Abstract
The ethanol extracts of Gracilaria lemaneiformis that have inhibitory effects on Karenia mikimotoi and Skeletonema costatum were separated by liquid-liquid extraction using different polar solvents into five fractions with antialgal activities (petroleum ether, chloroform, ethyl acetate, n-butanol, and water-soluble fractions). These fractions were chromatographed on silica gel to give, after repeated preparative thin-layer chromatography (PTLC) purification processes, 1-β-d-ribofuranosyluracil (1), 3-hydroxymethyl-pyrrolopiperazine-2,5-dione (2), benzene-1,2-propanoic acid (3), 1-O-palmitoyl-2-O-palmitoleoyl-3-O-β-d-galactopyranosyl glycerol (4), 7-oxabicyclo[4.1.0]-heptan-3-ol (5), linoleic acid (6), 3,4-dimethoxy-6-(methoxymethyl)-tetrahydro-2H-pyran-2,5-diol (7), and 3,7,11,16-tetramethyl -2-heptadecen-1-ol (8). Five of them, natural products 1, 2, 5, 7, and 8, were isolated from Gracilaria lemaneiformis for the first time, and three natural products (3, 5, and 8) were isolated from marine macroalgae for the first time. Among them, natural products (1, 2, 3, 4, and 6) showed the most obvious inhibition activities to the growth of Karenia mikimotoi and Skeletonema costatum at the concentration of 80 μg/mL. Therefore, antialgal activities of these five natural products against Amphidinium carterae, Heterosigma akashiwo, Karenia mikimotoi, Phaeocystis globosa, Prorocentrum donghaiense, and Skeletonema costatum were further tested at different concentrations (0.4, 2, 10, and 50 μg/mL). This was the first report of antialgal activities of five natural products (1, 2, 3, 4, and 6) to these six red tide microalgae. They showed significantly selective antialgal activities against all tested red tide microalgae. At the concentration of 50 μg/mL, the growth of Amphidinium carterae, Heterosigma akashiwo, Karenia mikimotoi, and Phaeocystis globosa was obviously inhibited; for Karenia mikimotoi, natural products 1, 2, and 6 have significant antialgal activities; the growth inhibition of Skeletonema costatum that was exposed to natural products 1, 3, and 4 was remarkable. Furthermore, by analyzing and comparing EC50–96 h values, it has been determined that natural product 3 (natural product 4) showed the superior application potential than potassium dichromate and some reported natural products (such as gossonorol isolated from Porphyra yezoensis, trehalose purified from Ulva pertusa) as a characteristic antialgal agent against Amphidinium carterae (Phaeocystis globosa). In addition, natural products 1 and 3 also showed good superiority than some reported natural products in inhibiting Skeletonema costatum; however, it was a pity that they were inferior to potassium dichromate in the inhibiting this red tide microalgae. Taken together, it is not hard to conclude that Gracilaria lemaneiformis was a good source of natural products with antialgal activities against some red tide microalgae.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Inhibitory effects of marine macroalgae on the growth of marine microalgae have been studied for more than 60 years (Alamsjah et al. 2005; Anderson et al. 1996; Fltcher 1975; Lee and Olsen 1985; Marshall and Orr 1949; McLachlan and Craigie 1964; Sun et al. 2018a, b, c), and have been fruitful especially in the past 30 years. The inhibition activities of some marine macroalgae against red tide microalgae, such as Alexandrium tamarense (Wang et al. 2007), Chattonella marina (Tang and Gobler 2011), Cochlodinium polykrikoides (Chowdhury et al. 2014; Jeong et al. 2000; Oh et al. 2010; Xu et al. 2005a, b), Heterosigma akashiwo (Alamsjah et al. 2005; Chowdhury et al. 2014; Hirao et al. 2012; Sun et al. 2016; Wang et al. 2009), Karenia mikimotoi (Nagayama et al. 2003; Sun et al. 2017, 2018a, b, c), Ostreopsis cf. ovata (Accoroni et al. 2015), Prorocentrum minimum (Li et al. 2007; Tang and Gobler 2011), Skeletonema costatum (An et al. 2008; Bie et al. 2011; Cui 2014; Gao et al. 2018; Jin 2005; Lu 2011), and other marine microalgae (Accoroni et al. 2015; Alamsjah et al. 2005; Ben Gharbia et al. 2017; Cai 2016; Cho et al. 1999; Konig et al. 1999; Manilal et al. 2010; El Hattab et al. 2015; Takedai et al. 2003; Tang and Gobler 2011; Tian 2009; Wang et al. 2007, 2012a, b; Wu 2016; Ye and Zhang 2013; Yu et al. 2010), have been reported. In our previous work, researches between marine macroalgae and red tide microalgae were analyzed using CiteSpace, and it was found that allelopathic interaction between them has been developed into an active research area during the last 20 years (Sun et al. 2019). Further, based on these reports have been published in the Web of Science, Springer, Google Scholar, and CNKI between 1990 and 2019, we also found that more than 120 species of marine macroalgae have antialgal activities against red tide microalgae (Fig. 1a), and red macroalgae in Rhodophyta have highest proportion among them. According to different orders of these red macroalgae, we summarize their classification and found that orders Gracilariales and Gigartinales have the most species with antialgal activities (Fig. 1b).
Gracilaria lemaneiformis, a red macroalgae belong to order Gracilariales, occurs naturally in coastal areas of Shandong Peninsula in northern China. Researchers have found a lot of solvent extracts from Gracilaria lemaneiformis have antimutagenic (Chen et al. 2005; Zhang et al. 2005), antitumor (Mei et al. 2006; Zhang et al. 2005), antioxidant (Chen et al. 2005, 2008), antialgal (Lu 2011; Sun et al. 2011), and other biological activities (Chen et al. 2007, 2008). In fact, antialgal effects of Gracilaria lemaneiformis on growth of Prorocentrum donghaiense and Alexandrium tamarense in co-culture were studied in early time (Wang et al. 2006). Also, Liu (2006) reported that antialgal interaction between Gracilaria lemaneiformis and three red tide microalgae (Chaetoceros curvisetus, Skeletonema costatum and Scrippsiella trochoidea). Shao et al. (2011) and Lei et al. (2010) showed the inhibitory effects of Gracilaria lemaneiformis on the growth of Heterosigma akashiwo and Prorocentrum micans. Methanol and aqueous extracts of Gracilaria lemaneiformis were found to inhibit the growth of Karenia mikimotoi and Skeletonema costatum (Sun et al. 2011). However, a few antialgal substances from Gracilaria lemaneiformis were structurally elucidated (Lu 2011; Sun et al. 2017). Lu (2011) reported that linoleic acid isolated from Gracilaria lemaneiformis was inhibitory allelochemical against Skeletonema costatum. Our group has already isolated gossonorol and other six compounds from Gracilaria lemaneiformis (Sun et al. 2017), for the interpretation of antialgal activities of methanol extracts of this marine macroalgae (Sun et al. 2011, 2012).
A previous experiment provided evidence for inhibitory effects of different solvent extracts (petroleum ether, chloroform, and ethyl acetate) from Gracilaria lemaneiformis on Karenia mikimotoi (Sun et al. 2012), but the nature of the antialgal active compound(s) was not investigated. Different extraction and isolation methods are likely to get different results; therefore, we were likely to find some compounds with antialgal activities that were different from those reported. Hopefully, present paper purified eight natural products, and six of them have not been characterized before. Based on that, this work was to evaluate their antialgal activities against six species of red tide microalgae (Amphidinium carterae, Heterosigma akashiwo, Karenia mikimotoi, Phaeocystis globosa, Prorocentrum donghaiense, and Skeletonema costatum) and obtain important parameters, e.g., EC50–96 h values for future practical HAB control. Gracilaria lemaneiformis was, assuredly, a rich source of natural products with antialgal activities. It can be used to develop antialgal inhibitor against red tide microalgae in future research.
Materials and methods
HAB algae and macroalgae
Amphidinium carterae, Heterosigma akashiwo, Karenia mikimotoi, Phaeocystis globosa, Prorocentrum donghaiense, and Skeletonema costatum were cultured aseptically in Guillard’s f/2 medium (Guillard and Ryther 1962), 20 °C, and 60 μmol photons/m2/s using fluorescent lamps with a 16/8 dark/light cycle.
Gracilaria lemaneiformis collected in July 2017 from the coast of Fujian, China, was purchased from a wholesaler, identified by Prof. Binlun Yan (Jiangsu Key Laboratory of Marine Biotechnology, Jiangsu Ocean University), and washed with a brush to carefully remove attached organisms. And then, these materials were cut into small pieces (ca. 2.0 cm of length) and freeze-dried. After freeze-drying treatment, these pieces were ground to make powder using a blender for 1 min.
Extraction, isolation, purification, and identification
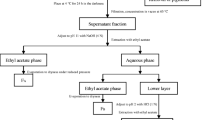
The milled sample (500 g) was extracted twice with 100% ethanol (2.5 L, 36 h, and 1.5 L, 12 h). The extracts were concentrated to 300 mL under reduced pressure in an evaporator and filtered through a Whatman GF/C filter. The filtrates were added to H2O (400 mL), stayed overnight in the refrigerator at 4 °C, and centrifuged for 10 min at 7000×g under low temperature. The supernatants were freeze-dried, and then, the alcoholic extracts of Gracilaria lemaneiformis (AEGL, 58.775 g) were obtained. The extracts (AEGL) were further isolated by the following methods (Fig. 2).
Brown mixed solution could be obtained by the leaching process of the extracts with a two-liquid phase solvent system (400 mL of petroleum ether/H2O (1:3, v:v)) at room temperature (20 °C) for 24 h in the darkness under agitation with mill speed of 30 r/min. The above leaching process was repeated three times. Extraction liquid was combined and filtered. And then, brown mixed solution was transferred to a 2000-mL separation funnel, shaken for 5 min, and allowed to stand for 30 min. Aqueous phase and petroleum ether phase were got, respectively. Petroleum ether phase was kept. Aqueous phase was evaporated to remove petroleum ether and pretreated using liquid-liquid extraction based on chloroform. This extraction was repeated three times. The resultant mixture was transferred to a 2000-mL separation funnel, shaken for 5 min, and allowed to stand for 30 min. Chloroform phase was retained, and upper layer was evaporated to remove chloroform to obtain aqueous phase. Then, 100 mL of ethyl acetate was added, and aqueous phase was extracted three times with ethyl acetate. Ethyl acetate phase was retained. The raffinate was evaporated to remove ethyl acetate, and 100 mL of n-butanol was added. This was followed by separation using a separation funnel and repeated three times. N-butanol phase and bottom layer were collected, respectively. In this way, five fractions (4.072 g petroleum ether fraction, 0.589 g chloroform fraction, 0.689 g ethyl acetate fraction, 9.85 g n-butanol fraction, and 16.58 g water-soluble fraction) were separated from the alcoholic extracts. All of the fractions were collected separately, evaporated to dryness, weighed, re-dissolved with ethanol (10 mg/mL approximately), and filtered through a 0.22-micron syringe filter as a stock solution before bioassays.
Isolation and purification of five fractions mentioned above were performed as follows. First, five fractions were applied to silica gel (200–300 mesh) column (3.0 × 100 cm) (mobile phase: chloroform/acetone/formic acid = 18:1:1 (v:v:v) and chloroform/methanol = 16:1 (v:v), alternatively), respectively. Ten microliter of sample was injected onto the top of the column and then eluted with eluent at a flow rate of 2.0 mL/min. The collection of the 10-mL sub-fraction was started immediately after the addition of eluent. Antialgal activities of sub-fractions were determined by a bioassay using Karenia mikimotoi and Skeletonema costatum, and then, they were pooled and concentrated, respectively. Secondly, sub-fractions with antialgal activities were respectively loaded on silica gel (100–200 mesh) column (1.5 × 50 cm) and washed with chloroform/acetone/formic acid (15:3:2, v:v:v) at a flow-rate of 1.0 mL/min. Collection of the 5-mL elution component was started immediately. Further, in turn, the active elution components were subjected to preparative TLC on silica gel G plates (500 μm, Merck) and collected according to Rf value under UV light (UV 254 nm). PTLC was repeated two times and carried out in repetitive way. Finally, active components were purified by Sephadex LH-20 column (1.0 × 30 cm) (mobile phase: methanol), respectively. Systemic solvent separation, two phase solvent extraction, silica gel column chromatography, PTLC and Sephadex LH-20 column chromatography methods were applied to eight natural products. Finally, each natural product was dissolved in ethanol at a concentration of 5 mg/mL, filtered through a 0.22-micron syringe filter, and assessed antialgal activity to tested red tide microalgae. At the same time, the structural identification of these natural products was carried out by comparison of HR-ESI-MS, 1H-NMR, and 13C-NMR with spectral data.
Growth assays
Crude extracts and multiple isolated constituents were monitored by a bioassay using Karenia mikimotoi and Skeletonema costatum. Natural seawater was obtained from the coast of Lianyungang, China, collected in precleaned polyethylene tanks and aged. And then aged natural seawater was filtered through an acid-cleaned 0.22 μm Millipore membrane filter.
Growth assays of AEGL
The growth inhibition of AEGL was evaluated by algal bioassays with Karenia mikimotoi and Skeletonema costatum on the method reported by Sun et al. (2016) with some modifications. One hundred microliters of the solvent-partitioned fraction with different concentrations was added to Erlenmeyer flasks containing 5 mL of algal inoculant and 44.9 mL of culture medium (initial concentration of fraction in suspensions of two microalgae: 400, 800, 1200 μg/mL). Controls received the same volume of ethanol. The initial cell numbers were set at 9 × 104 cells/mL for Karenia mikimotoi and 21 × 104 cells/mL for Skeletonema costatum. The tested red tide microalgae were cultured under 60 μmol photons/m2/s (light-dark = 16–8 h) at 20 °C for 10 days. There were four replicates for every treatment used in this experiment.
Growth assays of isolated constituents in the process of isolation and purification
Ten microliters of each isolated constituent was added to teat glass containing 0.5 mL of algal inoculant and 4.49 mL of culture medium (initial concentration of isolated constituent in suspensions of tested red tide microalgae 80 μg/mL); their growth effects on tested red tide microalgae (Karenia mikimotoi and Skeletonema costatum) were measured on the method reported by Sun et al. (2016) with some modifications. Controls received the same volume of ethanol. The initial cell numbers were set at 22 × 104 cells/mL for Karenia mikimotoi and 26 × 104 cells/mL for Skeletonema costatum. There were four replicates for every treatment used in this experiment, and the culture conditions were the same as mentioned above. This experiment lasted for 4 days.
The evaluation of antialgal activities of five natural products
Five natural products with antialgal activities were obtained from our research. Antialgal ability of each natural product (0.4, 2, 10, and 50 μg/mL) against six red tide microalgae (Amphidinium carterae, Heterosigma akashiwo, Karenia mikimotoi, Phaeocystis globosa, Prorocentrum donghaiense, and Skeletonema costatum) was measured. Potassium dichromate was used as a positive control. The culture conditions were conducted as described above. After 4-day culture, the cells were counted. On day 4, the growth inhibition of each natural product against tested red tide microalgae was determined according to the method mentioned in our previous work (Sun et al. 2012). EC50–96 h was calculated as the method reported by Marklund and Marklun (1974).
Data process and statistical analysis
Cell numbers of red tide microalgae were counted by hemocytometer. All the data of the growth assays in this study were analyzed by ANOVA and Tukey’s test.
Results
Solvent fractions of AEGL from Gracilaria lemaneiformis
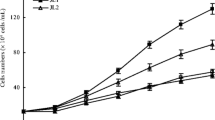
AEGL (alcoholic extracts of Gracilaria lemaneiformis) showed significant (P < 0.05) inhibitory activities against the growth of Karenia mikimotoi and Skeletonema costatum, and the inhibitory effects to these two red tide microalgae were above 50% at the concentration of 800 μg/mL on day 10 (Fig. 3). Further, five fractions are gained in AEGL by using systematical solvent separation method. Among all these fractions, the dark brown water-soluble fraction (WS, 16.58%) showed the highest yield followed by deep yellow n-butanol fraction (BN, 9.85%), invisible green petroleum ether fraction (PE, 6.93%), the yellowy ethyl acetate fraction (EA, 0.689%), and the reseda chloroform fraction (CF, 0.589%). Their inhibitory effects on the growth of Karenia mikimotoi and Skeletonema costatum are shown in Table 1. Results exhibited that they restrained these two tested red tide microalgae and inhibitory activities of BN and WS against Karenia mikimotoi and Skeletonema costatum were stronger than those of other three fractions. Then, isolation and purification of the above-mentioned five fractions were conducted.
Isolation, purification, and identification
PE (3.0 g) and CF (0.4 g) were respectively eluted through silica gel column chromatography with mixtures of chloroform/acetone/formic acid (18:1:1, v:v:v) and further purified by PTLC (twice, mobile phase: chloroform/acetone/formic acid (15:3:2, v:v:v)) to yield three samples with antialgal activities: FPE11 (0.232 g), FCF11 (0.024 g), and FCF21 (0.025 g) (Table 2).
EA (0.4 g), BN (5 g), and WS (5 g) were respectively subjected to silica gel column chromatography using chloroform/methanol (16:1, v:v) as eluent and PTLC (twice, mobile phase: chloroform/acetone/formic acid (15:3:2, v:v:v) to yield five samples with antialgal activities: FEA21 (0.012 g), FEA31 (0.017 g), FEA32 (0.062 g), FBN11 (0.713 g), and FWS11 (0.384 g) (Table 2).
All eight samples showed antialgal activities against Karenia mikimotoi and Skeletonema costatum. The difference was as follows: FPE11, FCF11, FCF21, FEA21, and FEA32 significantly inhibited the growth of these two test red tide microalgae, and other three samples only have weak antialgal activities against Karenia mikimotoi and Skeletonema costatum (Table 2). Therefore, these eight samples were finally purified through Sephadex LH-20 column chromatography to obtain eight natural products with antialgal activities, namely FPE111 (0.218 g, yield 0.077%, dw basis of AEGL), FCF111 (0.017 g, yield 0.043%), FCF211 (0.018 g, yield 0.063%), FEA211 (0.009 g, yield 0.037%), FEA311 (0.009 g, yield 0.015%), FEA321 (0.051 g, yield 0.105%), FBN111 (0.685 g, yield 2.165%), and FWS111 (0.363 g, yield 2.014%) (Table 2).
On the basis of the above experiments, the structures of eight natural products were identified. FPE111, FCF111, FCF211, FEA211, FEA311, FEA321, FBN111, and FWS111 were as follows: 1-β-d-ribofuranosyluracil (1), 3-hydroxymethyl-pyrrolopiperazine-2,5-dione (2), benzene-1,2-propanoic acid (3), 1-O-Palmitoyl-2-O-palmitoleoyl-3-O-β-d-galactopyranosyl glycerol (4), 7-Oxabicyclo[4.1.0]-heptan-3-ol (5), linoleic acid (6), 3,4-dimethoxy-6-(methoxymethyl)-tetrahydro-2H-pyran-2,5-diol (7), and 3,7,11,16-tetramethyl-2-heptadecen-1-ol (8). Their NMR spectroscopic data are listed in Tables 3, 4, 5, and 6. Due to these, eight natural products were known compounds, so the specific process of structural identification was omitted in this paper. And the structures of these natural products are shown in Fig. 4.
Overall, eight natural products with antialgal activities were isolated from Gracilaria lemaneiformis. And among them, five natural products 1, 2, 3, 4, and 6 have higher inhibitory activities against Karenia mikimotoi and Skeletonema costatum at the concentration of 80 μg/mL. So far, there was no study on inhibition activities of these five natural products against red tide microalgae. Thus, antialgal activities of natural products 1, 2, 3, 4, and 6 were tested against the common red tide microalgae Amphidinium carterae, Heterosigma akashiwo, Karenia mikimotoi, Phaeocystis globosa, Prorocentrum donghaiense, and Skeletonema costatum in this work.
Antialgal activity evaluation of five natural products against six red tide microalgae
Amphidinium carterae, Heterosigma akashiwo, Karenia mikimotoi, Phaeocystis globosa, Prorocentrum donghaiense, and Skeletonema costatum were employed for the inhibition tests. In Figs. 5a, b, d, and e, inhibition effects of five natural products (1, 2, 3, 4, and 6) on Amphidinium carterae, Heterosigma akashiwo, Phaeocystis globosa, and Prorocentrum donghaiense grow more and more with their increasing concentration (0.4~50 μg/mL). There were significant (P < 0.05) differences between cell numbers of control groups and treated groups with added five natural products. At 50 μg/mL, all natural products for 50% inhibition of growth of these four red tide microalgae were found (Fig. 6b). However, among them, only natural products 1 and 2 significantly (P < 0.05) restrained Karenia mikimotoi (Fig. 5c) and growth inhibition of these two natural products to this red tide microalgae was 53.4% and 76.1%, respectively (Fig. 6b); in addition to natural products 2 and 6, other three natural products strongly (P < 0.05) inhibited Skeletonema costatum (Figs. 5f and 6b). These five natural products have selective inhibitory activities against tested six red tide microalgae.
(a) Effects of natural products (1, 2, 3, 4, and 6) on the growth of Amphidinium carterae. (b) Effects of natural products (1, 2, 3, 4, and 6) on the growth of Heterosigma akashiwo. (c) Effects of natural products (1, 2, 3, 4, and 6) on the growth of Karenia mikimotoi. (d) Effects of natural products (1, 2, 3, 4, and 6) on the growth of Prorocentrum donghaiense. (e) Effects of natural products (1, 2, 3, 4, and 6) on the growth of Phaeocystis globosa. (f) Effects of natural products (1, 2, 3, 4, and 6) on the growth of Skeletonema costatum.
Antialgal ability of potassium dichromate against six species of red tide microalgae increased significantly (P < 0.05) with its increasing concentration (Fig. 6a). At 16 μg/mL, growth inhibition of potassium dichromate on Amphidinium carterae, Heterosigma akashiwo, Karenia mikimotoi, Phaeocystis globosa, Prorocentrum donghaiense, and Skeletonema costatum was 83.5%, 42.9%, 48.9%, 39.7%, 76.9%, and 95.0%, respectively.
Based on antialgal activity evaluation, EC50–96 h values of five natural products abovementioned for six red tide microalgae were determined for the first time (Table 7). In OECD (1984), EC50–96 h value of tested compound could directly indicate its toxicity to the test algae, namely, EC50–96 h value was in the range < 1 μg/mL, 1~10 μg/mL, 10~100 μg/mL, and > 100 μg/mL; the corresponding toxicity was extremely high toxic, high toxic, medium toxicity, and low toxicity, respectively. And potassium dichromate was used as positive control in algae test standard according to the recommendation of this reference. Advantages of algal inhibition activities of potassium dichromate and five natural products were analyzed, by comparing their toxicity and EC50–96 h values for each tested red tide microalgae, in order to select more potential compounds from them in further application. According to these standard mentioned above (OECD 1984), for Amphidinium carterae, potassium dichromate, natural products 2 and 3 exhibited high toxicities, and other three natural products showed medium toxicities. And EC50–96 h value of natural product 3 for this microalgae was significant (P < 0.05) lower than that of potassium dichromate; EC50–96 h values of five natural products for Heterosigma akashiwo and Phaeocystis globosa were significant (P < 0.05) less than those of potassium dichromate. Among them, other natural products showed high toxicities for Heterosigma akashiwo except natural product 1 and potassium dichromate, four natural products exhibited high toxicities for Phaeocystis globosa besides natural product 5 and potassium dichromate which only showed medium toxicity; natural products 2, 4, and 6 had high toxicities for Karenia mikimotoi, and their EC50–96 h values were significant (P < 0.05) lower than those of potassium dichromate; natural product 2 and potassium dichromate for Prorocentrum donghaiense, natural products 1, 3, 4, and potassium dichromate for Skeletonema costatum were high toxic; and other natural products for these two microalgae were medium or low toxic. It was a pity that EC50–96 h values of these five natural products for Prorocentrum donghaiense and Skeletonema costatum were significant (P < 0.05) higher than those of potassium dichromate. Based on the above analysis, we obtained several natural products with demonstrably antialgal superior than potassium dichromate against Amphidinium carterae, Heterosigma akashiwo, Karenia mikimotoi, and Phaeocystis globosa. These results were as follows: natural product 3 to Amphidinium carterae; natural products 1, 2, 3, 4, and 6 for Heterosigma akashiwo and Phaeocystis globosa; and natural products 2, 4, and 6 against Karenia mikimotoi.
Discussions
Some red seaweeds which have shown notable inhibitory or algicidal activities to red tide microalgae that can prevent the development of microalgae or even kill them (Alamsjah et al. 2005; An et al. 2008; Jeong et al. 2000; Luyen et al. 2009; Suzuki et al. 1998; Tanabe et al. 1993; Xu 2008; Xu 2005; Xu et al. 2005a, b). For example, lectin which prepared from Gracilaria verrucosa was investigated for its activity against the growth of Chattonella antiqua and found that this toxic red tide microalgae was completely suppressed at 50 μg/mL (Tanabe et al. 1993). Suzuki et al. (1998) reported that ethanol extracts of Lithophyllum spp. exhibited significant antialgal activities against Heterosigma akashiwo. Jeong et al. (2000) investigated extracts of 10 red seaweeds from the coast of Korea on the growth of Cochlodinium polykrikoides and found that methanol extracts of Corallina pilulifera, Gigartina intermedia, Grateloupia prolongata and Porphyra yezoensis showed algicidal activities at the concentration of 200 μg/mL. Inhibition of Heterosigma akashiwo that resulted from methanol extracts of 9 species of red macroalgae Asparagopsis taxiformis, Bangia atropurpurea, Demonema pulvinatum, Gelidium japonicum, Gracilaria chorda, Gracilaria gigas, Gracilaria vermiculophylla, Hypnea pannosa, and Symphyocladia marchantioides was also found (Alamsjah et al. 2005). Xu et al. (2005a) screened methanol extracts of 10 red macroalgae, such as Ahnfeltiopsis flabelliformis, Chondrus ocellatus, Gelidium amansii, Gracilaria bursa-pastoris, Grateloupia filicina, Gymnogongrus flabelliformis, Halymenia floresia, Plocamium telfairiae, Polysiphonia japonia, and Symphyocladia latiuscula, and pointed out all 9 red macroalgae inhibited the growth of Heterosigma akashiwo besides Chondrus ocellatus. Several red macroalgae, such as Gracilaria lemaneiformis (Xu et al. 2005b), Chondracanthus intermedius (Xu 2008), Symphyocladia marchantioides (Xu 2008), Porphyra tenera (An et al. 2008), Lithophyllum yessoense (Luyen et al. 2009), Chondria crassicaulis (Jin 2011), and Grateloupia ramosiss (Jin 2011), have been found to inhibit some red tide microalgae, Alexandrium tamarense, Heterosigma akashiwo and Skeletonema costatum. Recently, the potential of red seaweeds as a source of active compounds against red tide microalgae has been confirmed in different studies (Hirao et al. 2012; Li et al. 2012; Lu et al. 2011a, b; Sun et al. 2016, 2017, 2018a, b; Tang et al. 2015; Ye and Zhang 2013).
Previous work showed that methanol extracts of Gracilaria lemaneiformis dry powder inhibited Amphidinium hoefleri and Alexandrium tamarense at the concentration of 2000 μg/mL; at a concentration of 1000 μg/mL, methanol extracts exhibited strongest antialgal active against Karenia mikimotoi (Sun et al. 2011). In this study, ethanol extracts of Gracilaria lemaneiformis also have significant inhibitory activities against Karenia mikimotoi and Skeletonema costatum at the concentration of 800 μg/mL (Fig. 3). The growth of red tide microalga Cochlodinium polykrikoides was significantly restrained by methanol extracts of four red seaweeds (Corallina pilulifera, Gigartina intermedia, Grateloupia prolongata, and Porphyra yezoensis) at a concentration of 200 μg/mL (Jeong et al. 2000). All these results showed that the intensity of inhibitory activities of red seaweeds extracts is related not only to algae species but also to red tide microalgae species. The remediation from red macroalgae of Gracilariales in eutrophic water bodies has been reported in China (Tang et al. 2003; Xu et al. 2004; Yu et al. 2017). Our term has pointed that ten species of Gracilariales showed growth inhibition to some red tide microalgae up to now (Sun et al. 2019), such as Gracilaria asiatica (Wang et al. 2012a, b), Gracilaria bursa-pastoris (Xu et al. 2005a, b), Gracilaria chorda (Alamsjah et al. 2005), Gracilaria chouae (Alamsjah et al. 2005), Gracilaria gigas (Alamsjah et al. 2005), Gracilaria lemaneiformis (Lu 2011; Sun et al. 2017; Xu et al. 2005a, b), Gracilaria tenuistipitata (Ye and Zhang 2013), Gracilaria tenuistipitata var. liui (Tang et al. 2003), Gracilaria vermiculophylla (Alamsjah et al. 2005), and Gracilaria verrucosa (Tanabe et al. 1993). Hence, they have drawn great attention. However, there was little information about isolation and identification of antialgal compounds in red macroalgae of Gracilariales. Except Gracilaria lemaneiformis (Lu 2011; Sun et al. 2017), antialgal compounds in other red macroalgae of Gracilariales not have been reported. However, in terms of antialgal compounds in Gracilaria lemaneiformis, their research was far away from enough.
Several researchers have isolated and/or identified some chemical constituents from Gracilaria lemaneiformis (Chen et al. 2004; Lu 2011; Lu et al. 2009, 2011a, b; Mei et al. 2006; Sun et al. 2017, 2018a; Zhang et al. 2012), such as 8-hydroxy-4E,6E-octadien-3-one (Lu 2011), 1,3-dipalmitin (Lu et al. 2011a), linoleic acid (Lu et al. 2011b), and oleic acid (Chen et al. 2004; Lu et al. 2009; Mei et al. 2006; Zhang et al. 2012; Zhang 2011). In order to clearly reflect the differences in results between these reported literatures and our this work, the isolation (or analysis) process and identified compounds are summarized in Table 8. In our current study, we have purified from ethanol extracts of Gracilaria lemaneiformis 1-β-d-ribofuranosyluracil (1), 3-hydroxymethyl-pyrrolopiperazine-2,5-dione (2), benzene-1,2-propanoic acid (3), 7-oxabicyclo[4.1.0]-heptan-3-ol (5), 3,4-dimethoxy-6-(methoxymethyl)-tetrahydro-2H-pyran-2,5-diol (7) and 3,7,11,16- tetramethyl -2-heptadecen-1-ol (8) besides benzene-1,2-propanoic acid (3), 1-O-palmitoyl-2-O-palmitoleoyl-3-O-β-d-galactopyranosyl glycerol (4) and linoleic acid (6) that previously isolated from Gracilaria lemaneiformis (Lu 2011; Lu et al. 2011a; Sun et al. 2018a) (Fig. 4). It could be seen very clearly that five natural products 1, 2, 5, 7 and 8 were isolated from this marine macroalgae for the first time. Further, after searching the metabolites from marine macroalgae, we found that natural products 3, 5, and 8 were isolated from marine macroalgae for the first time. There were at least 80 chemical constituents in Gracilaria lemaneiformis (Chen et al. 2004; Lu 2011; Lu et al. 2009, 2011a, b; Mei et al. 2006; Zhang et al. 2012), but no more than 30 chemical components could be isolated and purified (Table 8). And among these components which isolated and purified from Gracilaria lemaneiformis, the components with antialgal activity were less than 20 (Lu 2011; Lu et al. 2011b; Sun et al. 2017).
Antialgal activity evaluation showed that five natural products (1, 2, 3, 4, and 6) have selective antialgal activities against tested six red tide microalgae (Figs. 5a–f, 6b, and 12; Table 7). Further, we compared the EC50–96 h values of five natural products obtained in this work and all natural products reported, such as gossonorol isolated from Porphyra yezoensis (Sun et al. 2018b), trehalose (Sun et al. 2018b), 1,2-benzenedicarboxylic acid, butyl 2-methylpropyl ester (Kang 2006) purified from Ulva pertusa, and 8-hydroxy-4E,6E-octadien-3-on obtained from Gracilaria lemaneiformis (Lu 2011; Sun et al. 2017), and found that natural product 3 (natural product 4) showed the superior application potential than potassium dichromate and other reported natural products as a characteristic antialgal agent against Amphidinium carterae (Phaeocystis globosa).
In conclusion, fifteen natural products (8 in this work (Fig. 4), 7 in our previous study (Sun et al. 2017)) with antialgal activities were obtained from Gracilaria lemaneiformis, and they exhibit a range of antialgal activities including inhibition effects on Amphidinium carterae, Heterosigma akashiwo, Karenia mikimotoi, Phaeocystis globosa, Prorocentrum donghaiense, and Skeletonema costatum. Their inhibitory activities were evaluated and it was found that five natural products (1, 2, 3, 4, and 6) purified from this study showed good superiority than these seven natural products isolated from our previous research in inhibiting Amphidinium carterae; growth inhibition of these natural products against Heterosigma akashiwo, Karenia mikimotoi, Prorocentrum donghaiense, and Skeletonema costatum was very close; for Phaeocystis globosa, seven natural products except glycerol monopalmitate isolated in our previous research (Sun et al. 2017) have stronger inhibition effects than these five natural products purified in this study. These results give a conclusion that Gracilaria lemaneiformis was a new source of natural products with antialgal activities.
Conclusions
We now report the purification of the eight natural products and a study of their antialgal effects on the growth of the red tide microalgae, Amphidinium carterae, Heterosigma akashiwo, Karenia mikimotoi, Phaeocystis globosa, Prorocentrum donghaiense, and Skeletonema costatum. And it clearly pointed out that two compounds, benzene-1,2-propanoic acid (3) and 1-O-palmitoyl-2-O-palmitoleoyl-3-O-β-d-galactopyranosyl glycerol (4), are expected to be developed into environment-friendly antialgal agent against Amphidinium carterae or Phaeocystis globosa.
Recently, the isolation of natural products with antialgal activities from marine macroalgae has been regarded as an environmentally friendly alternative approach for controlling red tide microalgae in marine systems (Accoroni et al. 2015; Alamsjah et al. 2005; An et al. 2008; Ben Gharbia et al. 2017; Jeong et al. 2000; Nan et al. 2004; Sun et al. 2015; Tang and Gobler 2011). These natural products include a variety of bioactive molecules such as monoterpenes (Konig et al. 1999), bromide (Ohsawa et al. 2001), polyphenol (Gross 2003), phenylpropanoids (Sun et al. 2018a), miscellaneous compounds (Macίas et al. 2008; Sun et al. 2016, 2017, 2018a, b, c), and other not yet identified compounds (Accoroni et al. 2015; An et al. 2008; Ben Gharbia et al. 2017; Chowdhury et al. 2014; Sun et al. 2016; Tang and Gobler 2011; Wang et al. 2007), and etc. However, this is far from enough for biological control of red tide microalgae using natural products with antialgal activities isolated from marine macroalgae. Therefore, it is urgent for researchers to screen and isolate antialgal compounds from more species of marine macroalgae.
Data availability
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
References
Accoroni S, Percopo I, Cerino F, Romagnoli T, Totti C (2015) Allelopathic interactions between the HAB dinoflagellate Ostreopsis cf. ovata and macroalgae. Harmful Algae 49:147–155
Alamsjah MA, Hirao S, Ishibashi F, Fujita Y (2005) Isolation and structure determination of algicidal compounds from Ulva fasciata. Biosci Biotechnol Biochem 69:2186–2192
An Z, Wang ZY, Li FM, Tian ZJ, Hu HY (2008) Allelopathic inhibition on red tide microalgae Skeletonema costatum by five macroalgal extracts. Front Environ Sci Eng China 2(3):297–305 (in Chinese)
Anderson RJ, Monteiro PMS, Levitt GJ (1996) The effect of localised eutrophication on competition between Ulva lactuca (Ulvaceae, Chlorophyta) and a commercial resource of Gracilaria verrucosa (Gracilariaceae, Rhodophyta). Hydrobiologia 326–327(1):291–296
Ben Gharbia H, Kéfi-Daly Yahia O, Cecchi P, Masseret E, Amzil Z, Herve F, Rovillon G, Nouri H, M’Rabet C, Couet D, Triki HZ, Laabir M (2017) New insights on the species-specific allelopathic interactions between macrophytes and marine HAB dinoflagellates. PLoS One 12(11):1–28
Bie CC, Li FM, Li YY, Zhao YH, Wang ZY (2011) Inhibitory effects of 6 macroalgae extracts on Skeletonema costatum and isolation of allelochemicals. Period Ocean Univ China 41(7/8):107–112 (in Chinese)
Cai SY (2016) Effects of the massive cultivation of Gracilaria lemaneiformis on plankton community structure and its inhibition on microalgal bloom. Dissertation, Jinan University (in Chinese)
Chen MZ, Zhang YY, Yu J, Xie XB (2004) Studies on separation and scavenging activities on free radicals of phycobiliproteins from Gracilaria lemaneiformis. Food Sci 25(3):159–162 (in Chinese)
Chen MZ, Yu J, Long ZJ, Luo QB (2005) Studies on antimutagenic and the free radical scavenging effect of polysaccharide from Gracilaria lemaneiformis. Food Sci 26(7):219–222 (in Chinese)
Chen MZ, Ge AS, Yu J, Yang WJ (2007) Studies of antitumor activity of phycoerythrin from Gracilaria lemaneiformis. Chin J Mar Drugs 26(4):27–31 (in Chinese)
Chen MZ, Yu J, Liao ZH, Wang X (2008) Study on the antitumor activity and antioxidation of polysaccharide from Gracilaria lemaneiformis in sarcoma180 bearing mice. Chin J Mar Drugs 27(2):46–49 (in Chinese)
Cho JY, Jin HJ, Lim HJ, Whyte JNC, Hong YK (1999) Growth activation of the microalga Isochrysis galbana by the aqueous extract of the seaweed Monostroma nitidum. J Appl Phycol 10(6):561–567
Chowdhury MTH, Bangoura I, Kang JY, Cho JY, Joo J, Choi YS, Hwang DS, Hong YK (2014) Comparison of Ecklonia cava, Ecklonia stolonifera and Eisenia bicyclis for phlorotannin extraction. J Environ Biol 35:713
Cui F (2014) Studies on allelopathy effects of Ulva prolifera on red tide microalgae and allelochemicals identification. Dissertation, Shanghai Ocean University. (in Chinese)
El Hattab M, Genta-Jouve G, Bouzidi N, Ortalo-Magné A, Hellio C, Maréchal J-P, Piovetti L, Thomas OP, Culioli G (2015) Cystophloroketals A-E, unusual phloroglucinol-meroterpenoid hybrids from the brown alga Cystoseira tamariscifolia. J Nat Prod 78:1663–1670
Fletcher RL (1975) Heteroantagonism observed in mixed algal cultures. Nature 253(5492):534–535
Gao H, Zhou FF, Tang HJ, Shi XY, Su RG (2018) Allelopathy of extracts of Ulva prolifera on green tides in the Yellow Sea and the identification of the allelochemicals. Acta Oceanol Sin 240(12):11–20 (in Chinese)
Gross EM (2003) Allelopathy of aquatic autotrophs. Crit Rev Plant Sci 22:313–339
Guillard RRL, Ryther JH (1962) Studies of marine planktonic diatom. I. Cyclotella nana Hustedt and Detonula confervacea (Cleve) Gran. Can J Microbiol 17:309–314
Hirao S, Tara K, Kuwan K, Tanaka J, Ishibashi F (2012) Algicidal activity of glycerolipids from brown alga Ishige sinicola toward red tide microalgae. Biosci Biotechnol Biochem 76(2):372–374
Jeong JH, Jin HJ, Sohn CH, Suh KH, Hong YK (2000) Algicidal activity of the seaweed Corallina pilulifera against red tide microalgae. J Appl Phycol 12:37–43
Jin Q (2005) Studies on the allelopathic effects of macroalga Ulva pertusa on red tide microalgae and isolation and characterization of its allelochemicals. Dissertation, Ocean University of China. (in Chinese)
Jin HL (2011) Studies on the inhibition activity of Ulva intestinalis on red tide microalgae and the isolation and identification of the algicidal compounds. Dissertation, Ningbo University
Kang K (2006) Isolation and characterization of allelochemicals from Chlorophyta Ulva pertusa. Dissertation, Ocean University of China
Konig GM, Wright AD, Linden A (1999) Plocamium hamatum and its monoterpenes: chemical and biological investigation of the tropical marine red alga. Phytochemistry 52:1047–1053
Lee VC, Olsen S (1985) Eutrophication and management initiatives for the control of nutrient inputs to rhode island Coastal Lagoons. Estuaries 8(2):191
Lei GY, Yang YF, Li X (2010) Inhibitory effects of Gracilaria lemaneiformis on growth of Heterosigma akashiwo and Prorocentrum micans. Mar Environ Sci 29(1):27–31 (in Chinese)
Li B, Cai HJ, Liu CF (2007) Allelopathic effect of sea weed Chondrus ocellatus on algae Karenia mikimotoi and Prorocentrum minimum. J Dalian Ocean Univ 27(1):27–31 (in Chinese)
Li B, Cai HJ, Liu CF (2012) Allelopathic effect of sea weed Chondrus ocellatus on algae Karenia mikimotoi and Prorocentrum minimum. J Dalian Ocean Univ 27(1):27–31 (in Chinese)
Liu TT (2006) Study on inhibitory effects of Gracilaria lemaneiformis on the three red tide microalgae. Master's thesis, Jinan University. (in Chinese)
Lu HM (2011) Chemical constituents of the seaweed Gracilaria lemaneiformis and their allelopathic effects on Skeletonema costatum. Dissertation, Jinan University. (in Chinese)
Lu JF, Yang WG, Yan WH, Zhou XY (2009) Purification and composition of polysaccharide from Gracilaria lemaneiformis. Oceanol ET Limnol Sin 40(4):484–488 (in Chinese)
Lu CY, Deng Y, Mei L, Guo DL (2011a) Studies on chemical constituents of Gracilaria lemaneiformis. Chin Tradit Herb Drugs 42(6):1069–1071 (in Chinese)
Lu HM, Xie HH, Yang YF, Wei XY (2011b) Chemical constituents from the macroalga Gracilaria lemaneiformis. J Tropic Subtropic Botany 19(2):166–170 (in Chinese)
Luyen HQ, Cho JY, Choi JS, Kang JY, Park NG, Hong YK (2009) Isolation of algal spore lytic C17 fatty acid from the crustose coralline seaweed Lithophyllum yessoense. J Appl Phycol 21:423–427
Macίas FA, Galindo JLG, Garcίa-Dίaz MD, Galindo JCG (2008) Allelopathic agents from aquatic ecosystems: potential biopesticides models. Phytochem Rev 7:155–178
Manilal A, Sujith S, Sabarathnam B, Kiran GS, Selvin J, Shakir C, Lipton AP (2010) Bioactivity of the red algae Asparagopsis taxiformis collected from the southwestern coast of India. Braz J Oceanogr 58:93–100
Marklund S, Marklun G (1974) Involvement of superoxide anion radical in the auto-oxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:169–174
Marshall SM, Orr AP (1949) Further experiments on the fertilization of a sea loch (Loch Craiglin). J Mar Biol Assoc UK 27:360–379
McLachlan J, Craigie JS (1964) Algal inhibition by yellow ultraviolet-absorbing substances from fucus vesiculosus. Can J Bot 42(3):287–292
Mei WL, Dai HF, Xu JT (2006) Composition and cytotoxic activity of the organic acids from Gracilaria lemaneiformis. Chin J Mar Drugs 25(2):45–47 (in Chinese)
Oh MY, Lee SB, Jin DH, Hong YK, Jin HJ (2010) Isolation of algicidal compounds from the red alga Corallina pilulifera against red tide microalgae. J Appl Phycol 22(4):453–458
Nagayama K, Shibata T, Fujimoto K, Honjo T, Nakamura T (2003) Algicidal effect of phlorotannins from the brown alga Ecklonia kurome on red tide microalgae. Aquaculture 218(1–4):601–611
Nan CR, Zhang HZ, Zhao GQ (2004) Allelopathic interactions between the macroalga Ulva pertusa and eight microalgal species. J Sea Res 52:259–268
OECD (1984) Alga growth inhibition test. Test Guideline No. 201. OECD Guidelines for Testing of Chemicals. Organization for Economic Cooperation and Development, Paris
Ohsawa N, Ogata Y, Okada N, Itoh N (2001) Physiological function of bromoperoxidase in the red marine alga, Corallina pilulifera: production of bromoform as an allelochemical and the simultaneous elimination of hydrogen peroxide. Phytochemistry 58:683–692
Shao MW, Sun X, Xu NJ (2011) Inhibitory effects of marine red algae Gracilaria lemaneiformis on two HAB algae and the relationship with environmental factors. J Mar Sci 29(2):100–106 (in Chinese)
Sun YY, Zhang J, Liu HJ, Li C, Wang CH (2011) Effects of macroalga Gracilaria lemaneiformis on the growth of the three species of red tide microalgae under laboratory conditions. Mar Sci Bull 30(3):328–333 (in Chinese)
Sun YY, Zhang J, Xu SZ, Li WH, Wang CH (2012) Growth inhibition of Karenia mikimitoi by extracts from Gracilaria lemaneiformis using five solvents. Inform Technol Agric Eng 134:199–210
Sun YY, Wang H, Guo GL, Pu YF, Yan BL, Wang CH (2015) Green alga Ulva pertusa—a new source of bioactive compounds with antialgal activity. Environ Sci Pollut Res 22(13):10351–10359
Sun YY, Wang H, Guo GL, Pu YF, Yan BL, Wang CH (2016) Isolation, purification and identification of antialgal substances in green alga Ulva prolifera for antialgal activity against the common harmful red tide microalgae. Environ Sci Pollut Res 23(2):1449–1459
Sun YY, Meng K, Su ZX, Guo GL, Pu YF, Yan BL, Wang CH (2017) Isolation and purification of antialgal compounds from the red alga Gracilaria lemaneiformis for activity against common harmful red tide microalgae. Environ Sci Pollut Res 24(5):4964–4972
Sun YY, Zhou WJ, Guo GL, Pu YF, Su ZX (2018a) Isolation and purification of phenylpropanoid antialgal substances from Gracilaria lemaneiformis and their growth inhibition effects on six species of red tide microalgae. J Fish China 42(7):1019–1025
Sun YY, Xing JZ, Zhang JS, Zhou WJ, Pu YF (2018b) Sesquiterpenoids with antialgal activity against the common red tide microalgae from marine macroalgae Porphyra yezoensis. Environ Sci Pollut Res 25(8):7844–7859
Sun YY, Zhou WJ, Guo GL, Su ZX, Pu YF (2018c) Antialgal compounds with antialgal activity against the common red tide microalgae from a green algae Ulva pertusa. Ecotox Environ Safe 157:61–66
Sun YY, Dong SS, Zhou WJ, Guo L, Guo GL, Zhang X (2019) A comprehensive review of secondary metabolites with antialgal activity from marine macroalgae against red tide microalgae. J Coast Res (SI) 93:1–14
Suzuki Y, Takabayashi T, Kawaguchi T (1998) Isolation of allelopathic substance from the crustose coralline algae, Lithophyllum spp. and its effect on the brown Algae, Laminaria religiosa miyabe (phaeophyta). J Exp Mar Biol Ecol 225:69–77
Takedai F, Sakamaki T, Xu K, Chiba N, Nishimura O, Sudo R (2003) Effect of potential allelochemicals extracted from Sargassum horneri on the growth of red tide microalgae. J Environ Syst Eng 748:25–32
Tanabe H, Kamishima H, Yoshinari K (1993) Inhibitory effect of red alga lectin and skipjack fat on the growth of the red tide plankton Chattonella antiqua. J Ferment Bioeng 75(5):387–388
Tang YZ, Gobler CJ (2011) The green macroalga, Ulva lactuca, inhibits the growth of seven common harmful algal bloom species via allelopathy. Harmful Algae 10:480–488
Tang KX, Yuan DX, Lin SB, Lin YS, You XP, Shen DY, Chen ME, Hong WS (2003) Depression and affect of red tide on main water quality index by Gracilaria tenuistipitata. Mar Environ Sci 22(2):24–27 (in Chinese)
Tang YZ, Kang Y, Berry D, Gobler CJ (2015) The ability of the red macroalga, Porphyra purpurea (Rhodophyceae) to inhibit the proliferation of seven common harmful microalgae. J Appl Phycol 27:531–544
Tian ZJ (2009) Inhibition effect of allelochemicals from large seaweeds on Gymnodinium breve. Master of science and engineering thesis, Ocean University of China. (in Chinese)
Wang Y, Yu ZM, Song XX, Zhang SD (2006) Effects of macroalgae on growth of 2 species of bloom microalgae and interactions between these microalgae in laboratory culture. Environ Sci 27(2):274–280 (in Chinese)
Wang RJ, Xiao H, Zhang PY, Qu L, Cai HJ, Tang XX (2007) Allelopathic effects of Ulva pertusa, Corallina pilulifera and Sargassum thunbergii on the growth of the dinoflagellates Heterosigma akashiwo and Alexandrium tamarense. J Appl Phycol 19(2):109–121
Wang Y, Zhou B, Tang XX (2009) Effects of two species of macroalgae-Ulva pertusa and Gracilaria lemaneiformis-on growth of Heterosigma akashiwo (Raphidophyceae). J Appl Phycol 21(4):375–385
Wang YH, Shen SF, Sun QH, Fen MX, Zheng Y (2012a) Antialgal and antibiotic action of lectins from two species of Gracilaria. J Fujian Normal Univ 28(4):94–98 (in Chinese)
Wang R, Wang Y, Tang X (2012b) Identification of the toxic compounds produced by Sargassum thunbergii to red tide microalgae. Chin J Oceanol Limnol 30:778–785 (in Chinese)
Wu L (2016) Effects of temperature and extracts of macroalgae and seagrass on the growth and carbohydrate yield of marine benthic dinoflagellates. Dissertation, Jinan University. (in Chinese)
Xu Y (2005) Studies on the allelopathic effects of Enteromorpha linza on Heterosigma akaskiwo. Dissertation, Ocean University of China. (in Chinese)
Xu FH (2008) The interaction between macro-algae and micro-algae and its responses to enriched CO2. Master's thesis, Qingdao University. (in Chinese)
Xu YJ, Qian LM, Jiao NZ (2004) Nitrogen nutritional character of Gracilaria as bioindicators and restoral plants of eutrophication. J Fish Sci China 11(3):276–280
Xu Y, Dong SL, Jin Q (2005a) Study on inhibitory effects of nine macroalgae on the growth of Heterosigma akashiwo. Period Ocean Univ China 35(3):475–477 (in Chinese)
Xu YJ, Qian LM, Jiao NZ (2005b) Influences of adding macroalgae Gracilaria lemaneiformis to Skeletonema costatum’s bloom. J Oceanogr Taiwan Strait 24(4):533–539
Ye CP, Zhang MC (2013) Allelopathic effect of macroalga Gracilaria tenuistipitata (Rhodophyta) on the photosynthetic apparatus of red-tide causing microalga Prorocentrum micans. IERI Procedia 5:209–215
Yu J, Lu HM, Yang YF (2010) Effect of the extracts from Gracilaria lemaneiformis on the growth and ultrastructure of Scippsiella trochoidea. J Shenzhen Univ Sci Eng 27(2):199–205 (in Chinese)
Yu J, Dai XL, Zhang ZL, Zhang ZY, Chen RZ, Huang GQ, Su Q, Li WH (2017) Characteristics of nutrients and eutrophication assessment in water quality of Gracilaria asiatica and Gracilaria tenuistipitata growing sea area. J South Agric 48(8):1511–1517 (in Chinese)
Zhang M (2011) Primary studies on metabolic composition and metabolic mechanism of five economic seaweeds. Dissertation, Suzhou University. (in Chinese)
Zhang YY, Chen MZ, Yu J, Lin YX (2005) Studies on the antimutagenic and antitumour effects of phycobiliproteins from Gracilaria lemaneiformis. Chin J Mar Drugs 24(3):159–162 (in Chinese)
Zhang M, Li RX, Yi JF, Shen SD, Hu CM, Ying SY, Tang J, Zhang T, Xu P (2012) Analysis of the fatty acid composition of four economic seaweeds. Mar Sci 36(4):7–12 (in Chinese)
Funding
This work was supported by Jiangsu Province fifth “333 project” training funding (BRA2020263); Special Foundation for A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions; Excellent Young Teachers and Principals Program of Jiangsu Province, China; Haiyan project of Lianyungang City; and Innovation Training Program for College Students of Jiangsu Ocean University.
Author information
Authors and Affiliations
Contributions
Ying-ying Sun performed the data analyses and wrote the manuscript; Jing Zhou contributed significantly to analysis and manuscript preparation; Xiu Han, Zi-xuan Yang, and Xin Zhang performed the experiment; Nai-sheng Zhang helped perform the analysis with constructive discussions.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethical approval
Not applicable.
Consent to participate
All the authors agreed to participate in the study.
Consent to publish
Written informed consent for publication was obtained from all participants.
Additional information
Responsible Editor: Vitor Manuel Oliveira Vasconcelos
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sun, Yy., Zhou, J., Han, X. et al. Several natural products isolated from a red alga Gracilaria lemaneiformis and its evaluation of antialgal activity against six common red tide microalgae. Environ Sci Pollut Res 28, 22409–22426 (2021). https://doi.org/10.1007/s11356-020-11755-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-11755-3