Abstract
Using copper nitrate trihydrate as the copper source, TiO2@Cu-MOF nanocomposites were prepared by a one-step crystallization method, and the effect of the amount of TiO2 loaded on the adsorption of rhodamine B was studied. X-ray diffraction (XRD), scanning electron microscope (SEM), energy spectrometer (EDS), N2 adsorption-desorption (BET), and infrared spectroscopy (FTIR) were used to characterize the microstructure and surface properties of composite materials. The results show that the composite material not only has a good degradability for rhodamine B, the decolorization rate reaches 98.03% after 120 min, but it also maintains a good cycle performance. Fitting the first-order kinetic equation to the reaction process, under the optimal conditions, R2 = 0.98, indicating that the reaction process conforms to the first-order kinetic equation. Therefore, the catalyst has good catalytic degradation and cycle performance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Environmental pollution is becoming more and more prominent with increase in the activities of industries. Textile, cosmetic, paper, leather, and food processing industries produce a lot of wastewater that is polluted with significantly high amount of synthetic dyes. Most of these synthetic dyes are chemically and thermally stable, non-biodegradable, and quite toxic. Among the common wastewater pollution, dye wastewater pollution is toxic, deep in color, and very difficult to degrade (Ruan et al. 2010). The methods to solve the traditional wastewater such as chemical method, physical method, and biodegradation method are not effective. These are the reasons why the removal of organic dyes from wastewater remains an environment challenge that attracts attention of the research community (Li et al. 2016).
Titanium dioxide (TiO2), as a kind of conventional photocatalytic nanomaterial with strong oxidation resistance, high stability, and non-toxicity, is considered to be the most promising photocatalyst, and is expected to be widely used in organic wastewater treatment (Jiang et al. 2014; Yu et al. 2019; Kiwi and Rtimi 2018). However, there are some disadvantages in the photocatalytic degradation of pure TiO2, such as low photocatalytic efficiency, fast photocatalysis carrier hole recombination, poor dispersion, and so on (Xing et al. 2016; Rtimi et al. 2016; Zeghioud et al. 2019). Metal organic framework—MOF—has also developed rapidly in recent years. This kind of material mainly consists of two parts: organic ligand (generally containing O, N, S coordination atoms) and inorganic metal center. It is a kind of crystalline porous material formed by self-assembly (Li et al. 2012). MOFs have a porous structure and diverse functions, so they have important application potential in the fields of sensing, biopharmaceuticals, catalysis, and separation (Farha and Hupp 2010; Meilikhov et al. 2010). As a new porous material, MOFs have potential application value in photocatalysis.
Herein, TiO2@Cu-MOF composite catalyst was prepared by one-step crystallization method. The prepared TiO2@Cu-MOF was characterized using some classical characterization methods and rhodamine B (RhB) was selected as a model dye and used in evaluating the TiO2@Cu-MOF adsorption performance in aqueous solutions. In the results obtained from adsorption process, it takes a little time to reach the adsorption equilibrium and achieves a good adsorption efficiency. In addition, the TiO2@Cu-MOF can be reused for many times under recycle activation.
Experimental
Reagents
Rhodamine B and N,N-dimethylformamide (DMF, AR) were procured from Tianjin Kemiou Chemical Reagent Development Center, China. 1,3,5-Benzenetricarboxylic acid (H3TBC, 98%), TiO2 (99.8%), and ethyl alcohol absolute were procured from Shanghai McLean Biochemical Technology Co., Ltd. Copper nitrate, H2SO4 (98%), and NaOH (AR) were procured from Tianjin Fengchuan Chemical Reagent Technology Co., Ltd.
Test instrument
The following are the test instruments: electric heating blast drying oven (DHG-9245A, Shanghai-Heng Scientific Instrument Co., Ltd., China), electronic balance (FA2004, Shanghai Jinping Scientific Instrument Co., Ltd., China), low-speed desktop centrifuge DT5-2 (Beijing Times Beili Centrifuge Co., Ltd., China Company), multi-head magnetic stirrer (HJ-4A, China Jiangsu Kexi Instrument Co., Ltd.), Xenon light source (MICROSOLAR300, China Beijing Bofeilai Technology Co., Ltd.), low-temperature coolant circulation pump (GKDL-20, Gongyi City, China) Yu Gaoke Instrument Factory), CNC ultrasonic cleaner (KQ-300DE, Kunshan Ultrasonic Instrument Co., Ltd., China), and 100-ml high-pressure hydrothermal reactor (Anhui Kemi Machinery Technology Co., Ltd., China).
Preparation of TiO2@Cu-MOF
First, take 1.039 g of Cu (NO3)2 • 3H2O and 7.5 mL of deionized water in a beaker and stir vigorously at room temperature for 30 min. The Cu (NO3)2 • 3H2O is dissolved and recorded as A. Then, take 7.5 mL each of DMF, C2H6O, and H3TBC and stir for 30 min that is completely dissolved and recorded as B. Add solution A and B together and continue to stir, and record as solution C. Weigh TiO2 according to the Cu/Ti weight ratio, add it to solution C, and continue vigorous stirring for 1 h. Then, the mixed solution was transferred to a 100 mL polytetrafluoroethylene-lined autoclave and hydrothermally heated at 120 °C for 12 h. The prepared liquid was centrifuged and dried at 80 °C to obtain a TiO2@Cu-MOF nanocomposite sample (Fig. 1).
Characterization of the adsorbents
The specific surface and porosity of the samples were analyzed using Autosorb-iQ-MP low-temperature nitrogen adsorption instrument. The surface morphology of the samples was studied by means of scanning electron microscope (SEM, Fena World Corporation Quanta 250). The crystal morphology of the samples was analyzed by the patterns of the X-ray diffraction (XRD), which were recorded on a Japan Smart Lab diffractometer (40 kV, 200 mA) using filtered Cu Ka radiation. Diffraction data were collected in the 2θ range of 10–80°. Using Bruker Tensor27 Fourier infrared spectrometer (FTIR) functional groups on the surface of the composite material, wave number range 4000 ~ 400 cm−1.
Adsorption experiments
A certain concentration of RhB solution was charged into the reactor, and the prepared TiO2@Cu-MOF catalyst was added. The pH value of the mixed solution was adjusted using H2SO4 and NaOH. Firstly, the mixed solution was stirred with a magnetic stirrer and adsorbed for 30 min under the condition of no light, until the solution reached the physical adsorption equilibrium; Secondly, the solution is placed under the irradiation of ultraviolet and visible light, and the temperature is controlled by circulating water through a low-temperature coolant circulating pump; Finally, turn on the power supply, wait until the reference current and the reference light intensity are recorded as the starting point, and then take samples at 15 min, 30 min, 60 min, and 120 min respectively, take the supernatant, and use the ultraviolet spectrophotometer to measure the absorbance of each sample at 554 nm.
The decolorization rate D is calculated according to Eq. (1).
where A0 (mg/L) denote the initial dye concentration; At (mg/L) represents the residual dye concentration at time t.
Results and discussion
Characterization of the adsorbents
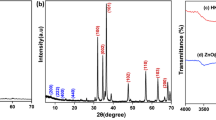
Figure 2 shows the XRD patterns of Cu-MOF and TiO2@Cu-MOF catalysts. The two samples showed diffraction peaks at 2θ = 10°, 15°, and 37°. These are the characteristic diffraction peaks of Cu-MOF. It can be seen from the figure that the nanocomposites of TiO2@Cu-MOF also have new diffraction peaks at 2θ = 25.3°, 37.8°, 48°, and 55.2°. Compared with the XRD standard card (jcpds78-1996), the new peaks correspond to the 101, 004, 200, and 211 diffraction peaks of anatase TiO2. The results show that TiO2 in the composite is mainly anatase crystal. In addition, the characteristic peaks of CuO and Cu2O are not seen in the figure, which indicates that the Cu-MOF material is basically pure after washing treatment, without CuO and Cu2O impurities. Studies have shown that, compared with rutile TiO2, the surface of anatase TiO2 has a stronger ability to adsorb oxygen, which makes it have the function of capturing photoelectrons in the photocatalytic process, thereby achieving the purpose of improving the photocatalytic activity of the catalyst. In addition, the diffraction peak of Cu-MOF was clear and sharp after TiO2 was loaded on Cu-MOF, indicating that the skeleton structure of Cu-MOF did not change after TiO2 loading.
Figure 3 shows SEM images of Cu-MOF and TiO2@Cu-MOF samples. It can be clearly seen that the surface of Cu-MOF is a smooth octahedron, and the surface of the octahedron in the sample picture of TiO2@Cu-MOF is rough, which may be caused by the crystallization of TiO2 on the surface of Cu-MOF. From the EDS energy spectrum analysis in Table 1, it can be seen that the main elements of TiO2@Cu-MOF are Cu, Ti, and O, and their mass percentages are 27.66%, 43.14%, and 29.2%, and the atomic percentages are 13.92%, 28.98%, and 57.1%. It is proved that Ti element exists in the catalyst, which is confirmed by XRD results.
According to the classification method of IUPAC adsorption isotherms, the adsorption isotherms of TiO2@Cu-MOF nanocomposites and Cu-MOF materials are typical type IV isotherms, indicating that there are a large number of medium pores with a pore size of 2–50 nm in these two materials, as shown in Fig. 4. The specific surface area of the TiO2@Cu-MOF composite material is 495.55 m2/g−1, which is an increase over the Cu-MOF specific surface area of 425 m2/g−1. It can be seen that not only new voids are formed in the material but also the mesoporosity is also increased. Growth and special structure make the distribution of TiO2 on Cu-MOF material more uniform. Larger specific surface area and higher mesoporous structure can increase the catalyst surface adsorption performance and generate more active sites, which is more conducive to the occurrence of degradation reactions.
Figure 5 shows the FTIR spectra of Cu-MOF and TiO2@Cu-MOF.The Cu-MOF spectrum shows that the sample contains asymmetric stretching peaks of –COOH at 1637 cm−1, 1444 cm−1, and 1373 cm−1, and the C–H bond bending vibration peak at 728 cm−1. The bending vibration peak of the C–H bond at 938 cm−1 and the absorption peak at a wave number of about 3428 cm−1 are mainly caused by the adsorption of water in the test sample. Comparing the two bands, it is found that the TiO2@Cu-MOF does not show the Ti–O–Ti bond and Ti–O–C bond stretching vibration peak in the range of 945–400 cm−1. The reason for the above results may be as follows: TiO2 is through adsorption and some chemical bonds were formed on the surface of the Cu-MOF instead of Cu-MOF.
Batch adsorption studies
Influence of different reaction systems on decolorization effect
In this experiment, under the optimal conditions of light irradiation, pH = 6, initial concentration of RhB solution is 20 mg/L, reaction temperature is 30 °C, catalyst dosage is 0.3 g/L, and RhB solution reacts for 120 min, which proves that the high-efficiency degradation of RhB is produced by the interaction of various reaction conditions.
The experimental results are shown in Fig. 6. The decolorization rate of TiO2 added under visible light is 33.33%; When Cu-MOF is added and given a certain amount of light, the decolorization rate is 35.23%. This is because the developed pores on the MOF have a certain adsorption capacity; When the catalyst is added alone, the decolorization rate is 22.36%, indicating that the composite has a certain adsorption performance; When the conditions of light and catalyst (Cu:Ti = 1:2/1:4/1:6) are both satisfied in the reaction system, the decolorization rates after 120 min stabilization are 95.89%, 98.03%, and 96.99%. This is because the photo-generated electrons and holes generated when the TiO2@Cu-MOF composite is excited by light can be in the conduction and valence bands of the two semiconductors. However, the decolorization rate of rhodamine B does not always increase with the increase of the amount of TiO2. When the catalyst (Cu:Ti = 1:4), the decolorization rate of RhB can reach 98.03%, and the decolorization effect is the best. Therefore, the following tests all use TiO2@Cu-MOF composite material (molar mass ratio Cu:Ti = 1:4) as the photocatalyst.
Effect of the solution pH on adsorption of RhB
Figure 7 shows the relationship between solution pH and decolorization rate. According to the results, it can be seen that there is an increase in solution pH from 2.4 to 10, and the decolorization first increases and then decreases. When the pH is 6, the decolorization rate of RhB solution reaches the maximum of 98.03%. The reason may be that TiO2@Cu-MOF composites have different acid-base balances at different pH values, and the change in pH of the solution will affect the efficiency of photocatalytic degradation of dyes.
Effect of initial dye concentration on adsorption of RhB
As the initial concentration of the RhB solution increases, the degradation effect becomes difficult, degradation of high concentration RhB solution is one of the main purposes of our experimental exploration. This experiment performed degradation experiments on RhB solutions of different concentrations, mainly from 20 mg/L, 40 mg/L, 60 mg/L, and 80 mg/L (Fig. S1). With the increase of the initial concentration of RhB solution, the degradation effect is getting lower and lower. The degradation rate and decolorization rate of the experiment decreased significantly from the initial 98.03% after 120-min degradation was stable. The reason may be that under the same conditions of the catalyst amount, the amount of hydroxyl radicals generated in a certain period of time is constant, and the increase in the initial concentration of RhB solution makes the relative yield of hydroxyl radicals insufficient, which reduces decolorization rate of RhB. In addition, the larger the initial concentration of RhB, the darker the solution color and the smaller the light transmittance, making the reaction system unable to fully absorb and utilize the light source. This will reduce the photocatalytic efficiency and the dye decolorization rate.
Effect of catalyst dosage on adsorption of RhB
The amount of catalyst is also the main factor that affects the degradation effect of RhB. In this experiment, 0.1 g/L, 0.2 g/L, 0.25 g/L, 0.3 g/L, and 0.4 g/L were used as the catalyst addition amounts. The results are shown in Fig. 9. It can be seen from Fig. S2 that the decolorization rate of 120 min is 69.89%, 79.22%, 91.25%, 98.03%, and 96.95% respectively, and the decolorization rate increases with the increase of catalyst dosage. Therefore, the optimal mass concentration of the composite photocatalyst should be 0.3 g/L. This may be due to the increase of the photocatalyst mass concentration and the increase of reactive sites, which is conducive to the catalyst’s adsorption of dye molecules and absorption of light quantum, which increases the decolorization rate of RhB, but the reaction solution becomes cloudy with the increase of the amount of catalyst, The light transmittance is not enough to continuously improve the decolorization rate of RhB. Therefore, choosing a suitable photocatalyst mass concentration can save the amount and obtain a good catalytic effect.
Kinetic adsorption
In order to investigating the adsorption mechanism, in this paper, the adsorption kinetics of RhB dye onto TiO2@Cu-MOF was studied by pseudo-first-order kinetic models (Lopes et al. 2003). The parameters for pseudo-first order were calculated by Eq. (2) respectively.
First-order kinetic equation fitting during reaction:
where A0 (mg/L) denote the initial dye concentration; At (mg/L) represents the residual dye concentration at time t; K1 (min−1) represents the reaction rate; t (min) represents the degradation time.
The experimental results are shown in Fig. 8. The rate constant of TiO2 added under visible light is 0.0015 min−1; When Cu-MOF is added and given a certain amount of light, the rate constant is 0.0016 min−1; When the catalyst is added alone, the rate constant is 0.0009 min−1; When the conditions of light and catalyst (Cu:Ti = 1:2/1:4/1:6) are both satisfied in the reaction system, the rate constants after 120 min stabilization are the rate constants 0.0125 min−1, 0.0144 min−1, and 0.0139 min−1. However, the rate constant increases as the decolorization rate increases. Therefore, in the TiO2@Cu-MOF composite material (molar mass ratio Cu:Ti = 1:4), the decolorization rate as a photocatalyst is as high as 98.03%. The first-order kinetic equation was fitted to the reaction process. and R2 = 0.98 under the optimal condition, which indicated that the reaction process obeys the first-order kinetic equation.
Cyclic stable performance
Disposal of waste adsorbents may cause environmental pollution and significant increase in economic costs. Therefore, it is crucial to treat the used adsorbent and re-use it to reduce its disposal and at same time reduce economic costs which could in turn increase its sustainability.
Compared with other catalysts, the catalyst in this experiment is easier to recycle and reuse. After the TiO2@Cu-MOF catalyst is degraded for 120 min, it was centrifuged at 5000r/min and then dried in a 90 °C drying box for 3 h. The decolorization rate reached 85.03% after 4 cycles (Fig. 9). This shows that the catalyst has good cycle performance. In addition, we can compare the infrared spectrum images of catalyst before and after use (Fig. 10), which shows that the catalyst has good stability. It was also proved that the degradation of RhB by the catalyst prepared in this experiment was mainly chemical decomposition, not physical adsorption.
The above analysis shows that the TiO2@Cu-MOF nanocomposite catalyst prepared in this study has the ability to improve the photocatalytic performance of TiO2. The composite catalyst prepared in this paper is simple in operation, cheap in materials, green and environmentally friendly, and has very stable recyclability, which also shows that the catalyst has good stability. The optimal ratio of TiO2@Cu-MOF nanocomposite prepared in this research has certain advantages in the photocatalytic degradation of RhB.
Conclusion
-
1.
This article uses copper nitrate trihydrate as the copper source to synthesize TiO2@Cu-MOF nanocomposites by one-step crystallization. The results of photocatalytic RhB showed that it had a good ability to degrade RhB after 120-min reaction under mild conditions. Because the catalyst provides more active sites and electron holes; the deethylation reaction and the ring-opening oxidation reaction of the benzene ring occur simultaneously during the degradation process. Effectively removed the color and organic pollutant content of RhB.
-
2.
TiO2@Cu-MOF nanocomposite catalyst is a typical mesoporous material with a specific surface area of 495.55 m2/g−1, and the pore size is mainly distributed between 2 and 10 nm. The crystal form is uniformly dispersed on the surface of Cu-MOF. TiO2@Cu-MOF nanocomposite has excellent photocatalytic degradation performance, and its degradation rate of rhodamine B can reach 98.03% under 120-min visible light, and R2 = 0.98 under the optimal condition,which indicated that the reaction process obeys the first-order kinetic equation.
-
3.
The TiO2@Cu-MOF nanocomposite catalyst prepared in this paper has the characteristics of simple operation, cheap materials, and environmental protection during the preparation process, and it can also be used in a stable cycle after being centrifuged and dried. This catalyst has good stability.
This study focuses on the preparation of an innovative TiO2/Cu catalyst to degrade RhB under light and reports this surface properties and the degradation kinetics of this dye.
Data availability
The data is true and reliable.
References
Farha OK, Hupp JT (2010) Rational design, synthesis, purification and activation of metal-organic framework materials. Acc Chem Res 43(8):1166
Jiang P, Ren D, He D et al (2014) An easily sedimentable and effective TiO2 photocatalyst for removal of dyes in water. Sep Purif Technol 122:128–132
Kiwi J, Rtimi S (2018) Mechanisms of the antibacterial effects of TiO2–FeOx under solar or visible light: Schottky barriers versus surface Plasmon resonance. Coatings 8:391
Li J-R, Sculley J, Zhou H-C (2012) Metal-organic frameworks for separations. Chem Rev 112(2):869–932
Li H, An N, Liu G, Li J, Liu N, Jia M, Zhang W, Yuan X (2016) Adsorption behaviors of methyl orange dye on nitrogen-doped mesoporous carbon materials. J Colloid Interface Sci 466:343e351
Lopes ECN, Dos Anjos FSC, Vieira EFS, Cestari AR (2003) An alternative Avrami equation to evaluate kinetic parameters of the interaction of Hg(II) with thin chitosan membranes. J Colloid Interface Sci 263:542e547
Meilikhov M, Yusenko K, Esken D et al (2010) Metals@ MOFs, loading MOFs with metal nanoparticles for hybrid functions. Eur J Inorg Chem 24:3701
Rtimi S, Pulgarin C, Sanjines R, Kiwi J (2016) Accelerated self-cleaning by Cu promoted semiconductor binary-oxides under low intensity sunlight irradiation. Appl Catal B Environ 180:648–655
Ruan X, Liu M, Zeng Q et al (2010) Degradation and decolorization of reactive red X-3B aqueous solution by ozone integrated with internal micro electrolysis. Sep Purif Technol 74(2):195–201
Xing BL, Shi CL, Zhang CX et al (2016) Preparation of TiO2/activated carbon composites for photocatalytic degradation of RhB under UV light irradiation. J Nanomater 2016:1–10
Yu J, Kiwi J, Wang T, Pulgarin C, Rtimi S (2019) Evidence for a dual mechanism in the TiO2/CuxO photocatalyst during the degradation of sulfamethazine under solar or visible light: critical issues. J Photochem Photobiol 375:270–279
Hichem Zeghioud, Aymen Amine Assadi, Nabila Khellaf et al. Photocatalytic performance of CuxO/TiO2 deposited by HiPIMS on polyester under visible light LEDs: oxidants, ions effect, and reactive oxygen species investigation . Materials 12 (2019) 412
Funding
This work was supported by the key research projects of colleges and universities in Henan Province (19A530002), Key Laboratory of carbon Green Conversion in Henan Province, China (662413/002/001 and CGCF201804), and Distinguished Foreign Scientist Workshop on carbon Green Conversion (GZS20200012).
Author information
Authors and Affiliations
Contributions
This manuscript was contributed by all authors. All the authors have ratified the manuscript. Xue Xiaoxiao compiled the manuscript; Yang Shicheng, Zhang Zhengting, and Meng Shihang collected data and information; Yi Guiyun verified the availability of the data and information; Weng Yujing and Zhang Yulong reviewed the manuscript and provided sources of funding. All authors have participated in the work of this manuscript and agreed to the publication of this paper in your journal.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable
Consent to participate
All authors agree to participate in the editing of the paper.
Consent to publish
All authors agree to publish this manuscript in your journal.
Additional information
Responsible editor: Sami Rtimi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 61 kb)
Rights and permissions
About this article
Cite this article
Xue, X., Weng, Y., Yang, S. et al. Effect of Cu-based metal organic framework (Cu-MOF) loaded with TiO2 on the photocatalytic degradation of rhodamine B dye. Environ Sci Pollut Res 28, 15883–15889 (2021). https://doi.org/10.1007/s11356-020-11805-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-11805-w