Abstract
In this study, municipal sewage sludge (MSS) is converted simultaneously into renewable biofuels (bio-oil, syngas) and high value-added products (biochar) using a fixed bed pyrolyzer. This work examines the combined effect of two factors: final pyrolysis temperature (°C) and MSS moisture content (%) on pyrogenic product yields and characteristics. A centered composite experimental design (CCD) is established for pyrolysis process optimization by adopting the response surface methodology (RSM). The statistical results indicate that the optimal conditions considering all studied factors and responses are 550 °C as final pyrolysis temperature and 15% as MSS moisture content. In these optimal conditions, biofuels yield is around 48 wt%, whereas biochar yield is about 52 wt%. The pyrolysis products characterizations reveal that (i) pyrolytic oil has a complex molecular composition rich with n-alkanes, n-alkenes, carboxylic acids, and aromatic compounds; (ii) bio-oil presents a high-energy content (high heating value HHV around 30.6 MJ/kg); (iii) syngas mixture has a good calorific value (HHV up to 8 MJ/kg), which could be used as renewable energy vector or for pyrolysis reactor heating; and (iv) biochar residue has good aliphatic and oxygenated group contents favoring its application as biofertilizer. These findings suggest that MSS conversion into biofuels and biochar is an appropriate approach for MSS treatment. MSS-to-energy could be proposed as an element for circular economy concept due to its effectiveness in producing high value-added and sustainable products and reducing environmental problems linked to MSS disposal.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As a residual product of urban wastewater treatment, municipal sewage sludge (MSS) is a heterogeneous biomass with a relatively high organic matter content. MSS thermochemical conversion, particularly via pyrolysis, could contribute significantly to produce green energy sources (biofuels) and sustainable biofertilizers (biochar) and, thus, reduce environmental problems related to conventional fuel consumption and chemical fertilizer import.
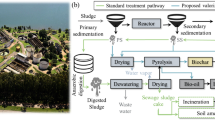
Tunisia is generating yearly around 2.5 million tons of MSS (ONAS 2016) and has an energy demand around 3857 ktep in 2016 (ONE 2017) and a chemical fertilizer consumption around 127.376 kg/ha of arable land (FAO 2016). Fulfilling this high-energy demand and ensuring a sustainable supply of fertilizer are serious challenges in developing countries like Tunisia. From another side, regarding the huge quantities produced yearly and the organic character of MSS, these biosolids could be a resource if we can efficiently convert them into green bioenergy and eco-friendly fertilizer. The aim of this study is to investigate experimentally the MSS pyrolysis as an innovative process that should enable the combination of environmentally friendly MSS disposal and bioproduct recovery. This is a promising approach for MSS disposal owing to its effectiveness in decreasing the MSS volume and in generating valuable and sustainable products. It could be the best way to implement circular economy concept in Tunisia.
Actually, there are various options for MSS treatment: landfilling, anaerobic digestion, agricultural composting, incineration, gasification, pyrolysis, microwave pyrolysis, torrefaction, supercritical oxidation, and thermal hydrolysis (Karaca et al. 2018; Gao et al. 2020; Schnell et al. 2020). Most of these methods were used to enable MSS hygienization (toxic compounds limitation) and volume reduction. However, the rising concerns over waste management and the increasing focus on hygiene combined with the associated legal changes highlight the limitations of such traditional methods for MSS disposal and the relevance of alternative technologies for safe treatment through sustainable conversion into green products (biofuels and biofertilizers).
Biofuel and bioproduct generation from MSS is an effective non-traditional method for MSS treatment since bioenergy and bioagriculture are attracting public and scientific attention, driven by some factors such as the necessity of increased energy and food security and the concern over the growing emissions of greenhouse gas from the fossil fuels and synthetic fertilizer consumption. MSS can be converted into bio-oil, syngas, and biofertilizer by an environment-friendly, cost-effective, and safe technology called pyrolysis (Alvarez et al. 2016; Karaca et al. 2018; Gao et al. 2020). Pyrolysis is an emerging and a promising technique due to its efficiency in MSS volume reduction, in pathogen neutralization, and in recovery of organic compounds in forms of energy (liquid and gaseous biofuels) and into valuable bioproducts (solid biochar) (Fonts et al. 2012; Gao et al. 2020).
Pyrolysis is the thermochemical decomposition of organic materials at temperature range between 300 and 800 °C, in the absence of oxygen, producing volatile compounds which are usually recovered as condensable tars and non-condensable gases and a remaining solid residue (biochar) (Fonts et al. 2012; Ben Hassen-Trabelsi et al. 2014; Gao et al. 2020). Key operational parameters (end temperature, heating rate, residence time) and some feedstock properties (namely raw material moisture content) are key factors that should be considered due to the sensitivity of the pyrolysis technology. By adjusting the operating parameters, pyrolysis reactions can lead to obtain mainly char, liquid, or gas. One of the benefits of using MSS pyrolysis process is that the produced bio-oil and syngas could be used as energy vectors (Domínguez et al. 2006; Fonts et al. 2012; Djandja et al. 2020). The most common applications of bio-oil/biocrude include the utilization as an alternative fuel for transportation and the valorization as feedstock for valuable chemical products and especially as a source of active molecules. Syngas is a combustible gaseous mixture which can be applied widely to produce electricity, for industrial processes (drying for example) or for liquid fuels generation through Fischer-Tropsch reactions or also as a heating source for pyrolysis reactor. Depending on raw feedstock composition, the remaining biochar contains also high amounts of nutrients and can be applied for agronomic purposes as bio-organic fertilizer (Fan et al. 2020; Praspaliauskas et al. 2020).
Most of the previous works dealing with sewage sludge pyrolysis (Trabelsi et al. 2017; Karaca et al. 2018; Fan et al. 2020) were conducted to explore the sludge-to-energy potential and to investigate the pyrolytic products properties. A part of these works focused on the potential of converting sewage sludge into solid biochar valuable as soil enricher or as adsorbent via pyrolysis (Agrafioti et al. 2013; Fan et al. 2020; Praspaliauskas et al. 2020). The other parts studied the conversion of MSS into useful forms of energy (liquid and gaseous biofuels) (Fonts et al. 2012; Trabelsi et al. 2017; Gao et al. 2020). Ben Hassen Trabelsi et al. (2017) conducted pyrolysis experiments on Tunisian MSS and highlighted the great potential of these waste to produce hydrogen-rich syngas with a high calorific value, whereas Sobek and Werle (2020) investigated the gaseous biofuels production from the sewage sludge and highlighted the good “sludge-to-energy” potential and its importance as an element of the circular economy concept. Most of these studies, although useful, were conducted as pointed research work, focusing on some key process operating parameters and leading to the production of one or more energy vectors without paying attention to the economic efficiency of pyrolysis process. Moreover, many works focused on the investigation of pyrolysis reactions using thermogravimetric scale for sewage sludge (Shao et al. 2008; Languer et al. 2020; Sobek and Werle 2020) and few studies prove the pyrolysis process feasibility to produce, simultaneously and experimentally, useful biofuels and sustainable bioproducts.
The objective of this study is to investigate the feasibility of MSS valorization via pyrolysis towards biochar and energy production. The novelty of this work is in the experimental identification using an RSM methodology, of the combined effect of two key parameters (final pyrolysis temperature and feedstock moisture content) on pyrolysis products distribution and qualities, with a focus on converting MSS totally into renewable biofuels and value-added bioproducts with economic interest. This study was basically performed to evaluate experimentally the applicability of moderate pyrolysis (500–600 °C) to MSS at laboratory scale (TRL3/TLR4), in order to advance technical proofs about its economical efficiency as an environment-friendly route to up-cycle MSS into energy and biochar.
Therefore, the specific objective of this study is the experimental investigation at TRL3/TL4, through the collection of experimental proofs of MSS pyrolysis in a laboratory fixed-bed reactor. The ultimate objective of this work is to demonstrate that MSS energy/material recovery is a sustainable valorization system as an element of the circular economy concept and as an innovative eco-social business model.
Materials and methods
Raw feedstock characterization
The MSS samples used were collected from an urban wastewater treatment Plant in Rades (Northern Tunisia). A pre-treatment process by dewatering and then open air-drying was applied to reduce MSS moisture content. Pre-dried MSS was characterized for its ultimate and proximate properties. Ultimate analysis was performed using PerkinElmer 2400 CHN elemental analyzer. The sulfur (S) content was measured via Horiba Jobin Yvon elemental sulfur analyzer (ASTM method D3176-74, 1997). The oxygen amount was obtained by difference as the following:
The raw MSS high heating value (HHV) and low heating value (LHV) calculation were obtained based on CHN-O elemental composition using Eq. 2 for HHV established by Sheng and Azevedo (2005) and Eq. 3 for LHV reported by Oyebanji et al. (2018):
Proximate properties were determined using standard methods for moisture content (AFNOR XP CEN/TS 14774-3), for ash content (AFNOR XP CEN/TS 14775), and for volatile matter content (ASTM method D-1762-84, 1990). Fixed carbon percentage was obtained by difference (Eq. 4):
The raw MSS crystalline nature was studied using XRD technique with a PANalytical X’Pert Pro X-ray diffractometer equipped with a Cu Kα radiation (λ = 1.540562 Å) in the 2θ range from 3 to 100°. FTIR spectroscopy analysis of pre-dried MSS was conducted using KBr pellets at a ratio of 5:100 (sample/KBr), in these conditions: (i) Equipment: PerkinElmer Spectrum BX® Model spectrophotometer, (ii) spectral range from 400 to 4000/cm, (iii) number of accumulations: 15 scans. The compounds identification was accomplished based on previous data reported in the literature (Ben Hassen-Trabelsi et al. 2014).
Thermogravimetric analysis TGA of pre-dried MSS was performed using a Setaram thermogravimetric analyzer type Labsys® thermobalance. The measurements of mass loss were achieved in these conditions: (i) inert atmosphere: N2 nitrogen gas flow; (ii) temperature range: from 25 to 900 °C; (iii) heating rate: 10 °C/min. The data were taken and recorded every 0.4 s (Zaafouri et al. 2016).
MSS pyrolysis optimization
Pyrolysis facility and product recovery
The pyrolysis experiments were conducted in a poor O2 atmosphere (constant flow rate of nitrogen N2 as a carrier gas), under atmospheric pressure, using a laboratory-scale reactor in the Research and Technology Centre of Energy (CRTEn-Tunisia) (Kraiem et al. 2017). Briefly, the pyrolysis plant consists of a stainless steel batch-type cylindrical reactor (L 125 mm; Ø 80 mm), heated by an electric furnace. The pyrolysis reactor is equipped with (i) a thermocouple K-type which records the axial temperature inside the reactor, (ii) a temperature PID controller, (iii) a series of condensers with a recovery unit, and (iv) a cryostat for cold water (glycolated water) circulation. The pyrolysis experimental conditions are as follows: 10 °C/min of heating rate and 30 min of residence time. During experiments, the reactor was continuously flushed with N2 in order to guarantee an inert medium for thermochemical degradation and thus releasing vapors produced during cracking reactions.
Taking into consideration the heterogeneous nature of the raw MSS, pyrolysis experiments were carried out in duplicate. At the end of the experiment, the collected liquid fraction (bio-oil) and solid biochar were weighed separately, and their yields were calculated using Eqs. 5 and 6. Syngas yield was calculated by difference following Eq. 7.
CCD
A centered composite experimental design (CCD) has been used to optimize the MSS pyrolysis to produce both bioenergy (biofuels) and biomaterial (biochar). The CCD is composed of three parts: two-level complete or fractional factorial plane, central points of the study interval that can be repeated several times, and the star plane; the points of this plane are located at the same distance from the center of the study interval. These star tests are additional in the case that the objective is to establish a model of second degree. In this work, a study of the effect of the two factors, X1 (final pyrolysis temperature) and the X2 factor (MSS moisture content), on the MSS pyrolysis was performed in order to optimize the pyrolytic product generation in terms of quantity and quality. For the purpose, a CCD has been established by neglecting the points in stars, which makes it possible to study the interactions between the various factors, thus checking the central points with repetitions. Each studied factor has three levels: low (-) and high (+). The levels of each factor and the theoretical matrix are shown in Tables 1 and 2, whereas the experimental matrix is given in Table 3. Besides, the mathematical models and the statistical analysis of the responses were determined using Minitab® software version 17.
The bio-oil yield (wt%), the biochar yield (wt%), the syngas yield (wt%), the CH4 content (vol%), the H2 content (vol%), the CnHm content (vol%), the CO content (vol%), and the CO2 content (vol%) are the studied responses in this experimental design. The CCD usually follows a second-degree mathematical model that links the factors to the responses studied. For all the responses, the mathematical model is written as follows:
- b 0 :
-
The intercept coefficient
b1, b2
Linear coefficients of the two factors X1, X2
b11, b22
quadric coefficients of the two factors X1, X2
b 12
interaction coefficient of the two factors X1, X2
- X 1 2 X 2 2 :
-
quadric terms of the two factors X1, X2
- X 1 :
-
final pyrolysis temperature (°C)
X 2
MSS moisture content (%).
Statistical analysis and mathematic models
The statistical analysis of the output variables obtained after running the experimental design and the calculation of the coefficients was done with Minitab® software version 17. The obtained maps studying the interaction between the studied factors on the final responses of the CCD experimental design were drawn using the Nemrod-W® software version 9901.
Pyrolysis product analyses
Pyrolytic oil
Produced bio-oil obtained at experimental conditions leading to a maximum of liquid products (600 °C as final pyrolysis temperature and 20% as MSS moisture content) was characterized by measuring basic fuel properties including density at 15 °C (AFNOR standard method NFT 60-101/ISO 3675), kinematic viscosity at 40 °C (AFNOR standard method NFT 60-148), calorific value HHV (AFNOR standard method NF M 07-030 ), moisture content (AFNOR standard method NF T 60–113), acidity index (NF ISO 6618 standard method), flash point (NF M07- 182 019 standard method), ash content (NF T 183 60–111 standard method), and color (NF ISO 2909 standard method). The GC-MS bio-oil analyses were performed using a HP 6890 gas chromatography system with a mass selective detector HP 5973. The GC-MS conditions are as follows: (i) a HP-5MS capillary column (L 30 m, internal diameter 0.25 mm, 0.25 μm film thickness); (ii) He carrier gas with a flow rate of 0.7 ml/min; (iii) an injector temperature of 320 °C; (vi) an oven temperature program as follows: 42 °C for 4 min followed by a temperature increase up to 300 °C with 3 °C/min as heating rate and 30 min as hold time at final temperature.
The pyrolytic oil FTIR spectroscopic analyses were performed in the same conditions used for raw feedstock FTIR characterization.
Pyrochar
Remaining pyrochar obtained in the optimal conditions (550 °C as final pyrolysis temperature and 15% as MSS moisture content) was characterized using proximate analyses and FTIR in the same conditions as for raw feedstock characterization.
Synthetic gas
Syngas chemical composition was determined for all pyrolysis experiments using an online gas analyzer (GEIT 3160 model) equipped with (i) an IRD for CO, CO2, CH4, light hydrocarbons CnHm analyses, (ii) a TCD to quantify H2, and (iii) an electrochemical cell for O2 measurement. Before analyses, non-condensable gases were first, cleaned using a series of gas filters, a water bubbler for tars elimination, and an activated carbon column for dusts and particulates removing.
Results and discussion
Main characteristics of the MSS
The main MSS properties given in Table 4 indicate that this waste presents a high VM content (54.5%) giving it a great potential to be used as a renewable energy source through pyrolysis. The as-received MSS moisture content is very high (around 86.6%). It is usually considered as a constraint for thermochemical processes. For these reasons, we performed air drying to reduce moisture content (up to 10, 15, and 20%) before pyrolysis experiments. The selection of these 3 levels (10, 15, 20%) of MSS moisture content is based on previous studies on MSS pyrolysis (Fonts et al. 2009; Trabelsi et al. 2017; Agar et al. 2018) which showed that biosolids can be effectively pyrolyzed at moisture content around 10%. Moreover, Eke et al. (2020) highlighted that thermochemical technologies require feedstock with less than 15 wt% moisture. In this context, 10% was chosen as low level moisture content and the two higher levels (15% and 20%) were selected in order to exanimate the effect of MSS moisture content on thermal degradation and on pyrolysis products properties and to optimize the MSS pre-treatment step (by reducing the drying duration).
The MSS ash content is around 30.8 % indicating the high inorganics content whereas fixed carbon content is around 6.5%. The MSS ash is formed mostly of minerals such as quartz, calcite, or microline, which comprise Fe, Ca, K, and Mg elements. These inorganic species can enhance some pyrolytic reactions due to their catalytic effect (Fonts et al. 2012; Djandja et al. 2020).
The atomic ratios H/C (2.37), O/C (0.18), and energy contents (HHV around 20.80 and LHV around 19.02 MJ/kg) of studied MSS are also given in Table 4. Due to the great heterogeneity of MSS, heating values are very variable from one study to another (for example: LHV between 22 and 28 MJ/kg, Inguanzo et al. 2002, and HHV around between 3.4 and 12.2 MJ/kg; Thipkhunthod et al. 2007). Nevertheless, the MSS energy content is relatively high compared with other biomasses, confirming its use as feedstock for biofuels production via thermochemical processes.
The pre-dried MSS FTIR spectroscopy pattern is given in Fig. 1. The FTIR obtained results reflect the high oxygenated groups content (large band at 3393/cm attributed to hydroxyl groups (OH) contained in alcohols, phenols and organic acids) and the aromatic character (a large band at 1640/cm corresponding to aromatic groups of C=C) of these raw materials. The results of TGA/DTA of pre-dried MSS, given in Fig. 2, indicate that the MSS thermal decomposition occurs in two major stages: the first one occurring between 50 and 100 °C is related to a drying phase (with mass loss around 4.64 %) and the second step of mass loss (estimated to 46.43%) took place between 200 and 600 °C related to the decomposition of major organic components contained in MSS. After 600 °C, the weight loss, estimated to 3.16% of the MSS original mass, is attributed to the decomposition of inorganic materials. The XRD spectrum of the studied MSS sample given in Fig. 3 highlighted the occurrence of key minerals such as quartz (SiO2), calcite (Ca CO3), dolomite (CaMg(CO3)2), and sugilite (KNa2Fe3+2(Li3Si12)O30).
Optimization of MSS pyrolysis
Analysis of the coefficients of the centered composite experimental design
The linear and quadratic coefficients corresponding to the mathematical models that describe the responses are given in Table 5. The obtained mathematical models for the bio-oil yield (wt%), the biochar yield (wt%), the syngas yield (wt%), the CH4 content (vol%), the H2 content (vol%), the CnHm content (vol%), the CO content (vol%), and the CO2 content (vol%) can be written as follows:
with H: MSS moisture content (%) and T: final pyrolysis temperature (°C).
Analysis of the contour plots
An analysis of the eight responses studied in the experimental design was conducted, and the contour plots of each response are presented in Figs. 4, 5, and 6.
Pyrolysis product yields
For bio-oil production (Fig. 4a), the statistical results reveal that the maximum bio-oil yield (around 29 wt%) is obtained in a temperature range between 580 and 600 °C and a moisture content between 18 and 20%. This result is consistent with experimental data where the pyrolytic oil yield is around 28 wt% under these operating conditions: 600 °C as a final pyrolysis temperature and 20% for moisture content (Table 3).
For biochar production (Fig. 4b), the statistical results indicate that the maximum biochar yield (around 59.4 wt%) is obtained in a temperature range between 500 and 530 °C and a moisture content about 10%. This result is also consistent with experimental data where the char yield is around 58 wt% under these operating conditions: 500 °C as a final temperature and 10% for moisture content (Table 3).
For syngas production (Fig. 4c), the statistical results indicate that the maximum syngas yield (around 29 wt%) is obtained in a temperature range between 500 and 600 °C and a moisture content about 10%. This result is consistent with experimental data where the syngas yield is around 29.2 wt% under these operating conditions: 600 °C as a final temperature and 10% for moisture content (Table 3).
Syngas composition
For CH4 content (Fig. 5a), the statistical results indicate that the maximum CH4 content (around 19%) is obtained at 600 °C and a moisture content about 15%. This result is consistent with experimental data where the yield of CH4 is around 21% under these operating conditions: 600 °C as a final temperature and 20% for moisture content (Table 3).
For H2 and CnHm contents (Fig. 5b, c), the statistical results indicate that the maximum H2 and CnHm contents, around 6% and 9% respectively, are obtained at 600 °C and a moisture content about 20%. These results are consistent with experimental data where the H2 and CnHm yields are around 9% under these operating conditions 600 °C as a final temperature and 20% for moisture content (Table 3).
For CO and CO2 contents (Fig. 6a, b), the statistical results indicate that the maximum CO and CO2 contents, around 9% and 70%, respectively, are obtained at 600 °C and for a moisture content about 20%. These results are consistent with experimental data for CO yield with 9.59% under these operating conditions: 600 °C as a final temperature and 20% for moisture content (Table 3).
Considering all studied responses and factors, the optimal conditions given by Minitab17 are as follows: maximum pyrolysis temperature 550 °C and MSS moisture content 15%.
Pyrolysis product characterization
Bio-oil properties
The GC-MS total ion chromatogram (TIC) of bio-oil fraction obtained at experimental conditions leading to maximum of liquid products (600 °C as final pyrolysis temperature and 20% as MSS moisture content) is given in Fig. 7 and the various identified compounds are given in Table 6. The chemical compound identification highlights the richness of studied bio-oil with hydrocarbons with atom number ranging from C12 to C29. Among the identified compounds, the most abundant are the following: (i) linear saturated hydrocarbons including heptadecane, octadecane; (ii) linear unsaturated hydrocarbons including pentadecene, hexadecene; (iii) carboxylic acids including heptadecenoic acid, linoleic acid, 1,2-benzendicarboxylic acid, hexadecanoic acid, octadecadienoic acid; (iv) cyclic hydrocarbons including cyclopropane, cyclotetracosane; (v) alcohols such as bicyclo[2,2,1]heptan-2-ol and dehydrocholesterol; (vi) ketones such as methylcyclopentanone; (vii) aldehydes such as tetradecanal. These results highlight the high complexity of the composition of bio-oils obtained from MSS pyrolysis due to the heterogeneous character of studied raw materials and to the occurrence of several chemical reactions (condensation, polycondensation or polymerization of the hydrocarbon fragments) with temperature increase. Regarding the high complexity of the chemical composition, the direct use of obtained pyrolytic oil in petroleum-based plants is still difficult. Therefore, we can suggest their application as source of active molecule or for the extraction of chemicals through appropriate refining methods.
These findings are comparable with those reported by Languer et al. (2020) who demonstrated that GC-MS results of bio-oil obtained from MSS pyrolysis indicate high concentrations of monoaromatics and aliphatic hydrocarbon compounds, confirming the potential of these liquid products as biofuels and valuable chemicals. Adhikari et al. (2018) compared the GC-MS molecular composition of some bio-oils samples produced from solid waste and concluded that several chemical organic groups including acids, esters, alcohols, ketones, phenols, aldehydes, alkenes, alkanes, furans, and sugars are usually present in pyrolysis oils and their amounts depend on biomass type and pyrolysis conditions. Besides, in their investigation of GC-MS composition of bio-oil produced from sewage sludge using microwave pyrolysis, Domínguez et al. (2006) identified eight main classes of monoaromatics, aliphatics, acids, esters, amides, nitriles, steroids, and polycyclic aromatic compounds, with a dominance of aromatic compounds (22%) and of carboxylic acids (14%).
Table 7 shows basic fuel properties of obtained bio-oil at experimental conditions leading to maximum of liquid products (600 °C as final pyrolysis temperature and 20% as MSS moisture content), with comparison with those of Tunisian and European Diesel oils (Ben Hassen-Trabelsi et al. 2014) and heavy fuel oil (Weerachanchai et al. 2007). From these results, it can be seen that:
-
Bio-oil density at 15 °C is around 982 kg/m3 which is in the range of conventional crude oils density (around 1000 kg/m3) but lower than the density value of soybean oil cake pyrolysis bio-oil (density at 15 °C around 1107 kg/m3) (Şensöz and Kaynar 2006) and that of bio-oil produced from date seeds (density about 1020 kg/m3) (Bharath et al. 2020). Fonts et al. (2009) reported comparable density values (between 972 and 975 kg/m3) for bio-oils produced from three sewage sludge samples. The bio-oil API degree is around 12.6 which qualify it as a “heavy crude” (< 20° API = heavy crude and > 30° API = light crude).
-
Bio-oil viscosity at 40 °C is about 1.43 cSt and is in the range of viscosity values of petroleum oils (1.5–1.8 cSt for crude oils), but it is lower than the heavy fuel oil viscosity (around 180 cSt; Weerachanchai et al. 2007). Similar viscosity values measured at 25 °C were reported by Bharath et al. (2020) for bio-oils obtained from date tree mixture (1.36 cSt) and for date seeds (1.41 cSt), but very high viscosity values (around 81 cSt) were reported by Wang and Yu (2012) for pyrolytic oil produced from Castor seed and also for bio-oils produced from sewage sludge (between 7.92 and 16.91; Fonts et al. (2009).
-
Bio-oil moisture content is relatively high (18%) compared with conventional fuels but this value complies with standards (ASTM D7544 for pyrolysis bio-oil requirements) which suggests that bio-oil moisture content must be less than 30% on a weight basis. The moisture content of produced bio-oil (18%) is directly linked to raw MSS moisture content (20% in these conditions). Several bio-oils produced from lignocellulosic waste such us oil palm empty fruit bunches, rice husk exhibit moisture contents from 18 to 28.3% (Azri Sukiran et al. 2009; Huang et al. 2020), whereas bio-oils obtained from sewage sludge present moisture contents between 27 and 47% (Fonts et al. 2009). Bio-oil moisture content is associated to moisture content in raw MSS but is usually enhanced by dehydration reactions during thermal cracking (Adhikari et al. 2018). High moisture content in pyrolytic oil is an inconvenience for its direct use as fuel (low calorific value, damage in burners, blockage of filters), but it could be an advantage since few amounts of water could upgrade the fluidity (giving lower viscosity) of the bio-oil.
-
Bio-oil acidity index is around 2.82 mg KOH/g of sample and is in the same range of those obtained for petroleum Diesel (between 0.5 and 5 mg KOH/g of sample). The acidity index of bio-oils obtained from date stones pyrolysis is about 85 mg KOH/g of sample (Fadhil et al. 2017) while acidity index values between 124 and 138 mg KOH/g of sample were reported by Ben Hassen-Trabelsi et al. (2014) for bio-oils produced from fatty animal waste. The bio-oil acidity is associated to the occurrence of acidic functions contained in acids, phenols, sugars, and extractives, and it is usually associated to its oxygen content (Adhikari et al. 2018).
-
Bio-oil energy content: the studied bio-oil HHV is 30.6 MJ/kg. This energy content is slightly lower than those of conventional petroleum fuels (between 46 and 62 MJ/kg; Table 7), but it is higher than the minimum heating value (HHV of 15 MJ/kg) required by the pyrolytic oil specifications (ASTM D7544). The HHV of bio-oil obtained from MSS pyrolysis is in the range of those of bio-oils produced from various biomasses: 33.6 MJ/kg for bio-oil produced from soybean oil cake pyrolysis (Şensöz and Kaynar 2006), 37.38 MJ/kg for pyrolytic oil obtained from date stones (Fadhil et al. 2017), 21.41 MJ/kg for bio-oil obtained from oil palm empty fruit bunches (Azri Sukiran et al. 2009), and 24.35 and 29.06 MJ/kg for bio-oil produced from date tree mixture and date seeds, respectively (Bharath et al. 2020). Fonts et al. (2009) reported HHV values between 30 and 32 MJ/kg for pyrolytic oils obtained from various sewage sludge pyrolysis.
-
Bio-oil flash point is around 43 °C, which still under the minimum flash point value (45 °C) required by pyrolysis bio-oil requirements (ASTM D7544) and also lower than the petroleum diesel, which is between 55 and 156 °C. Bio-oils from agricultural residues exhibit high flash point value: 63 °C for bio-oil produced from soybean oil cake pyrolysis (Şensöz and Kaynar 2006) and 87 °C for pyrolytic oil produced from castor seeds (Fadhil et al. 2017). The flash point determination is essential to establish the maximum temperature of storage or handling to avoid a fire hazard (Adhikari et al. 2018).
As compared with conventional fuels, the restrictive properties of bio-oil produced from MSS are high moisture content, low calorific value, and instability under storage and heating conditions, hindering its direct use as biofuel. Further treat and refinement processes of the crude pyrolytic oil are required to enhance its quality and stability.
FTIR pattern of bio-oil obtained from MSS pyrolysis in the optimum conditions (600 °C and 20% of moisture content) is given in Fig. 8. The FTIR results reveal the abundance of oxygenated compounds revealed mainly by the intense peak at 3416/cm attributable to O–H and N–H stretching vibrations (Alvarez et al. 2016). The O–H groups are related to the presence of moisture and hydroxyl functionalities contained in alcohols or carboxylic acids, whereas the N–H function is associated to amines and amides from the degradation of proteins contained in MSS (Pokorna et al. 2009; Alvarez et al. 2016). The band at 1710/cm which corresponds to the stretching bands of the C=O confirms the richness of bio-oil with carboxylic acids. The aliphatic character of crude pyrolytic oil is revealed by the band around 1402/cm (vibrations of CH2 and CH3 groups). The small peaks between 1600 and 1500/cm confirms the presence of amides in the studied bio-oil (Domínguez et al. 2006; Pokorna et al. 2009). These results are consistent with those reported in the literature, i.e., comparable FTIR patterns have been reported by Domínguez et al. (2006), Pokorna et al. (2009), and Alvarez et al. (2016) for bio-oils obtained from sewage sludge using conventional and other pyrolysis technologies such as microwave and flash pyrolysis. Due to the abundance of oxygenated groups contained in phenols, carboxylic acids, esters, aldehydes, ketones, sugars, and bio-oil chemical composition give rise to heterogeneous fuel unstable over time, hindering its widespread applications.
Biochar properties
Biochar produced from MSS in optimal conditions (550 °C as final pyrolysis temperature and 15% as MSS moisture content) presents low moisture content (1.66%), high volatile matter content (38%), and relatively high ash content (55%) due to the high inorganic matter content in raw MSS. Compared with other biochars produced from agricultural biomass such as wheat straw, sweet potato vine, corn stalk, and peanut shell, which exhibit ash contents between 8 and 24% (Sun et al. 2017), the high ash content of studied biochar gives it unattractive properties to be used as solid biofuel. However, due to its high organic matter content and to its carbon sequestration potential, produced biochar from MSS can serve as a soil additive where it acts for stimulating several biological processes and soil moisture retention and thus promoting plant growth and dynamics of soil microorganisms.
The FTIR spectrum of the obtained biochar in optimal conditions (550 °C as final pyrolysis temperature and 10% of moisture content) is given in Fig. 9. The IR pattern reveals the richness of biochar with organic compounds and namely with aliphatic and oxygenated functional groups (1420 and 3424 and 1112/cm, respectively). The IRTF pattern of biochar obtained from MSS pyrolysis reveals also the presence of typical patterns of alumina-silicates (stretching vibration at 3500–3300/cm, 1650–1600/cm, 1100–1000/cm, and 550–450/cm) and shows also the specific absorption bands around 1420 for carbonates (Pokorna et al. 2009). Many small overlapped peaks between 875 and at 700/cm are usually assigned to CaCO3 (Pokorna et al. 2009). The characterization of biochar obtained from MSS reveal substantial promise for using it for soil restoration, as a fertilizer in replacement of raw MSS which is highly concentrated with pathogens and toxic compounds. Since biochar properties are associated to feedstock and production conditions, and considering the specificity of raw feedstock (MSS), further characterization for produced biochar produced from MSS as specific amendment and deep investigations of soil application and in improving soil quality and stability may be necessary to achieve positive outcomes.
Syngas chemical composition
Figure 10 gives the non condensable gases composition variation with pyrolysis increasing temperature. At the maximum cracking temperature (550 °C), the syngas is formed by around 9.04 vol% of CO, 45.81 vol% of CO2, 15.43 vol% of CH4, 4.2 vol% of H2, and 6.980 of CnHm, with an energetic value around 8 MJ/m3. The high CH4 and CO2 content and the relatively low H2 and CO contents of the gas at 550 °C could be attributed to the fact that exothermic reactions (Eqs. 8 and 7) were mainly favored by the carbon dioxide and hydrogen (Domínguez et al. 2006):
These reactions are at the origin of the observed change in gas composition as a function pyrolysis time. Similar gas composition has been observed during the MSS pyrolysis using microwave technology (Domínguez et al. 2006). In their study, while comparing syngas composition using conventional and microwave pyrolysis, they reported that syngas (H2 + CO mixture) yield is much higher in microwave than in conventional pyrolysis. The high content of methane and light hydrocarbons gave to the produced gaseous mixture a good calorific value. Regarding its good heating value, the obtained gas mixture could be used for MSS drying process or as a renewable source of heating for the pyrolysis reactor.
Conclusions
MSS effective management, renewable bioenergy resource exploitation, and sustainable biofertilizer use are among key challenge in many developing countries like Tunisia. This study demonstrates that pyrolysis technology could be presented as an alternative method to replace the conventional MSS treatment in Tunisia (mechanical dehydration and landfilling), in terms of MSS volume reduction and of energy recovery with the valorization of all obtained products (bio-oil as liquid biofuel, syngas for pyrolysis reactor heating, and biochar as biofertilizer). Under optimum conditions of 550 °C and 15% of MSS moisture content, moderate pyrolysis (15 °C/min for heating rate) proved to be an innovative process which leads to an energy recuperation of 48% as biofuels (bio-oil and syngas). The remaining 52% is composed of solid pyrochar which could be applied for agronomic purposes as amendment. Further investigations of the biochar application as soil amendment and the evaluation of soil properties change need to be performed, but initial results have been promising towards the generation of biofuels and bio-products from MSS in Tunisia. This paper provides a useful reference to help future decisions for the implementation of large scale pyrolysis plants within MSS-to-energy industry, as an element of circular economy approach, which will guarantee the generation of economic and environmental benefits such as MSS management problems, pollution, and fossil fuel and chemical fertilizer consumption.
Data availability
Not applicable.
References
Adhikari S, Nam H, Chakraborty JP (2018) Conversion of solid wastes to fuels and chemicals through pyrolysis. Waste Biorefinery 8:239–263. https://doi.org/10.1016/B978-0-444-63992-9.00008-2
Agar DA, Kwapinska M, Leahy JJ (2018) Pyrolysis of wastewater sludge and composted organic fines from municipal solid waste: laboratory reactor characterisation and product distribution. Environ Sci Pollut Res 25:35874–35882. https://doi.org/10.1007/s11356-018-1463-y
Agrafioti E, Bouras G, Kalderis D, Diamadopoulos E (2013) Biochar production by sewage sludge pyrolysis. J Anal Appl Pyrolysis 101:72–78. https://doi.org/10.1016/j.jaap.2013.02.010
Alvarez J, Lopez G, Amutio M, Artetxe M, Barbarias I, Arregi A, Bilbao J, Olazar M (2016) Characterization of the bio-oil obtained by fast pyrolysis of sewage sludge in a conical spouted bed reactor. Fuel Process Technol 149:169–175. https://doi.org/10.1016/j.fuproc.2016.04.015
Azri Sukiran M, Chin CM, Abu Bakar NK (2009) Bio-oils from pyrolysis of oil palm empty fruit bunches Malaysian palm oil board, No. Am J Appl Sci 6:869–875
Ben Hassen-Trabelsi A, Kraiem T, Naoui S, Belayouni H (2014) Pyrolysis of waste animal fats in a fixed-bed reactor: production and characterization of bio-oil and bio-char. Waste Manag. 34:210–218. https://doi.org/10.1016/j.wasman.2013.09.019
Bharath G, Hai A, Rambabu K, Banat F, Jayaraman R, Taher H, Bastidas-Oyanedel JR, Ashraf MT, Schmidt JE (2020) Systematic production and characterization of pyrolysis-oil from date tree wastes for bio-fuel applications. Biomass and Bioenergy 135:105523. https://doi.org/10.1016/j.biombioe.2020.105523
Djandja OS, Wang Z, Wang F, Xu YP, Duan PG (2020) Pyrolysis of municipal sewage sludge for biofuel production: a review. Ind Eng Chem Res. 59:16939–16956. https://doi.org/10.1021/acs.iecr.0c01546
Domínguez A, Menéndez JA, Inguanzo M, Pís JJ (2006) Production of bio-fuels by high temperature pyrolysis of sewage sludge using conventional and microwave heating. Bioresour Technol 97:1185–1193. https://doi.org/10.1016/j.biortech.2005.05.011
Eke J, Onwudili JA, Bridgwater AV (2020) Influence of moisture contents on the fast pyrolysis of trommel fines in a bubbling fluidized bed reactor. Waste and Biomass Valorization 11:3711–3722. https://doi.org/10.1007/s12649-018-00560-2
Fadhil AB, Alhayali MA, Saeed LI (2017) Date (Phoenix dactylifera L.) palm stones as a potential new feedstock for liquid bio-fuels production. Fuel 210:165–176. https://doi.org/10.1016/j.fuel.2017.08.059
Fan J, Li Y, Yu H, Li Y, Yuan Q, Xiao H, Li F, Pan B (2020) Using sewage sludge with high ash content for biochar production and Cu(II) sorption. Sci Total Environ 713:136663. https://doi.org/10.1016/j.scitotenv.2020.136663
FAO (2016) http://www.fao.org/faostat/en/#data/
Fonts I, Azuara M, Gea G, Murillo MB (2009) Study of the pyrolysis liquids obtained from different sewage sludge. J Anal Appl Pyrolysis 85:184–191. https://doi.org/10.1016/j.jaap.2008.11.003
Fonts I, Gea G, Azuara M, Abrego J, Arauzo J (2012) Sewage sludge pyrolysis for liquid production: a review. Renew Sustain Energy Rev 6:2781–2805. https://doi.org/10.1016/j.rser.2012.02.070
Gao N, Kamran K, Quan C, Williams PT (2020) Thermochemical conversion of sewage sludge: a critical review. Prog Energy Combust Sci 79:100843
Huang Y, Li Y, Han X, Zhang J, Luo K, Yang S, School JW (2020) Investigation on fuel properties and engine performance of the extraction phase liquid of bio-oil/biodiesel blends. Renew Energy 147:1990–2002. https://doi.org/10.1016/j.renene.2019.10.028
Inguanzo M, Domínguez A, Menéndez JA, Blanco CG, Pis JJ (2002) On the pyrolysis of sewage sludge: the influence of pyrolysis conditions on solid, liquid and gas fractions. J Anal Appl Pyrolysis 63:209–222
Karaca C, Sözen S, Orhon D, Okutan H (2018) High temperature pyrolysis of sewage sludge as a sustainable process for energy recovery. Waste Manag 78:217–226. https://doi.org/10.1016/j.wasman.2018.05.034
Kraiem T, Ben HA, Belayouni H, Jeguirim M (2017) Production and characterization of bio-oil from the pyrolysis of waste frying oil. Environ Sci Pollut Res. 24:9951–9961. https://doi.org/10.1007/s11356-016-7704-z
Languer MP, Batistella L, Alves JLF, Da Silva JCG, Filho VFS, Di Domenico M, Moreira RFPM, José HJ (2020) Insights into pyrolysis characteristics of Brazilian high-ash sewage sludges using thermogravimetric analysis and bench-scale experiments with GC-MS to evaluate their bioenergy potential. Biomass and Bioenergy 138:105614. https://doi.org/10.1016/j.biombioe.2020.105614
ONAS (2016) Office National d’Assainissement, Rapport Annuel 2016, 26 pages. www.onas.nat.tn
ONE (2017) Observatoire National de l’Energie, Conjoncture énergétique, Rapport mensuel, Edition janvier 2017, 26 pages. http://data.industrie.gov.tn/
Oyebanji JA, Okekunle PO, Lasode OA, Oyedepo SO (2018) Chemical composition of bio-oils produced by fast pyrolysis of two energy biomass. Biofuels 9:479–487. https://doi.org/10.1080/17597269.2017.1284473
Pokorna E, Postelmans N, Jenicek P, Schreurs S, Carleer R, Yperman J (2009) Study of bio-oils and solids from flash pyrolysis of sewage sludges. Fuel 88:1344–1350. https://doi.org/10.1016/j.fuel.2009.02.020
Praspaliauskas M, Žaltauskaitė J, Pedišius N, Striūgas N (2020) Comprehensive evaluation of sewage sludge and sewage sludge char soil amendment impact on the industrial hemp growth performance and heavy metal accumulation. Ind Crops Prod 150:112396. https://doi.org/10.1016/j.indcrop.2020.112396
Schnell M, Horst T, Quicker P (2020) Thermal treatment of sewage sludge in Germany: a review. J Environ Manage 263:110367
Şensöz S, Kaynar I (2006) Bio-oil production from soybean (Glycine max L.); fuel properties of bio-oil. Ind Crops Prod 23:99–105. https://doi.org/10.1016/j.indcrop.2005.04.005
Shao J, Yan R, Chen H, Wang B, Lee D, Liang D (2008) Pyrolysis characteristics and kinetics of sewage sludge by thermogravimetry Fourier transform infrared analysis†. Energy & Fuels 22:38–45. https://doi.org/10.1021/ef700287p
Sheng C, Azevedo JLT (2005) Estimating the higher heating value of biomass fuels from basic analysis data. Biomass and Bioenergy 28:499–507. https://doi.org/10.1016/j.biombioe.2004.11.008
Sobek S, Werle S (2020) Solar pyrolysis of waste biomass: part 2 kinetic modeling and methodology of the determination of the kinetic parameters for solar pyrolysis of sewage sludge. Renew Energy 153:962–974. https://doi.org/10.1016/j.renene.2020.02.061
Sun J, He F, Pan Y, Zhang Z (2017) Effects of pyrolysis temperature and residence time on physicochemical properties of different biochar types. Acta Agric Scand Sect B Soil Plant Sci 67:12–22. https://doi.org/10.1080/09064710.2016.1214745
Thipkhunthod P, Meeyoo V, Rangsunvigit P, Rirksomboon T (2007) Describing sewage sludge pyrolysis kinetics by a combination of biomass fractions decomposition. J Anal Appl Pyrolysis 79:78–85. https://doi.org/10.1016/j.jaap.2006.10.005
Trabelsi ABH, Ghrib A, Zaafouri K, Friaa A, Ouerghi A, Naoui S, Belayouni H (2017) Hydrogen-rich syngas production from gasification and pyrolysis of solar dried sewage sludge: experimental and modeling investigations. Biomed Res Int. https://doi.org/10.1155/2017/7831470
Wang L, Yu H (2012) Biodiesel from Siberian apricot (Prunus sibirica L.) seed kernel oil. Bioresour Technol 112:355–358. https://doi.org/10.1016/j.biortech.2012.02.120
Weerachanchai P, Tangsathitkulchai C, Tangsathitkulchai M (2007) Fuel properties and chemical compositions of bio-oils from biomass pyrolysis. JSAE 20077087:1224–1231
Zaafouri K, Ben Hassen Trabelsi A, Krichah S, Ouerghi A, Aydi A, Claumann CA, Wüst CA, Naoui S, Bergaoui L, Hamdi M (2016) Enhancement of biofuels production by means of co-pyrolysis of Posidonia oceanica (L.) and frying oil wastes: experimental study and process modeling. Bioresour Technol 207:387–398. https://doi.org/10.1016/j.biortech.2016.02.004
Acknowledgments
The authors thank the National Sanitation Office in Tunisia (ONAS) for kindly providing the MSS samples for this study. All authors acknowledge Mrs. Rym Zayoud for her contribution in the pyrolysis experiments and in RSM result interpretation.
Funding
The research leading to these results received funding from the “Ministry of Higher Education and Scientific Research in Tunisia (MESRST)” and the National Research Project: “Thermo-chemical conversion of solid waste into renewable biofuels and bio-products.” Project Leader: Aïda Ben Hassen Trabelsi.
Author information
Authors and Affiliations
Contributions
ABHT: verified the analytical results, supervised the findings of this work, and has a major contribution in the redaction of the manuscript. KZ: conceived and planned the experiments by building CCD model, analyzed RSM results, and contributed in writing manuscript. AF: carried out pyrolysis experiments, interpreted RSM results with supports of KZ, and contributed in writing manuscript. SA: contributed in the raw materials and pyrolysis product analyses. SN and FJ: helped in pyrolysis facility setup and monitoring. All authors discussed the results, provided critical feedback, and helped shape the research, analysis, and manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: Ta Yeong Wu
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Trabelsi, A.B.H., Zaafouri, K., Friaa, A. et al. Municipal sewage sludge energetic conversion as a tool for environmental sustainability: production of innovative biofuels and biochar. Environ Sci Pollut Res 28, 9777–9791 (2021). https://doi.org/10.1007/s11356-020-11400-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-11400-z