Abstract
There is an increasing interest in product recovery, closed-loop supply chains, and reverse logistics (RL) for mitigating environmental impairment. Although RL is becoming a mandatory policy in developed countries, it is still in an embryonic stage in some industrial sectors of emerging economies. The purpose of this study is twofold: (1) identify the critical factors to the successful implementation of RL in the Brazilian pharmaceutical care process (PCP) and (2) determine the cause-and-effect relationships among them. We use snowball sampling to select the relevant RL studies and deductive reasoning and classification to identify the critical factors and a grey decision-making trial and evaluation laboratory (DEMATEL) to evaluate the cause-and-effect relationships among them. The study revealed management, collaboration, information technology, infrastructure, policy, financial and economic, end-of-life management practices, and logistic performance factors as the most relevant factors to the successful implementation of RL in the Brazilian PCP. The end-of-life management practices were identified as the most critical factor, and information technology was identified as the least critical factor. We further determined the end-of-life management practices and policy have the strongest casual relationship. The municipal PCP coordinators can use the findings of this study to formulate mitigating strategies to identify and eliminate barriers to the successful implementation of RL in the Brazilian PCP.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The sustainable management and conservation of resources require sustainable strategic initiatives by business and government (Scavarda et al. 2019; Prakash and Barua 2016). Reverse logistics (RL) is a strategic initiative that contributes to the conservation of natural resources. Guarnieri et al. (2020) have argued RL improves competitiveness, reduces waste, provides greater profitability, and improves customer relationships. Other seminal publications reinforce the economic and social values provided by RL (Rogers and Tibben-lembke 2001; Prahinski and Kocabasoglu 2006).
Paula et al. (2019) highlighted the environmental impacts of the pharmaceutical supply chains as solid waste and wastewater generation (caused by incorrect discharges), human well-being (increased aging and demographic change leading to an escalating dependency on medicines), and social equality (contrasting with lack of access, high prices, and losses in supply chains). RL practices have been an integral part of sustainable development strategies, and it is an important approach for the efficient use of resources, minimizing waste from end-of-use/end-of-life (EOU/EOL) products, and being driven by regulatory issues (Viegas et al. 2019). Although many countries face difficulties in RL implementation, the problem is more serious in developing countries (Chauhan et al. 2018; Scavarda et al. 2019), where the rate of economic growth and urbanization is quickly increasing while the legislation is often incipient (Bouzon et al. 2016). When it comes to unused EOU/EOL medicine, disposal has been a high priority problem and a concern for public health professionals, governments, and society (Aquino et al. 2018; Kelly et al. 2018; Vellinga et al. 2014).
Pharmaceutical waste has undesirable effects with significant economic and environmental consequences, particularly when it is discarded illegally in landfills, rivers, or oceans. Thus, the implementation of collection strategies requires greater attention (Bungau et al. 2018; Guirguis 2010). With regard to the disposal of unused and expired EOU/EOL medicine, a study conducted by Al-Shareef et al. (2016) in Saudi Arabia, revealed that 79.15% of medication users dispose of their unwanted medication through household waste; 7.04% dispose of them into the toilet or sink; 4% do not know what to do with them; 3.71% send them for hazardous waste collection; 1.70% returned them to their doctor; 1.70% return them to the pharmacy; 1.60% give them to someone else; 0.70% never dispose them; and 0.60% discarded them some other ways. Similar results were found in Illinois, in a study by (Wieczorkiewicz et al. 2013).
Pinto et al. (2014) conducted a behavior survey with undergraduate students to understand what practices are adopted for the disposal of EOU/EOL medicine in Brazil. Results reveal that 62% of the students disposed of their unwanted medicine as ordinary garbage, 19% have a habit of discarding them in running water, 10% returned the medication to a health center, pharmacy, or community center, 4% disposed everything as recyclable waste, and 5% adopted other disposal practices. In many cases, pharmaceutical professionals recognize health waste collection as a new pharmaceutical service in addition to their daily responsibilities (Manojlović et al. 2015).
The pharmaceutical care process (PCP) implemented by the Brazilian Health Ministry is a structured set of procedures, part of the broader pharmaceutical supply chain, which includes selection, programming, acquisition, storage, distribution, and dispensation (SPASDD), supplying medicines to Brazilian citizens (Brazil 2001). While the health agencies have regularly forbidden the recovery of EOU/EOL medicine in the PCP, many professionals, government workers, and system users have questioned this policy for a long time. The 12,305/10 Solid Residues Act (Brazilian Solid Waste Policy 2010) has played an important role in maintaining the subject on the front burner. The disposal of medicines may be a complex discussion, albeit necessary, that requires efforts from the entire society, as well as various private and public sectors. There is an urgent need to accelerate the discussion and implement pilot drug collection programs (Medeiros et al. 2014). Campos et al. (2017) have identified regulatory and stakeholders’ educational perspective by calling attention to the need for professional awareness throughout the pharmaceutical supply chain. These authors mention that, ideally, pharmacists occupy a privileged position in the PCP for collecting unused medications and educating the population.
Due to its complexity, different countries do not have guidelines for the proper disposal of unused medicines (Tong et al. 2011). Moreover, the disposal of unused medicine becomes challenging as medication use increases, and the pharmaceutical return process encounters several barriers along the RL supply chain (Yazdani et al. 2020; Abbas and Farooquie 2013; Viegas et al. 2019; Van Der Wiel et al. 2012). According to Pereira et al. (2017), the lack of agreement for the pharmaceutical sector may lead to serious environmental and public health challenges. Therefore, the identification and classification of these critical factors are prerequisites to the successful implementation of RL (Kumar and Dixit 2018; Prakash and Barua 2016).
Different issues may inhibit effective healthcare solid waste management, such as political, governmental, financial, and technological issues, among others (Yazdani et al. 2020). The rationale behind this study is to empirically understand the critical factors that prevent the implementation of pharmaceutical waste management in the public health sector, specifically in the PCP, so that it is possible to create guidelines for addressing these barriers. We formulate the following research questions in this study:
-
Research question 1: What are the critical factors to the successful implementation of RL in the PCP?
-
Research question 2: What are the cause-and-effect relationships among these critical factors?
For this purpose, we propose a multi-criteria decision analysis (MCDA) model using the grey decision-making trial and evaluation laboratory (grey-DEMATEL).
The remainder of this manuscript is organized as follows. The second section presents the literature review on the RL critical factors. The third section presents an explanation of the grey-DEMATEL method. The fourth section shows the application of the proposed method, and the fifth section discusses the results. Finally, the last section closes the paper by presenting the concluding remarks and suggestions on future research directions.
Theoretical references
Abbas and Farooquie (2013) and Prajapati et al. (2019) argue every RL implementation faces obstacles, and it is vital to identify and eliminate these barriers for a successful application, especially in the pharmaceutical supply chains comprising the disposal of unused medicine. They also show that MCDA tools and techniques are most uniquely effective in identifying the barriers to the successful implementation of RL. In this section, we review the MCDA applications in RL.
MCDA Applications in RL
Rahman and Subramanian (2012) proposed a framework for EOL computer recycling operations by identifying the critical factors in implementing recycling operations. They investigated the causal relationship between factors that influence recycling operations using DEMATEL. The results indicate that resource availability, coordination and integration of recycling tasks, and volume and quality of recyclables are critical to computer recycling operations. Factors such as government legislation, incentive, and customer demand were identified as the main drivers.
Prakash et al. (2015) proposed a fuzzy analytic hierarchy process (FAHP) to identify and weigh the importance of the barriers to RL implementation. They also used the technique for order of preference by similarity to ideal solution (TOPSIS) to classify these barriers and formulate strategies for their elimination. Prakash and Barua (2016) presented a FAHP-based MCDA method for prioritizing barriers to the adoption of RL in the electronic industry. They identified 38 barriers through literature review and expert opinions. These barriers were classified into seven main categories. The result of the study indicated that customer acuity in RL, lack of coordination/collaboration with third-party logistic providers, and uncertain quality and return time are the three most significant barriers to RL adoption. Xia et al. (2015) employed the grey-DEMATEL method to analyze the internal barriers in the automotive parts industry. They showed the cause/effect relationships could be used by the remanufacturers to eradicate the main internal barriers and increase productivity.
Govindan et al. (2016) investigated the key barrier to remanufacturing and addressed crucial interrelations and interdependencies in this industry. The data obtained were processed using two solution methodologies, interpretive structural modeling (ISM), and fuzzy analytic network process. They showed the higher cost and the lack of acceptance by the customer were the key barriers in the automotive industry. Chauhan et al. (2018) used the combination of MCDA, ISM, and DEMATEL to identify the barriers that deter the establishment of waste recycling units. Their study suggests that the lack of financial resources, raw material, and government subsidies are the most critical barriers to the successful implementation of waste recycling infrastructure. Agrawal et al. (2016) reviewed and prioritized the critical success factors (CSFs) for the successful implementation of RL in the electronic industry. Twelve CSFs were identified through a literature review, and a discussion with industry experts, and the following four factors were identified as CSFs: top management awareness, resource management, economic factors, and contract terms and conditions. They also identified process capabilities and skilled workers as the two least critical factors. The findings were useful for successful RL implementation in the electronic industry. The authors have employed the TOPSIS to prioritize the CSFs, and they affirmed that the factors prioritized in their investigation were critical to RL implementation. Similarly, Mangla et al. (2016) used the AHP and DEMATEL methods to evaluate the CSFs in the adoption of RL in manufacturing. Their results show the importance of global competitiveness in RL.
Waqas et al. (2018) studied the barriers to RL implementation in the manufacturing industry by combining the Delphi methodology and structural equation modeling. They identified high cost of RL adoption, lack of skilled professionals, lack of supporting policies, lack of organizational culture, lack of human resources, lack of knowledge of environmental laws, lack of community pressure, and company policies as the most critical barriers. Sivakumar et al. (2018) used DEMATEL to evaluate the barriers to sustainable practices for EOL products. They evaluated the strength of the relationships between 18 barriers in the used plastic industry and identified the most prevalent barrier. Sirisawat and Kiatcharoenpol (2018) focused on the classification and ranking of barriers to RL implementation in the electronic industry and proposed a methodology based on fuzzy AHP and fuzzy TOPSIS, in which fuzzy AHP was used to obtain the weights of each barrier and TOPSIS was used for the final ranking of the RL implementation barriers. Kumar and Dixit (2018) used DEMATEL to identified the barriers that should be addressed urgently to manage the waste issue in consumer electronics. They also recommended that a combination of the method with structural equation modeling could be used to validate the proposed model statistically. Bouzon et al. (2018) evaluated the barriers to RL implementation in the Brazilian consumer electronic industry from the stakeholders’ perspective. They used grey-DEMATEL and identified organizational barriers as the most dominating barrier in the Brazilian consumer electronic industry. RL can no longer be treated as an auxiliary strategy, especially in industries susceptible to product recall and perishable products such as the pharmaceutical industry (Ali and Abdelsalam 2017). We summarize by emphasizing on the importance of RL in the pharmaceutical care process with EOU/EOL medical products and medicine.
Critical factors in the implementation of pharmaceutical RL
Abdulrahman et al. (2014) and Mangla et al. (2016) argue that “critical barriers” and “critical factors” are synonymous in the context of RL implementation of RL. We use the term critical factors in this study. We use a literature review with the construction of a bibliographic portfolio to identify the critical factors in the PCP. Initially, a literature review is conducted for the period between 2000 and 2019 with the keywords “reverse logistics” AND “critical barriers” OR “critical factors”; “reverse logistics” AND “implementation” in the following databases: Web Science, Science Direct, Emerald, and Google Scholar. We used the Snowball procedure (Biernacki and Waldorf 1981; Wohlin 2014) and selected a portfolio of 78 papers related to the implementation of RL in different sectors. There is an increase in the number of papers from 2008 on, and no publication was identified in 2019. The distribution of papers by year is presented graphically in Appendix Fig. 3. We pooled the following eight factors by using deductive reasoning and classification method proposed by Abdulrahman et al. (2014) and Mangla et al. (2016): management factor (MF), collaboration factor (CF), information technology factor (ITF), infrastructure factor (IF), policy factor (PF), financial and economic factor (FEF), EOL management practices (EOL-MP), and logistic performance factor (LPF). Table 1 shows a brief description of these factors.
Table 1 summarizes the content analysis and the classification of the critical factors for the 78 papers reviewed. As shown in this table, MF, CF, ITF, PF, and FEF factors were more frequently mentioned in the literature. These critical factors and their attributes are presented in Appendix Table 7.
Proposed framework
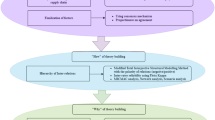
In this section, we present the grey-DEMATEL framework proposed in this study to analyze the barriers to the implementation of RL in the PCP. The proposed framework depicted in Fig. 1 is composed of three distinct phases. In phase 1, we identify a comprehensive list of the critical factors through literature review (snowball procedure) and qualitative interviews with experts (see Appendix Table 7). In phase 2, we use the grey-DEMATEL approach to select the most critical factors for the successful implementation of RL in the pharmaceutical care process. In phase 3, we analyze and synthesize our findings and propose the managerial and practical implications of our study.
Context of application
The Brazilian Unified Health System - Sistema Único de Saúde (SUS) is one of the most intricate public health systems in the world, with services ranging from simple treatment to more complex cases (Brazilian Ministry of Health 2019). This healthcare network is structured with the primary health care (PHC), which consists of basic health units, mobile care service community agents, emergency care units or Unidade de Pronto Atendimento (UPA), and medium and high complexity care provided in hospitals. The secondary and the tertiary care levels, not the scope of this investigation, are generally provided in hospitals and consist of services with higher technological density and greater complexity (Mendes 2011). There is a clear need for efficiency in the regional health management systems (Zare et al. 2019).
The PCP cycle is a part of the pharmaceutical supply chain and comprises drug selection, scheduling, procurement, storage, distribution, and dispensing operations not only to serve the citizens by the entire network of UPAs but also to serve public and private hospitals. The city of Porto Alegre in Brazil has 1.6 million citizens with access to medications, for free, at ten municipal pharmacies and 145 UPAs, which receive medications from the city medicine inventory called Pharmaceutical Supply Center. In the context of the public health system, drugs are procured by bidding, based on the municipal list of essential drugs, buy means of the PCP cycle.
Data collection
In the first step of data collection, we interviewed the pharmacists who coordinate each of the ten municipal pharmacy and on-site observations to become familiar with the PHC direct and reverse flows (see Fig. 1). In the second step, after the development of the critical factors matrix (Appendix Table 8), new interviewees, including professionals that were involved with the working group for the development of the solid waste management plan for the municipality, were contacted. In total, 14 interviews were conducted. We limited the size of the decision-making group to a minimum of five and a maximum of fifty members, as suggested by Ranjan et al. (2016), and used the data collection instrument presented in Appendix Table 8.
The complete team of experts was made up of managers from different municipal pharmacies and health clinics in the public health system, waste managers from public hospitals, the municipal public cleaning department, and the sanitary surveillance of the city of Porto Alegre, Brazil. More information on these expert profiles is provided in Table 2.
All the interviewees and responding experts in the pairwise comparison matrix have direct or indirect experience in pharmaceutical or hospital waste management, and most of them were involved with the working group. The sample was considered a reasonable representative of the PCP since each expert performed a different role in the system. The interviews were administered face-to-face and on an individual basis, within 2 months, depending on the availability of the interviewees. The representatives from the public urban cleaning company were included in the process to represent the opinions of this stakeholder group that impacts the reverse flow of medicine in the PCP cycle.
Justification for choosing the grey-DEMATEL method in the study context
Bai and Sarkis (2013) argue DEMATEL is a useful method for uncovering causal knowledge from the causal analysis. The uncovered causal knowledge is instrumental in advancing the quality of decision-making and thus enabling the process of converting strategic objectives into practical actions. DEMATEL is a powerful MCDA method developed by the Geneva Research Centre of the Battelle Memorial Institute (Gabus and Fontela 1972) for collecting knowledge from a group of experts and visualizing the interrelationships with a cause/effect relationship diagram. Shaik and Abdul-Kader (2014) justify the applications of DEMATEL by highlighting its strengths as follows:
-
1.
Providing a graphical output and presenting the reciprocal relationship of the factors under study numerically
-
2.
Visualizing the feedback relationships among the factors at every level (the same, upper, and lower levels)
-
3.
Presenting the importance weight of each factor in comparison with the influence of all other factors in the system
Therefore, DEMATEL is considered a versatile method. When compared with interpretive structural modeling (ISM), DEMATEL allows for greater discrimination of measures (ISM uses binary levels, while DEMATEL permits variations in the strength of relationships). Moreover, unlike fuzzy cognitive mapping (FCM), ISM, and other casual maps, DEMATEL allows two-way relationships (Bai and Sarkis 2013). Compared with the AHP method, DEMATEL allows for possible multiple directional relationships, whereas AHP utilizes a unidirectional relationship and various separate matrices which require integration (Zhu et al. 2011).
However, even considering its many advantages, DEMATEL has its weaknesses (Bouzon et al. 2020). For instance, DEMATEL is unable to deal with uncertainty and lack of information (Bai and Sarkis 2013). In order to overcome this limitation, we integrated the grey system theory with DEMATEL because RL is not currently used in the PCP flows due to legal and safety reasons, and the experts’ opinions are naturally uncertain. The level of uncertainty in the responses was inherent and concern, the application sector had data that could be inaccurate, since experts were contacted at different times. When compared with the fuzzy set theory, the major benefit of a grey system is its flexible capability in pattern detection and low necessity on sample data (Yang and John 2003). Therefore, the integration of the grey system theory with DEMATEL allows for the representation of vague, imprecise, and incomplete information (Xia et al. 2015). Thus, grey-DEMATEL is used in scenarios where there is uncertainty in expert responses concerning the relationships among the factors. The grey-DEMATEL steps are described in the next section.
Steps of the grey-DEMATEL approach
The grey system theory was initially proposed by (Deng 1982) for representing vague, imprecise, and uncertain information. The grey-DEMATEL method comprises the following steps (Xia et al. 2015; Zhu et al. 2011).
-
Step 1:
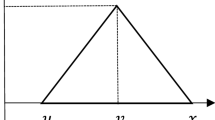
The experts are asked to indicate the influence exerted by each critical factor when compared with other critical factors using a scale ranging from 0 to 4 (where 0 indicates no influence, 1 indicates low influence, 2 indicates moderate influence, 3 indicates high influence, and 4 indicates very high influence). In this case, n represents the total number of experts. The grey scales used to represent these linguistic values are defined in Table 3.
-
Step 2:
An 8 × 8 grey-direct relation matrix X was defined for the grey pairwise influence relation comparison (⊗\( {x}_{ij}^k \)), which was constructed based on the experts’ judgments, generating 14 matrices with 64 comparisons for each expert. The diagonal values in the matrix are represented by 0 because there is no interrelation between a factor and itself. \( \otimes {X}_{ij}^k \) represents this grey-direct relation matrix:
where \( \otimes {x}_{ij}^k \) represents the grey number from an evaluator k who assesses the influence of barrier i on barrier j.
Each expert is given a brief description of the relevant factors with each pairwise matrix. Zhu et al. (2011) proposed a three-stage procedure to convert the grey numbers into crisp numbers using the modified communication function classification system, as demonstrated in Eqs. (1) to (4):
-
Step 3:
Normalize the values:
where \( \underline{\otimes}{\overline{x}}_{ij}^k \) and \( \overline{\otimes}{\overline{x}}_{ij}^k \)are the lower and upper bounds of ⊗X, respectively, and \( {\Delta }_{\mathrm{min}}^{\mathrm{max}}=\underset{j}{\max}\overline{\otimes}{x}_{ij}^k-\underset{j}{\min}\underline{\otimes}{x}_{ij}^k \).
-
Step 4:
Determine the total normalized crisp value:
-
Step 5:
Determine the final crisp value:
-
Step 6:
Develop a crisp direct relation matrix for each expert and transforming matrix X into a crisp matrix denominated Z. It is possible to derive an average matrix N from a group of experts’ direct matrices.
- 1
-
Step 8:
Determine the total relation matrix (T) by using Eq. (7):
where I is denoted as an identity matrix and T as a total-relation matrix.
-
Step 9:
Determine the row Ri and column Dj sums for row i and column j in the total relation matrix using Eqs. (8) and (9):
-
Step 10:
Determine the overall importance or prominence (Pi) and net effect (Ei) of each critical factor. In this case Pi, the overall prominence or importance of critical factor i, is described regarding the overall relationships with other critical factors. According to Tzeng et al. (2007), the larger the value of Pi is, the greater the overall prominence of critical factor i will be. If the net effect Ei > 0, then critical factor i is a net cause for the other critical factor. If the net effect Ei < 0, the critical factor relies on the (net effect of) operation of the other critical factor.
-
Step 11:
Determine the strongest relationships between the critical factors and calculate the threshold value θ by constructing the digraph relationship for each critical factor (relative to other critical factors) using the total relation matrix T. In this case, the relations with threshold value plus 1 standard deviation is plotted on the digraph as shown in italic font in Table 5.
Results
This section presents the results of each step of the grey-DEMATEL method, which was applied considering the perspectives of 14 experts in the context of PCP.
Interrelations of the group of factors
Bearing in mind the context of PCP cycle selection, scheduling, procurement, storage, distribution, and dispensing operations, the experts should analyze how the critical factors presented might have impacted pharmaceutical RL in this process. Data related to the opinion of each expert were obtained in the form of an 8 × 8 matrix, using a pairwise comparison matrix. The total and non-negative matrices of the experts consulted are presented in Appendix Fig. 4.
The factors presented for analysis were the following: MF, CF, ITF, IF, PF, FEF, EOL-MP, and LPF. Table 4 shows the values that were derived from the normalized direct influence matrix (see the “Steps of the grey-DEMATEL approach” section).
Subsequently, to develop the cause and net effect diagram, the sum of the values of matrix T was calculated and multiplied by the number of factors squared. We considered relations with a threshold θ greater than 0.4083 when creating the causal diagram. These relations can be seen in Table 5 in italic.
The relationship Ri + Dj represents the total influence that a criterion exerts and receives in relation to the others The relationship Ri − Dj represents the net influence that a criterion exerts on the set of criteria, that is, the causal relationship. Positive values indicate that the critical factor exerts more influence than it receives from other factors. If this value is negative, it indicates that it receives more influence than exerts on the others. Table 6 shows the degrees of prominence and net cause/effect.
These critical factors, listed according to their relative weight and order of classification, are EOL-MP > LPF > FEF > MF > PF > CF > IF> ITF >. Figure 2 shows the interrelationship map, and the relationships that were plotted on the graph were those previously highlighted in bold. In Table 4, the critical factors that exert greater influence were identified from the established threshold value, and it was possible to filter the effects that had irrelevant importance.
Results from Fig. 2 are discussed in the “Discussion” section.
Discussion
The grey-DEMATEL framework proposed in this study considered uncertainties and systematically organized the critical RL implementation factors into two cause and effect cluster groups, as shown in Table 5 and Fig. 2.
The first major cause is “financial and economic factor.” This finding is consistent with the previous studies conducted by Abdulrahman et al. (2014), Agrawal et al. (2016), Kumar and Dixit (2018), Mangla et al. (2016), and Sivakumar et al. (2018). This critical factor represents the economic aspects of RL implementation, including the level of economic resources required to make improvements to the pharmaceutical care information system. Aspects regarding the availability of financial resources for sorting out, collecting, and properly disposing of pharmaceutical waste were also surveyed and presented in Table 1. Considering the public environment of the PCP cycle, in which resources are scarce, this can be a major inhibitor, making it difficult to implement pharmaceutical RL. Despite the fact that there is a clear public policy for the distribution of resources in more than 5000 municipalities in Brazil, the funding of PCP is mainly directed towards forward logistics instead of RL. Moreover, unfortunately, public health management has been impacted by corruption and bureaucracy in Brazil. Apart from this local contextual description, in a study by Chauhan et al. (2018), the lack of funds was considered to exert the greatest influence in RL in the waste disposal industry. Furthermore, economic barriers were also described in Kaviani et al. (2020) as being of great importance.
The second major cause is the “management factor.” This critical factor represents the management collaboration with the RL initiative and activities, technical knowledge of the management in motivating and leading the employees in this initiative, and the ability and familiarity of the management with capacity building and training. The number of employees to perform the PCP direct flow is already limited, and the RL flow might be the target of outsourcing strategies. This finding is consistent with Prakash and Barua (2016), which found a lack of management coordination and collaboration as the most important barriers. It is also consistent with Abdulrahman et al. (2014), which found the lack of RL management expertise and commitment most impactful, and Agrawal et al. (2016), which found the top management awareness plays an important role in the implementation of RL.
The third major cause is the “policy factor.” This critical factor represents government policies, laws, and legislation on public health waste management, as well as the supervision of waste management practices by the municipal bodies. This finding is consistent with the studies by Kumar and Dixit (2018), Mangla et al. (2016), Sirisawat and Kiatcharoenpol (2018), and Abdulrahman et al. (2014), which revealed policy and regulatory barriers as the most important barriers to the implementation waste in electrical and electronic equipment management. The difficulties in implementing the sectoral agreement on pharmaceutical RL illustrate the importance of this barrier.
The fourth major cause is “information and technological factor.” This factor represents the information systems used in the pharmaceutical care for daily activities such as dispensing, relocation, registration of expired drugs, or registration of expired drugs delivered by patients in the public health system. This finding is consistent with the studies conducted by Kumar and Dixit (2018) and Sivakumar et al. (2018), which found the technological factors as one of the main causes of RL implementation.
Our study found two major effects. The first major effect is “EOL-MP.” This effect represents the daily actions of professionals in “sorting waste, monitoring the expiry date of drugs” and “stock supplies” and “the practices related to third parties hired to collect and properly dispose of the waste generated within the pharmaceutical health process.” The second major effect was the critical factor connected with logistic performance. This effect represents elements related to drug dispensation to the public health patients, drug relocation, when there are cases of excess in health facilities, inventory control, amount of expired medicines, and order delivery times are considered for this critical factor. As the RL has not been structured yet and, considering the current forward logistics, an entirely new group of management practices (EOL-MP) and logistic performance factors (LPF) would be necessary. The collaboration factor (CF), including information exchange and sharing, reliability, and cooperation among the parties involved, are frequently associated with ITF, as demonstrated by Paula et al. (2019), but was not revealed similarly in this study. The last major effect was infrastructure (IF). Considering the overall rankings presented in Table 6, CF > IF > ITF are the bottom-ranked factors.
Practical implications
The Brazilian case of pharmaceutical waste is extraordinary because of the availability of medicine through the public PCP network, a large number of pharmacies and drugstores, judicialization of healthcare, and self-medication. Despite these forms of access to medications, only local return programs run by private companies or institutions, called voluntary delivery points, are available to the public without a nationwide return program (Gracia-Vásquez et al. 2014; Pereira et al. 2012; Thach et al. 2013). In addition, there is a lack of awareness among the population and professionals about the impact of medication on the environment when disposed of incorrectly (Glassmeyer et al. 2009; Saravanan and Manoj 2016; Vellinga et al. 2014).
Bouzon et al. (2018) have performed an evaluation of the interrelationship between the barriers of implementation of RL considering three perspectives: governmental, organizational, and consumer. Using the grey-DEMATEL, the authors evaluated a set of 20 barriers and revealed that the stakeholders mentioned most of the main barriers. They have mentioned 10 of the 13 main barriers to the implementation of RL.
This research was developed in the medication dispensing sector of the public health network. The grey-DEMATEL method allowed the identification/prioritization of the factors considered to be the primary barriers to the implementation of RL in the public health sector. Our study presents important findings for managers, especially for pharmaceutical assistance coordination. Despite the fact this type of investigation is rarely conducted in the public health sector, the contribution of this study is specific to a sector not studied by Bouzon et al. (2018). It is important to mention that although interviewees were evaluating the interdependencies among the factors, each subfactor was clearly explained at the time of the interview to ensure the interviewees are completely familiar with the factors presented in Table 1.
Recently, the federal decree number 10,388 was approved in June 2020, regulating the environmentally acceptable disposal of drugs and packaging in the entire production and consumption chain (Brazil 2020). In this context, the RL of EOU/EOL medication policy should gain public support and put pressure on the PCP network management. These facts are synergic with the former concerns of health professionals about the relationship between the EOU/EOL medicine and the environment and the recent pandemic crisis worldwide. This is especially important in Brazil since health residues become potentially propagators source of virus transmission.
Another issue that is also related to drugs provided by the public health system is that the major concern is often with drug dispensation only. Pharmacists are often aware of the impact of drugs on the environment, but there is no structure in place for drug collection to become a reality. As a result, from the perspective of the interviewed public health sector experts, findings of this research may help managers to understand the critical factors that need to be addressed before RL implementation throughout the pharmaceutical health process.
Practicing managers should take into consideration the barriers identified in this study to create alternatives to bypass the financial and economic policies and management causes. Possible outsourcing of PCP RL is an example of a mitigating strategy. Nevertheless, the third party should be selected carefully, considering its ability to efficiently and effectively collect unwanted medicine. An adequate information technology infrastructure and performance indicators are prerequisites to the successful RL implementation in the PCP.
Concluding remarks
This study was conducted with the PCP cycle in the city of Porto Alegre, Brazil. The study used a grey-DEMATEL approach to consider a group of eight factors and identify the factors critical to the RL implementation in the PCP. The contribution of this study is twofold: (1) the results suggest how the PCP public health sector, which plays an important role in the Brazilian health system, can efficiently and effectively address and eliminate these critical factors to the successful implementation of RL in the PCP and (2) we have identified and prioritized the critical factors to RL implementation in the PCP by considering each step of the SPASDD cycle. Therefore, the municipal PCP coordinator may use this knowledge to formulate strategies and articulate actions to eliminate these barriers.
The scope of this investigation is limited to the understanding of the critical factors hindering RL implementation from the perspective of the public health sector experts. Encouraged by the growth of the pharmaceutical industry and the excessive drug consumption worldwide, future endeavors may include studying the problem from within the pharmaceutical industry, which supplies the PCP with medicines in a broader scope of the pharmaceutical supply chain. Other critical factors may arise from the complex relationship between the pharmaceutical industry and the government agencies in charge of public health. Subsequently, it is important to confirm the results by means of an analysis of the critical factors equipped with statistical validation empowered by structural equation modeling.
References
Abahussain E, Waheedi M, Koshy S (2012) Practice, awareness and opinion of pharmacists toward disposal of unwanted medications in Kuwait. Saudi Pharm J 20:195–201. https://doi.org/10.1016/j.jsps.2012.04.001
Abbas H, Farooquie JA (2013) Return and disposal of unused medicines: a customer perspective of reverse logistics. Int J Bus Manag Invent 2(11):2319–8028
Abbas H, Farooquie JA (2018) Reverse logistics operations in a pharmaceutical retail environment. Int J Logist Econ Glob 7:1–12
Abdullah N, Yaakub S (2014) Reverse logistics: pressure for adoption and the impact on firm’s performance. Int J Bus Soc 15(1):151–170
Abdulrahman MD, Gunasekaran A, Subramanian N (2014) Critical barriers in implementing reverse logistics in the Chinese manufacturing sectors. Int J Prod Econ 147:460–471. https://doi.org/10.1016/j.ijpe.2012.08.003
Agrawal S, Singh RK, Murtaza Q (2015) A literature review and perspectives in reverse logistics. Resour Conserv Recycl 97:76–92. https://doi.org/10.1016/j.resconrec.2015.02.009
Agrawal S, Singh RK, Murtaza Q (2016) Prioritizing critical success factors for reverselogistics implementation using fuzzy-TOPSIS methodology. J Ind Eng Int 12(1):15–27. https://doi.org/10.1007/s40092-015-0124-8
Akici A, Aydin V, Kiroglu A (2017) Assessment of the association between drug disposal practices and drug use and storage behaviors. Saudi Pharm J 26:7–13. https://doi.org/10.1016/j.jsps.2017.11.006
Ali C, Abdelsalam A (2017) Analyzing pharmaceutical reverse logistics barriers: an interpretive structural modeling approach. Bus Manag Rev 8(5):88–99. https://doi.org/10.4018/IJAL.2017010102
Ali SM, Arafin A, Moktadir MA, Rahman T, Zahan N (2018) Barriers to reverse logistics in the computer supply chain using interpretive structural model. Glob J Flex Syst Manag 19(1):53–68. https://doi.org/10.1007/s40171-017-0176-2
Al-Shareef F, El-Asrar SA, Al-Bakr L, Al-Amro M, Alqahtani F, Aleanizy F, Al-Rashood S (2016) Investigating the disposal of expired and unused medication in Riyadh. Saudi Arabia : a cross-sectional study International Journal of Clinical Pharmacy 38:822–828. https://doi.org/10.1007/s11096-016-0287-4
Aquino S, Spina GA, Antonietta M (2018) Reverse logistics of postconsumer medicines: the roles and knowledge of pharmacists in the municipality of São Paulo. Brazil Sustainability. https://doi.org/10.3390/su10114134
Badenhorst A (2016) Prioritising the implementation of practices to overcome operational barriers in reverse logistics. J Transport Supply Chain Manag. https://doi.org/10.4102/jtscm.v10i1.240
Bai C, Sarkis J (2013) A grey-based DEMATEL model for evaluating business process management critical success factors. Int J Prod Econ 146(1):281–292. https://doi.org/10.1016/j.ijpe.2013.07.011
Bashaar M, Thawani V, Hassali MA, Saleem F, (2017). Disposal practices of unused and expired pharmaceuticals among general public in Kabul 1–8. https://doi.org/10.1186/s12889-016-3975-z
Biernacki P, Waldorf DAN (1981) Snowball sampling problems and techniques of chain referral sampling. Sociol Methods Res 10(2):141–163
Bouzon M, Govindan K, Rodriguez CMT, Campos LMS (2016) Identification and analysis of reverse logistics barriers using fuzzy Delphi method and AHP. Resour Conserv Recycl 108:182–197. https://doi.org/10.1016/j.resconrec.2015.05.021
Bouzon M, Govindan K, Taboada CM (2018) Evaluating barriers for reverse logistics implementation under a multiple stakeholders’ perspective analysis using grey decision making approach. Resour Conserv Recycl 128:315–335. https://doi.org/10.1016/j.resconrec.2016.11.022
Bouzon M, Govindan K, Taboada Rodriguez CM (2020) Grey DEMATEL technique for evaluating product return drivers: a multiple stakeholders’perspective. Environ Eng Manag J 19:19–36
Brazil (2001) Ministry of Health. Health Policy Secretariat. Department of Primary Care Pharmaceutical Assistance: technical instructions for your organization. Ministry of Health, Brasilia Available at: http://bvsms.saude.gov.br/bvs/publicacoes/cd03_15.pdf. (Accessed March 2020)
Brazil (2020) Diário Oficial da União. Decreto N° 10,388 de 5 de Junho 843 de 2020. Regulamenta o § 1° do caput do art. 33 da Lei n° 12.305, de 844 2 de agosto de 2010, e institui o sistema de logística reversa de 845 medicamentos. Available at: https://www.planalto.gov.br/ccivil_03/_ato2019-2022/2020/decreto/d10388.htm. Accessed Sept 2020
Brazilian Ministry of Health (2019). Sistema Único de Saúde (SUS): estrutura, princípios e como funciona. Available at: https://www.saude.gov.br/sistema-unico-de-saude. (Accessed March 2020).
Brazilian Policy of Solid Waste, (2010). Law 12,305, August 2nd 2010. Brazil. Available at: http://www.planalto.gov.br/ccivil_03/_ato2007-2010/2010/lei/l12305.htm . (Accessed September 2018)
Bungau S, Tit Mirela D, Fodor K, Cioca G, Agop M, Iovan C, … Bustea, C. (2018). Aspects regarding the pharmaceutical waste management in Romania, 1–14. https://doi.org/10.3390/su10082788
Campos EAR, Paula IC, Pagani RN, Guarnieri P (2017) Reverse logistics for the end-of-life and end-of-use products in the pharmaceutical industry: a systematic literature review. Supply Chain Manag 22(4):375–392. https://doi.org/10.1108/SCM-01-2017-0040
Chan HK (2007) A pro-active and collaborative approach to reverse logistics - a case study. Prod Plan Control 18:350–360. https://doi.org/10.1080/09537280701318736
Chan FTS, Chan HK (2008) A survey on reverse logistics system of mobile phone industry in Hong Kong. Manag Decis 46:702–708. https://doi.org/10.1108/00251740810873464
Chauhan A, Singh A, Jharkharia S (2018) An interpretive structural modeling (ISM) and decision-making trail and evaluation laboratory (DEMATEL) method approach for the analysis of barriers of waste recycling in India. J Air Waste Manag Assoc 68(2):100–110. https://doi.org/10.1080/10962247.2016.1249441
Chileshe N, Rameezdeen R, Lehmann S, Hosseini MR (2014) Reverse logistics (RL) implementation among contractors in Australia: practices and barriers. 30th Annu. Assoc. Res. Constr. Manag. Conf. ARCOM 2014:83–92
Chileshe N, Rameezdeen R, Hosseini MR (2015) Barriers to implementing reverse logistics in South Australian construction organisations. Supply Chain Manag 20:179–204. https://doi.org/10.1108/SCM-10-2014-0325
Chileshe N, Rameezdeen R, Hosseini MR, Lehmann S, Udeaja C (2016) Analysis of reverse logistics implementation practices by South Australian construction organisations. Int J Oper Prod Manag 36:332–356. https://doi.org/10.1108/IJOPM-01-2014-0024
Chiou CY, Chen HC, Yu CT, Yeh CY (2012) Consideration factors of reverse logistics implementation - a case study of Taiwan’s electronics industry. Procedia Soc Behav Sci 40:375–381. https://doi.org/10.1016/j.sbspro.2012.03.203
Cline A, LeMay S, Helms MM (2015) A framework for reverse logistics: the case of post-consumer carpet in the US. Int J Commer Manag 25:466–489. https://doi.org/10.1108/IJCoMA-02-2013-0013
Cole R, Lindsay CF, Barker F (2018) Reverse exchange of healthcare devices: the case of hearing aid equipment in the UK. Prod Plan Control 29:1045–1057. 0, 1–13. https://doi.org/10.1080/09537287.2018.1506892
Deng J-L (1982) Control problems of grey systems. Syst Control Lett 1(5):288–294. https://doi.org/10.1016/S0167-6911(82)80025-X
Diabat A, Govindan K (2011) An analysis of the drivers affecting the implementation of green supply chain management. Resour Conserv Recycl 55:659–667. https://doi.org/10.1016/j.resconrec.2010.12.002
Diabat A, Khreishah A, Kannan G, Panikar V, Gunasekaran A (2013) Benchmarking the interactions among barriers in third-party logistics implementation: an ISM approach. Benchmarking 20:805–824. https://doi.org/10.1108/BIJ-04-2013-0039
Drohomeretski E, Da Costa SG, De Lima EP (2014) Green supply chain management: drivers, barriers and practices within the Brazilian automotive industry. J Manuf Technol Manag 25:1105–1134. https://doi.org/10.1108/JMTM-06-2014-0084
El Baz J, Frei R, Laguir I (2018) Reverse supply chain practices in developing countries: the case of Morocco. J Manuf Technol Manag 29:198–216. https://doi.org/10.1108/JMTM-04-2017-0068
Erol I, Velioǧlu MN, Şerifoǧlu FS, Büyüközkan G, Aras N, Çakar ND, Korugan A (2010) Exploring reverse supply chain management practices in Turkey. Supply Chain Manag 15:43–54. https://doi.org/10.1108/13598541011018111
Gabus A, Fontela E (1972) World problems, an invitation to further thought within the framework of DEMATEL. Battelle Geneva Research Center, Geneva
Glassmeyer ST, Hinchey EK, Boehme SE, Daughton CG, Ruhoy IS, Conerly O, Daniels RL, Lauer L, McCarthy M, Nettesheim TG, Sykes K, Thompson VG (2009) Disposal practices for unwanted residential medications in the United States. Environ Int 35(3):566–572. https://doi.org/10.1016/j.envint.2008.10.007
González‐Torre P, Álvarez M, Sarkis J, Adenso‐Díaz D et al (2010) Barriers to the implementation of environmentally oriented reverse logistics: evidence from the automotive industry sector. Br J Manag. https://doi.org/10.1111/j.1467-8551.2009.00655.x
Govindan K, Bouzon M (2018) From a literature review to a multi-perspective framework for reverse logistics barriers and drivers. J Clean Prod 187:318–337. https://doi.org/10.1016/j.jclepro.2018.03.040
Govindan K, Madan Shankar K, Kannan D (2016) Application of fuzzy analytic network process for barrier evaluation in automotive parts remanufacturing towards cleaner production - a study in an Indian scenario. J Clean Prod 114:199–213. https://doi.org/10.1016/j.jclepro.2015.06.092
Gracia-Vásquez SL, Ramírez-Lara E, Camacho-Mora IA, Cantú-Cárdenas LG, Gracia-Vásquez YA, Esquivel-Ferriño PC, Ramírez-Cabrera MA, Gonzalez-Barranco P (2014) An analysis of unused and expired medications in Mexican households. Int J Clin Pharm 37(1):121–126. https://doi.org/10.1007/s11096-014-0048-1
Guarnieri P, Cerqueira-streit JA, Batista LC (2020) Reverse logistics and the sectoral agreement of packaging industry in Brazil towards a transition to circular economy. Resour Conserv Recycl 153(October 2019):104541. https://doi.org/10.1016/j.resconrec.2019.104541
Guirguis K (2010) Medications collected for disposal by outreach pharmacists in Australia. Pharm World Sci 32(1):52–58. https://doi.org/10.1007/s11096-009-9340-x
Jabbour CJC, De Sousa Jabbour ABL, Govindan K, De Freitas TP, Soubihia DF, Kannan D, Latan H (2016) Barriers to the adoption of green operational practices at Brazilian companies: effects on green and operational performance. Int J Prod Res 54:3042–3058. https://doi.org/10.1080/00207543.2016.1154997
Jindal A, Sangwan KS (2011) Development of an interpretive structural model of barriers to reverse logistics implementation in Indian industry. Int J Bus Perform Supply Chain Model. 5:448–453. https://doi.org/10.1007/978-3-642-19692-8
Jindal A, Sangwan KS (2013) Development of an interpretive structural model of drivers for reverse logistics implementation in Indian industry. Int J Bus Perform Supply Chain Model 5(4):325–342. https://doi.org/10.1504/IJBPSCM.2013.058201
Kapetanopoulou P, Tagaras G (2011) Drivers and obstacles of product recovery activities in the Greek industry. Int J Oper Prod Manag 31:148–166. https://doi.org/10.1108/01443571111104746
Kaviani MA, Tavana M, Kumar A, Michnik J, Niknam R, Campos EAR (2020) An integrated framework for evaluating the barriers to successful implementation of reverse logistics in the automotive industry. J Clean Prod 272:122714. https://doi.org/10.1016/j.jclepro.2020.122714
Kelly F, Mcmillan S, Spinks J, Bettington E, Wheeler AJ (2018) ‘You don’t throw these things out :’ an exploration of medicines retention and disposal practices in Australian homes. BMC Public Health 18(1026):1–12
Khan A, Subzwari M (2009) Reverse logistics in Pakistan’s pharmaceutical sector. South Asian J Manag Sci 3:27–36
Kinrys G, Gold AK, Nierenberg AA, Nierenberg AA (2016) Proper drug disposal: studying a solution to household prescription and over-the- counter drug abuse. J Drug Abus 2:1–7. https://doi.org/10.21767/2471-853X.100027
Kissling R, Coughlan D, Fitzpatrick C, Boeni H, Luepschen C, Andrew S, Dickenson J (2013) Success factors and barriers in re-use of electrical and electronic equipment. Resour Conserv Recycl 80:21–31. https://doi.org/10.1016/j.resconrec.2013.07.009
Kongar E, Haznedaroglu E, Abdelghany O, Bahtiyar MO (2015) A novel IT infrastructure for reverse logistics operations of end-of-life pharmaceutical products. Inf Technol Manag 16:51–65. https://doi.org/10.1007/s10799-014-0195-z
Kumar A, Dixit G (2018) Evaluating critical barriers to implementation of WEEE management using DEMATEL approach. Resour Conserv Recycl 131:101–121. https://doi.org/10.1016/j.resconrec.2017.12.024
Laribi L, Dhouib D, (2015). Barriers to implementing reverse logistics in Tunisian companies. 10th IEEE Int. Conf. Serv. Oper. Logist. Informatics, SOLI 2015 - conjunction with ICT4ALL 2015 145–153. https://doi.org/10.1109/SOLI.2015.7367609
Lau KH, Wang Y (2009) Reverse logistics in the electronic industry of China: a case study. Supply Chain Manag 14:447–465. https://doi.org/10.1108/13598540910995228
Liu X, Tanaka M, Matsui Y (2006) Electrical and electronic waste management in China: progress and the barriers to overcome. Waste Manag Res 24:92–101. https://doi.org/10.1177/0734242X06062499
Luthra S, Kumar V, Kumar S, Haleem A (2011) Barriers to implement green supply chain management in automobile industry using interpretive structural modeling technique - an Indian perspective. J Ind Eng Manag 4:231–257. https://doi.org/10.3926/jiem.2011.v4n2.p231-257
Mangla SK, Govindan K, Luthra S (2016) Critical success factors for reverse logistics in Indian industries: a structural model. J Clean Prod 129:608–621. https://doi.org/10.1016/j.jclepro.2016.03.124
Manojlović J, Jovanović V, Georgiev AM, Tesink JG, Arsić T, Marinković V (2015) Pharmaceutical waste management in pharmacies at the primary level of health care in Serbia situation analysis. Indian J Pharm Educ Res 49(2):106–111. https://doi.org/10.5530/ijper.49.2.5
Massoud MA, Fayad R, El-Fadel M, Kamleh R (2010) Drivers, barriers and incentives to implementing environmental management systems in the food industry: a case of Lebanon. J Clean Prod 18:200–209. https://doi.org/10.1016/j.jclepro.2009.09.022
Massoud MA, Makarem N, Ramadan W, Nakkash R (2015) Environmental management practices in the Lebanese pharmaceutical industries: implementation strategies and challenges. Environ Monit Assess 187:107. https://doi.org/10.1007/s10661-015-4290-3
Massoud MA, Chami G, Al-Hindi M, Alameddine I (2016) Assessment of household disposal of pharmaceuticals in Lebanon: Management options to protect water quality and public health. Environ Manag 57(5):1125–1137. https://doi.org/10.1007/s00267-016-0666-6
Mathiyazhagan K, Govindan K, NoorulHaq A, Geng Y (2013) An ISM approach for the barrier analysis in implementing green supply chain management. J Clean Prod 47:283–297. https://doi.org/10.1016/j.jclepro.2012.10.042
Mathiyazhagan K, Govindan K, Noorul Haq A (2014) Pressure analysis for green supply chain management implementation in Indian industries using analytic hierarchy process. Int J Prod Res 52(1):188–202
Medeiros MSG, Moreira LMF, Lopes CCGO (2014) Descarte de medicamentos: Programas de recolhimento e novos desafios. Revista de Ciencias Farmaceuticas Basica e Aplicada 35(4):651–662
Mendes EV (2011) As redes de atenção à saúde, 2ed edn. Organização Pan-Americana da Saúde, Brasília
Meyer A, Niemann W, Mackenzie J, Lombaard J (2017) Drivers and barriers of reverse logistics practices: a study of large grocery retailers in South Africa. J Transp Supply Chain Manag 11:1–16. https://doi.org/10.4102/jtscm.v11i0.323
Moktadir A, Mithun S, Rajesh R, Kumar S (2018) Modeling the interrelationships among barriers to sustainable supply chain management in leather industry. J Clean Prod 181:631–651. https://doi.org/10.1016/j.jclepro.2018.01.245
Muduli K, Govindan K, Barve A, Geng Y (2013a) Barriers to green supply chain management in Indian mining industries: a graph theoretic approach. J Clean Prod 47:335–344. https://doi.org/10.1016/j.jclepro.2012.10.030
Muduli K, Govindan K, Barve A, Kannan D, Geng Y (2013b) Role of behavioural factors in green supply chain management implementation in Indian mining industries. Resour Conserv Recycl 76:50–60. https://doi.org/10.1016/j.resconrec.2013.03.006
Narayana SA, Elias AA, Pati RK (2014) Reverse logistics in the pharmaceuticals industry: a systemic analysis. Int J Logist Manag 25:379–398. https://doi.org/10.1108/IJLM-08-2012-0073
Ngwuluka NC, Ochekpe NC, Odumosu PO (2011) An assessment of pharmaceutical waste management in some Nigerian pharmaceutical industries. Afr J Biotechnol 10:11259–11268. https://doi.org/10.5897/AJB10.1194
Paula IC, Campos EAR, Pagani RN, Guarnieri P, Kaviani MA (2019) Are collaboration and trust sources for innovation in the reverse logistics? Insights from a systematic literature review. Supply Chain Manag: An International Journal 25:176–222. https://doi.org/10.1108/SCM-03-2018-0129
Pereira AL, Silva JTM, Teixeira LAA (2012) Healthcare waste reverse logistics: a case study of Brazilian public hospitals. Int Bus Manag 6(2):95–98. https://doi.org/10.3923/ibm.2012.95.98
Pereira AL, de Vasconcelos Barros RT, Pereira SR (2017) Pharmacopollution and Household Waste Medicine (HWM): how reverse logistics is environmentally important to Brazil. Environ Sci Pollut Res 24(31):24061–24075. https://doi.org/10.1007/s11356-017-0097-9
Pérez-Belis V, Bovea MD, Ibáñez-Forés V (2015) An in-depth literature review of the waste electrical and electronic equipment context: trends and evolution. Waste Manag Res 33:3–29. https://doi.org/10.1177/0734242X14557382
Pinto GMF, da Silva KR, de Pereira RFAB, Sampaio SI (2014) Estudo do descarte residencial de medicamentos vencidos na região de Paulínia (SP), Brasil. Engenharia Sanitaria e Ambiental 19(3):219–224. https://doi.org/10.1590/S1413-41522014019000000472
Prahinski C, Kocabasoglu C (2006) Empirical research opportunities in reverse supply chains. Omega 34(6):519–532
Prajapati H, Kant R, Shankar R (2019) Prioritizing the solutions of reverse logistics implementation to mitigate its barriers: a hybrid modified SWARA and WASPAS approach. J Clean Prod 240:118219. https://doi.org/10.1016/j.jclepro.2019.118219
Prakash C, Barua MK (2016) A multi-criteria decision-making approach for prioritizing reverse logistics adoption barriers under fuzzy environment: case of Indian electronics industry. Glob Bus Rev 17(5):1107–1124. https://doi.org/10.1177/0972150916656667
Prakash C, Barua MK, Pandya KV (2015) Barriers analysis for reverse logistics Implementation in Indian electronics industry using fuzzy analytic hierarchy process. Procedia Soc Behav Sci 189:91–102. https://doi.org/10.1016/j.sbspro.2015.03.203
Pumpinyo S, Nitivattananon V (2014) Investigation of barriers and factors affecting the reverse logistics of waste management practice: a case study in Thailand. Sustain. 6:7048–7062. https://doi.org/10.3390/su6107048
Rahman S, Subramanian N (2012) Factors for implementing end-of-life computer recycling operations in reverse supply chains. Int J Prod Econ 140(1):239–248. https://doi.org/10.1016/j.ijpe.2011.07.019
Rameezdeen R, Chileshe N, Hosseini MR, Lehmann S (2016) A qualitative examination of major barriers in implementation of reverse logistics within the South Australian construction sector. Int J Constr Manag 16:185–196. https://doi.org/10.1080/15623599.2015.1110275
Ranjan R, Chatterjee P, Chakraborty S (2016) Performance evaluation of Indian railway zones using DEMATEL and VIKOR methods. Benchmarking: An International Journal 23(1):78–95
Ravi V, Shankar R, Raci V, Shankar R (2005) Analysis of interactions among the barriers of reverse logistics. Technol Forecast Soc Change 72:1011–1029. https://doi.org/10.1016/j.techfore.2004.07.002
Rogers DS, Tibben-lembke R (2001) An examination of reverse logistitics practices. J Bus Logist 22:129–148. https://doi.org/10.1002/j.2158-1592.2001.tb00158.x
Saravanan S, Manoj TM (2016) Reverse logistic disposal practices of household pharmaceutical medicines and its impact on environment in Trichy, Tamilnadu, India. Int Res J Pharm 7(6):66–70. https://doi.org/10.7897/2230-8407.07666
Sasu S, Kümmerer K, Kranert M (2012) Assessment of pharmaceutical waste management at selected hospitals and homes in Ghana. Waste Manag Res 30:625–630. https://doi.org/10.1177/0734242X11423286
Scavarda A, Daú GL, Scavarda LF, Korzenowski AL (2019) A proposed healthcare supply chain management framework in the emerging economies with the sustainable lenses: the theory, the practice, and the policy. Resour Conserv Recycl 141(November 2018):418–430. https://doi.org/10.1016/j.resconrec.2018.10.027
Shaharudin MR, Zailani S, Tan KC (2015) Barriers to product returns and recovery management in a developing country: investigation using multiple methods. J Clean Prod 96:220–232. https://doi.org/10.1016/j.jclepro.2013.12.071
Shaik MN, Abdul-Kader W (2014) Comprehensive performance measurement and causal-effect decision making model for reverse logistics enterprise. Comput Ind Eng 68(1):87–103. https://doi.org/10.1016/j.cie.2013.12.008
Sharma SK, Panda BN, Mahapatra SS, Sahu S (2011) Analysis of barriers for reverse logistics an Indian perspective. Int J Model Opt 1(2):101–106
Sirisawat P, Kiatcharoenpol T (2018) Fuzzy AHP-TOPSIS approaches to prioritizing solutions for reverse logistics barriers. Comput Ind Eng 117(April 2017):303–318. https://doi.org/10.1016/j.cie.2018.01.015
Sivakumar K, Jeyapaul R, Vimal KEK, Ravi P (2018) A DEMATEL approach for evaluating barriers for sustainable end-of-life practices. J Manuf Technol Manag. https://doi.org/10.1108/JMTM-08-2017-0164
Škapa R (2011) Reverse logistics in the Czech Republic: barriers to development. Acta Univ Agric Silvic Mendelianae Brun LIX 59:363–370
Starostka-Patyk M, Zawada M, Pabian A, Abed M (2013) Barriers to reverse logistics implementation in enterprises. 2013 Int. Conf. Adv. Logist. Transp. ICALT 2013:506–511. https://doi.org/10.1109/ICAdLT.2013.6568510
Thach AV, Brown CM, Pope N (2013) Consumer perceptions about a community pharmacy-based medication take back program. J Environ Manag 127:23–27. https://doi.org/10.1016/j.jenvman.2013.04.025
Tingley DD, Cooper S, Cullen J (2017) Understanding and overcoming the barriers to structural steel reuse, a UK perspective. J Clean Prod 148:642–652. https://doi.org/10.1016/j.jclepro.2017.02.006
Tong AYC, Peake BM, Braund R (2011) Disposal practices for unused medications around the world. Environ Int 37:292–298. https://doi.org/10.1016/j.envint.2010.10.002
Tzeng GH, Chiang CH, Li CW (2007) Evaluating intertwined effects in e-learning programs: a novel hybrid MCDM model based on factor analysis and DEMATEL. Expert Syst Appl 32(4):1028–1044. https://doi.org/10.1016/j.eswa.2006.02.004
Van Der Wiel A, Bossink B, Masurel E (2012) Reverse logistics for waste reduction in cradle-to-cradle-oriented firms: waste management strategies in the Dutch metal industry. Int J Technol Manag 60(1/2):96. https://doi.org/10.1504/IJTM.2012.049108
Vellinga A, Cormican S, Driscoll J, Furey M, O’Sullivan M, Cormican M (2014) Public practice regarding disposal of unused medicines in Ireland. Sci Total Environ 478:98–102. https://doi.org/10.1016/j.scitotenv.2014.01.085
Viegas CV, Bond A, Vaz CR, Bertolo RJ (2019) Reverse flows within the pharmaceutical supply chain: a classificatory review from the perspective of end-of-use and end-of-life medicines. J Clean Prod 238:117719. https://doi.org/10.1016/j.jclepro.2019.117719
Waqas M, Dong Q, Ahmad N, Zhu Y (2018) Critical barriers to implementation of reverse logistics in the manufacturing industry: a case study of a developing country. Sustainability 10(November):1–25. https://doi.org/10.3390/su10114202
Wieczorkiewicz SM, Kassamali Z, Danziger LH (2013) Behind closed doors: medication storage and disposal in the home. Ann Pharmacother 47:482–489. https://doi.org/10.1345/aph.1R706
Wohlin C (2014). Guidelines for snowballing in systematic literature studies and a replication in software engineering. In Proceedings of the 18th international conference on evaluation and assessment in software engineering (pp. 1-10).
Xia X, Govindan K, Zhu Q (2015) Analyzing internal barriers for automotive parts remanufacturers in China using grey-DEMATEL approach. J Clean Prod 87:811–825. https://doi.org/10.1016/j.jclepro.2014.09.044
Xie Y, Breen L, Cherrett T, Zheng D, Allen CJ (2016) An exploratory study of reverse exchange systems used for medical devices in the UK National Health Service (NHS). Supply Chain Manag 21:194–215. https://doi.org/10.1108/SCM-07-2015-0278
Yang Y, John R (2003). Grey systems and interval valued fuzzy sets. EUSFLAT Conf. p.193-197.
Yang C, Doshi M, Mason N (2015) Analysis of medications returned during a medication Take-back event. Pharmacy 3:79–88. https://doi.org/10.3390/pharmacy3030079
Yazdani M, Tavana M, Pamučar D, Chatterjee P (2020) A rough based multi-criteria evaluation method for healthcare waste disposal location decisions. Comput Ind Eng 143:106394
Zare H, Tavana M, Mardani A, Masoudian S, Saraji MK (2019) A hybrid data envelopment analysis and game theory model for performance measurement in healthcare. Health Care Manag Sci 22(3):475–488. https://doi.org/10.1007/s10729-018-9456-4
Zhu Q, Sarkis J, Geng Y (2011) Barriers to environmentally-friendly clothing production among Chinese apparel companies. Asian Bus Manag 10(3):425–452. https://doi.org/10.1057/abm.2011.15
Availability of data and materials
Not applicable
Funding
This research was funded by the Brazilian Ministry of Education - Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) process no. 88882.346482/2019-01. Dr. Madjid Tavana is grateful for the partial support he received from the Czech Science Foundation (GAˇCR19-13946S) for this research.
Author information
Authors and Affiliations
Contributions
Elaine Aparecida Regiani de Campos (conceptualization, formal analysis, methodology, and writing—original Draft); Madjid Tavana (methodology, formal analysis, project administration, supervision, visualization, and writing—review and editing); Carla Schwengber ten Caten (supervision, conceptualization, review, investigation, and resources); Marina Bouzon (review, resources, and software); and Istefani Carísio de Paula (review, investigation, and software).
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethics approval
This study was approved by the Research Ethics Committee, the Federal University of Rio Grande do Sul, and Municipal Health Department under number CAAE 82571018.6.0000.5347, and opinion no. 3.272.380. It was conducted in the pharmaceutical care process (PCP) cycle that is under the Pharmaceutical Assistance Coordination (PAC) in the city of Porto Alegre, Brazil.
Consent to participate
Not applicable
Consent for publication
Not applicable
Additional information
Responsible editor: Eyup Dogan
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Rights and permissions
About this article
Cite this article
de Campos, E.A.R., Tavana, M., ten Caten, C.S. et al. A grey-DEMATEL approach for analyzing factors critical to the implementation of reverse logistics in the pharmaceutical care process. Environ Sci Pollut Res 28, 14156–14176 (2021). https://doi.org/10.1007/s11356-020-11138-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-11138-8