increase (Pérez-Iglesias et al. 2016; De Oliveira et al. 2017; Pérez-Iglesias et al. 2019) or a decrease (Regnault et al. 2014; Fanali et al. 2017, 2018). MMs are associated with detoxifying pollutants (Pérez-Iglesias et al. 2016) and neutralizing free radicals (Agius and Roberts 2003; Barni et al. 1999); hence, the decrease in melanin may be an initial stress caused by fipronil, whereas the increase may be a physiological adaptation of the cell—trying to reestablish the normal condition of the organ. This fact is corroborated by the 16-day group, which did not present any significant difference in the melanin area.
Blood cells reflect all the physical and chemical alterations in organisms which are exposed to insecticides and pesticides (Ambali et al. 2011). The L. catesbeianus tadpoles exposed to fipronil had an increase in the total rate of nuclear abnormalities after 4 (0.04–0.08 mg/L), 8 (0.04–0.08–0.4 mg/L), and 16 days (0.08–0.4 mg/L). Some studies also evaluated the systemic effects of both an acute and a chronic exposure of aquatic vertebrates to fipronil. In the experiment carried out by Ghisi et al. (2011) in the catfish (Rhamdia quelen), the results suggest that only the highest concentrations of fipronil cause damage in erythrocytes, but none of the concentrations (0.05–0.23 μg/L) were sufficient to alter the DNA or the morphology of the gill cells. The authors suggest the need for studies using higher concentrations, other exposure times and other tissues. Further studies in carp fish (Cyprinus carpio) exposed for 96 h to sub-lethal concentrations of fipronil (400 μg/L) reported morphological injuries (Qureshi et al. 2016). These injuries in carp fish included erythrocyte abnormalities which included necrosis, micronucleus, and hyperchromatosis; histopathological changes in the gills, liver, and kidney. Particularly, in the liver, these effects occur as karyorrhexis, hepatocellular hypertrophy, nuclear hypertrophy, melanomacrophage aggregates, and central vein contraction. The toxicity in fish (concentrations of 0.3–0.4 mg/L) was also reported by Fredianelli et al. (2019), which included a clinical condition of anemia, alterations in liver enzyme levels, nuclear erythrocyte changes and liver, gill, and kidney damage. In this study, the rate of abnormalities was higher for the 4-day group and decreased as the experiment advanced. Such results highlight the recycling dynamics of erythrocytes with alterations at short time intervals, which makes it difficult to observe the genotoxic effects during prolonged times at low concentrations of fipronil.
Based on our data, we demonstrated that the higher the melanin quantity, both in the skin and the liver, the lower the rate of nuclear abnormalities observed in erythrocytes. This comparative approach between genotoxic responses and morphological properties of visceral (hepatic) and somatic (cutaneous) melanin is in accordance with the assumptions by De Oliveira et al. (2017), who identified melanin as having a protective role for tissues, due to the toxicity of environmental chemical contaminants.
Our experimental design, particularly the concentrations of the compound, was based on the most recent studies of these aquatic vertebrates. In addition to the LC50 for these aquatic vertebrates, was to obtain systemic effects comparable with the data in the literature on amphibians. There are some studies with tadpoles of Neotropical anurans having been exposed to higher concentrations of fipronil (0.5 and 1.5 mg/L); these are related to biochemical biomarkers in Scinax fuscovarius (Margarido et al. 2013) and related to antioxidant enzyme responses and the metamorphosis in Physalaemus nattereri (Boscolo et al. 2017). Some studies are very close to our contamination levels of fipronil (0.04, 0.08, 0.4 mg/L): showing oxidative stress and neurotoxic effects (at concentrations 0.026, 0.05, 0.1, 0.5, 1.5 mg/L) in Physalaemus gracilis tadpoles (Rutkoski et al. 2020) and lipid peroxidation and enzymatic antioxidant defense in Eupemphix nattereri tadpoles exposed to intermediary concentrations in water and sediment (0.035, 0.12, 0.18 mg/kg) (Gripp et al. 2017).
For Neotropical amphibians, evidence showed that the higher concentrations of fipronil (0.5 and 1.5 mg/L) was responsible for alterations in antioxidant enzymes of both Scinax fuscovarius tadpoles (Margarido et al. 2013) and Physalaemus nattereri tadpoles (Boscolo et al. 2017). As both direct and indirect effects, there are increases in the levels of oxidative damage of the cellular components due to an increase of the susceptibility of the animals to oxidative stress. Gripp et al. (2017) reported similar conclusions about fipronil and its degradation products promoting oxidative stress in Eupemphix nattereri tadpoles exposed to environmentally relevant concentrations (0.035 to 0.18 mg/kg), which decreased physiological performance in the long-term. Rutkoski et al. (2020) observed changes that suggest the occurrence of oxidative damage and alterations in the central nervous system at concentrations ranging from 0.026 to 1.5 mg/L in Physalaemus gracilis tadpoles. As a result, we hypothesize that the sum of each specific effect can trigger systemic alterations, leading to detrimental results at the population level.
Conclusion
Fipronil has systemic effects based on its concentration (0.04, 0.08, and 0.4 mg/L) and exposure time (4, 8, 12, and 16 days). Such alterations are related to the pigment alterations and rate of abnormality in erythrocytes, which altogether, result in morphological and physiological effects which may compromise the behavior and survival of the organism.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Agius C (1980) Phylogenetic development of melano-macrophage centres in fish. J Zool 191:11–31
Agius C, Roberts RJ (2003) Review: melano-macrophage centres and their role in fish pathology. J Fish Biol 26:499–509. https://doi.org/10.1046/j.1365-2761.2003.00485.x
Agrofit (2020) Sistema de Agrotóxicos Fitossanitários. Regent 800 WG: Relatório de produtos formulados. Ministério da Agricultura. Pecuária e Abastecimento. Brasília, 3p
Albuquerque AF, Ribeiro JS, Kummrow F, Nogueira AJA, Montagner CC, Umbuzeiro GA (2016) Pesticides in Brazilian freshwaters: a critical review. Environ Sci Process Impacts 18:779–787. https://doi.org/10.1039/C6EM00268D
Allen TD, Awasthi A, Rana SVS (2004) Fish chromatophores as biomarkers of arsenic exposure. Environ Biol Fish 71:7–11. https://doi.org/10.1023/B:EBFI.0000043145.58953.86
Amaral RB (2012) Investigação do comportamento eletroquímico do inseticida fipronil e desenvolvimento de metodologia eletroanalítica. Thesis, Universidade de São Paulo, São Carlos
Ambali SF, Ayo JO, Kan E, Ojo SA (2011) Hemotoxicity induced by chronic chlorpyrifos exposure in Wistar rats: mitigating effect of vitamin C. Vet Med Int. https://doi.org/10.4061/2011/945439
Anvisa ((2019)) Agência Nacional de Vigilância Sanitária. Accessed 12 October 2020
Aspengren S, Hedberg D, Sköld HN, Wallin M (2009) New insights into melanossome transport in vertebrate pigment cells. Int Rev Cell Mol Biol 272:245–302. https://doi.org/10.1016/S1937-6448(08)01606-7
Bach NC, Marino DJG, Natale GS, Somoza GM (2018) Effects of glyphosate and its commercial formulation, Roundup® Ultramax, on liver histology of tadpoles of the neotropical frog, Leptodactylus latrans (Amphibia: Anura). Chemosphere 202:289–297. https://doi.org/10.1016/j.chemosphere.2018.03.110
Barni S, Bertone V, Croce AC, Bottiroli G, Bernini F, Gerzeli G (1999) Increase in liver pigmentation during natural hibernation in some amphibians. J Anat 195:19–25. https://doi.org/10.1046/j.1469-7580.1999.19510019.x
Bernabò I, Guardia A, Macirella R, Sesti S, Crescente A, Brunelli E (2016) Effects of long-term exposure to two fungicides, pyrimethanil andtebuconazole, on survival and life history traits of Italian tree frog (Hyla intermedia). Aquat Toxicol 172:56–66. https://doi.org/10.1016/j.aquatox.2015.12.017
Boone MD (2008) Examining the single and interactive effects of three insecticides on amphibian metamorphosis. Environ Toxicol Chem 27:1561–1568. https://doi.org/10.1897/07-520.1
Boscolo CNP, Felício AA, Pereira TSB, Margarido TCS, Rossa-Feres DC, Almeida EA, Freitas JS (2017) Comercial insecticide fipronil alters antioxidant enzymes response and accelerates the metamorphosis in Physalaemus nattereri (Anura: Leiuperidae) tadpoles. Eur J Zool Res 5:1–7
Brodeur JC, Candioti JV (2017) Impacts of agriculture and pesticides on amphibian terrestrial life stages: potential biomonitor/bioindicator species for the pampa region of Argentina. In: Larramendy ML (ed) Ecotoxicology and genotoxicology: non-traditional terrestrial models. Royal Society of Chemistry, Cambridge, pp 163–194
Brunelli E, Bernabò I, Berg C, Lundstedt-Enkel K, Bonacci A, Tripepi S (2009) Environmental relevant concentrations of endosulfan impair development, metamorphosis and behavior in Bufo bufo tadpoles. Aquat Toxicol 91:135–142. https://doi.org/10.1016/j.aquatox.2008.09.006
Çakici Ö (2015) Histopathologic changes in liver and kidney tissues induced by carbaryl in Bufotes variabilis (Anura: Bufonidae). Exp Toxicol Pathol 67:237–243. https://doi.org/10.1016/j.etp.2014.12.003
Cauble K, Wagner RS (2005) Sublethal effects of the herbicide glyphosate on amphibian metamorphosis and development. Bull Environ Contam Toxicol 75:429–435
Césarini JP (1996) Melanins and their possible roles through biological evolution. Adv Space Res 18:35–40. https://doi.org/10.1016/0273-1177(96)00025-7
Daiwile AP, Naoghare PK, Giripunje MD, Rao PP, Ghosh TK, Krishnamurthi K, Sivanesan S (2015) Correlation of melanophore index with a battery of functional genomic stress indicators for measurement of environmental stress in aquatic ecosystem. Environ Toxicol Pharmacol 39:486–495. https://doi.org/10.1016/j.etap.2014.12.006
De Oliveira C, Franco-Belussi L, Fanali LZ, Santos LR (2017) Use of melanin-pigmented cells as a new tool to evaluate effects of agrochemicals and other emerging contaminants in Brazilian anurans. Section II: terrestrial vertebrates as experimental models. In: Larramendy ML (ed) Ecotoxicology and genotoxicology: non-traditional terrestrial models. Royal Society of Chemistry, Cambridge, pp 125–139
Denver RJ, Crespi EJ (2006) Stress hormones and human development plasticity: lessons from tadpoles. NeoReviews 7:183–188. https://doi.org/10.1542/neo.7-4-e183
Fanali LZ, Valverde BSL, Franco-Belussi L, Provete DB, Oliveira C (2017) Response of digestive organs of Hypsiboas albopunctatus (Anura: Hylidae) to benzo[a]pyrene. Amphibia-Reptilia 38:175–185. https://doi.org/10.1163/15685381-00003101
Fanali LZ, Franco-Belussi L, Bonini-Domingos CR, Oliveira C (2018) Effects of benzo[a]pyrene on the blood and liver of Physalaemus cuvieri and Leptodactylus fuscus (Anura: Leptodactylidae). Environ Pollut 237:93–102. https://doi.org/10.1016/j.envpol.2018.02.030
Fent GM (2014) Fipronil. Encyclopedia of toxicology, 3rd edn, 2:596-597
Ferreira M, Oliveira PR, Denardi SE, Bechara GH, Mathias MIC (2012) Action of the chemical agent fipronil (active ingredient of acaricide frontline1) on the liver of mice: an ultrastructural analysis. Microsc Res Tech 75:197–205
Foit A, Chatzinotas M, Liess K (2010) Short-term disturbance of a grazer has long-term effects on bacterial communities-relevance of trophic interactions for recovery from pesticide effects. Aquat Toxicol 99:205–211. https://doi.org/10.1016/j.aquatox.2010.04.019
Franco-Belussi L, Skold HN, Oliveira C (2016) Internal pigment cells respond to external UV radiation in frogs. J Exp Biol 219:1378–1283. https://doi.org/10.1242/jeb.134973
Franco-Belussi L, Nilsson Sköld H, De Oliveira C (2018) Regulation of eye and jaw colouration in three-spined stickleback (Gasterosteus aculeatus). J Fish Biol 92:1788–1804. https://doi.org/10.1111/jfb.13620
Franco-Belussi L, Provete DB, Borges RE, De Oliveira C, Santos LRS (2020) Idiosyncratic liver pigment alterations of five frog species in response to contrasting land use patterns in the Brazilian Cerrado. PeerJ 8:e9751
Fredianelli AC, Pierin VH, Uhlig SC, Galeb LAG, Rocha DCC, Ribeiro DR, Anater A, Pimpão CT (2019) Hematologic, biochemical, genetic, and histological biomarkes for the evaluation of the toxic effects of fipronil for Rhamdia quelen. Turk J Vet An Sci 43:54–59
Ghisi NC, Ramsdorf WA, Ferraro MVM, De Almeida MIM, De Oliveira Ribeiro CA, Cestari MM (2011) Evaluation of genotoxicity in Rhamdia quelen (Pisces, Siluriformes) after sub-chronic contamination with Fipronil. Environ Monit Assess 180:589–599. https://doi.org/10.1007/s10661-010-1807-7
Gibbons D, Morrissey C, Mineau P (2015) A review of the direct and indirect effects of neonicotinoids and fipronil on vertebrate wildlife. Environ Sci Pollut Res 22:103–118. https://doi.org/10.1007/s11356-014-3180-5
Gregorio LS, Franco-Belussi L, Gomes FR, De Oliveira C (2016) Flutamide effects on morphology of reproductive organs and liver of Neotropical Anura, Rhinella schneideri. Aquat Toxicol 176:181–189. https://doi.org/10.1016/j.aquatox.2016.04.022
Gripp HS, Freitas JS, Almeida EA, Bisnoti MC, Moreira AB (2017) Biochemical effects of fipronil and is metabolites on lipid peroxidation and enzymatic antioxidan defense in tadpoles (Eupemphix natteteri: Leiuperidae). Ecotoxicol Environ Saf 136:173–179. https://doi.org/10.1016/j.ecoenv.2016.10.027
Hildenbrandt A, Guillamon M, Lacore S, Tauler R, Barcelo D (2008) Impact of pesticides used in agriculture and vineyards to surface and groundwater quality (North Spain). Water Res 42:3315–3326. https://doi.org/10.1016/j.watres.2008.04.009
Josende ME, Tozetti AM, Alalan MT, Mathies Filho V, Ximenez SS, Silva Júnior FMR, Martins SE (2015) Genotoxic evaluation in two amphibian species from Brazilian subtropical wetlands. Ecol Indic 49:83–87. https://doi.org/10.1016/j.ecolind.2014.10.007
Konstantinou LK, Hela DG, Albanis TA (2006) The status of pesticide pollution in surface waters (rivers and lakes) of Greece. Part I. Review on occurrence and levels. Environ Pollut 141:555–570. https://doi.org/10.1016/j.envpol.2005.07.024
Lopes CVA, Albuquerque GSCD (2018) Agrotóxicos e seus impactos na saúde humana e ambiental: uma revisão sistemática. Saúde em debate 42:518–534
Lüdecke D (2016) sjPlot: data visualization for statistics in social science. R package version 2.1.2. Available from: https://CRAN.R-project.org/package=sjPlot
Mann RM, Hyne RV, Choung CB, Wilson SP (2009) Amphibians and agricultural chemicals: review of the risks in a complex environment. Environ Pollut 157:2903–2927. https://doi.org/10.1016/j.envpol.2009.05.015
Margarido TCS, Felício AA, Rossa-Feres DC, Almeida EA (2013) Biochemical biomarkers in Scinax fuscovarius tadpoles exposed to a commercial formulation of the pesticide fipronil. Mar Environ Res 91:61–67. https://doi.org/10.1016/j.marenvres.2013.02.001
MMA - Ministério do Meio Ambiente, Brasil (2018) Agrotóxicos. Accessed 15 February 2020
Montagna W, Carlisle K (1991) The architecture of black and white facial skin. J Am Acad Dermatol 24:929–937. https://doi.org/10.1016/0190-9622(91)70148-U
Oliveira CR, Fraceto LF, Rizzi GM, Salla RF, Abdalla FC, Costa MJ, Silva Zacarin ECM (2016) Hepatic effects of the clomazone herbicide in both its free form and associated with chitosan-alginate nanoparticles in bullfrog tadpoles. Chemosphere 149:304–313. https://doi.org/10.1016/j.chemosphere.2016.01.076
Pandey AK, Tomar V (1985) Melanophores in Bufo melanostictus (Schneider) tadpoles following exposure to the insecticide dimethoate. Bull Environ Contam Toxicol 35:796–801. https://doi.org/10.1007/BF01636590
Pérez-Iglesias JM, Franco-Belussi L, Moreno L, Tripole S, Oliveira C, Natale GS (2016) Effect of glyphosate on hepatic tissue evaluating melanomacrophages and erythrocytes responses in neotropical anuran Leptodactylus latinasus. Environ Sci Pollut Res 23:9852–9861. https://doi.org/10.1007/s11356-016-6153-z
Pérez-Iglesias JM, Franco-Belussi L, Natale GS, Oliveira C (2019) Biomarkes at different levels of organisation after atrazine formulation (SIPTRAN 500SC®) exposure in Rhinella schineideri (Anura: Bufonidae) Neotropical tadpoles. Environ Pollut 244:733–746. https://doi.org/10.1016/j.envpol.2018.10.073
Pochini KM, Hoverman JT (2017) Reciprocal effects of pesticides and pathogens on amphibian hosts: the importance of exposure order and timing. Environ Pollut 221:359–366. https://doi.org/10.1016/j.envpol.2016.11.086
Provete DB, Franco-Belussi L, Santos LRS, Zieri R, Moresco RM, Martins IA, De Oliveira C (2012) Phylogenetic signal and variation of visceral pigmentation in eight anuran families. Zool Scr 41:547–556
Qureshi IZ, Bibi A, Shahid S, Ghazanfar M (2016) Exposure to sub-acute doses of fipronil and buprofezin in combination or alone induces biochemical, hematological, histopathological and genotoxic damage in common carp (Cyprinus carpio L.). Aquat Toxicol 179:103–114. https://doi.org/10.1016/j.aquatox.2016.08.012
R Core Team (2017) R: a language for and environment for statistical computing, version 3.4.0
Regnault C et al (2014) Impaired liver function in Xenopus tropicalis exposed to benzo[a]pyrene: transcriptomic and metabolic evidence. BMC Genomics 15:666–681
Reynaud S, Worms IAM, Veyrenc S, Portier J, Maitre A, Miaud C, Raveton M (2012) Toxicokinetic of benzo[a]pyrene and fipronil in female green frogs (Pelophlax kl. esculentus). Environ Pollut 161:206–214. https://doi.org/10.1016/j.envpol.2011.10.029
Ribeiro ACA, Dores EFGC, Amorim RSS, Lourencetti C (2013) Resíduos de pesticidas em águas superficiais de área de nascente do rio São Lourenço-MT: validação de método por 72 extração em fase sólida e cromatografia líquida. Quím Nova 36
Roberts JR, Reigart JR (2013) Recognition and management of pesticide poisonings, 6th edn. USEPA, Washington 227 p
Rutkoski CF, Macagnan N, Folador A, Skovronski VJ, do Amaral AM, Leitemperger JW, Hartmann MT (2020) Cypermethrin-and fipronil-based insecticides cause biochemical changes in Physalaemus gracilis tadpoles. Environ Sci Pollut Res:1–11. https://doi.org/10.1007/s11356-020-10798-w
Santos LRS, Franco-Belussi L, Zieri R, Borges RE, Oliveira C (2014) Effects of thermal stress on hepatic melanomacrophages of Eupemphix nattereri (Anura). Anat Rec 297:864–875. https://doi.org/10.1002/ar.22884
Scaia MF, Gregorio LS, Franco-Belussi L, Succi-Domingues M, Oliveira C (2019) Gonadal, body color, and genotoxic alterations in Lithobates catesbeianus tadpoles exposed to nonylphenol. Environ Sci Pollut Res 26:22209–22219. https://doi.org/10.1007/s11356-019-05403-8
Shaw G (1802) General zoology or systematic natural history. Volume III, Part 1. Amphibia. Thomas Davison, London
Silva MS, Concenza DS, Grillo R, Melo NFS, Tonello PS, Oliveira LC, Cassimiro DL, Rosa AH, Fraceto LF (2011) Paraquat-loaded alginate/chitosan nanoparticles: preparation, characterization and soil sorption studies. J Hazard 190:366–374. https://doi.org/10.1016/j.jhazmat.2011.03.057
Simon-Delso N, Amaral-Rogers V, Belzunces LP, Bonmatin JM, Chagnon M, Downs C, Goulson D (2015) Systemic insecticides (neonicotinoids and fipronil): trends, uses, mode of action and metabolites. Environ Sci Pollut Res 22:5–34. https://doi.org/10.1007/s11356-014-3470-y
Sköld HN, Aspengren S, Cheney KL, Wallin M (2016) Fish chromatophores—from molecular motors to animal behavior. Int Rev Cell Mol Biol 321:171–219. https://doi.org/10.1016/bs.ircmb.2015.09.005
Svensson C, Kundzewicz WZ, Maurer T (2005) Trend detection in river flow series: 2. Flood and low-flow index series. Hydrol Sci J 50:811–824. https://doi.org/10.1623/hysj.2005.50.5.811
Thompson T, Fawell J, Kunikane S, Jackson D, Appleyard S, Callan P (2007) Chemical safety of drinking water: assessing priorities for risk management. WHO World Health Organization
Toffoli AL, da Mata K, Bisinoti MC, Moreira AB (2015) Development, validation, and application of a method for the GC-MS analysis of fipronil and three of its degradation products in samples of water, soil, and sediment. J Environ Sc Healt B 50:753–759. https://doi.org/10.1080/03601234.2015.1058091
Tomiazzi JS, Judai MA, Nai GA, Pereira DR, Antunes PA, Favareto APA (2018) Evaluation of genotoxic effects in Brazilian agricultural workers exposed to pesticides and cigarette smoke using machine-learning algorithms. Environ Sci Pollut Res 25:1259–1269. https://doi.org/10.1007/s11356-017-0496-y
Wagner N, Müller H, Viertel B (2016) Effects of a commonly used glyphosate based herbicide formulation on early developmental stages of two anuran species. Environ Sci Pollut Res 24:1495–1508. https://doi.org/10.1007/s11356-016-7927-z
Zhao X, Salgado VL (2010) The role of GABA and glutamate receptors in susceptibility and resistance to chloride channel blocker insecticides. Pestic Biochem Physiol 97:153–160. https://doi.org/10.1016/j.pestbp.2009.10.002
Zieri R, Franco-Belussi L, Santos LRS, Taboga SR, Oliveira C (2015) Sex hormones change visceral pigmentation in Eupemphix nattereri (Anura): effects in testicular melanocytes and hepatic melanomacrophages. Anim Biol 65:21–32. https://doi.org/10.1163/15707563-00002457
Zuasti A, Jara JR, Ferre C, Solano F (1989) Occurrence of melanin granules and melanosynthesis in the kidney of Sparus auratus. Pigment Cell Res 2:93–100. https://doi.org/10.1111/j.1600-0749.1989.tb00168.x
Acknowledgments
Lara S. Gregorio, Lara Z. Fanali, Maysa S. Domingues, and Luciana Trevizan helped with experimental procedures.
Funding
CO has been continuously supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (# 304552/2019-4) and BSLV received a CAPES-DS scholarship. This study was supported by Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP) (grant # 2018/01078-7) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil (CAPES) (Finance Code 001).
Author information
Authors and Affiliations
Contributions
ATS: investigation, methodology. BSLV: conceptualization, investigation, methodology, writing original draft, and review. LFB: conceptualization, methodology, formal analysis, writing original draft, and review and editing. CO: conceptualization, resources, writing original draft, and review and editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
All manipulation and care of animal’s procedures were previously approved by the Ethical Committee and Animal Experimentation from IBILCE/UNESP (CEUA #199/2018).
Competing interests
The authors declare that they have no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Santos, A.T., Valverde, B.S.L., De Oliveira, C. et al. Genotoxic and melanic alterations in Lithobates catesbeianus (anura) tadpoles exposed to fipronil insecticide. Environ Sci Pollut Res 28, 20072–20081 (2021). https://doi.org/10.1007/s11356-020-11948-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-11948-w