Abstract
The main goals of conservation agriculture are to enhance soil fertility and reduce soil degradation, especially through erosion. However, conservation agriculture practices can increase the risk of contamination by pesticides, mainly through vertical transfer via water flow. Better understanding of their sorption and degradation processes is thus needed in conservation agriculture as they control the amount of pesticide available for vertical transfer. The purpose of our study was to investigate the sorption and degradation processes of nicosulfuron in soil profiles (up to 90 cm deep) of a Vermic Umbrisol and a Stagnic Luvisol managed either in conventional or in conservation agriculture. Two laboratory sorption and incubation experiments were performed. Low sorption was observed regardless of the soil type, agricultural management or depth, with a maximum value of 1.3 ± 2.0 L kg−1. By the end of the experiment (91 days), nicosulfuron mineralisation in the Vermic Umbrisol was similar for the two types of agricultural management and rather depended on soil depth (29.0 ± 2.3% in the 0–60-cm layers against 7.5 ± 1.4% in the 60–90 cm). In the Stagnic Luvisol, nicosulfuron mineralisation reached similar value in every layer of the conservation agriculture plot (26.5% ± 0.7%). On the conventional tillage plot, mineralisation decreased in the deepest layer (25–60 cm) reaching only 18.4 ± 6.9% of the applied nicosulfuron. Regardless of the soil type or agricultural management, non-extractable residue formation was identified as the main dissipation process of nicosulfuron (45.1 ± 8.5% and 50.2 ± 7.0% under conventional and conservation agriculture respectively after 91 days). In our study, nicosulfuron behaved similarly in the Vermic Umbrisol regardless of the agricultural management, whereas the risk of transfer to groundwater seemed lower in the Stagnic Luvisol under conservation agriculture.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
To enhance the sustainability of agricultural systems and decrease the impacts of conventional agriculture, alternative cultivation practices such as conservation agriculture have been developed. Conservation agriculture is defined as a combination of three main interconnected soil-conservation techniques: (i) little or no soil disturbance, (ii) permanent soil cover by crop residues and/or cover crops and (iii) diversification of plant species in the crop rotation (FAO 2016). The main goals of conservation agriculture are to reduce soil degradation due to erosion but also the contamination of surface water by runoff or transfer of sorbed agrochemicals on eroded soil particles (Holland 2004; Hobbs et al. 2008).
Compared with conventional agriculture that includes soil tillage (sometimes with inversion of topsoil layers), conservation agriculture enhances faunal activity, especially earthworm activity, which results in a dense biomacropore network (Shipitalo et al. 2000). Moreover, because the topsoil is disturbed less, it usually contains a greater continuity of vertically oriented macropores, which leads to higher hydraulic conductivity than that of tilled soils (Wahl et al. 2004). Increasing vertical transfer is one way to reduce runoff and thus surface water contamination, but in return, it increases the risk of deep vertical transfer to groundwater. This is a major drawback of conservation agriculture, especially as reducing tillage intensity generally increases weed pressure, which often leads to an increase in herbicide use (Alletto et al. 2010). Better understanding of sorption and degradation processes in such agricultural systems is thus critically needed as they control the amount of pesticide available for vertical transfer.
One of the main changes of not inverting topsoil layers, as in conservation agriculture, is the accumulation of dead residues or mulch at the soil surface, which enriches the first few centimetres of soil in organic carbon. The carbon content then decreases with soil depth, reaching contents less than or equal to those of conventionally tilled soil (Doran 1980; Kay and VandenBygaart 2002). For many pesticides, sorption can be related to soil organic matter content (Gaston et al. 2007; Đurović et al. 2009; Sadegh-Zadeh et al. 2017). Thus, at an equivalent depth in the topsoil, pesticide sorption is generally higher under conservation agriculture than conventional agriculture because of the former’s higher carbon content (Gaston et al. 2003; Locke et al. 2005). Deeper in the soil, sorption decreases because the carbon content is lower, and it tends to follow the decrease in carbon content (Clay and Koskinen 2003). In addition, the accumulation of organic matter (and fertilisers) under conservation agriculture generally acidifies the topsoil (Thompson and Whitney 2000; Thomas et al. 2007; Obour et al. 2017). Weak acids and ionisable molecules dissociate to their anionic form as soil pH increases, which lowers adsorption (Dyson et al. 2002). More acidic conditions in the topsoil may then increase pesticide adsorption under conservation agriculture. For instance, Alletto et al. (2013) observed a negative correlation between pH and S-metolachlor sorption in soils under conservation agriculture.
Because of soil organic matter accumulation and less soil disturbance by agricultural operations, microbial biomass and microbial activity in the topsoil is generally greater under conservation agriculture than conventional agriculture (Kandeler et al. 1999). This increase in microbial activity can sometimes increase pesticide degradation (Locke et al. 2005; Zablotowicz et al. 2007), but pesticide degradation does not always reflect soil microbial activity (Mahía et al. 2007; Okada et al. 2019). Decrease in substrate availability due to higher adsorption is often mentioned as an explanation for low degradation under conservation agriculture (Zablotowicz et al. 2000). Adaptation of the microbial community can also increase pesticide degradation (Barriuso et al. 1996). Both may explain the lack of correlation between total microbial activity and pesticide mineralisation (Alletto et al. 2013). On the other hand, microbial activity and biomass are often correlated with soil pH, which strongly influences pesticide degradation in soils (Kah et al. 2007). Acidification of the topsoil under conservation agriculture may thus modify pesticide degradation. In the subsoil (> 25–30 cm deep), pesticide degradation is generally lower, generally due to less microbial activity and/or biomass related to lower carbon content, temperature and oxygenation (Fomsgaard 1995; Bending and Rodríguez-Cruz 2007).
We studied the sorption and degradation of nicosulfuron in soil profiles (up to 90 cm deep) of two soils, a Stagnic Luvisol and a Vermic Umbrisol, managed for more than 10 years under two types of agricultural management: conventional agriculture (tillage, bare soil and monoculture) and conservation agriculture (no tillage, cover crops and crop rotations). Nicosulfuron is a post-emergence herbicide used to control grass species in maize. It is considered environmentally safe because of low application rates (usually < 100 g ha−1) (Regitano and Koskinen 2008). Nicosulfuron is however generally in anionic form in soils and is considered to be highly mobile, which could result in groundwater contamination (Gonzalez and Ukrainczyk 1996). In addition, nicosulfuron generally has low sorption and fast degradation rates (Olivera et al. 2001; Poppell et al. 2002; Azcarate et al. 2018; PPDB 2020). Recently, Cueff et al. (2020) reported high nicosulfuron leaching and a high degree of preferential flow in undisturbed columns sampled in the same soils as in the present study.
Materials and methods
Sites and types of agricultural management
The two sites of this study are located in south-western France, in the Pyrénées-Atlantiques (43.516532 N, –0.260186 W) and Gers (43.410980 N, 0.284574 E) departments. Both sites are subjected to an altered oceanic climate.
The soil at the Pyrénées-Atlantiques site is a thick humic acid soil developed from Quaternary silty alluvial deposits that is classified as a Vermic Umbrisol according to the World Reference Base for Soil Resources (IUSS Working Group WRB 2007) and as a veracrisol according to the French Soil Classification (Référentiel Pédologique 2008) (locally called “Touyas”). It has a rich, deep (50–80 cm deep) and well-structured organic horizon (Table 1). Its texture is composed mainly of fine silt (> 450–500 g kg−1), with a low proportion of sand (< 100–120 g kg−1). Clay minerals are mainly kaolinite, illite and vermiculite (Arrouays et al. 1992). It has a high agronomic potential, especially for maize (mean yield ≈ 13–15 t ha−1) and soya bean (mean yield ≈ 3.7–4.2 t ha−1) (most not irrigated). Hereafter, this site is referred to as the VER (veracrisol) site.
The soil at the Gers site is classified as a Stagnic Luvisol (IUSS Working Group WRB 2007) and a luvisol redoxisol (Référentiel Pédologique 2008) (locally called “Boulbènes”), with a loamy topsoil layer (fine + coarse silt ≈ 450–500 g kg−1) and an illuvial clay horizon that appears at ca. 45–55 cm (Table 1). Clay minerals are mainly illite and smectite (Michel et al. 2003). The substratum is an alluvial pebbly layer with low permeability that appears at ca. 60–80 cm. With low organic carbon contents (usually < 7–8 g kg−1) in the topsoil and high silt contents, this soil is strongly sensitive to crusting. Because of these properties (especially hydromorphic conditions at low depth), it has a moderate agronomic potential for mainly irrigated crops (mean maize yields ≈ 10–12 t ha−1). Hereafter, this site is referred to as the LUV (luvisol) site.
Each site consists of two adjacent plots managed under conventional (TILL) and conservation (CONS) practices. The conventional plots have been cultivated under maize monoculture for more than 40 years, and the soil is left bare in winter. Tillage depth with a mouldboard plough is ca. 22–23 cm at the VER site and 30 cm at the LUV site. In both conservation plots (VER and LUV), agricultural management consists of (i) no-tillage practices, (ii) permanent soil surface cover by mulch and cover crop mixtures (phacelia and faba bean at the VER site, two cereals and faba bean at the LUV site) sown during fallow periods and (iii) a diversified crop rotation (maize, soya bean, cereals and sometimes intercrops of cereals and legumes). These conservation agriculture practices have been performed since 2006 and 2000 at the VER and LUV sites, respectively.
Soil sampling and conditioning
In each plot, bulk soil was sampled from soil profiles collected at three locations (considered replicates) at ca. 20-m intervals along a transect parallel to the border between the TILL and CONS plots. To minimise the spatial variability in soil properties between the two plots as much as possible, the transects lay 20 m from the border between the plots. Four soil layers (0–10, 10–25, 25–60 and 60–90 cm) and three soil layers (0–10, 10–25 and 25–60 cm) were collected in each profile at the VER and LUV site, respectively. Soils were air-dried and sieved at 5 mm and stored in a cold chamber (4 °C) before analysis.
Experimental study
Soil organic carbon and carbon stock
Carbon content was measured according to the NF ISO 10694 standard (AFNOR 1995) based on CO2 emission of the sample following dry combustion. Results were corrected for carbonates present in the sample.
In each soil profile, we used 250 cm3 (8 cm diameter, 5 cm high) cylindrical cores to collect undisturbed soil samples by hand using gentle pressure. Soil cores were dried in an oven (105 °C, 48 h), and bulk density (g cm−3) was determined to estimate the carbon stock in each soil layer.
Sorption experiments
Few studies about nicosulfuron sorption are available in the literature. In most cases, Freundlich exponent was found to be close to 1. Four studies covering different soil types and textures (including similar textures as soils from our study) reported n values ranging mainly between 0.9 and 1.1 (only three n values were higher than 1.1, with a maximum of 1.4, on the 33 soils studied in the literature) (Gonzalez and Ukrainczyk 1996; EFSA 2008; Regitano and Koskinen 2008; Caceres-Jensen et al. 2020). Azcarate et al. (2015) reported no effect of the initial solution concentration and assimilated the sorption coefficient to a Kd. In light of the results from the literature, only one initial concentration was applied in our study in order to determine a Kd.
Nicosulfuron (98.4 % purity) was purchased from Cluzeau Info Labo (Ste Foy, France). Batch sorption experiments were conducted with a 1:3 soil/solution ratio (m/v) with a solution of nicosulfuron concentrated at 19.8 μg L−1. Nicosulfuron concentration was determined by ultra-performance liquid chromatography coupled with tandem mass spectrometry (UHPLC-MS/MS Acquity-TQD, Waters Corp., Milford, MA, USA) after 24-h equilibrium. A detailed measurement protocol was published by Cueff et al. (2020). Limit of quantification (LOQ) was determined in blank soil extracts spiked with low concentrations of nicosulfuron, as the concentration that led to a signal to noise ratio of 10. Limit of detection (LOD) was calculated from LOQ as LOD = LOQ / 3. For nicosulfuron, LOD and LOQ were equal to 0.016 and 0.05 μg/L, respectively.
Incubation experiments
A soil sample (10 g dry weight) from each soil layer was placed in a Falcon tube (50 mL, Corning, NY, USA) and adjusted to 80% of soil field capacity (− 33 kPa), which corresponds to pF 2.5 (with pF = log10|h|, h in cm). For each soil sample, water content at field capacity was determined using a pressure plate (Klute 1986). Each Falcon tube was placed in a hermetically sealed incubation jar that was large enough to prevent formation of anoxic conditions in the soil. Then, 0.5 mL of a solution of 14C-nicosulfuron with a mean activity of 609,235 DPM/mL was added to the soil. In addition, a tube of 10 mL of water (to maintain constant relative humidity in the jar) and a tube of 6 mL of 1 N sodium hydroxide (NaOH) solution were placed into the jar. The NaOH solution trapped all the CO2 (12C–CO2 and 14C–CO2) released by the mineralisation. The NaOH trap was replaced periodically until the end of the experiment to avoid saturating it. Degradation of 14C-nicosulfuron was monitored for 91 days, in the dark, at 28 ± 0.5 °C. Triplicate samples for each soil layer of each site were run for five incubation times: 0, 7, 14, 35, and 91 days.
The distribution of 14C-nicosulfuron activity among the mineralised, water-extractable, methanol-extractable, and non-extractable-residue fractions was determined at the five incubation times. The mineralised fraction and total CO2 released were also measured in all of the intermediate NaOH solutions. The water-extractable fraction of 14C was determined by CaCl2 extraction (30 mL, 10−2 M). Then, the methanol-extractable fraction of 14C was determined by two successive methanol extractions (30 mL). For each extraction, the tubes were left for 22 h in an end-over-end shaker and then centrifuged at 14,000×g for 10 min. The 14C activity was measured by adding 10 mL of a scintillating liquid (Ultima Gold XR, Perkin Elmer, Waltham, MA, USA) to a 1-mL aliquot of the aqueous phase of all three successive extractions. The non-extractable-residue fraction of 14C was determined after drying and grinding the soil remaining after the extractions. The remaining 14C activity was measured by combusting the ground soil in an oxidizer (Biological Oxidizer, OX 700, Zinsser Analytic, Frankfurt, Germany). 14C-CO2 released from the combustion (900 °C, 5 min) of the soil samples was trapped in Oxysolve 400 solution (Zinsser Analytic), in which the 14C activity was directly measured. The mineralised fraction of 14C was determined by adding 10 mL of scintillating liquid to a 2-mL aliquot of the NaOH trap solution. 14C activity of each fraction was measured by liquid scintillation counting for 10 min in a Tri-Carb 2100TR scintillation counter (Packard Instruments, Meriden, CT, USA).
In addition, mineralisation of soil organic carbon was determined by measuring the 12C-CO2 trapped by the NaOH using a colorimetric method (Colorimetric Skalar Analyzer, Breda, Netherlands). Microbial biomass was determined by the fumigation-extraction method (Wu et al. 1990) using a total organic carbon analyser (Shimadzu TOC 5050A).
Statistical analysis
Cumulative soil carbon mineralisation (12C–CO2) and cumulative nicosulfuron mineralisation (14C–CO2) for each soil sample were fitted to an exponential model that increases to a maximum:
where, respectively, yOC and yN are the modelled mineralisation of 12C–CO2 and 14C–CO2 (expressed as % of initial organic carbon and of applied nicosulfuron), aOC and aN are the final cumulative mineralisation (%), bOC and bN are the first-order rate constant (days−1) and t is time (days).
The decrease in the water-extractable fraction of 14C over time was fitted to a first-order kinetics model:
where yWE is the water-extractable fraction modelled at time t (%), aWE is the initial water-extractable fraction measured at t0 (%) and bWE is the first-order rate constant (days−1).
The increase in the non-extractable-residue (NER) fraction of 14C over time was fitted to an exponential model that increases to a maximum:
where yNER is the NER fraction modelled at time t (%), y0 is the initial NER fraction measured at t0 (%), aNER is the final cumulative percentage of the NER fraction (%) and bNER is the first-order rate constant (days−1). As the methanol-extractable fraction did not follow any particular dynamics, it was not fitted to a model.
Spearman’s correlation analysis was performed in order to explore the relationships between soil properties, sorption coefficients, 14C fractions and model parameters for the two sites and the two agricultural managements. Correlation analysis and correlation matrix were done using the “psych” and “corrplot” R packages.
Analysis of variance (ANOVA) was performed for carbon stock, microbial biomass, Kd and all parameters fitted to models to identify effects of agricultural management and soil depth. When possible, an effect of an interaction between agricultural management and soil depth was evaluated. The Shapiro-Wilk normality test and Levene’s homoscedasticity test were applied to the residues of the ANOVA to verify application conditions. When the conditions were not met, logarithmic or square root transformations were applied. When transformed data could not meet the conditions, the Kruskal-Wallis test was performed.
Results
Soil organic carbon content and mineralisation
At both sites, mean organic carbon content did not differ significantly between the two surface layers (0–10 and 10–25 cm) and decreased with depth (Table 2). Nevertheless, in the CONS plot at the LUV site, mean organic carbon content was 31% higher in the 0–10-cm layer than in the 10–25-cm layer. Moreover, mean organic carbon content was generally slightly higher throughout the soil profile in the CONS plot than in the TILL plot at the LUV site and was highest in the two surface layers in both the CONS and TILL plots at the VER site. Differences in organic carbon content according to agricultural management were significant only at the LUV site (P < 0.01).
Carbon stock increased with depth in both plots but varied more at the VER site. Carbon stock was similar for the two types of agricultural management at the VER site, whereas it was higher in the CONS plot than in the TILL plot (especially for the 0–10-cm and 25–60-cm layers) at the LUV site (P < 0.01).
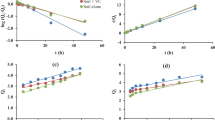
Microbial biomass decreased strongly with depth in both plots (Table 2), in relation to the decrease in organic carbon content (r = 0.71: Fig. 1). It was also negatively correlated with soil pH (r = − 0.50). Microbial biomass was higher in the CONS plot than in the TILL plot at both sites (P < 0.01) due to the high microbial biomass in the 0–10-cm layer of the CONS plots (570 ± 29 and 710 ± 98 mg C kg−1 dry soil at the VER and LUV site, respectively).
Cumulative organic carbon mineralisation (12C–CO2 t91: Fig. 1) was positively correlated with microbial biomass (r = 0.48). At each site, it was higher in the 0–10-cm layer of the CONS plot than in any other layer of either plot (Fig. 2). The difference was larger at the LUV site, with aOC reaching up to 20.1 ± 8.5%, while it reached only 8.1 ± 1.4% at the VER site (Table 3). Mineralisation data fit the exponential model well (Eq. 1) for all soil layers in both plots (R2 = 0.96 ± 0.16), and aOC generally decreased with depth, except for the 25–60-cm layer of the CONS plot at the VER site. Both agricultural management (P < 0.01) and depth (P < 0.05) had a significant effect on aOC at the LUV site, whereas only agricultural management had a significant effect (P < 0.05) at the VER site. The first-order rate constant was highest in the deepest layer in both plots at the VER site (Table 3). In the upper three layers, bOC had similar values in the TILL plot, but bOC was highest in the 0–10-cm layer in the CONS plot. At the LUV site, bOC was highest in the 25–60-cm layer in the TILL plot and in the 0–10-cm layer in the CONS plot. Only depth had a significant effect on bOC at the VER site (P < 0.01) and the LUV site (P < 0.05).
Sorption experiments
Sorption of nicosulfuron was low at both sites (mean of 0.7 ± 0.6 L kg−1). Kd was highest (1.3 ± 2.0 L kg−1) in the surface layer (0–10 cm) of the TILL plot at the LUV site (Table 2). With similar values of nicosulfuron sorption of between surface and deep layers, no clear trend with depth was observed, even though organic carbon content differed significantly among soil layers (Table 2). These results suggest that nicosulfuron has moderate affinity for organic carbon, which is confirmed by the KOC values, which were generally highest in the deepest layer, except in the CONS plot at the LUV site (Table 2). KOC values of many pesticides are commonly higher in deep layers, even for pesticides with stronger affinity for organic carbon. Kd values did not differ significantly by site, agricultural management or depth, but KOC values differed significantly by depth (P < 0.001), indicating the difference between the 60–90-cm layer and all other layers at the VER site.
Incubation experiments
VER site
At the VER site, a mean of 96.5 ± 3.2% of the initially applied 14C-nicosufluron was recovered after quantifying the mineralised, water-extractable, methanol-extractable and non-extractable-residue fractions of 14C for the five incubation durations. At t0, most of the radioactivity was found in the water-extractable fraction (mean of 76.1 ± 5.9% of applied 14C). The rest of the radioactivity was distributed between the methanol-extractable (9.1 ± 4.7%) and non-extractable-residue fractions (15.5 ± 2.6%) as mineralisation had not yet started. In most layers, the non-extractable-residue fraction of 14C was 2–3 times as large as the methanol-extractable fraction at t0, except in the 0–10-cm layer of the CONS plot, in which these fractions were similar (17.0 ± 1.9% and 19.9 ± 6.3%, respectively). The methanol-extractable fraction in this layer was the largest found at t0 at the VER site.
14C-nicosulfuron started to mineralise within 3 days after it was applied. Cumulative mineralisation increased throughout the experiment and fit the exponential model well (Eq. 2) (R2 = 0.99 ± 0.00 for the upper three soil layers (0–60 cm). In the deepest layer (60–90 cm), nicosulfuron mineralisation was low, and the observed 14CO2 dynamics did not fit the exponential model well (a linear model was used instead). After 91 days, mean cumulative nicosulfuron mineralisation differed significantly (P < 0.001) between the upper three layers (ca. 29.0 ± 2.3%) and the deepest layer (60–90 cm) (7.5 ± 1.4%) in both the CONS and TILL plots (Fig. 3). While nicosulfuron mineralisation decreased progressively (but non-significantly) with depth (from 0 to 60 cm) in the TILL plot (Fig. 3), it ultimately followed the reverse order of depth in the CONS plot, with the highest cumulative mineralisation observed after 50 days in the 25–60-cm layer. In the TILL plot, model parameters indicated a trend similar to that for measured data, with equivalent values of the first-order rate constant bN (Table 3). In the CONS plot, however, the modelled final cumulative mineralisation aN in the 25–60-cm layer was higher than the measured data (Table 3; Fig. 3), indicating that nicosulfuron in this soil horizon may continue to mineralise beyond the 91-day incubation period of the experiment. The first-order rate constant followed a similar trend and was much lower in the 25–60-cm layer of the CONS plot than in the other layers.
The measured water-extractable fraction fit the first-order kinetics model well (Eq. 3) (R2 = 0.91 ± 0.04) (Fig. 4a). The initial water-extractable fraction aWE was slightly (but not significantly) higher in the CONS plot. It then decreased strongly during the experiment, especially during the first 7 days (decrease of 22–45 percentage points). The decrease was much stronger in the 60–90-cm layer, which had a much higher first-order rate constant kWE than the upper three layers in both plots (P < 0.001). This resulted in a small amount of 14C-nicosulfuron remaining in the water-extractable fraction at the end of the experiment, which the model always underestimated (e.g. estimating 0% when the measured value was 7–16%).
The methanol-extractable fraction varied little in the upper three layers (0–60 cm) in the TILL plot but decreased strongly in the 0–10-cm layer in the CONS plot (Fig. 4b). In both plots, due to its strong increase during the first 7 days, the methanol-extractable fraction in the 60–90-cm layer remained higher than in the upper three layers (P < 0.001) at t91. In addition, the methanol-extractable fraction in this layer became the second largest after the NER fraction after 14 days, whereas the water-extractable fraction exceeded the methanol-extractable fraction in the upper three layers.
The measured NER fraction data fit the exponential model well (Eq. 4) (R2 = 0.98 ± 0.01) (Fig 4c). The NER fraction at t0 (y0) was high in each layer and plot but higher in the TILL plot than in the CONS plot (P < 0.001). It increased throughout the experiment, with a large increase (22.0 ± 3.6 percentage points) during the first 14 days. The exponential model always underestimated the final NER fraction aNER, which was higher in the CONS plot than in the TILL plot (P < 0.01). NER formed faster in the 60–90-cm layer and slightly faster in the TILL plot than in the CONS plot, as indicated by the first-order rate constant bNER (Table 3). However, neither agricultural management nor depth had a significant effect on bNER.
LUV site
Like at the VER site, satisfying 14C-nicosulfuron mass balances were reached, with ca 95.6 ± 3.4% of the initially applied radioactivity that was recovered after quantifying all of the fractions. At t0, most of the radioactivity was found in the water-extractable fraction (mean of 81.3 ± 5.4%), regardless of the agricultural management and depth. Because of a particularly large water-extractable fraction, the methanol-extractable fraction at t0 was especially low in the TILL plot (mean of 1.0 ± 1.0%) (Fig. 4b). However, in the surface layers (0–10 and 10–25 cm), the methanol-extractable fraction was much greater in the CONS plot than in the TILL plot. Except for the 10–25-cm layer of the TILL plot, the NER fraction at t0 was larger than the methanol-extractable fraction.
As at the VER site, 14C-nicosulfuron began to mineralise within 3 days after it was applied (Fig. 3). Cumulative mineralisation data fit the exponential model well (Eq. 2) (R2 = 0.99 ± 0.00). After 35 days, mineralisation of 14C-nicosulfron in the 0–10 and 10–25-cm layers was higher in the TILL plot than in the CONS plot. During the incubation period, nicosulfuron mineralisation in the TILL plot remained lower in the deepest layer (25–60 cm) than in the two surface layers, with final cumulative mineralisation reaching 18.4% ± 6.9% of applied 14C in the 25–60-cm layer vs. 33.2 ± 2.7% and 30.5 ± 1.6% in the 0–10 cm and 10–25-cm layers, respectively. In the CONS plot, however, final cumulative nicosulfuron mineralisation at t91 was similar throughout the entire soil profile (reaching a mean of 26.5 ± 0.7% of applied 14C) (Fig. 3). As for the VER site, the modelled final cumulative 14C–CO2 aN in the 25–60-cm layer of the CONS plot was higher than the measured data. For the rest of the profile, aN matched the final cumulative 14C–CO2 measured, indicating that most of the nicosulfuron mineralised during the 91-day incubation period. In the TILL plot, aN was slightly (but not significantly) higher in the surface layers than in the 25–60-cm layer, whereas in the CONS plot, it was significantly higher in the 25–60-cm layer than the same layer in the TILL plot. The first-order rate constant bN indicated faster mineralisation in the surface layers (P < 0.001), especially in the CONS plot (P < 0.01).
The measured water-extractable fraction data fit the first-order kinetics model well (Eq. 3) (mean R2 = 0.93 ± 0.07), but, like at the VER site, the model underestimated the final fractions of radioactivity in the water extracts, particularly in the 0–10 and 10–25-cm layers (Fig. 4a). The initial water-extractable fraction aWE was slightly (but not significantly) higher in the TILL plot than in the CONS plot (Table 3). As observed at the VER site, this fraction decreased strongly early in the incubation before slowing down. The exponential decay was faster in the CONS plot (P < 0.05), especially in its surface layers (P < 0.01), as indicated by the first-order rate constant kWE (Table 3). Small amounts were ultimately recovered in these surface layers at t91 in both plots, while in the deeper soil layers (25–60 cm), the water-extractable fraction of 14C in the still represented 22.6 ± 7.5% in the CONS plot and nearly 46.0 ± 1.7% in the TILL plot (P < 0.05).
The methanol-extractable fraction of 14C increased slightly until day 35 and then generally decreased to its initial value in all layers in the TILL plot (Fig. 4b). In the CONS plot, it decreased in the surface layers, especially at t91, whereas it increased in the 25–60-cm layer (reaching 5.3 ± 1.5% of applied 14C). The final methanol-extractable fraction of 14C was similar in all plots and layers.
The NER fraction increased until the end of the experiment, with a strong increase during the first 14 days of incubation (21.0 ± 6.6%) (Fig. 4c). The data fit the exponential model well (Eq. 4) (R2 = 0.96 ± 0.06). Despite similarly high initial NER fractions among layers, the final fraction at t91 was lowest in the 25–60-cm layer (P < 0.001), especially in the TILL plot. This was due to faster formation of NER in the surface layer (P < 0.05), as indicated by the first-order rate constant bNER (Table 3). Mean bNER was slightly (but not significant) higher in the surface layer than in the other layers in the CONS plot. At the end of the experiment, the NER fraction contained most of the radioactivity in the surface layers (≈ 50% of applied 14C). It was higher in the CONS plot than in the TILL plot (P < 0.01) and lowest in the 25–60-cm layer of both plots (P < 0.001).
Discussion
Nicosulfuron behaviour in soil
Adsorption of nicosulfuron was low regardless of the agricultural management or depth, never exceeding 1.3 ± 2.0 L kg−1 (Table 2). The few studies on nicosulfuron available also generally reported low sorption, with sorption coefficients (Kd or Kf, with n usually close to 1) ranging from 0.02 to 1.8 L kg−1 in soils from the USA, Brazil, Argentina and Europe (EFSA 2008; Regitano and Koskinen 2008; Azcarate et al. 2015). To our knowledge, only two studies reported relatively high values of nicosulfuron Kf : up to 8.8 in a silt-loam soil in Iowa, USA (Gonzalez and Ukrainczyk 1996) and up to 16.1, in volcanic ash-derived soils, in Chile (Caceres-Jensen et al. 2020). Nicosulfuron is a weak acid (pKa of 4.78 and 7.58; EFSA 2008), which results in a mostly ionic form in most agricultural soils and thus little interaction with soil components, which can explain the low sorption. Nicosulfuron sorption was correlated with soil organic carbon content (r = − 0.03) in either soil type, as suggested by the KOC values; this result agrees with a previous study (Olivera et al. 2001). Ukrainczyk and Rashid (1995), however, reported that nicosulfuron sorption may depend instead on clay content, especially smectites, on which it could be rapidly and irreversibly sorbed. Such high sorption of nicosulfuron could strongly decrease leaching to groundwater (Gonzalez and Ukrainczyk 1999). In our study, however, nicosulfuron sorption was not correlated with clay content (r = 0.25). Caceres-Jensen et al. (2020) also reported irreversibility of the nicosulfuron sorption, and this could be related to sorption mechanisms, specific to volcanic soils. In the same soil type as in the present study, we recently showed a high potential for nicosulfuron leaching up to 30 cm in undisturbed soil columns (Cueff et al. 2020).
Large amounts of nicosulfuron residues were still available in the water-soluble fraction at the end of incubation, especially in the deep soil layers, which probably resulted from the combination of low sorption and low mineralisation. Based on previous studies, nicosulfuron often mineralised more than other pesticides, such as acifluorfen, bentazon and chlorimuron, at an equivalent depth and incubation time (Reddy et al. 1995; Gaston et al. 1996; Gaston and Locke 2000). Boivin et al. (2004) reported that only 11% of applied bentazon (with low adsorption and a half-life similar to that of nicosulfuron) mineralised after 160 days in a Stagnic Luvisol (north-eastern France). Thus, in a similar soil type and with a longer incubation period, the mineralised fraction of bentazon was one-third that of nicosulfuron. We also identified strong and rapid formation of NER in both soils, which represented the main dissipation pathway for nicosulfuron. NER likely originated from biodegradation of nicosulfuron rather than from physical or chemical bonding given the low interaction of nicosulfuron with the soil matrix. It has been suggested for several pesticides that most NER have a biogenic origin (Nowak et al. 2011, 2013), as suggested by the relatively strong correlation (r = 0.62) between the first-order rate constant of nicosulfuron mineralisation (bN) and the modelled final cumulative NER fraction (aNER). Despite uncertainties about the reversibility of this dissipation process, NER formation is generally assumed to decrease the bioavailability of pesticides for a long time (Barriuso et al. 2008; Schäffer et al. 2018). Several studies indicated that most sulfonylurea herbicides had high potential to contaminate groundwater (Martins and Mermoud 1999; Sondhia 2009; Azcarate et al. 2015). Nicosulfuron’s relatively low application rate (< 100 g ha−1) and relatively rapid dissipation by NER formation and mineralisation in surface layers could indicate little risk of transfer to groundwater. However, the low sorption in surface layers and slower dissipation in deeper layers suggest that groundwater contamination cannot be excluded, especially in situations of preferential transport.
Influence of soil type on nicosulfuron behaviour
We studied the fate of nicosulfuron at several depths in two soil types. Despite a strong decrease in microbial activity and biomass with depth, nicosulfuron mineralisation varied little in the 0–60-cm layer but was lower below 60 cm. Observing mineralisation in the 25–60-cm layer similar to that in surface layers in both soil types was surprising, as mineralisation of several pesticides has been observed to decrease with depth in agricultural soils, in particular under the plough layer (0–30 cm) (Larsen et al. 2000; Rodríguez Cruz et al. 2008). Pesticide mineralisation is generally assumed to be a function of soil organic carbon content, which influences microbial activity and biomass (Holden and Fierer 2005). However, in our study, nicosulfuron mineralisation was not correlated with microbial biomass (r = 0.09) or microbial activity (r = 0.02).
Differences between microbial activity and pesticide mineralisation can be due to development of a microbial community that specialises in degrading a regularly applied pesticide. Several microbial strains that can degrade nicosulfuron directly have been identified in the literature (Lu et al. 2012; Zhang et al. 2012; Song et al. 2013; Zhao et al. 2015; Carles et al. 2017). Higher mineralisation potential in soil previously exposed to a given pesticide has already been observed for several pesticides (Sørensen and Aamand 2003; Zablotowicz et al. 2006). Although this phenomenon has yet to be observed for nicosulfuron, specific degradation is a likely hypothesis to explain the nicosulfuron mineralisation in our study, since it was applied regularly to the studied fields.
Influence of agricultural management
In both soil types, higher microbial activity was measured in the topsoil layer (0–10 cm) in the CONS plot than in the TILL plot and in the deeper soil layers. As mentioned by several authors, this difference in microbial activity probably resulted from accumulation of organic carbon at the soil surface in untilled soils (Kandeler et al. 1999). Despite much higher carbon content at the VER site and similar microbial biomass at both sites, microbial activity (illustrated here by soil carbon mineralisation) appeared to be much higher at the LUV site. At the LUV site, total mineralisation was strongly correlated with organic carbon (r = 0.90) and microbial biomass (r = 0.93). At the VER site, however, the correlations were much weaker (non-significant for organic carbon and r = 0.66 for microbial biomass), which may indicate high organo-mineral interactions at the VER site that limited microbial access to carbon for mineralisation (Dungait et al. 2012). Conservation agriculture generally favours these interactions (Veloso et al. 2019).
Although surface layers had higher microbial activity and biomass, nicosulfuron mineralisation in them was no higher than that in the 25–60-cm layer in the CONS plot at both sites. Lower pesticide mineralisation in the topsoil under conservation agriculture than in deeper layers, or than in topsoil layers under conventional agriculture, is generally attributed to a decrease in bioavailability. This decrease is related to higher sorption of pesticides due to an increase in organic carbon content at the soil surface and the presence of crop residues that can intercept pesticides and reduce their availability to degrading microorganisms (Reddy and Locke 1998; Zablotowicz et al. 2000; Rodríguez Cruz et al. 2006; Alletto et al. 2010). Although we observed low sorption of nicosulfuron in the soil layers studied, the higher methanol-extractable fraction in the surface layers in CONS plots than in TILL plots from the beginning of incubation could indicate a lower nicosulfuron availability in the soil solution, thus limiting its mineralisation by microorganisms.
The NER fraction was always the largest dissipation pathway for nicosulfuron, especially under conservation agriculture, while the water-extractable fraction was always lower than that under conventional agriculture. In particular, a large water-extractable fraction was observed in the 25–60-cm layer in the TILL plot at the LUV site. This suggested low dissipation (through mineralisation and NER formation) of nicosulfuron, even 3 months after application. If nicosulfuron reaches this depth under field conditions, the TILL plot is likely to leach more of it than the CONS plot. At the LUV site, the CONS plot’s formation of more NER, greater potential for nicosulfuron mineralisation in the soil profile (0–60 cm) and lower water-available fractions suggest that conservation agriculture may have lower risk of nicosulfuron leaching than conventional agriculture. As mentioned, however, this risk may still exist under preferential flow. Nevertheless, the VER site, due to similarities in nicosulfuron behaviour of the two types of management, conventional agriculture did not exhibit higher risks than conservation agriculture.
Conclusion
We studied sorption and degradation processes of nicosulfuron in two soil types from south-western France managed under either conservation or conventional agriculture. Sorption and incubation experiments were performed under controlled laboratory conditions using 14C-nicosulfuron and used soil samples collected at several depths in the two soil types under both types of agricultural management. Little was known about the environmental behaviour of nicosulfuron, especially under contrasting types of agricultural management. Our results confirmed the low sorption affinity of nicosulfuron for soil components, which resulted in no significant difference in its sorption behaviour between the two types of agricultural management studied. Nicosulfuron mineralisation was influenced by mainly soil depth. For both soil types and types of agricultural management, the 25–60-cm layer had the same mineralisation as that in the topsoil layers but much lower microbial activity, suggesting development of a specific nicosulfuron-degrading microbial community. Formation of non-extractable residues is one of the main nicosulfuron dissipation pathways, especially for conservation agriculture systems. For these reasons, little nicosulfuron may be transferred to groundwater under conservation agriculture, especially in a Stagnic Luvisol. However, due to a generally higher proportion of macropores in these systems, the risk of preferential flow cannot be ignored.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
AFNOR (1995) NF ISO 10694: Determination of organic and total carbon after dry combustion (elementary analysis)
Alletto L, Coquet Y, Benoit P, Heddadj D, Barriuso E (2010) Tillage management effects on pesticide fate in soils. Agron. Sustain. Dev. 30:367–400
Alletto L, Benoit P, Bolognési B, Couffignal M, Bergheaud V, Dumény V, Longueval C, Barriuso E (2013) Sorption and mineralisation of S-metolachlor in soils from fields cultivated with different conservation tillage systems. Soil Tillage Res. 128:97–103. https://doi.org/10.1016/j.still.2012.11.005
Arrouays D, Baize D, Hardy M, Le Lay C (1992) Les sols de touyas issus d’alluvions anciennes des gaves pyrénéens : veracrisols. Intégration au Référentiel Pédologique. Sci. du Sol 30:227–247
Azcarate MP, Montoya JC, Koskinen WC (2015) Sorption, desorption and leaching potential of sulfonylurea herbicides in Argentinean soils. J. Environ. Sci. Heal Part B 50:229–237. https://doi.org/10.1080/03601234.2015.999583
Azcarate MP, Papiernik SK, Montoya JC, Koskinen WC (2018) Sorption-desorption of rimsulfuron, nicosulfuron, and metabolites in soils from Argentina and the USA. Agric. Environ. Lett. 3:1–5. https://doi.org/10.2134/ael2018.09.0048
Barriuso E, Calvet R, Schiavon M, Soulas G (1996) Les pesticides et les polluants organiques des sols. Transformations et dissipation. Etude Gest. des sols 3:279–296
Barriuso E, Benoit P, Dubus IG (2008) Formation of pesticide nonextractable (bound) residues in soil: magnitude, controlling factors and reversibility. Environ. Sci. Technol. 42:1845–1854
Bending GD, Rodríguez-Cruz MS (2007) Microbial aspects of the interaction between soil depth and biodegradation of the herbicide isoproturon. Chemosphere 66:664–671. https://doi.org/10.1016/j.chemosphere.2006.07.099
Boivin A, Cherrier R, Perrin-Ganier C, Schiavon M (2004) Time effect on bentazone sorption and degradation in soil. Pest Manag. Sci. 60:809–814. https://doi.org/10.1002/ps.889
Caceres-Jensen L, Rodriguez-Becerra J, Escudey M, Joo-Nagata J, Villagra CA, Dominguez-Vera V, Neira-Albornoz A, Cornejo-Huentemilla M (2020) Nicosulfuron sorption kinetics and sorption/desorption on volcanic ash-derived soils: proposal of sorption and transport mechanisms. J. Hazard. Mater. 385:121576. https://doi.org/10.1016/j.jhazmat.2019.121576
Carles L, Joly M, Bonnemoy F, Leremboure M, Batisson I, Besse-Hoggan P (2017) Identification of sulfonylurea biodegradation pathways enabled by a novel nicosulfuron-transforming strain Pseudomonas fluorescens SG-1: toxicity assessment and effect of formulation. J. Hazard. Mater. 324:184–193. https://doi.org/10.1016/j.jhazmat.2016.10.048
Clay SA, Koskinen WC (2003) Effect of variability of soil properties as a function of depth on pesticide sorption-desorption. In: terrestrial field dissipation studies: purpose, design, and interpretation; Arthur, E.L., Barefoot, A.C., Clay, V.E., Eds.; ACS Symposium Series. American Chemical Society, Washington, DC, pp 102–116
Cueff S, Alletto L, Bourdat-Deschamps M, Benoit P, Pot V (2020) Water and pesticide transfers in undisturbed soil columns sampled from a Stagnic Luvisol and a Vermic Umbrisol both cultivated under conventional and conservation agriculture. Geoderma 377:377. https://doi.org/10.1016/j.geoderma.2020.114590
Doran JW (1980) Soil microbial and biochemical changes associated with reduced tillage. Soil Sci. Soc. Am. J. 44:765–771
Dungait JAJ, Hopkins DW, Gregory AS (2012) Soil organic matter turnover is governed by accessibility not recalcitrance. Glob. Chang. Biol. 18:1781–1796. https://doi.org/10.1111/j.1365-2486.2012.02665.x
Đurović R, Gajić-Umiljendić J, Đorđević T (2009) Effects of organic matter and clay content in soil on pesticide adsorption processes. Pestic. i fitomedicina 24:51–57
Dyson JS, Beulke S, Brown CD, Lane MCG (2002) Adsorption and degradation of the weak acid mesotrione in soil and environmental fate implications. J. Environ. Qual. 31:613–618
European Food Safety Authority (EFSA) (2008) Conclusion regarding the peer review of the pesticide risk assessment of the active substance nicosulfuron. EFSA Journal 6(1):RN–120, 91. https://doi.org/10.2903/j.efsa.2008.120r
FAO (2016) Conservation agriculture, in: save and grow in practice. Maize, Rice and Wheat
Fomsgaard IS (1995) Degradation of pesticides in subsurface soils, unsaturated zone-a review of methods and results. Int. J. Environ. Anal. Chem. 58:231–245. https://doi.org/10.1080/03067319508033127
Gaston LA, Locke MA (2000) Acifluorfen sorption, degradation, and mobility in a Mississippi delta soil. Soil Sci. Soc. Am. J. 64:112–121
Gaston LA, Locke MA, Zablotowicz RM (1996) Sorption and degradation of bentazon in conventional-and no-till Dundee soil. J. Environ. Qual. 25:120–126
Gaston LA, Boquet DJ, Bosch MA, Fluometuron NNN (2003) Fluometuron sorption and degradation in cores of silt loam soil from different tillage and cover crop systems. Soil Sci. Soc. Am. J. 67:747–755
Gaston L, Locke M, Mcdonald J, Dodla S, Liao L, Putnam L, Udeigwe T (2007) Effects of tillage on norflurazon sorption, degradation and mobility in a mississippi delta soil. Soil Sci. 172:534–545. https://doi.org/10.1097/SS.0b013e318053dbb8
Gonzalez JM, Ukrainczyk L (1996) Adsorption and desorption of nicosulfuron in soils. J. Environ. Qual. 25:1186–1192. https://doi.org/10.2134/jeq1996.00472425002500060003x
Gonzalez J, Ukrainczyk L (1999) Transport of nicosulfuron in soil columns. J. Environ. Qual. 28:101–107. https://doi.org/10.2134/jeq1999.00472425002800010011x
Hobbs PR, Sayre K, Gupta R (2008) The role of conservation agriculture in sustainable agriculture. Philos. Trans. R. Soc. B Biol. Sci. 363:543–555. https://doi.org/10.1098/rstb.2007.2169
Holden PA, Fierer N (2005) Microbial processes in the vadose zone. Vadose Zo. J. 4:1–21
Holland JM (2004) The environmental consequences of adopting conservation tillage in Europe : reviewing the evidence. Agric. Ecosyst. Environ. 103:1–25. https://doi.org/10.1016/j.agee.2003.12.018
IUSS Working Group WRB (2007) World Reference Base For Soil Resources 2006, first update. A framework for international classification, correlation and communication. FAO, Food and Agriculture Organization of the United Nations, Rome
Kah M, Beulke S, Brown CD (2007) Factors influencing degradation of pesticides in soil. J. Agric. Food Chem. 55:4487–4492. https://doi.org/10.1021/jf0635356
Kandeler E, Tscherko D, Spiegel H (1999) Long-term monitoring of microbial biomass, N mineralisation and enzyme activities of a Chernozem under different tillage management. Biol. Fertil. Soils 28:343–351
Kay BD, VandenBygaart AJ (2002) Conservation tillage and depth stratification of porosity and soil organic matter. Soil Tillage Res. 66:107–118
Klute (1986) Water retention: laboratory methods. Methods of soil analysis: Part 1 Physical and mineralogical methods 5:635–662
Larsen L, Sørensen SR, Aamand J (2000) Mecoprop, isoproturon, and atrazine in and above a sandy aquifer: vertical distribution of mineralization potential. Environ. Sci. Technol. 34:2426–2430. https://doi.org/10.1021/es9911723
Locke MA, Zablotowicz RM, Bauer PJ, Steinriede RW, Gaston LA (2005) Conservation cotton production in the southern United States: herbicide dissipation in soil and cover crops. Weed Sci. 53:717–727
Lu XH, Kang ZH, Tao B, Wang YN, Dong JG, Zhang JL (2012) Degradation of nicosulfuron by Bacillus subtilis YB1 and Aspergillus niger YF1. Appl. Biochem. Microbiol. 48:460–466. https://doi.org/10.1134/S0003683812050079
Mahía J, Martín A, Carballas T, Díaz-Raviña M (2007) Atrazine degradation and enzyme activities in an agricultural soil under two tillage systems. Sci. Total Environ. 378:187–194. https://doi.org/10.1016/j.scitotenv.2007.01.036
Martins JMF, Mermoud A (1999) Transport of rimsulfuron and its metabolites in soil columns. Chemosphere 38:601–616
Michel JC, Tessier D, Favrot JC (2003) Comportement et organisation de sols de Boulbène dans le Tarn - Quelques éléments sur le passage d’une structure prismatique à une structure vertique dans les horizons enrichis en argile. Etude Gest. des Sols 10:71–80
Nowak KM, Miltner A, Gehre M, Schäffer A, Kästner M (2011) Formation and fate of bound residues from microbial biomass during 2, 4-D degradation in soil. Environ. Sci. Technol. 45:999–1006
Nowak KM, Girardi C, Miltner A, Gehre M, Schäffer A, Kästner M (2013) Contribution of microorganisms to non-extractable residue formation during biodegradation of ibuprofen in soil. Sci. Total Environ. 445–446:377–384. https://doi.org/10.1016/j.scitotenv.2012.12.011
Obour AK, Mikha MM, Holman JD, Stahlman PW (2017) Changes in soil surface chemistry after fi fty years of tillage and nitrogen fertilization. Geoderma 308:46–53. https://doi.org/10.1016/j.geoderma.2017.08.020
Okada E, Costa JL, Bedmar F (2019) Glyphosate dissipation in different soils under no-till and conventional tillage. Pedosphere 29:773–783. https://doi.org/10.1016/S1002-0160(17)60430-2
Olivera RS, Koskinen WC, Ferreira FA (2001) Sorption and leaching potential of herbicides on Brazilian soils. Weed Res. 41:97–110
Poppell CA, Hayes RM, Mueller TC (2002) Dissipation of nicosulfuron and rimsulfuron in surface soil. J. Agric. Food Chem. 50:4581–4585. https://doi.org/10.1021/jf020172a
PPDB (2020) PPDB - an international database for pesticide risk assessments and management. Hum. Ecol. Risk Assess. An Int. J. 22:1050–1064. https://doi.org/10.1080/10807039.2015.1133242
Reddy KN, Locke MA (1998) Sulfentrazone sorption, desorption, and mineralization in soils from two tillage systems. Weed Sci. 46:494–500
Reddy KN, Zablotowicz RM, Locke MA (1995) Chlorimuron adsorption, desorption, and degradation in soils from conventional tillage and no-tillage systems. J. Environ. Qual. 24:760–767
Référentiel Pédologique (2008) Association française pour L'étude des Sols. Baize and Girard, coordinators. Ed. Quae
Regitano JB, Koskinen WC (2008) Characterization of nicosulfuron availability in aged soils. J. Agric. Food Chem. 56:5801–5805
Rodríguez Cruz MS, Jones JE, Bending GD (2006) Field-scale study of the variability in pesticide biodegradation with soil depth and its relationship with soil characteristics. Soil Biol. Biochem. 38:2910–2918. https://doi.org/10.1016/j.soilbio.2006.04.051
Rodríguez Cruz MS, Jones JE, Bending GD (2008) Study of the spatial variation of the biodegradation rate of the herbicide bentazone with soil depth using contrasting incubation methods. Chemosphere 73:1211–1215. https://doi.org/10.1016/j.chemosphere.2008.07.044
Sadegh-Zadeh F, Wahid SA, Jalili B (2017) Sorption, degradation and leaching of pesticides in soils amended with organic matter: a review. Adv. Environ. Technol. 3:119–132. https://doi.org/10.22104/AET.2017.1740.1100
Schäffer A, Kästner M, Trapp S (2018) A unified approach for including non-extractable residues (NER) of chemicals and pesticides in the assessment of persistence. Environ. Sci. Eur. 30:1–14. https://doi.org/10.1186/s12302-018-0181-x
Shipitalo MJ, Dick WA, Edwards WM (2000) Conservation tillage and macropore factors that affect water movement and the fate of chemicals. Soil Tillage Res. 53:167–183
Sondhia S (2009) Leaching behaviour of metsulfuron in two texturally different soils 111–115. https://doi.org/10.1007/s10661-008-0381-8
Song J, Gu J, Zhai Y, Wu W, Wang H, Ruan Z, Shi Y, Yan Y (2013) Biodegradation of nicosulfuron by a Talaromyces flavus LZM1. Bioresour. Technol. 140:243–248. https://doi.org/10.1016/j.biortech.2013.02.086
Sørensen SR, Aamand J (2003) Rapid mineralisation of the herbicide isoproturon in soil from a previously treated Danish agricultural field. Pest Manag. Sci. 59:1118–1124. https://doi.org/10.1002/ps.739
Thomas GA, Dalal RC, Standley J (2007) No-till effects on organic matter, pH, cation exchange capacity and nutrient distribution in a Luvisol in the semi-arid subtropics. Soil Tillage Res. 94:295–304. https://doi.org/10.1016/j.still.2006.08.005
Thompson CA, Whitney DA (2000) Effects of 30 years of cropping and tillage systems on surface soil test changes. Commun. Soil Sci. Plant Anal. 31:241–257. https://doi.org/10.1080/00103620009370433
Ukrainczyk L, Rashid N (1995) Irreversible sorption of nicosulfuron on clay minerals. J Agric. Food Chem. 43:855–857. https://doi.org/10.1021/jf00052a001
Veloso MG, Cecagno D, Bayer C (2019) Legume cover crops under no-tillage favor organomineral association in microaggregates and soil C accumulation. Soil Tillage Res. 190:139–146. https://doi.org/10.1016/j.still.2019.03.003
Wahl NA, Bens O, Buczko U, Hangen E, Hüttl RF (2004) Effects of conventional and conservation tillage on soil hydraulic properties of a silty-loamy soil. Phys. Chem. Earth 29:821–829. https://doi.org/10.1016/j.pce.2004.05.009
Wu J, Joergensen RG, Pommerening B, Chaussod R, Brokkes PC (1990) Measurment of soil microbial biomass C by fumigation-extraction - an automated procedure. Soil Biol. Biochem 22:1167–1169
Zablotowicz RM, Locke MA, Gaston LA, Bryson CT (2000) Interactions of tillage and soil depth on fluometuron degradation in a Dundee silt loam soil. Soil Tillage Res. 57:61–68
Zablotowicz RM, Weaver MA, Locke MA (2006) Microbial adaptation for accelerated atrazine mineralization/degradation in Mississippi Delta soils. Weed Sci. 54:538–547
Zablotowicz RM, Locke MA, Gaston LA (2007) Tillage and cover effects on soil microbial properties and fluometuron degradation. Biol. Fertil. Soils 44:27–35. https://doi.org/10.1007/s00374-007-0175-0
Zhang H, Mu W, Hou Z, Wu X, Zhao W, Zhang X, Pan H, Zhang S (2012) Biodegradation of nicosulfuron by the bacterium Serratia marcescens N80. J. Environ. Sci. Heal. 47:153–160. https://doi.org/10.1080/03601234.2012.632249
Zhao W, Wang C, Xu L, Zhao C, Liang H, Qiu L (2015) Biodegradation of nicosulfuron by a novel Alcaligenes faecalis strain ZWS11. J. Environ. Sci. 35:151–162. https://doi.org/10.1016/j.jes.2015.03.022
Acknowledgements
We thank Déborah Mouquet, Yolaine Delaunay, Valérie Bergheaud, Marjolaine Bourdat-Deschamps, Valentin Serre, Nathalie Bernet and Ghislaine Delarue for helping with the experiment.
Funding
This study was financially supported by the Agence de l’Eau Adour-Garonne and Occitanie Region (BAG’AGES and BAG’AGES CISOL projects).
Author information
Authors and Affiliations
Contributions
SC, LA, PB and VP conceived and designed research. SC and VD conducted the chemical analysis. SC, LA, PB and VP wrote the manuscript. All the authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing interests
Ethical approval and consent to participate
Not applicable
Consent to publish
Not applicable
Additional information
Responsible editor: Kitae Baek
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cueff, S., Alletto, L., Dumény, V. et al. Adsorption and degradation of the herbicide nicosulfuron in a stagnic Luvisol and Vermic Umbrisol cultivated under conventional or conservation agriculture. Environ Sci Pollut Res 28, 15934–15946 (2021). https://doi.org/10.1007/s11356-020-11772-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-11772-2